Abstract
Neuroinflammation associated with advanced HIV-1 infection is often exacerbated in cocaine-abusing, HIV-infected patients. The underlying mechanisms could, in part, be attributed to the increased impairment of blood brain barrier integrity in the presence of cocaine. Platelet-derived growth factor (PDGF) has been implicated in several pathologic conditions, specifically attributable to its potent mitogenic effects. Its modulation by drug abuse, however, has received very little attention. In the present study, we demonstrated cocaine-mediated induction of PDGF-BB in human brain microvascular endothelial cells through the binding to its cognate σ receptor. Furthermore, this effect was mediated, with subsequent activation of mitogen-activated protein kinases and Egr-1 pathways, culminating ultimately into increased expression of PDGF-BB. Cocaine exposure resulted in increased permeability of the endothelial barrier, and this effect was abrogated in mice exposed to PDGF-BB neutralizing antibody, thus underscoring its role as a vascular permeant. In vivo relevance of these findings was further corroborated in cocaine-treated mice that were administered neutralizing antibody specific for PDGF-BB as well as in Egr-1−/− mice. Understanding the regulation of PDGF-BB expression may provide insights into the development of potential therapeutic targets for neuroinflammation associated with HIV infection and drug abuse.
Introduction
Although the advent of combined antiretroviral therapy has significantly decreased the incidence of HIV-associated neurocognitive disorders (HANDs), the prevalence of the disease is actually increasing. Drug abuse has been implicated as a contributing risk factor for increased neuroinflammation associated with HIV-1 infection. Intriguingly, cocaine has been shown to open up the blood-brain barrier (BBB).1–3 BBB is critical for the maintenance of central nervous system (CNS) homeostasis and for the regulation of the neuronal microenvironment. However, under conditions of barrier disruption as in HIV-1 infection, virus can enter the CNS through infected leukocytes likely via the Trojan horse mechanism.
Zhang et al3 have demonstrated that cocaine-mediated effects on the BBB are complex, including both direct proapoptotic effects on the endothelial cells as well as indirect paracrine effects that are manifested by proinflammatory modulators such as chemokines and cytokines. Cocaine plays a critical role in HIV-1 neuroinvasion, thus accelerating the progression of HAND.1–3
Because the chemokine/growth factor platelet-derived growth factor (PDGF) has been shown in previous reports to be up-regulated in the areas around the blood vessels in the brains of macaques with simian immunodeficiency virus infection,4 we hypothesized that cocaine-mediated disruption of the endothelial barrier could involve PDGF. Adding further validity to this hypothesis was a recent report by Su et al5 suggesting the role of a novel mediator of the PDGF family as a cerebrovascular permeant in ischemic stroke. Other investigators have also demonstrated the effects of PDGF on endothelial cells.6–8
PDGF comprises a family of proteins that are the products of 4 genes (A-D) that are highly conserved throughout the animal kingdom.9 Members of this family are disulfide-bonded polypeptides that have multifunctional roles ranging from embryonic development to wound healing.10–12 PDGF signaling has been implicated in several pathologic disease states9,13–17; however, its role in HAND, specifically as it pertains to drug abuse–related changes in barrier permeability, has not been elucidated.
In the present study we explored the mechanisms by which cocaine mediates the induction of PDGF in human brain microvascular endothelial cells (HBMECs) and its functional implications in associated increased neuroinflammation. Understanding the regulation of the isomeric form of PDGF-B (PDGF-BB) expression and its functional relevance by cocaine may provide insights into potential therapeutic targets for neuroinflammation that is correlated with drug abuse.
Methods
Animals
C57BL/6N mice were purchased from (Charles River Laboratories Inc). Egr-1−/− mice purchased from Taconic; before purchase, they were backcrossed 10 generations to a C57BL/6N inbred background. All of animals were housed under conditions of constant temperature and humidity on a 12-hour light/12-hour dark cycle, with lights on at 7:00 am. Food and water were available ad libitum. Animals were deeply anesthetized by overdose of isoflurane followed by pneumothorax before perfusion. All animal procedures were performed according to the protocols approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center.
Cell culture
Primary HBMECs were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum, 10% Nu-Serum (BD Biosciences), 2mM glutamine, 1mM pyruvate, penicillin (100 units/mL), streptomycin (100 μg/mL), essential amino acids, and vitamins. All cell- culture dishes were coated with rat tail collagen type I (R&D Systems Inc).
