Abstract
Promoter DNA melting, culminating in the loading of the single-stranded DNA template into the RNA polymerase active site, is a key step in transcription initiation. Recently, the first transcription inhibitors found to block distinct steps of promoter melting were characterized. Here, the impact of these studies is discussed with respect to the current models of transcription initiation.
Key words: σ subunit, switch-2 region, transcription inhibitors, promoter melting, lipiarmycin, myxopyronin
Bacterial RNA polymerase (RNAP) is a complex molecular machine, composed of the catalytic core (subunits 2αββ'ω) and one of the promoter-specific σ factors directing promoter recognition and melting. Transcription initiation, governed by the interplay between a panoply of promoter sequences and a number of σ factors, is modulated by numerous transcriptional activators, repressors and small regulatory molecules. Such a network provides the basis for the fine-tuning of bacterial gene expression. Understanding transcriptional regulation requires the characterization of the intermediates and checkpoints in the initiation pathway leading to a transcriptionally active RNAP-promoter complex, generically referred to as RPo. The antibiotics lipiarmycin (Lpm) and myxopyronin (Myx), which target two distinct steps in forming RPo,1–3 open up new perspectives for fundamental studies of transcriptional regulation and for medical research to identify new drug target sites.
Three Steps to the Open Promoter Complex
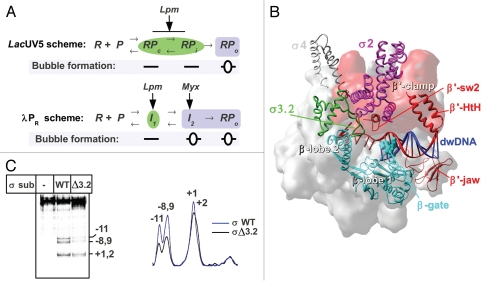
Transcription initiation starts with the reversible promoter binding that results in the formation of the “closed complex” (RPc or I1), containing straight doublestranded DNA (Fig. 1A). The closed complex isomerizes to form the transcriptionally competent “open complex” (RPo), in which the promoter DNA is bent ∼90° and ∼13 bp of DNA (positions −11 to +2) around the transcription start site are melted to form a transcription bubble. The formation of the transcription bubble starts from the nucleation of melting at the −10 element, followed by the downstream propagation toward position +1.4–6 The slow isomerization from RPc to RPo involves several intermediate complexes (RPi or I2) and includes large scale conformational changes in RNAP.7–10 Kinetic studies performed primarily using the two promoters lacUV5 and λPR indicate that there is a general three-step scheme for open complex formation (Fig. 1A). It should be kept in mind that the structures of the kinetically significant intermediates for these two schemes are different: for the lacUV5 promoter, the RPi is “closed” and stable,4 whereas the RPi complex for the λPR promoter is “open” and unstable.10
Figure 1.
(A) Kinetics scheme of RPo formation on the lacUV5,7 and λPR promoters.9 R: RNAP, P: promoter. Complexes with open DNA marked by blue boxes; closed complexes marked by green ellipses. (B) Structural model of RNAP in complex with dwDNA fragment.15 The structure of T. thermophilus RNAP12 is shown as a molecular surface colored in gray. The β lobes (in cyan), β' switch-2 (sw2) (Ser602-Lys621), β' jaw (Arg1266-Gly1328) and β' B-helix-turn-helix (HtH) (Leu469-Pro506)18 are shown as ribbons, the β-gate loop (Arg243-Pro248)12 is shown in CPK. Numbering corresponds to T. thermophilus. The β' clamp part of the RNAP surface is colored in red. DNA is shown in red (template) and blue (non-template). The σ subunit is shown as ribbons with the structural domains 2, 3 and 4 in magenta, green and gray, respectively. (C) Influence of the σ3.2HL deletion on lacUV5 promoter melting. KMnO4 probing of the open complexes formed between end-labeled lacUV5 promoter and the RNAP carrying either wild type σ70 or σ70 with deletion of the region 3.2 hairpin loop (Asp513-Leu519).3 Positions of the template strand thymines reactive to KMnO4 are indicated. The scan of the gel is shown on the right.
