Abstract
B and T lymphocyte attenuator (BTLA) is a coinhibitory receptor that interacts with herpesvirus entry mediator (HVEM), and this interaction regulates pathogenesis in various immunologic diseases. In graft-versus-host disease (GVHD), BTLA unexpectedly mediates positive effects on donor T-cell survival, whereas immunologic mechanisms of this function have yet to be explored. In this study, we elucidated a role of BTLA in GVHD by applying the newly established agonistic anti-BTLA monoclonal antibody that stimulates BTLA signal without antagonizing BTLA-HVEM interaction. Our results revealed that provision of BTLA signal inhibited donor antihost T-cell responses and ameliorated GVHD with a successful engraftment of donor hematopoietic cells. These effects were dependent on BTLA signal into donor T cells but neither donor non-T cells nor recipient cells. On the other hand, expression of BTLA mutant lacking an intracellular signaling domain restored impaired survival of BTLA-deficient T cells, suggesting that BTLA also serves as a ligand that delivers HVEM prosurvival signal in donor T cells. Collectively, current study elucidated dichotomous functions of BTLA in GVHD to serve as a costimulatory ligand of HVEM and to transmit inhibitory signal as a receptor.
Introduction
Activation of T lymphocytes is regulated by 2 distinct signals: one is a primary signal delivered by T-cell receptor interaction with antigenic peptide/major histocompatibility complex (MHC), and the other is a cosignal delivered by interactions between cosignal receptors on T cells and their ligands on antigen-presenting cells.1,2 Cosignaling receptors transmit stimulatory or inhibitory signals according to characteristics of their intracellular signaling motifs, and a balance of cosignals defines the fate of T-cell responses (ie, optimal activation or deactivation/tolerance induction).3,4 Approaches to regulate cosignaling functions have been applied as novel and promising immunotherapies in various disorders, including cancer, infectious diseases, autoimmunity, organ transplantation, and graft-versus-host disease (GVHD).
B and T lymphocyte attenuator (BTLA) is a cosignaling molecule that structurally belongs to the immunoglobulin (Ig) superfamily, expressed on broad ranges of immune cells, including T cells, B cells, and dendritic cells (DCs).5–7 Intracellular domain of BTLA has 2 immunoreceptor tyrosine-based inhibition motifs, to which SH2 domain-containing protein tyrosine phosphatase-1 and tyrosine phosphatase-2 are recruited.5,8,9 This signaling characteristic is consistent with its immune inhibitory functions, as BTLA gene-deficient mice exhibit an enhanced susceptibility to autoimmune diseases and increased inflammatory responses.5,10–14 BTLA coinhibitory signal is induced by interaction with its endogenous ligand herpesvirus entry mediator (HVEM), a member of tumor necrosis factor-receptor superfamily.8,15 In addition to BTLA, HVEM has 3 other binding partners, LIGHT (lymphotoxin-like, inducible expression, competes with herpes simplex virus glycoprotein D for HVEM, a receptor expressed by T lymphocytes), CD160 and lymphotoxin-α.16 LIGHT-HVEM interaction transmits HVEM-positive cosignal into T cells via activation of nuclear factor-κB (NF-κB) signaling pathway.16–18 HVEM interactions with BTLA and LIGHT are dependent on distinct extracellular regions of HVEM (ie, cysteine-rich domain-1 for BTLA while opposing cysteine-rich domain-2 and -3 sites for LIGHT binding), and it has been suggested that ternary LIGHT-HVEM-BTLA complex either augments or disrupts HVEM-BTLA interactions according to soluble or membrane form of LIGHT.19
In contrast to negative cosignaling functions of BTLA, recent studies also suggested prosurvival effects of BTLA. For instance, in nonirradiated parent-into-F1 GVHD model, transfer of BTLA-knockout (KO) donor T cells resulted in significantly diminished donor-antihost responses because of an impaired donor T-cell survival.20 In addition, intestinal inflammation induced by a transfer of BTLA-KO T cells into Rag-KO mice was not accelerated compared with wild-type (WT) T-cell transfer because of an impaired accumulation of BTLA-KO T cells in vivo.11 To interpret these puzzling findings, 3 possible mechanisms are hypothesized. First, in certain immunologic conditions including GVHD and inflammatory bowel disease, BTLA may serve as a positive cosignaling receptor that mediates prosurvival effects. This hypothesis is attributed to the fact that BTLA intracellular domain has a membrane-proximal tyrosine containing YDND motif that binds to growth receptor bound-2 and interacts with phosphatidylinositol 3-kinase.21 Second, BTLA may act as an agonistic ligand that delivers positive cosignal into HVEM receptor and enhances T-cell survival. This hypothesis is supported by recent findings that BTLA forms homo-oligomers on cell membrane and activates NF-κB by activating HVEM signal.22 Third, BTLA may promote T-cell survival as a consequence of the inhibitory effects on other negative cosignaling molecules. For instance, rejection of fully MHC-mismatched cardiac transplant in BTLA-KO recipients is delayed compared with those in WT recipients because of an enhanced expression of PD-1 on BTLA-KO host T cells.23 It remains unexplored which of these mechanisms operate, individually or cooperatively, for the prosurvival effects of BTLA.
To elucidate the prosurvival function of BTLA in GVHD, analyses using BTLA-KO mice might not be conclusive because homeostasis of immune cells, including T cells and DCs, is somewhat dysregulated in these mice.24,25 More importantly, they lose not only BTLA signal but also its potential effects as agonistic ligand of HVEM. For the same reason, anti-BTLA monoclonal antibody (mAb), which antagonizes HVEM-BTLA interaction, is not an ideal tool to selectively elucidate BTLA functions in GVHD. To address this unsolved question, we developed a mAb clone that is agonistic but not antagonistic to BTLA, thus promoting BTLA functions while sparing endogenous HVEM-BTLA interaction. By capitalizing on this unique property of our mAb, current studies delineated a role of HVEM-BTLA cosignaling pathway in the regulation of GVHD.
Methods
Mice
Female C57BL/6 (B6, H-2b), BALB/c (H-2d), DBA/2 (H-2d), and F1 (B6 x DBA/2) (BDF1; H-2bxd) mice were purchased from the National Cancer Institute (Frederick, MD). C3H.SW mice (C3.SW-H2b/SnJ), Thy1.1-congenic mice (B6.PL-Thy1a/CyJ), and NOD-SCID common γ-chain-KO mice (NOD.Cg-Prkdcscid Il2rgtm1wjl/Szj) were purchased from The Jackson Laboratory. B6-background HVEM-KO and BTLA-KO mice were kindly provided by Dr Wayne Hancock (Department of Pathology, University of Pennsylvania School of Medicine, Philadelphia, PA) and Dr Kenneth Murphy (Department of Pathology and Immunology, Washington University School of Medicine, St Louis, MO), respectively. Age- and sex-matched 6- to 8-week-old mice were used for all experiments. All the animal experiments described in this manuscript were approved by the Animal Care and Use Committee of the University of Maryland School of Medicine.