Reverse transcription and real-time PCR
The quantitative polymerase chain reaction (PCR) primers for mouse PDGF-BB were obtained from SABiosciences. Total RNA was extracted with Trizol reagent (Invitrogen) according to the manufacturer's instructions and our previous report.18
Short Interfering RNA transfection
HBMEC cells were transfected with short interfering RNA (siRNA) σ-1 receptor (σ-1R) from Dharmacon. The knockdown efficiency of siRNAs was determined after 2 days of transfection by Western blotting.
Western blotting
Treated cells were lysed by use of the Mammalian Cell Lysis kit (Sigma-Aldrich). Equal amounts of the proteins were electrophoresed in a sodium dodecyl sulfate-polyacrylamide gel (12%) under reducing conditions followed by transfer to polyvinylidene fluoride membranes. The blots were blocked with 5% nonfat dry milk in phosphate-buffered saline (PBS). The Western blots were then probed with antibodies recognizing the phosphorylated forms of extracellular signal-regulated kinase (ERK), JNK, p38, Akt (1:200, Cell Signaling), PDGF-BB (1:1000; Santa Cruz Biotechnology), Egr-1 (1:200; Santa Cruz Biotechnology), and β-actin (1:4000; Sigma-Aldrich). The secondary antibodies were alkaline phosphatase conjugated to goat anti mouse/rabbit IgG (1:5000) as described previously.19
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assay was performed according to the manufacturer's instructions (Millipore). After treatment the cells with cocaine for 1 hour, 18.5% fresh formaldehyde was added directly into the medium at a final concentration of 1% formaldehyde and incubated for 10 minutes at room temperature, followed by quenching with 10 × glycine. The cells were then scraped using 2 mL of prechilled PBS containing 1 × protease inhibitor cocktail. The cell pellet was harvested by spinning at 800g at 4°C followed by adding lysis buffer (provided in the kit) to harvest nuclei. DNA was sheared by sonication. A total of 50 μL of the sheared cross-linked chromatin was then mixed with 20 μL of protein A magnetic beads and 5 μg of the corresponding antibodies (anti-Egr-1, Anti-acetyl-Histone H3, or IgG) diluted in 450 μL of dilution buffer followed by incubation overnight at 4°C. The magnetic beads binding antibody/chromatin complex was then washed with 0.5 mL each of a series of cold wash buffers in the order of low salt buffer, high salt buffer, LiCl buffer, and finally with Tris-ethylenediaminetetraacetic acid buffer. The cross-linked protein/DNA complexes were reversed to free DNA by incubation at 62°C for 2 hours and purified by the use of DNA purification spin columns following the manufacturer's instructions. Finally, the purified DNA was amplified via PCR to identify promoter region containing Egr-1 binding site “GCG GGG GCG.” The sequence of the primers used to identify the PDGF-BB promoter bound by transcription factor Egr-1 was as follows: sense: 5′-gcagaggcctgagcgcctgatc-3′, anti-sense: 5′-cggagtcggcatgaatcgctgc-3′.
Cell permeability
Primary HBMECs at passages 10-12 were seeded on 6.5-mm polyester Transwell inserts (0.4-μm pore size) and grown to confluence. After confluency HBMECs monolayers were exposed to a mixture of cocaine (10μM) and neutralizing PDGF-BB antibody (500 ng/mL) or an isotype antibody for the time indicated. To detect changes in monolayer permeability, fluorescein isothiocyanate Dextran-4 (1 mg/mL; Sigma-Aldrich) was added to the upper chamber of the Transwell plates. Aliquots (100 μL) were collected from the lower chamber for fluorescent measurement using excitation and emission wavelengths of 480 and 530 nm, respectively.
Isolation of brain microvessels
Brain microvessels were isolated as described previously.20 Animals under anesthesia were perfused with saline; the brains were removed and immediately immersed in ice-cold isolation buffer A (103mM NaCl, 4.7mM KCl, 2.5mM CaCl2, 1.2mM KH2PO4,1.2mM MgSO4, and 15mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4, with Complete Protease Inhibitor). The choroid plexus, meninges, cerebellum, and brain stem were removed followed by homogenization of the brain in 5 mL of isolation buffer B (Buffer A with 25mM NaHCO3, 10mM glucose, 1mM Na pyruvate, and dextran [molecular weight 64 kD; 10 g/L], pH 7.4) with Complete Protease Inhibitor. Dextran (26%) was then added to the homogenates followed by centrifugation at 5800g for 20 minutes. Cell pellets were resuspended in isolation buffer B and filtered through a 70-μm mesh filter (Becton Dickinson). Filtered homogenates were repelleted by centrifugation and smeared on slides.