Role of the RNAP Clamp in Loading of Template into the Active Site
X-ray studies of multi-subunit RNAPs provide a framework for understanding the transcription mechanics. The structure of bacterial RNAP resembles a crab claw, with the pincers (or jaws) formed by the clamp domain (primarily the β' subunit) and β subunit lobes11–13 or the rope-swing region (T. thermophilus βAla132-Ser387),14 (Fig. 1B). The active site marked by the magnesium ion is deeply buried in the cleft between the pincers. The clamp domain is linked to the core by the five switch regions: β' switch-1, -2 and -5 and β switch-3 and -4.11 The β' clamp serves as a docking site for the σ subunit. The weakly conserved region 3.2 of σ forms an unfolded linker between the σ promoter recognition regions 2 and 4 and fills the RNA exit channel in the holoenzyme.13 It is likely that the σ region 3.2 hairpin loop (σ3.2HL, E. coli σ70 residues Glu508-His519) can function as a sensor of RNA length that triggers the initiation-to-elongation transition as soon as 10–11 nucleotides of RNA have been synthesized, but the mechanism of this transition is not yet understood.
To form the catalytically competent open complex, the +1 base of the single-stranded template DNA of the transcription bubble must be placed in the active site cleft, whereas the ∼15 bp DNA duplex downstream of the start site (dwDNA) should be locked by the pincers in the downstream channel.15 The β' switch-2 element contacts the template at positions −2 and −3 and assists in positioning the template in the RNAP active site.11,16 Additional elements of the β' clamp (B-helix-turn-helix and “jaw,” Fig. 1B) form a network of DNA-protein contacts that stabilizes the promoter and elongation complexes.15,17–19
However, in the holoenzyme, the active site cleft is too narrow (14–17 Å) to allow the entry of the DNA duplex (∼22 Å), but it can accommodate single-stranded DNA.1,13 Access to the active site is blocked by the “β-gate loop”12 and σ subunit region 1.1 (Fig. 2B). To bypass this barrier, several scenarios are possible: (1) the cleft opens (clamp or lobes swinging), (2) the DNA melts or (3) σ is displaced. In support of the “clamp swinging” mechanism, the comparison of bacterial and eukaryotic RNAP structures has revealed different positions of the clamp.11,13 Recently, an alternative model was suggested, in which the β subunit lobes must be transiently displaced to allow the dwDNA to enter to the dwDNA binding channel while the clamp remains static.14 Additionally, replacement of the region 1.1 by the dwDNA takes place during isomerization from RPi to RPo.20
Figure 2.
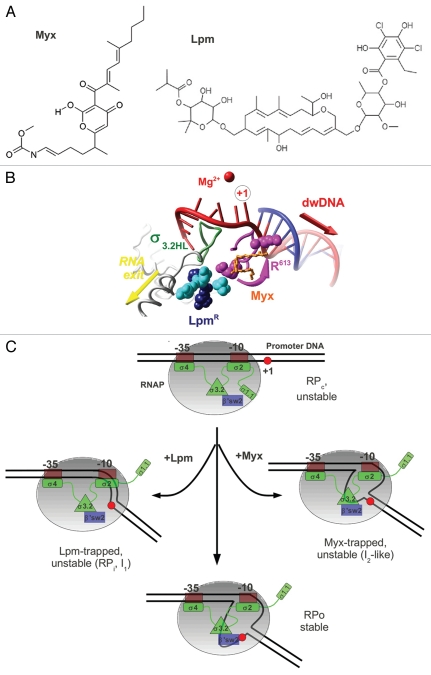
(A) Chemical structures of Myx and Lpm. (B) Overlap of the Lpm and Myx binding sites. Amino acids substitutions conferring resistance to Lpm27 are shown in CPK colored in cyan for β subunit (T. thermophilus Q1018, V1087, N1064) and magenta (R613) or dark blue (R87, P526) for β' subunit, σ3.2HL is shown as green ribbons and Myx is shown in ball-and-stick and orange. R613 is shown in two conformations—as in holoenzyme and as in the RNAP-Myx complex.1 (C) Model of the mechanism of Lpm and Myx action. RNAP core is shown as a gray ellipse. The σ subunit is shown in green, region 3.2 is shown as a green triangle, and the β' switch-2 is shown as a blue rectangle. The +1 base of the promoter is indicated by a red circle.