Cell lines, fusion proteins, enzyme-linked immunosorbent assay kits, and Abs
P815 mastocytoma (H-2d), EL4 T-cell lymphoma (H-2b), and A20 B-cell lymphoma (H-2d) were purchased from the ATCC. All cell lines were maintained in the complete medium under appropriate conditions. Mouse HVEM-mouse Ig and BTLA-human Ig fusion proteins were generated in our laboratory by a method previously reported.26 Specifically, extracellular domain sequence of HVEM or BTLA was fused in frame with constant region sequence of mouse IgG2a or human IgG1, and further cloned into expression vectors. COS or 293T cells were transfected with these vectors, and the fusion proteins were isolated from the culture supernatants by protein A or G affinity column. enzyme-linked immunosorbent assay kits for measurement of interferon-γ and interleukin-4 (IL-4) were purchased from eBioscience. Control rat IgG and human IgG were purchased from Sigma-Aldrich. Antimouse BTLA mAbs (clone; BYK-1 and BYK-2) were generated in our laboratory by a standard technique, as described previously.27
Flow cytometry
Fluorescein isothiocyanate–, phycoerythrin-, allophycocyanin-, or phycoerythrin-Cy5–conjugated mAbs to mouse IgG, CD3, CD4, CD8, B220, Thy1.1, Thy1.2, PD-1, and rat IgG were purchased from eBioscience. Fluorescein isothiocyanate– or phycoerythrin-conjugated mAbs to H-2Kd and Ly9.1 were purchased from BD Biosciences. Cells were preincubated with antimouse FcγR mAb 2.4G2 for 15 minutes to block nonspecific FcγR binding and then incubated with the relevant mAbs for 30 minutes. Finally, cells were washed twice and analyzed using an LSRII flow cytometer (BD Biosciences) and FlowJo software Version 8.8.6 (TreeStar).
T-cell isolation and proliferation assay
Mouse T cells were purified by magnetic beads separation using Pan T Cell Isolation kit (Miltenyi Biotec). Isolated T cells were cultured in 96-well flat-bottom culture plates precoated with anti-CD3 mAb (clone 145-2C11; BD Biosciences PharMingen). In some cases, the culture plates were also precoated with BTLA-Ig or control Ig. T-cell proliferation was measured by incorporation of 3H-thymidine. For detection of division, purified T cells were labeled with 5μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) and the dilution of CFSE was assessed by flow cytometry.
T-cell cytolytic assay
Cytolytic activity of donor T cells was measured by a standard 51Cr-release assay, as previously described.28 Specifically, recipient spleen cells containing activated donor T cells were incubated with 51Cr-labeled target cells (P815; H-2d or EL4; H-2b) for 4 hours at various effector-to-target ratios. Maximal and spontaneous release of target cells was determined by incubating them with 10% Triton-X (Sigma-Aldrich) or media alone, respectively. Release of 51Cr in supernatants was measured by a Wallac Microbeta TriLux counter (PerkinElmer Life and Analytical Sciences). The percentages of specific lyses were calculated as follows: [(sample release − spontaneous release)/(maximum release − spontaneous release)] × 100.
Parent-to-F1 transfer GVHD model
Nonirradiated or irradiated (9 Gy) BDF1 recipient mice received intravenous injection of spleen cells isolated from B6 WT or BTLA-KO mice, as previously described.28 In some experiments, donor spleen cells were labeled with 5μM CFSE before transfer to assess division of donor T cells in vivo.
Allogeneic BMT model in lethally irradiated recipient mice
Lethal GVHD was induced by MHC-matched, minor histocompatibility antigen-mismatched allogeneic bone marrow transplantation (BMT) as previously described.28 Specifically, B6 (H-2b, Ly9.1−) mice were exposed to lethal dose irradiation (12 Gy) and subsequently injected intravenously with 5 × 106 T cell–depleted BM cells from C3H.SW mice (H-2b, Ly9.1+) together with 3 × 106 spleen plus lymph node cells isolated from C3H.SW mice on day 0. The recipient mice were treated with intraperitoneal administration of 200 μg of BYK-1 or control Ig on days 0, 5, 10, 15, 20, and 25. Thereafter, the survival of recipient mice, changes in body weight and GVHD scores were monitored regularly. For scoring, 5 clinical parameters (ie, weight loss, posture, activity, fur texture, and skin integrity; 0-2 in each parameter, maximal score of 10) were used as previously described.29 In the recipient mice that survived more than 100 days, reconstitution of recipient hematopoietic system with donor cells was assessed by flow cytometry using double staining with Ly9.1 and CD3 or B220. The liver and skin of recipient mice were harvested for pathologic analysis by hematoxylin and eosin staining. Tissue images were captured using a Micromaster I microscope (Westover Scientific) with a VistaVision Microscope Camera (VWR International) controlled by uImage software Version 2.0 (XMPie). A Micromaster 10×/20 ocular lens and a 40×/0.65 or 10×/0.25 numeric aperture objective lens were used.
Construction and expression of BTLA lacking cytoplasmic signaling domain
To construct BTLA lacking a potential to deliver signal while sparing its function as a ligand to HVEM, cytoplasmic tail of BTLA was truncated by introducing stop codon at amino acid 236, before the first intracellular tyrosine residue. This construct, named BTLAΔCY, or wild-type BTLA was subcloned into pBMN-GFP (Orbigen) to express as a retroviral vector along with green fluorescent protein (GFP) regulated by internal ribosome entry site. As control, retroviral vector expressing GFP alone was also constructed. Retroviral particles were generated by transfecting these retroviral vectors to Plat-E packaging cells by Lipofectamine 2000 (Invitrogen). For retroviral transduction, T cells isolated from B6 BTLA-KO mice were first stimulated by immobilized anti-CD3 mAb (5 μg/mL) and anti-CD28 mAb (1 μg/mL) for 24 hours. Activated T cells were harvested, suspended with retrovirus-contained medium, centrifuged in the culture plated precoated with RetroNectin (25 μg/mL, Takara Bio) at 37°C for 2.5 hours, and then cultured with 100 IU/mL recombinant IL-2 and 10 ng/mL IL-7. The next day, the culture medium was replaced with fresh complete medium containing IL-2 and IL-7. After 2 days, GFP-positive T cells were sorted by FACSAria (BD Biosciences, purity > 90%) and transferred intravenously into sublethally irradiated (6 Gy) BDF1 mice. For in vitro survival assay, the sorted GFP-positive T cells (2 × 105 cells/well) were incubated in the presence of feeder cells (T cell–depleted B6 spleen cells, 2 × 105 cells/well) for 4 to 7 days. The number of survived T cells was assessed by flow cytometry as GFP-positive, propidium iodide-negative population.