For immunofluorescence staining, brain microvessels smeared on slides were fixed for 10 minutes at 95°C, followed by incubation with 3% formaldehyde in PBS for 10 minutes at 25°C. The slides were washed 5 times with PBS, permeabilized with 0.1% Triton X-100 for 30 minutes, rewashed 5 times in PBS, and blocked in 1% bovine serum albumin in PBS for 30 minutes at 25°C. Samples were then incubated with rabbit PDGF-BB (1:50; Santa Cruz Biotechnology) and Cav-1 (1:100; Santa Cruz Biotechnology) antibodies (1:100) overnight at 4°C. The slides were washed with PBS and incubated with AlexaFluor 594–conjugated anti–rabbit or AlexaFluor 488–conjugated anti–mouse immunoglobulin G (IgG) for 1 hour at room temperature. After a final washing with PBS, the slides were mounted with mounting medium (Prolong Gold Anti-fade Reagent; Invitrogen) was applied and coverslips were sealed with clear nail polish. Fluorescent images were acquired at room temperature on a Zeiss Observer. A Z1 inverted microscope was used (40×/0.3) oil objective. Images were processed with the use of AxioVs 40 Version 4.8.0.0 software (Carl Zeiss MicroImaging GmbH). Photographs were acquired with an AxioCam MRm digital camera.
Assessment of the BBB Integrity
Assay of BBB integrity was performed in C57BL/6 and Egr-1−/− mice. C57BL/6 mice were divided into 5 groups (n = 6): (a) saline, (b) cocaine, (c) cocaine plus anti-PDGF-BB neutralizing antibody, (d) cocaine plus isotype control antibody, and (e) mannitol. Egr-1−/− mice were treated with either saline or cocaine. Cocaine was injected at a dose of 20 mg/kg once daily on day 1 for 7 days intraperitoneally. In the antibody plus cocaine group, cocaine was first injected for 2 days followed by an intraperitoneal antibody injection on days 3 and 5 at a concentration of 25 μg/mouse/injection (anti-PDGF-BB neutralizing antibody; AF-220-NA, or isotype control goat IgG1; AB-108-C, R&D Systems). On the 8th day, animals were injected in the tail vein with 200 μL of sodium fluorescein (6 mg/mL; Sigma-Aldrich) in PBS that was allowed to circulate for 15 minutes. As a positive control, 200 μL of mannitol (2M) was injected through tail vein to circulate for 1 hour followed by sodium fluorescein injection. The mice were then anesthetized with isoflurane in oxygen and perfused with 30 mL of heparinized saline through the left ventricle. The brains were then harvested and homogenized in PBS (1:10 g/vol). The homogenates were precipitated in 15% trichloroacetic acid (1:1 vol/vol) and centrifuged at 1000g for 10 minutes. The pH was adjusted by adding 125 μL of 5M sodium hydroxide to 500 μL of supernatant aliquots, and fluorescence was detected with a fluorescence plate reader with excitation at 485 nm and emission at 530 nm, followed by quantification according to the previously described report.21
Statistical analysis
Statistical analysis was performed using one-way analysis of variance with a post hoc Student t test. Results were judged statistically significant if P < .05 by analysis of variance.