The “Bend-Load-Open” and “Open-Bend-Load” Models of RPo Formation
Two models have been proposed to explain the mechanism of template DNA loading in the RNAP active site cleft. In the first model, referred to as “bend-load-open,” the downstream part of the promoter (positions from −5 to +25) is bent over the σ region 2 and then loaded to the active site cleft before the formation of the fully open transcription bubble.10 The interaction of the RNAP clamp with dwDNA promotes the conformational transition of the core RNAP and the melting of the transcription start site within the active site cleft.8 This model is supported by the footprinting and cross-linking studies of the putative intermediate complexes formed at low temperature on the lacUV5, λPR, T7A1 and groE promoters.4,21–23 Whereas the equivalence of the “temperature-trapped” complexes to the real transcription intermediates remains in question, the real-time footprinting studies on λPR showed that the contacts with dwDNA are formed in closed intermediate RPc before the bubble opening occurs in RPi.10,24
The second model, referred to as “open-bend-load,” is based on real-time kinetic measurements on the T7A1 promoter5 and on the −10/−35 synthetic consensus promoter.6 In this model, melting precedes (or is even required for) the entry of the downstream DNA segment into the RNAP jaws. Computer-based Brownian dynamics simulations of the RPc→RPo transition have also suggested that DNA melts before entering the cleft.14 The apparent contradiction between the above models may indicate that the exact sequence of the events depends on the promoter type.5
σ3.2HL Forms a Single Functional Module with the β' Switch-2 Element
Several studies have suggested that the β lobe and the β' clamp play an active role in bubble propagation.25 Indeed, deletions in the β subunit lobe,26 or mutations in the β' switch-2,1,16 block the propagation of melting toward the transcription start site. Thus, RNAP acts as a helicase during the isomerization from RPc to RPo, unwinding the promoter DNA that was pre-melted by the σ subunit. The β' switch-2 contacts σ3.2HL, which likely forms a single functional module implicated in the RPo formation and template fitting at the active site. Accordingly, the substitutions in the E. coli RNAP β' switch-2 or the deletion of σ3.2HL, increase the Km for initiating nucleotides.16 Additionally, the deletion of σ3.2HL changes the relative KMnO4 sensitivity of the thymines within the lacUV5 promoter transcription bubble (Fig. 1C). Thus, even if σ3.2HL is not essential for melting, it can affect the template fitting at the active site.
β' Switch-2 and σ Region 3.2 are the Targets for Transcription Inhibitors
Transcription inhibitors are valuable tools to study the molecular mechanism of transcription. Most known inhibitors target the RNAP active site functions, and no inhibitors acting at the promoter complex formation step were known until recently. The first class of such inhibitors to be characterized was the β' switch-2-targeting antibiotics, produced by myxobacteria: Myx from Myxococcus fulvus, corallopyronin A from Corallococcus coralloides and ripostatin A from Sorangium cellulosum.1,2 Among these molecules, Myx was shown to block transcription start site melting.1 Recently, Tupin et al. have demonstrated that the antibiotic Lpm from actinomycetes (Actinoplanes deccanensis) represents a new class of RNAP inhibitor that blocks isomerization from the closed to the open promoter complex.3 Genetic analysis showed that Lpm targets not only β' switch-2 but also other functional regions of RNAP (the β' Zn-finger, the β' Lid and β switch-3).27 Importantly, the activity of Lpm displays strong dependence on the integrity of the σ3.2HL structure.3 Because σ3.2HL is not essential for Lpm binding, it was proposed that Lpm might disrupt communication between β' switch-2 and σ3.2HL.3 As a consequence, fitting and stabilization of template DNA in the active site cleft is abolished. Notably, σ3.2HL is highly variable28 and is absent in some alternative σ factors (σE) or has insertions (e.g., σ32); thus, the prediction is that the inhibition mediated by Lpm is highly selective and depends on the promoter. Indeed, bacteriophage σ-like proteins gp28 and gp34 lacking the region 3.2, renders RNAP resistant to Lpm.29
The putative binding site for Lpm is located upstream of the Myx binding site (relative to the +1 base of template), at the entry to the RNA exit channel (Fig. 2B). β' switch-2 refolding in the Myx-RNAP complex was proposed as the basis of inhibition.1 This refolding results in repositioning the R613 (E. coli R337) residue toward the active site (the distance change ∼12 Å), creating additional space in the cleft. Notably, the refolded conformation of β' switch-2 is more compatible with the size of Lpm (volume ∼940 Å3) that is larger than Myx (volume ∼390 Å3). A plausible hypothesis would be that the β' switch-2 is refolded in a similar manner in presence of Lpm.