Statistical analysis
For survival data, Kaplan-Meier survival curves were prepared and statistical differences were analyzed using the log-rank (Mantel-Cox) test.
Results
Generation of agonistic but not antagonistic anti-BTLA mAb, BYK-1
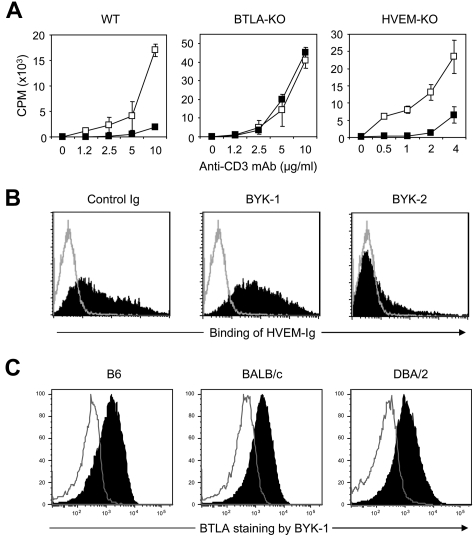
To explore enigmatic prosurvival effects of HVEM-BTLA pathway in GVHD, we first established a novel mAb clone BYK-1, which has agonistic, but not antagonistic, activity to mouse BTLA. When T cells isolated from WT B6 mice were stimulated with anti-CD3 mAb, their proliferation was significantly inhibited by addition of immobilized BYK-1 (Figure 1A). This inhibitory effect was completely abrogated when responder T cells were isolated from BTLA-KO mice, indicating that the effect of BYK-1 was mediated by its specific interaction with BTLA. Next, we examined antagonistic activity of BYK-1. In flow cytometric analysis, inclusion of BYK-1 did not inhibit the binding of HVEM-Ig fusion protein with BTLA-expressing CHO cells (Figure 1B). As an experimental control, this binding was blocked by another anti-BTLA mAb clone BYK-2, which has an antagonistic activity. This result indicated that BYK-1 is not antagonistic to BTLA-HVEM interaction and thus excluded a possibility that the inhibition of T-cell responses by BYK-1 was associated with a loss of HVEM-positive cosignal. This conclusion was further supported by our finding that proliferation of HVEM-KO T cells was attenuated by BYK-1 (Figure 1A), as this result indicated that BYK-1 mediated its effects, even in the absence of BTLA-HVEM interaction.
Figure 1.
Generation of agonistic, but not antagonistic, anti-BTLA mAb BYK-1. (A) T cells isolated from WT, BTLA-KO, or HVEM-KO mice were stimulated with indicated doses of anti-CD3 mAb in the presence of 20 μg/mL immobilized control rat IgG (□) or BYK-1 (■). Proliferative activity was assessed by 3H-thymidine incorporation during the last 18 hours of a 3-day culture. (B) BTLA-expressing CHO cells were cultured with HVEM-mouse Ig fusion protein in the presence of 10 μg control Ig, BYK-1, or BYK-2 (filled histogram). Background staining without HVEM-Ig is also shown (open histogram). Binding of HVEM-Ig fusion protein was detected by a staining with fluorescein isothiocyanate–conjugated antimouse Ig Ab. (C) CD4+ T cells isolated from B6, BALB/c, or DBA/2 mice were stimulated with 1 μg/mL antimouse CD3 mAb. After 48 hours, T cells were stained with BYK-1 (filled histogram) or control Ig (open histogram), and analyzed by flow cytometry. Data are representative of 3 independent experiments.
BTLA harbors polymorphisms within its extracellular domain, and 3 distinct allelic variants have been identified in mouse strains thus far.30 Therefore, we next examined whether BYK-1 binds selectively to these alleles represented by B6, BALB/c, and DBA/2 mice strains. CD4+ T cells isolated from these mouse strains were stimulated with anti-CD3 mAb and stained with BYK-1, as activated CD4+ T cells express high level BTLA on their cell surface.6 Activated CD4+ T cells of these strains were equally stained with BYK-1 (Figure 1C), showing its reactivity to all 3 alleles of BTLA. Taken together, we generated BYK-1, an agonistic, but not antagonistic, anti-BTLA mAb that recognizes all 3 alleles of mouse BTLA and mediates coinhibitory signal into T cells.
Inhibition of donor antihost allogeneic T-cell responses by BYK-1
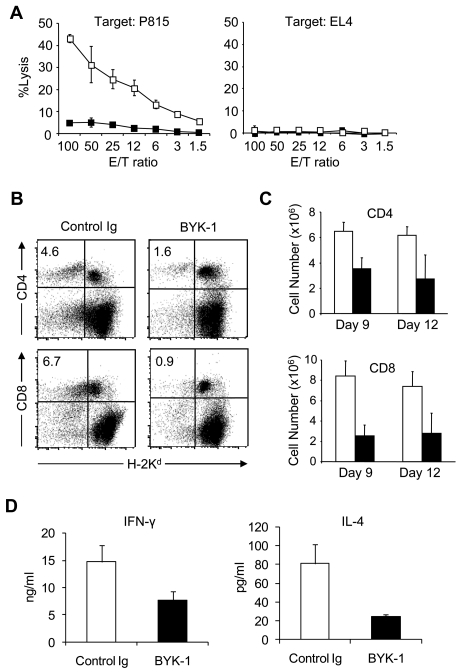
To address regulatory effects of BTLA signal in GVHD, we applied BYK-1 in a model in which B6 donor lymphocytes are transferred into BDF1 recipient mice.28,31 Administration of BYK-1 profoundly inhibited generation of donor antihost CTL, which was measured by cytolytic activity against P815, H-2d-positive host MHC-specific target cells (Figure 2A). These results indicated that BTLA cosignal mediated by agonistic BYK-1 mAb was capable of inhibiting the induction and/or maintenance of allogeneic CTL responses. To address immunologic mechanisms of this effect, we first monitored donor T-cell number after transfer into recipient mice. BYK-1 treatment significantly decreased the percentage and absolute number of H-2Kd–negative donor CD4+ and CD8+ T cells in recipient mice (Figure 2B-C). To exclude a possibility that this effect was associated with nonspecific depletion of BTLA-positive T cells by BYK-1, we transferred NOD T cells into T cell–deficient NOD-SCID common γ-chain-KO mice and injected BYK-1. This approach evaluates a potential of mAb to deplete T cells more strictly than simply injecting mAb into normal mice because the recipient mice lack T-cell generation capacity that could compensate T-cell number as they are depleted. The number of transferred T cells was not decreased by BYK-1 treatment in this model (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), indicating that BYK-1 is not a depletion mAb. In addition, BYK-1 treatment showed negligible effects on OT-I T-cell responses induced by injection of ovalbumin and polyinosinic-polycytidylic acid (supplemental Figure 1B), suggesting that the inhibitory effects of BYK-1 were rather specific to allogeneic T-cell responses. We next addressed cytokine production of donor T cells under BYK-1 treatment, as BTLA expression has been detected predominantly on Th1 cells but not Th2 cells.5,30 Donor CD4+ T cells from BYK-1–treated mice showed decreased productions of both interferon-γ and IL-4 (Figure 2D), suggesting that selective inhibition of Th1 was not responsible for the effect of BYK-1. In addition, because donor T-cell numbers were standardized per culture well in this assay, these results indicated that BYK-1 treatment inhibited donor T-cell functions at per cell basis. Collectively, these results indicated that BTLA cosignal triggered by agonistic BYK-1 mAb inhibited donor antihost allogeneic T-cell responses in GVHD without mediating selective inhibition of Th1 responses in donor T cells.