Results
Cocaine-mediated up-regulation of PDGF mRNA and protein in HBMECs
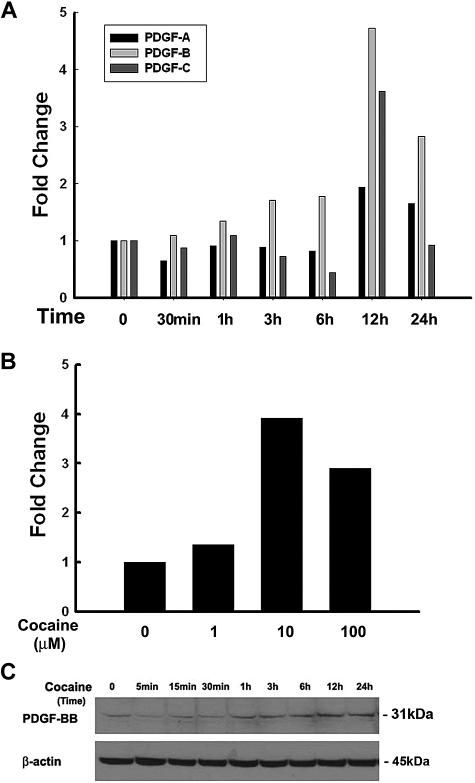
It is well documented that members of the PDGF family play critical roles as cerebrovascular permeants, especially in BBB damage.5 Very little is known, however, whether drugs of abuse such as cocaine that impair endothelial integrity exert their action through PDGF. In the present study HBMECs were serum-starved overnight followed by exposure to cocaine (10μM) for various time points. As an initial screen to identify which of the PDGF chains were regulated in response to cocaine, mRNA levels of PDGF-A, -B, and -C chains were determined by real-time PCR. As shown in Figure 1A, PDGF-B mRNA was maximally up-regulated (4.73- and 2.83-fold) at 12 and 24 hours, respectively, in HBMECs compared with other PDGF subtypes. Further studies were thus kept focused on PDGF-B chain primarily.
Figure 1.
Cocaine-mediated up-regulation of PDGF-BB in HBMECs. (A) Total RNA isolated from HBMECs was subjected to real-time PCR analysis by the use of primer sets specific for PDGF-A, PDGF-B, and PDGF-C. Cocaine dramatically up-regulated PDGF-B chain mRNA expression, with moderate change in PDGF-C and no change in expression of PDGF-A. (B) Dose curve of cocaine-mediated induction of PDGF-B RNA by real-time reverse transcription PCR in HBMECs. (C) Whole cell lysates from cocaine exposed cells were subjected to Western blot analysis with the use of antibodies specific for PDGF-BB. Cocaine induced time-dependent induction of PDGF-BB protein in HBMECs. All data are presented from 3 separate experiments.
Having determined that cocaine mediated increased expression of PDGF-B chain mRNA, the next step was to examine the optimal concentration of cocaine that resulted in up-regulation of PDGF. As shown in Figure 1B, HBMECs cultured in the presence of 10μM cocaine demonstrated increased up-regulation of PDGF-B chain mRNA with slightly lower levels in cells exposed to 100μM cocaine. For further studies we resorted to exposure of cells to 10μM cocaine. The rationale for choosing the range of cocaine (1-100μM) was determined on the basis of reports indicating these concentrations of cocaine in the brains of cocaine abusers after acute intoxication.22–24 To examine whether the mRNA up-regulation translated into increased protein expression, HBMECs were treated as described above, followed by cell lysis and subject to Western blotting. As shown in Figure 1C cocaine up-regulated PDGF-BB protein expression in a time-dependent manner, with a peak expression at 12 hours and a gradual decrease thereafter. Cumulatively, these data clearly demonstrate cocaine-mediated induction of PDGF-B chain RNA and PDGF-BB protein in HBMECs.
Cocaine-induced activation of MAPKs (ERK, JNK, p38) and PI3K-Akt pathways
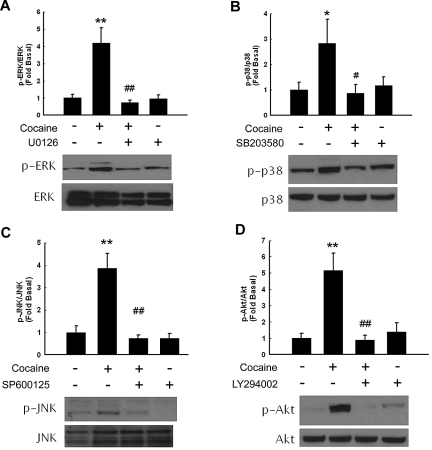
Because mitogen-activated protein kinases (MAPKs) and phosphoinositide 3-kinase (PI3K)–Akt pathways play critical roles in cocaine-mediated signaling pathways,18,25 we next examined the involvement of these pathways in cocaine-mediated induction of PDGF-BB. First, we examined the effect of cocaine on the activation of MAPKs. As shown in Figure 2, treatment of HBMECs with cocaine resulted in increased phosphorylation of ERK, JNK, p38, and Akt. The role of these signaling pathways was further assessed with the use of a pharmacologic approach to block their activation. Pretreatment of cells with either MEK (U0126), JNK (SP600125), p38 (SB203580), or PI3-K (LY294002) inhibitors resulted in amelioration of cocaine-mediated induction of ERK, JNK, p38, and Akt, respectively, thus underpinning their roles in the process.