Myx Mechanism and the Final Tuning of RPo Structure
Myx hinders the β' switch-2 function and prevents correct fitting of the template DNA strand into the RNAP active site cleft. So, which step of the open complex formation is targeted by the Myx? The Myx-induced block of the λPR promoter start-site melting correlated with the lack of a DNase I footprint at promoter positions +18 to +22.1 However, the major part of dwDNA can still be loaded into the cleft in the presence of Myx. Hence, the Myx-trapped promoter complex likely corresponds to an intermediate immediately preceding the first fully open unstable complex I2 observed at λPR10 (Figs. 1A and 2C). The lack of protection between +18 and +22 in the Myx-trapped complex could reflect the lack of “final tuning” in the RPo structure observed in real-time kinetics studies.5 This transition may include folding of the RNAP jaw domains8,19 or scrunching of the DNA due to bubble formation.14
Lpm Mechanism and RPi Isomerization
Strikingly, while targeting the β' switch-2, Lpm induces much more dramatic effects than Myx. Indeed, Lpm completely blocks the formation of the transcription bubble during RPo formation on different types of promoters3 and likely inhibits RNAP through a “multiple-hit” mechanism. Firstly, Lpm prevents the σ3.2/β' switch-2-dependent fitting of the +1 base into the active site (major mechanism) (Fig. 2C). Secondly, Lpm targets the β' Lid and β switch-3. Hence, these elements can also participate in the stabilization of the transcription bubble. Finally, Lpm may keep the β' clamp in an open position that prevents the stabilization of dwDNA binding. Indeed, a strong effect of Lpm on internal protection from DNase I (positions −20 to +15) was observed at lacUV5 but not at the λPR promoter.3 At the same time, no change in the border of downstream protection (+18) from exonuclease III on lacUV5 was observed in Lpm-trapped complexes. Hence, promoter dwDNA duplex is loaded to the active site cleft in presence of Lpm, while bubble formation is blocked. Transient opening of the RNAP pincers must occur to allow this loading.
Considering the kinetic schemes, the Lpm-trapped complex resembles the closed RPc complex on λPR scheme (dwDNA protection up to +20) and closed intermediate complexes (RPc or RPi) formed on lacUV5 at 14°C (Fig. 1A). The latter were characterized by the dwDNA footprint up to +20 and fast mobility on native gel electrophoresis.23,30 Since the kinetically significant RPi complex on lacUV5 is stable,4 the Lpm-trapped complex could correspond to the unstable closed intermediate that is transient on lacUV5 but kinetically significant on λPR. The instability likely reflects the dysfunction of β' switch-2.16 Real-time footprinting experiments are required to position the Lpm-trapped complexes in the pathway to RPo.
Conclusions and Perspectives
The studies on Lpm and Myx support the “bend-load-open” model and suggest that dwDNA can enter the active site channel in double-stranded form. Subsequent bubble formation takes place inside the channel and can be uncoupled from dwDNA binding.
Identifying antibiotics that act at the promoter-opening step in a σ-dependent manner raises a question about the role of these molecules in nature. It is tempting to speculate that such molecules cannot simply be killer molecules but might function as transcriptional regulators, repressing or even activating gene expression.31 Indeed, the effect of Lpm or Myx on the open complex resembles the effect of DksA/ppGpp on stringent promoters.32 Future structural and biochemical studies of different promoters and with alternative σ factors are required to understand the detailed mechanism of action and functional role of these molecules.
Acknowledgements
This work was supported by CNRS and INSERM. Author is grateful to Ruth Saecker and Bianca Sclavi for critical reading of the manuscript, to reviewers for their helpful suggestions and to Jean-Paul Leonetti for inspiration on the lipiarmycin study.