Figure 2.
Inhibition of donor antihost alloresponses by BYK-1 treatment. (A-C) BDF1 recipient mice were injected intravenously with 5 × 107 donor B6 spleen cells. The recipient mice were treated intraperitoneally with 200 μg of BYK-1 (■) or control Ig (□) on days 0, 3, and 6. (A) On day 9, recipient spleen cells were harvested and assessed for CTL activity against P815 (H-2d) and EL4 (H-2b) cells by a standard 4-hours 51Cr releasing assay. (B) On day 9, recipient spleen cells were stained with anti-H-2Kd mAb, together with either anti-CD4 or anti-CD8 mAb, and analyzed by flow cytometry. Percentages of donor CD4+ or CD8+ T cells (upper left quadrant) in the recipient spleen are shown. The experiment was repeated more than 3 times, and representative data are shown. P < .05 (control Ig vs BYK-1). (C) On days 9 and 12, absolute numbers of donor CD4+ and CD8+ T cells in the recipient spleen were assessed. Each column represents average plus or minus SD of donor T-cell number after the treatment with control Ig (white bar) or BYK-1 (black bar). (D) BDF1 recipient mice were exposed to irradiation (9 Gy) and subsequently injected intravenously with 5 × 106 donor B6 spleen cells. The recipient mice were injected intraperitoneally with 200 μg BYK-1 (black bar) or control Ig (white bar) on days 0 and 3. On day 6, mesenteric lymph node cells harvested from the recipient mice were standardized for numbers of CD4+ T cells (5 × 104 per well) and stimulated in vitro with 5 μg/mL immobilized anti-CD3 mAb and 2 μg/mL anti-CD28 mAb. After 24 hours, the culture supernatants were harvested, and the levels of interferon-γ (left panel) and IL-4 (right panel) were measured by enzyme-linked immunosorbent assay. Each column represents average plus or minus SD. P < .05 (control Ig vs BYK-1). Data are representative of 3 independent experiments.
Immunotherapeutic effects of BYK-1 in GVHD caused by allogeneic BMT
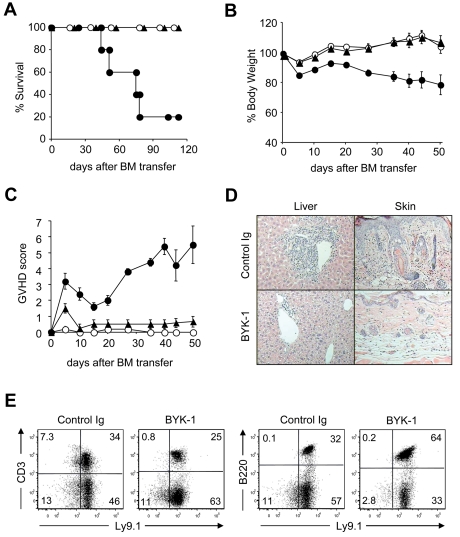
Although BYK-1 demonstrated profound inhibitory effect in the induction of donor antihost T-cell responses in nonirradiated parent into F1 GVHD, this model differs from actual clinical conditions in multiple aspects, including a lack of myeloablative preconditioning and a transfer of hematopoietic stem cells. Therefore, we next investigated whether BYK-1 shows therapeutic effects in a model that closely mimics clinical GVHD. We used a well-established C3H.SW into B6 model, in which lethally irradiated B6 recipient mice were injected with BM hematopoietic stem cells and peripheral T cells from MHC-matched, minor histocompatibility antigen-mismatched C3H.SW donor cells.32 The recipient mice were treated intraperitoneally with BYK-1 or control Ig every 5 days starting from the day of donor cell transfer until day 25, and the survival and GVHD severity of recipient mice were assessed as previously reported.28 Although only 20% of recipient mice survived by control Ig treatment, all recipient mice treated with BYK-1 survived more than 100 days after BMT (Figure 3A). BYK-1 treatment protected the recipients from weight loss associated with GVHD and almost completely alleviated GVHD clinical score (Figure 3B-C). Pathologic analysis also confirmed a therapeutic potential of BYK-1 treatment (Figure 3D). In liver, a massive infiltration of inflammatory cells in the portal tracts, a typical figure of hepatic GVHD was prevented by BYK-1 treatment. Skin epidermal hyperplasia, thickening of the dermis with massive cellular infiltration, was also prevented by BYK-1. In recipient mice that survived more than 100 days by BYK-1 treatment, hematopoietic cells, including T and B lymphocytes, were fully replaced with donor-derived cells expressing Ly9.1 marker (Figure 3E), indicating that BYK-1 treatment did not impair engraftment of donor hematopoietic system. Therapeutic effect of BYK-1 was also demonstrated in a GVHD model induced by an MHC-mismatched BMT model in which B6 donor BM and T cells were injected into lethally irradiated BDF1 recipient mice (supplemental Figure 2). Taken together, our results suggest that delivery of BTLA inhibitory signal by agonistic anti-BTLA mAb BYK-1 during the first 25 days after allogeneic BMT achieves long-term amelioration of GVHD with a successful donor cell engraftment.
Figure 3.
Therapeutic effects of BYK-1 in GVHD induced by MHC-matched, minor histocompatibility antigen-mismatched BMT. On day 0, B6 recipient mice were exposed to lethal irradiation (12 Gy) and transferred intravenously with 5 × 106 T cell-depleted BM cells together with 3 × 106 spleen plus lymph node cells isolated from C3H.SW donor mice. The recipient mice were treated intraperitoneally with 200 μg BYK-1 (▲) or control Ig (●) every 5 days from day 0 to day 25. As non-GVHD control, a group of mice were transferred with T cell–depleted BM cells alone (○). Survival of recipient mice (A), changes in body weight (B), and GVHD clinical scores (C) were monitored after BMT. (D) Pathologic features of recipient liver and skin were examined by hematoxylin and eosin staining (original magnification ×400 in the liver and ×100 in the skin). (E) Spleen cells of the recipient mice that had survived GVHD more than 100 days by BYK-1 treatment were assessed for the expression of Ly9.1 (donor cell marker) and CD3 or B220 by flow cytometry. Data are representative of 3 independently repeated experiments (n = 5 per group).