Figure 2.
Cocaine-induced activation of ERK, p38 JNK, MAPKs, and PI3K/Akt pathways in HBMECs. Western blot analysis of cocaine-mediated activation of ERK (A), p38 (B), JNK (C), and Akt (D). Pretreatment of HBMECs with MEK (U0126), p38 (SB203580), JNK (SP600125), or PI3K (LY294002) inhibitors ameliorated cocaine-mediated activation of the respective signaling pathways. All data are presented as mean ± SD of 3 separate experiments. *P < .05, **P < .01 vs control group; #P < .05, ##P < .01 vs cocaine-treated group.
ERK and JNK but not p38 or PI3K-Akt pathways are involved in cocaine-mediated Egr-1 expression in HBMECs
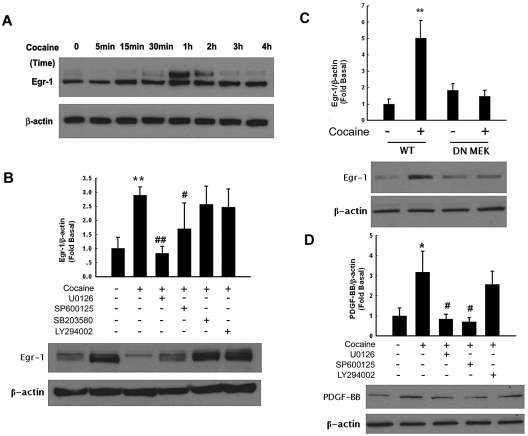
Having determined the activation of ERK, JNK and p38 MAPKs in cocaine-treated HBMECs and because PDGF promoter is known to have a Egr-1 binding site, we next sought to examine whether Egr-1 was the downstream mediator of the MAPKs pathway activated by cocaine. As shown in Figure 3A, cocaine up-regulated expression of Egr-1 in a time-dependent manner, with peak expression between 1 and 2 hours and a decrease thereafter. Together, these data clearly demonstrate that cocaine mediated the induction of Egr-1 expression in HBMECs.
Figure 3.
Egr-1 expression is up-regulated in HBMECs exposed to cocaine. (A) Cocaine induced time-dependent activation of Egr-1 expression in HBMECs. (B) Pharmacologic inhibition of ERK and JNK pathways by MEK (U0126) and JNK (SP600125) inhibitors resulted in amelioration of cocaine-mediated induction of Egr-1. (C) Transfection of cells with DN-MEK but not WT-MEK resulted in abrogation of cocaine-mediated induction of Egr-1. (D) Pretreatment of cells with MEK (U1026) and JNK (SP600125) but not PI3K (LY294002) inhibitors resulted in amelioration of cocaine-mediated induction of PDGF-BB. All data are presented as mean ± SD of 3 individual experiments. *P < .05, **P < .01 vs control group; #P < .05, ##P < .01 vs cocaine-treated group.
Next logic step then was to examine whether there existed a link that could tie the activation of ERK, JNK, p38 MAPKs, and PI3K/Akt to Egr-1. Similar to the studies on signaling molecules described previously, HBMECs were pretreated with MEK, JNK, p38, or PI3K inhibitors followed by treatment with cocaine and assessed for expression of Egr-1 by Western blotting. As shown in Figure 3B, MEK and JNK but not the p38 or PI3K inhibitors were able to inhibit cocaine-mediated activation of Egr-1.
Further validation of the involvement of ERK pathway in this process was confirmed by transfecting cells with either the wild-type (WT) or dominant-negative (DN) constructs of MEK followed by exposure to cocaine. Cocaine-mediated induction of Egr-1 was attenuated by DN-MEK but not by the WT-MEK construct (Figure 3C). Taken together, these findings thus underscore the involvement of ERK and JNK MAPKs but not p38 or PI3K/Akt cascade in cocaine-mediated induction of Egr-1 in HBMECs.