References
- 1.Belogurov GA, Vassylyeva MN, Sevostyanova A, Appleman JR, Xiang AX, Lira R, et al. Transcription inactivation through local refolding of the RNA polymerase structure. Nature. 2009;457:332–335. doi: 10.1038/nature07510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukhopadhyay J, Das K, Ismail S, Koppstein D, Jang M, Hudson B, et al. The RNA polymerase “switch region” is a target for inhibitors. Cell. 2008;135:295–307. doi: 10.1016/j.cell.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tupin A, Gualtieri M, Leonetti J, Brodolin K. The transcription inhibitor lipiarmycin blocks DNA fitting into the RNA polymerase catalytic site. EMBO J. 2010;29:2527–2537. doi: 10.1038/emboj.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spassky A, Kirkegaard K, Buc H. Changes in the DNA structure of the lac UV5 promoter during formation of an open complex with Escherichia coli RNA polymerase. Biochemistry. 1985;24:2723–2731. doi: 10.1021/bi00332a019. [DOI] [PubMed] [Google Scholar]
- 5.Rogozina A, Zaychikov E, Buckle M, Heumann H, Sclavi B. DNA melting by RNA polymerase at the T7A1 promoter precedes the rate-limiting step at 37{degrees}C and results in the accumulation of an off-pathway intermediate. Nucleic Acids Res. 2009;37:5390–5404. doi: 10.1093/nar/gkp560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroeder LA, Gries TJ, Saecker RM, Record MTJ, Harris ME, DeHaseth PL. Evidence for a tyrosineadenine stacking interaction and for a short-lived open intermediate subsequent to initial binding of Escherichia coli RNA polymerase to promoter DNA. J Mol Biol. 2009;385:339–349. doi: 10.1016/j.jmb.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buc H, McClure WR. Kinetics of open complex formation between Escherichia coli RNA polymerase and the lac UV5 promoter. Evidence for a sequential mechanism involving three steps. Biochemistry. 1985;24:2712–2723. doi: 10.1021/bi00332a018. [DOI] [PubMed] [Google Scholar]
- 8.Craig ML, Tsodikov OV, McQuade KL, Schlax PEJ, Capp MW, Saecker RM, et al. DNA footprints of the two kinetically significant intermediates in formation of an RNA polymerase-promoter open complex: evidence that interactions with start site and downstream DNA induce sequential conformational changes in polymerase and DNA. J Mol Biol. 1998;283:741–756. doi: 10.1006/jmbi.1998.2129. [DOI] [PubMed] [Google Scholar]
- 9.Saecker RM, Tsodikov OV, McQuade KL, Schlax PEJ, Capp MW, Record MTJ. Kinetic studies and structural models of the association of E. coli sigma (70) RNA polymerase with the lambda P(R) promoter: large scale conformational changes in forming the kinetically significant intermediates. J Mol Biol. 2002;319:649–671. doi: 10.1016/S0022-2836(02)00293-0. [DOI] [PubMed] [Google Scholar]
- 10.Gries TJ, Kontur WS, Capp MW, Saecker RM, Record MTJ. One-step DNA melting in the RNA polymerase cleft opens the initiation bubble to form an unstable open complex. Proc Natl Acad Sci USA. 2010;107:10418–10423. doi: 10.1073/pnas.1000967107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cramer P, Bushnell DA, Kornberg RD. Structural basis of transcription: RNA polymerase II at 2.8 angstrom resolution. Science. 2001;292:1863–1876. doi: 10.1126/science.1059493. [DOI] [PubMed] [Google Scholar]
- 12.Vassylyev DG, Sekine S, Laptenko O, Lee J, Vassylyeva MN, Borukhov S, et al. Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 A resolution. Nature. 2002;417:712–719. doi: 10.1038/nature752. [DOI] [PubMed] [Google Scholar]
- 13.Murakami KS, Masuda S, Darst SA. Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 A resolution. Science. 2002;296:1280–1284. doi: 10.1126/science.1069594. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Darst SA, Thirumalai D. Promoter melting triggered by bacterial RNA polymerase occurs in three steps. Proc Natl Acad Sci USA. 2010;107:12523–12528. doi: 10.1073/pnas.1003533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vassylyev DG, Vassylyeva MN, Perederina A, Tahirov TH, Artsimovitch I. Structural basis for transcription elongation by bacterial RNA polymerase. Nature. 2007;448:157–162. doi: 10.1038/nature05932. [DOI] [PubMed] [Google Scholar]