Essential role of BTLA on donor T cells in the inhibitory effect of BYK-1
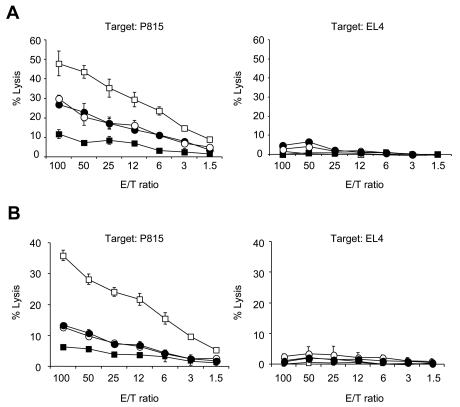
To explore immunologic mechanisms of BYK-1 in GVHD treatment, we attempted to identify cellular targets of BYK-1 using BTLA-KO cells as donor in B6 into BDF1 model. First, BDF1 mice were injected with either BTLA-KO or WT donor cells and treated with control Ig or BYK-1. Consistent with Figure 2A, BYK-1 treatment significantly inhibited antihost CTL activity when WT donor cells were transferred into BDF1 mice (Figure 4A). In sharp contrast, BYK-1 had no inhibitory effects on antihost CTL activity in BTLA-KO donor cells (Figure 4A), although the basal level of antihost CTL in BTLA-KO donor cells was lower than that of WT donor cells as reported previously.20 These results suggested that BYK-1 inhibited allogeneic CTL responses by targeting BTLA expressed on donor, but not recipient, cells.
Figure 4.
BTLA expressed on donor T cells as an essential target for inhibitory effects of BYK-1 in GVHD. (A) BDF1 mice were injected intravenously with 5 × 107 B6 WT or BTLA-KO mouse spleen cells on day 0 and treated intraperitoneally with 200 μg BYK-1 or control Ig on days 0, 3, and 6 in the following combinations: transfer of WT donor cells and control Ig treatment (□), transfer of WT donor cells and BYK-1 treatment (■), transfer of BTLA-KO donor cells and control Ig treatment (○), and transfer of BTLA-KO donor cells and BYK-1 treatment (●). On day 9, recipient spleen cells were harvested and analyzed for CTL activity against P815 (H-2d) and EL4 (H-2b) cells. (B) BDF1 mice were injected with mixture of T cells (2 × 107 cells) and non-T cells (4 × 107 cells) purified from either B6 WT or BTLA-KO mice and treated intraperitoneally with BYK-1 or control Ig on days 0, 3, and 6 in the following combinations: transfer of WT T cells plus BTLA-KO non-T cells followed by control Ig treatment (□), transfer of WT T cells plus BTLA-KO non-T cells followed by BYK-1 treatment (■), transfer of BTLA-KO T cells plus WT non-T cells followed by control Ig treatment (○), and transfer of BTLA-KO T cells plus WT non-T cells followed by BYK-1 treatment (●). On day 9, recipient spleen cells were harvested and analyzed for CTL activity against P815 (H-2d) and EL4 (H-2b). CTL level against P815 in BYK-1-treated WT T cells plus BTLA-KO non-T cells (■) was significantly lower than control Ig-treated BTLA-KO T cells plus WT non-T cells (○) at an effector/target (E/T) ratio of 100, 50, 25, and 12 (P < .05). Data are representative of 3 independently repeated experiments.
Considering an important role of donor antigen-presenting cells in GVHD pathogenesis and an ability of BTLA to transmit coinhibitory signal even in non-T immune cells including B cells and DCs,7,33 we next examined whether T cells or non-T cells in donor cells are targeted in the effect of BYK-1. BDF1 recipient mice were injected with donor cells composed of B6 WT T cells mixed with BTLA-KO non-T cells, or BTLA-KO T cells mixed with WT non-T cells, and subsequently treated with either control Ig or BYK-1. Although the level of antihost CTL activity was low when BTLA-KO T cells and WT non-T cells were transferred as donor cells, the inhibitory effect of BYK-1 was completely abrogated in this condition (Figure 4B). In sharp contrast, BYK-1 treatment profoundly inhibited CTL activity induced by donor cells of WT T cells mixed with BTLA-KO non-T cells. These results indicated that BTLA expressed on donor T cells, but not antigen-presenting cells, is a possible target for the inhibitory effects of BYK-1 in GVHD.