We next wanted to address the functional role of MAPKs and PI3K/Akt in PDGF-BB expression induced by cocaine. HBMECs were pretreated with inhibitors specific for the respective signaling pathways before stimulation with cocaine and assessed for expression of PDGF-BB. As shown in Figure 3D, pretreatment of cells with MEK (U0126, 20μM), JNK (SP600125, 20μM), but not p38 (SB203580, 20μM, data not shown) or PI3K (LY294002, 10μM) inhibitors resulted in amelioration of cocaine-mediated induction of PDGF-BB. Together, these findings confirm the involvement of ERK and JNK MAPKs but not p38 MAPK or PI3K/Akt cascade in cocaine-mediated induction of PDGF-BB in HBMECs.
Involvement of Egr-1 in cocaine-induced expression of PDGF-BB in HBMECs
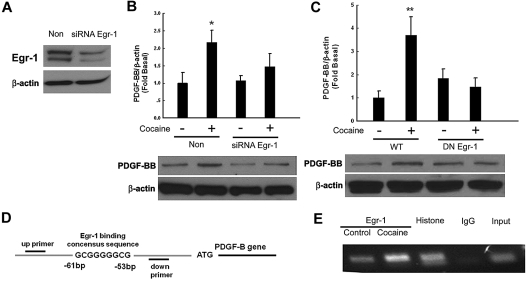
Because Egr-1 is a transcription factor implicated in the induction of PDGF-BB in arterial endothelial cells,26 we next examined its role in cocaine-mediated induction of PDGF-BB. The approach of silencing Egr-1 expression with the siRNA transfection was used. As shown in Figure 4A, transfection of HBMECs with Egr-1 siRNA resulted in efficient knockdown of Egr-1 protein as evidenced by Western blotting. Intriguingly, Egr-1 siRNA also significantly abrogated cocaine-mediated up-regulation of PDGF-BB expression (Figure 4B). To further validate the involvement of the Egr-1 in cocaine-induced regulation of PDGF-BB, cells were transfected with either WT or DN constructs of Egr-1, followed by exposure with cocaine. Cocaine-mediated inductions of PDGF-BB were attenuated by the DN-Egr-1 construct but not by the WT-Egr-1 construct (Figure 4C). Collectively, these findings thus underscored the role of Egr-1 in cocaine-mediated induction of PDGF-BB.
Figure 4.
Involvement of Egr-1 in cocaine-induced expression of PDGF-BB. (A) Whole cell lysates from HBMECs transfected with either Egr-1/nonsense (Non) siRNAs subject to Western blot analysis. (B) Egr-1 siRNA but not nonspecific siRNA inhibited cocaine-mediated induction of PDGF-BB. (C) DN-MEK but not WT-MEK inhibited cocaine-mediated induction of PDGF-BB. (D) Schematic illustration of Egr-1 binding consensus sequence on the PDGF-B promoter region. (E) ChIP assay demonstrating cocaine-mediated binding of Egr-1 to the PDGF-B promoter. Histone lane: positive control; IgG lane: negative control. All data are presented as mean ± SD of 3 separate experiments. *P < .05, **P < .01 vs control group.
The next step was to confirm the binding of Egr-1 to the PDGF-B promoter in its natural chromatin context. We resorted to the ChIP assay to reveal active sites on the promoter that were accessible to Egr-1. HBMECs were treated with cocaine for 1 hour followed by RNA extraction and processed by the use of the ChIP Analysis kit. These findings demonstrated increased binding of Egr-1 to the PDGF-B promoter in HBMECs exposed to cocaine (Figure 4D-E).