- 16.Pupov D, Miropolskaya N, Sevostyanova A, Bass I, Artsimovitch I, Kulbachinskiy A. Multiple roles of the RNA polymerase {beta}' SW2 region in transcription initiation, promoter escape and RNA elongation. Nucleic Acids Res. 2010;38:5784–5796. doi: 10.1093/nar/gkq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ederth J, Artsimovitch I, Isaksson LA, Landick R. The downstream DNA jaw of bacterial RNA polymerase facilitates both transcriptional initiation and pausing. J Biol Chem. 2002;277:37456–37463. doi: 10.1074/jbc.M207038200. [DOI] [PubMed] [Google Scholar]
- 18.Kul'bachinskii AV, Ershova GV, Korzheva NV, Brodolin KL, Nikiforov VG. [Mutations in beta'-subunit of the Escherichia coli RNA-polymerase influence interaction with downstream duplex DNA in the elongation complex] Genetika. 2002;38:1422–1427. [PubMed] [Google Scholar]
- 19.Kontur WS, Capp MW, Gries TJ, Saecker RM, Record MTJ. Probing DNA binding, DNA opening and assembly of a downstream clamp/jaw in Escherichia coli RNA polymerase-lambdaP(R) promoter complexes using salt and the physiological anion glutamate. Biochemistry. 2010;49:4361–4373. doi: 10.1021/bi100092a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mekler V, Kortkhonjia E, Mukhopadhyay J, Knight J, Revyakin A, Kapanidis AN, et al. Structural organization of bacterial RNA polymerase holoenzyme and the RNA polymerase-promoter open complex. Cell. 2002;108:599–614. doi: 10.1016/s0092-8674(02)00667-0. [DOI] [PubMed] [Google Scholar]
- 21.Cowing DW, Mecsas J, Record MTJ, Gross CA. Intermediates in the formation of the open complex by RNA polymerase holoenzyme containing the sigma factor sigma32 at the groE promoter. J Mol Biol. 1989;210:521–530. doi: 10.1016/0022-2836(89)90128-9. [DOI] [PubMed] [Google Scholar]
- 22.Schickor P, Metzger W, Werel W, Lederer H, Heumann H. Topography of intermediates in transcription initiation of E. coli. EMBO J. 1990;9:2215–2220. doi: 10.1002/j.1460-2075.1990.tb07391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Studitsky V, Brodolin K, Liu Y, Mirzabekov A. Topography of lacUV5 initiation complexes. Nucleic Acids Res. 2001;29:854–861. doi: 10.1093/nar/29.3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis CA, Bingman CA, Landick R, Record MTJ, Saecker RM. Real-time footprinting of DNA in the first kinetically significant intermediate in open complex formation by Escherichia coli RNA polymerase. Proc Natl Acad Sci USA. 2007;104:7833–7838. doi: 10.1073/pnas.0609888104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brodolin K, Zenkin N, Severinov K. Remodeling of the sigma70 subunit non-template DNA strand contacts during the final step of transcription initiation. J Mol Biol. 2005;350:930–937. doi: 10.1016/j.jmb.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 26.Nechaev S, Chlenov M, Severinov K. Dissection of two hallmarks of the open promoter complex by mutation in an RNA polymerase core subunit. J Biol Chem. 2000;275:25516–25522. doi: 10.1074/jbc.M002511200. [DOI] [PubMed] [Google Scholar]
- 27.Gualtieri M, Tupin A, Brodolin K, Leonetti J. Frequency and characterisation of spontaneous lipiarmycin-resistant Enterococcus faecalis mutants selected in vitro. Int J Antimicrob Agents. 2009;34:605–606. doi: 10.1016/j.ijantimicag.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Lonetto M, Gribskov M, Gross CA. The sigma 70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992;174:3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osburne MS, Sonenshein AL. Inhibition by lipiarmycin of bacteriophage growth in Bacillus subtilis. J Virol. 1980;33:945–953. doi: 10.1128/jvi.33.3.945-953.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brodolin KL, Studitsky VM, Mirzabekov AD. Conformational changes in E. coli RNA polymerase during promoter recognition. Nucleic Acids Res. 1993;21:5748–5753. doi: 10.1093/nar/21.24.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yim G, Wang HH, Davies J. Antibiotics as signalling molecules. Philos Trans R Soc Lond B Biol Sci. 2007;362:1195–1200. doi: 10.1098/rstb.2007.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutherford ST, Villers CL, Lee J, Ross W, Gourse RL. Allosteric control of Escherichia coli rRNA promoter complexes by DksA. Genes Dev. 2009;23:236–248. doi: 10.1101/gad.1745409. [DOI] [PMC free article] [PubMed] [Google Scholar]