BTLA function as a ligand to deliver HVEM costimulatory signal in GVHD
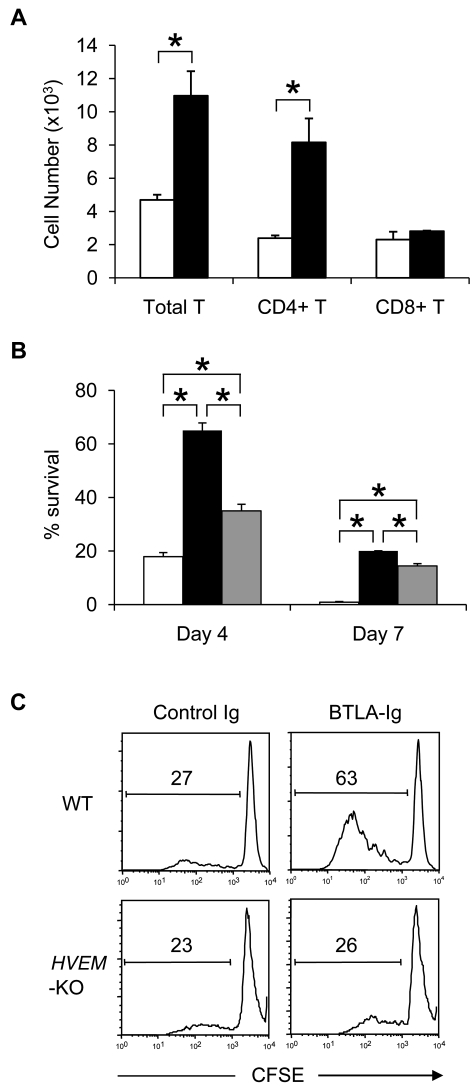
In our GVHD model, transfer of BTLA-KO donor cells resulted in an impaired antihost CTL responses (Figure 4). In addition, after transfer into BDF1 recipient mice, the number of BTLA-KO donor T cells was significantly lower than that of WT T cells, whereas the level of CFSE dilution was comparable between these donor T cells (supplemental Figure 3). Together with similar findings in previous studies, these results indicated the prosurvival effect of BTLA in late stage of GVHD, whereas its mechanisms have yet to be explored thus far.20 Our current findings revealed that BTLA signal induced by the agonistic anti-BTLA mAb BYK-1 inhibited antihost CTL responses and ameliorated GVHD (Figures 2–4). Therefore, the possibility that BTLA itself acts as a positive cosignaling receptor was excluded. In addition, we detected no difference of PD-1 expression levels between WT and BTLA-KO donor T cells or by BYK-1 treatment in our GVHD model (supplemental Figure 4), further excluding the possibility of PD-1-dependent secondary regulation of GVHD by BTLA signal, which was reported in an allogeneic heart transplantation model.23 Therefore, we examined the third possibility that BTLA acts as a functional ligand of HVEM and triggers prosurvival HVEM signal in donor T cells in GVHD. To address this question, we constructed mutated BTLA lacking cytoplasmic signaling domain (BTLAΔCY) and express it on BTLA-KO T cells to restore its functions as a ligand but not a receptor. When B6 BTLA-KO T cells expressing BTLAΔCY were transferred into BDF1 recipient mice, their survival was significantly enhanced compared with control BTLA-KO T cells in total and CD4+ donor T cells (Figure 5A). CD8+ BTLAΔCY T cells also showed survival benefit over BTLA-KO cells, although it was not statistically significant. These results suggested that the prosurvival effect of BTLA was dependent on its function as a ligand but not as a receptor. To further confirm this notion, we cultured BTLA-KO, BTLAΔCY-expressing, and wild-type BTLA-expressing T cells in vitro, and compared their survival 4 and 7 days later. Survival of BTLAΔCY-expressing T cells was significantly better than BTLA-KO T cells (Figure 5B), consistent with the result of in vivo transfer experiment. In addition, we found that wild-type BTLA-expressing T cells survived greater than BTLA-KO T cells but less than BTLAΔCY T cells. These results suggested that BTLA-HVEM interaction delivered positive and negative signals to HVEM and BTLA, respectively, whereas HVEM-mediated positive signal had predominant effects in our models. An ability of BTLA to stimulate HVEM-positive signal was further supported by our results that BTLA-Ig protein facilitated WT, but not HVEM-KO, T-cell proliferation induced by anti-CD3 mAb (Figure 5C). Taken together, these results demonstrated a predominant function of BTLA as a ligand to deliver prosurvival HVEM cosignal during GVHD, consistently with an ability of BTLA to activate NF-κB via HVEM signal.22
Figure 5.
BTLA functions as a ligand to deliver prosurvival signal in GVHD. (A) Activated BTLA-KO B6 T cells expressing BTLAΔCY-GFP (black bar) or GFP alone (white bar) were transferred intravenously into sublethally (6 Gy)-irradiated BDF1 mice (7 × 105 cells/mouse). After 24 hours, spleen was harvested from the recipient mice and the numbers of GFP-positive donor T cells (total, CD4+, and CD8+) were assessed by flow cytometry. *P < .05. (B) Activated BTLA-KO B6 T cells expressing BTLAΔCY-GFP (black bar), wild-type BTLA-GFP (gray bar), and GFP alone (white bar) were incubated in vitro. After 4 and 7 days, the number of live GFP-positive cells was counted and percentage survival is shown as average plus or minus SD of triplicate wells. *P < .05. (C) T cells isolated from spleen of WT or HVEM-KO mice were labeled with CFSE and stimulated with 2 μg/mL anti-CD3 mAb in the presence of 10 μg/mL immobilized BTLA-Ig or control Ig. After 3 days, the intensity of CFSE of the culture cells was analyzed by flow cytometry. The numbers in panels indicate percentage of cells undergone more than one division.
Demonstration of BTLA as a costimulatory ligand further raised a question whether BTLA on recipient cells also functions as a ligand to provide prosurvival signal to donor T cells because BTLA is expressed on antigen-presenting cells.6,7 When sublethally irradiated WT or BTLA-KO B6 recipient mice were injected with BALB/c donor T cells, induction of donor antihost CTL activity was comparable between these recipient mice (supplemental Figure 5). These results suggested that the BTLA function as a ligand to induce prosurvival signal in GVHD was associated with its expression on donor T cells but not on recipient cells, including antigen-presenting cells.
Discussion
In this study, we elucidated a role of BTLA cosignal functions in GVHD using the newly established anti-BTLA mAb that is agonistic, but not antagonistic, and thus drives BTLA signal without attenuating BTLA-HVEM interaction. Our results revealed that BTLA signal inhibited donor antihost T-cell responses and ameliorated GVHD caused by allogeneic BMT with a successful engraftment of donor hematopoietic cells. These effects were dependent on BTLA signal into donor T cells but neither donor non-T cells nor recipient cells. Despite the inhibitory potential of BTLA signal in GVHD, antihost responses caused by BTLA-KO donor cells were significantly decreased because of their impaired survival. This paradoxical result was explained by the function of BTLA as a ligand that provides prosurvival effects on donor T cells. Collectively, the current study elucidated the function of BTLA in GVHD as a costimulatory ligand of HVEM, whereas exogenous administration of agonistic anti-BTLA mAb still induces coinhibitory BTLA signal and ameliorates GVHD.
Although it has been reported that transfer of BTLA-KO donor cells or treatment with anti-BTLA blocking mAb resulted in an impaired donor antihost T-cell response, the mechanisms of this enigmatic phenomenon remained unexplored.20 Particularly, it was unclear whether positive effects of BTLA are mediated through its function as a receptor to recruit stimulatory adaptor proteins, such as growth receptor bound-2, or by its role as a ligand to induce the HVEM cosignal. The current study addressed this question and revealed that: (1) the BTLA signal triggered by agonistic anti-BTLA mAb inhibited donor antihost responses, and (2) expression of mutated BTLA lacking intracellular signaling domain on BTLA-KO donor T cells prolonged their survival. These results elucidated dichotomous functions of BTLA as a ligand to provide prosurvival signal to donor T cells and as a receptor to inhibit donor T-cell responses in GVHD. It has been reported that positive HVEM cosignal induced by BTLA ligation is dependent on their interaction in trans with adjacent cells, and the cis-interaction of these molecules rather interrupts HVEM signaling.22,34 Therefore, the prolonged survival of donor T cells expressing BTLAΔCY suggested that donor T-T cell interaction may play a responsible role in the positive effect of BTLA-HVEM pathway in GVHD. This notion was further supported by our finding that BTLA on recipient cells showed negligible effects on donor T-cell responses. A crucial role of T-T cell interaction is reminiscent of costimulatory functions of LIGHT-HVEM pathway in GVHD, as we previously reported.28 Other than this mechanism, our results demonstrated that PD-1 expression level was comparable between WT and BTLA-KO donor T cells in our model. This result indicated that secondary compensation of PD-1 cosignal in BTLA-KO cells was not responsible for their impaired GVHD activity, whereas such a mechanism was reported in other models.23
Although BTLA-HVEM interaction could induce opposing positive and negative cosignals into HVEM and BTLA, respectively, dominance of one signal over the other signal varies according to types of immune responses. As shown here and in previous studies, deficiency or blockade of BTLA resulted in impaired allogeneic responses in GVHD and organ transplantation, indicating a dominant role of HVEM-positive cosignal over BTLA signal in these models.20,23,35 In contrast, the BTLA-negative signal becomes dominant in autoimmune and inflammatory responses, as BTLA-KO mice showed enhanced susceptibility to these models.5,10–14 HVEM-KO mice also induced less severe GVHD but aggravated immune responses in hepatitis and experimental autoimmune encephalomyelitis models,28,36 confirming that these phenotypes are related to the BTLA-HVEM pathway. In addition, a dominant role of BTLA inhibitory cosignal also observed in homeostatic regulation of T cells, DCs, and immune tolerance.24,25,37 Taken together, the role of BTLA-HVEM pathway as a molecular switch to activate or inhibit T-cell responses could be affected by types of immune responses (eg, characteristics of Ag). Further studies are necessary to fully explore mechanisms by which dominance of dichotomous signals in the BTLA-HVEM pathway is regulated.
Whereas BTLA predominantly serves as a ligand to deliver prosurvival HVEM cosignal rather than as a coinhibitory receptor in GVHD, exogenous administration of agonistic anti-BTLA mAb BYK-1 was still capable of triggering coinhibitory BTLA signal and ameliorating GVHD as shown in this study. Although therapeutic effects of anti-BTLA mAb have been reported in GVHD and transplantation models, underlying mechanisms are different between clones used in the studies. For instance, clone 6A6 ameliorates GVHD and graft rejection in fully MHC-mismatched cardiac transplantation by antagonizing BTLA-HVEM prosurvival effects.20,23 On the other hand, clone 6F7 and PJ196 prolong islet allograft survival by depleting BTLA-positive T cells and down-regulating BTLA expression, respectively.35,38 The current study used a newly established clone BYK-1, which is agonistic but neither antagonistic nor depleting anti-BTLA mAb. Thus, we rigorously addressed a role of BTLA signal in GVHD and revealed its therapeutic potential by decreasing donor antihost T-cell number as well as inhibiting their activity at per cell basis. In addition, it is of interest that BYK-1 treatment did not inhibit anti-ovalbumin T-cell responses. Although immunologic mechanisms of the selective effects of BYK-1 in antialloresponses remain unclear, this unique property of BYK-1 could be applied to attenuation of GVHD without impairing graft-versus-leukemia and anti pathogen responses. Importantly, BYK-1 treatment achieved almost full engraftment of donor hematopoietic cells after BMT. This result suggests that BTLA signal has negligible effects on the process of lymphocyte reconstitution despite its expression on developing T and B cells in thymus and BM.6 Therapeutic effects of BYK-1 in GVHD could be enhanced by combination with LIGHT-HVEM blockade, as a synergy between enhancement of BTLA coinhibitory signal and attenuation of HVEM prosurvival signal is expected. Blockade of CD160-HVEM may be also beneficial for GVHD treatment because CD160 has been shown to induce the HVEM-dependent NF-κB activation.22,34
Taken together, current studies revealed the dichotomous functions of BTLA in GVHD as a ligand to deliver HVEM prosurvival cosignal and as a receptor to transmit coinhibitory signal, where the former seems predominant over the latter. Exogenous administration of agonistic anti-BTLA mAb was capable of inducing BTLA coinhibitory signal and ameliorating GVHD. Thus, our current studies elucidate BTLA functions in the pathogenesis of GVHD and open new avenues for GVHD treatment by targeting BTLA.
Supplementary Material
Acknowledgments
The authors thank Dr Lieping Chen, Dr Scott Strome, and Dr Kenneth Murphy for helpful discussions and Dr Wayne Hancock for providing HVEM-KO mice.
This work was supported by National Institutes of Health (grant HL088954).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y.S. participated in designing and performing the experiments; J.-J.P., Y.Z., A.K., D.G., Y.L., and E.D. contributed to the preparation of reagents and performing the experiment; and K.T. participated in designing the research, analyzing data, and writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Koji Tamada, 655 West Baltimore St, BRB 9-051, Baltimore, MD 21201; e-mail: ktamada@som.umaryland.edu.
References
- 1.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71(7):1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 2.Chambers CA, Allison JP. Co-stimulation in T-cell responses. Curr Opin Immunol. 1997;9(3):396–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 3.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4(5):336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 4.Subudhi SK, Alegre ML, Fu YX. The balance of immune responses: costimulation versus coinhibition. J Mol Med. 2005;83(3):193–202. doi: 10.1007/s00109-004-0617-1. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe N, Gavrieli M, Sedy JR, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4(7):670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 6.Han P, Goularte OD, Rufner K, Wilkinson B, Kaye J. An inhibitory Ig superfamily protein expressed by lymphocytes and APCs is also an early marker of thymocyte positive selection. J Immunol. 2004;172(10):5931–5939. doi: 10.4049/jimmunol.172.10.5931. [DOI] [PubMed] [Google Scholar]
- 7.Murphy KM, Nelson CA, Sedy JR. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat Rev Immunol. 2006;6(9):671–681. doi: 10.1038/nri1917. [DOI] [PubMed] [Google Scholar]
- 8.Sedy JR, Gavrieli M, Potter KG, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6(1):90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 9.Chemnitz JM, Lanfranco AR, Braunstein I, Riley JL. B and T lymphocyte attenuator-mediated signal transduction provides a potent inhibitory signal to primary human CD4 T cells that can be initiated by multiple phosphotyrosine motifs. J Immunol. 2006;176(11):6603–6614. doi: 10.4049/jimmunol.176.11.6603. [DOI] [PubMed] [Google Scholar]
- 10.Deppong C, Juehne TI, Hurchla M, et al. Cutting edge: B and T lymphocyte attenuator and programmed death receptor-1 inhibitory receptors are required for termination of acute allergic airway inflammation. J Immunol. 2006;176(7):3909–3913. doi: 10.4049/jimmunol.176.7.3909. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg MW, Turovskaya O, Shaikh RB, et al. A crucial role for HVEM and BTLA in preventing intestinal inflammation. J Exp Med. 2008;205(6):1463–1476. doi: 10.1084/jem.20071160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oya Y, Watanabe N, Owada T, et al. Development of autoimmune hepatitis-like disease and production of autoantibodies to nuclear antigens in mice lacking B and T lymphocyte attenuator. Arthritis Rheum. 2008;58(8):2498–2510. doi: 10.1002/art.23674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller ML, Sun Y, Fu YX. Cutting edge: B and T lymphocyte attenuator signaling on NKT cells inhibits cytokine release and tissue injury in early immune responses. J Immunol. 2009;183(1):32–36. doi: 10.4049/jimmunol.0900690. [DOI] [PubMed] [Google Scholar]
- 14.Iwata A, Watanabe N, Oya Y, et al. Protective roles of B and T lymphocyte attenuator in NKT cell-mediated experimental hepatitis. J Immunol. 2010;184(1):127–133. doi: 10.4049/jimmunol.0900389. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez LC, Loyet KM, Calemine-Fenaux J, et al. A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc Natl Acad Sci U S A. 2005;102(4):1116–1121. doi: 10.1073/pnas.0409071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Rio ML, Lucas CL, Buhler L, Rayat G, Rodriguez-Barbosa JI. HVEM/LIGHT/BTLA/CD160 cosignaling pathways as targets for immune regulation. J Leukoc Biol. 2010;87(2):223–235. doi: 10.1189/jlb.0809590. [DOI] [PubMed] [Google Scholar]
- 17.Mauri DN, Ebner R, Montgomery RI, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8(1):21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 18.Tamada K, Shimozaki K, Chapoval AI, et al. LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T-cell response. J Immunol. 2000;164(8):4105–4110. doi: 10.4049/jimmunol.164.8.4105. [DOI] [PubMed] [Google Scholar]
- 19.Murphy TL, Murphy KM. Slow down and survive: enigmatic immunoregulation by BTLA and HVEM. Annu Rev Immunol. 2010;28:389–411. doi: 10.1146/annurev-immunol-030409-101202. [DOI] [PubMed] [Google Scholar]
- 20.Hurchla MA, Sedy JR, Murphy KM. Unexpected role of B and T lymphocyte attenuator in sustaining cell survival during chronic allostimulation. J Immunol. 2007;178(10):6073–6082. doi: 10.4049/jimmunol.178.10.6073. [DOI] [PubMed] [Google Scholar]
- 21.Gavrieli M, Murphy KM. Association of Grb-2 and PI3K p85 with phosphotyrosile peptides derived from BTLA. Biochem Biophys Res Commun. 2006;345(4):1440–1445. doi: 10.1016/j.bbrc.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 22.Cheung TC, Steinberg MW, Oborne LM, et al. Unconventional ligand activation of herpesvirus entry mediator signals cell survival. Proc Natl Acad Sci U S A. 2009;106(15):6244–6249. doi: 10.1073/pnas.0902115106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao R, Wang L, Han R, et al. Differential effects of B and T lymphocyte attenuator and programmed death-1 on acceptance of partially versus fully MHC-mismatched cardiac allografts. J Immunol. 2005;175(9):5774–5782. doi: 10.4049/jimmunol.175.9.5774. [DOI] [PubMed] [Google Scholar]
- 24.Krieg C, Boyman O, Fu YX, Kaye J. B and T lymphocyte attenuator regulates CD8+ T cell-intrinsic homeostasis and memory cell generation. Nat Immunol. 2007;8(2):162–171. doi: 10.1038/ni1418. [DOI] [PubMed] [Google Scholar]
- 25.De Trez C, Schneider K, Potter K, et al. The inhibitory HVEM-BTLA pathway counter regulates lymphotoxin receptor signaling to achieve homeostasis of dendritic cells. J Immunol. 2008;180(1):238–248. doi: 10.4049/jimmunol.180.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chapoval AI, Zhu G, Chen L. Immunoglobulin fusion proteins as a tool for evaluation of T-cell costimulatory molecules. Mol Biotechnol. 2002;21(3):259–264. doi: 10.1385/MB:21:3:259. [DOI] [PubMed] [Google Scholar]
- 27.Wilcox RA, Flies DB, Zhu G, et al. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest. 2002;109(5):651–659. doi: 10.1172/JCI14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y, Flies AS, Flies DB, et al. Selective targeting of the LIGHT-HVEM costimulatory system for the treatment of graft-versus-host disease. Blood. 2007;109(9):4097–4104. doi: 10.1182/blood-2006-09-047332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
- 30.Hurchla MA, Sedy JR, Gavrieli M, Drake CG, Murphy TL, Murphy KM. B and T lymphocyte attenuator exhibits structural and expression polymorphisms and is highly induced in anergic CD4+ T cells. J Immunol. 2005;174(6):3377–3385. doi: 10.4049/jimmunol.174.6.3377. [DOI] [PubMed] [Google Scholar]
- 31.Tamada K, Shimozaki K, Chapoval AI, et al. Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nat Med. 2000;6(3):283–289. doi: 10.1038/73136. [DOI] [PubMed] [Google Scholar]
- 32.Reddy P, Negrin R, Hill GR. Mouse models of bone marrow transplantation. Biol Blood Marrow Transplant. 2008;14(1 suppl 1):129–135. doi: 10.1016/j.bbmt.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matte CC, Liu J, Cormier J, et al. Donor APCs are required for maximal GVHD but not for GVL. Nat Med. 2004;10(9):987–992. doi: 10.1038/nm1089. [DOI] [PubMed] [Google Scholar]
- 34.Cheung TC, Oborne LM, Steinberg MW, et al. T cell intrinsic heterodimeric complexes between HVEM and BTLA determine receptivity to the surrounding microenvironment. J Immunol. 2009;183(11):7286–7296. doi: 10.4049/jimmunol.0902490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Truong W, Plester JC, Hancock WW, et al. Negative and positive co-signaling with anti-BTLA (PJ196) and CTLA4Ig prolongs islet allograft survival. Transplantation. 2007;84(10):1368–1372. doi: 10.1097/01.tp.0000289995.70390.20. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Subudhi SK, Anders RA, et al. The role of herpesvirus entry mediator as a negative regulator of T cell-mediated responses. J Clin Invest. 2005;115(3):711–717. doi: 10.1172/JCI200522982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X, Alexiou M, Martin-Orozco N, et al. Cutting edge: a critical role of B and T lymphocyte attenuator in peripheral T cell tolerance induction. J Immunol. 2009;182(8):4516–4520. doi: 10.4049/jimmunol.0803161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Truong W, Plester JC, Hancock WW, et al. Combined coinhibitory and costimulatory modulation with anti-BTLA and CTLA4Ig facilitates tolerance in murine islet allografts. Am J Transplant. 2007;7(12):2663–2674. doi: 10.1111/j.1600-6143.2007.01996.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.