Engagement of σ-1R is critical for cocaine-induced PDGF-BB expression
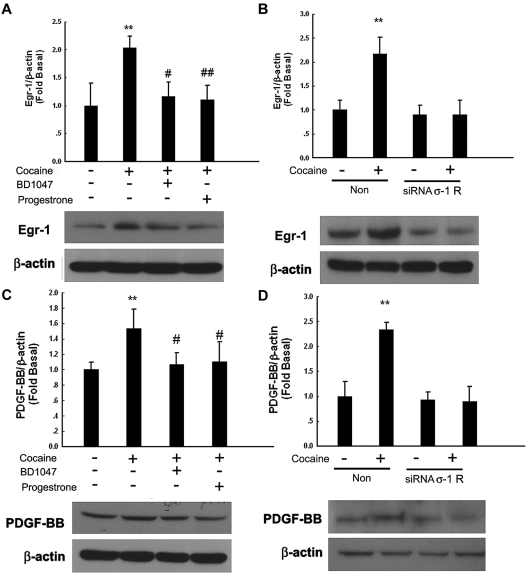
σ-1R, which belongs to the nonopioid receptor family, binds to a diverse class of psychotropic drugs, including cocaine.27 Cocaine-mediated induction of Egr-1 was significantly attenuated by pretreatment of cells with the known σ-1R antagonists BD104728 and progesterone29 (Figure 5A) or in cells transfected with σ-1R siRNA (Figure 5B). A similar trend was observed for PDGF-BB in cells treated with either the σ-1R antagonists (Figure 5C) or the σ-1R siRNA (Figure 5D), thereby underscoring the role of σ-1R in this process.
Figure 5.
Engagement of σ-1 receptor (σ-1R) is critical for cocaine-induced PDGF-BB expression. (A) Pretreatment of HBMECs with σ-1R antagonist BD1047 or progesterone inhibited cocaine-induced induction of Egr-1. (B) σ-1R siRNA but not nonspecific siRNA inhibited cocaine-mediated induction of Egr-1 expression. (C) Pretreatment of HBMECs with σ-1R antagonist BD1047 or progesterone inhibited cocaine-mediated induction of PDGF-BB expression. (D) σ-1R siRNA, but not nonspecific siRNA inhibited cocaine-mediated induction of PDGF-BB. All the data are presented as mean ± SD of 4 separate experiments. **P < .01 vs control group; #P < .05, ##P < .01 vs cocaine-treated group.
Cocaine-mediated disruption of zona occludens 1 expression involves PDGF-BB
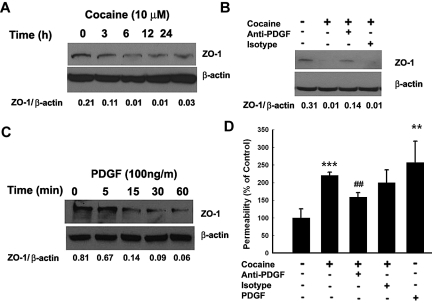
To examine whether cocaine induced disruption of BBB, HBMECs were exposed to cocaine and assessed for expression of tight junction protein zona occludens 1 (ZO-1). As shown in Figure 6A, cocaine exposure resulted in a sustained time-dependent decrease in ZO-1 expression. To assess whether this effect was mediated by cocaine-induced PDGF-BB, HBMECs were cocultured with cocaine with or without PDGF-BB neutralizing antibody followed by analysis of ZO-1 expression. As shown in Figure 6B, and as expected, exposure of cells to cocaine resulted in significant reduction in ZO-1 expression at 24 hours compared with untreated control cells. Intriguingly, treatment of cells with the PDGF-BB neutralizing antibody ameliorated cocaine-mediated reduction of ZO-1 expression, thereby underscoring the role of PDGF-BB in this process. Validation of the role of PDGF was also independently determined by treating HBMECs with PDGF-BB alone. As shown in Figure 6C, PDGF-BB exposure resulted in a rapid down-regulation of ZO-1 expression within as little as 1 hour.
Figure 6.
Cocaine-mediated disruption of ZO-1 expression involves PDGF-BB. (A) Exposure of HBMECs to cocaine resulted in time-dependent decrease in expression of ZO-1. (B) Cocaine-mediated decrease of ZO-1 expression was ameliorated by pretreating the cells with the PDGF-BB neutralizing antibody. (C) Exposure of HBMECs to PDGF-BB resulted in a time-dependent decrease in ZO-1 expression. (D) Cocaine-mediated increase in BBB permeability was ameliorated in HBMECs pretreated with the PDGF-BB neutralizing antibody. All the data are presented as mean ± SD of 4 separate experiments. **P < .01, ***P < .001 vs control group; ##P < .01 vs cocaine-treated group.
Functional relevance of PDGF-mediated down-regulation of ZO-1 was confirmed by permeability assays. As shown in Figure 6D, exposure of HBMECs monolayer to either cocaine or PDGF-BB resulted in increased monolayer permeability and this effect was time-dependent (data not shown). Furthermore, exposure of cells to neutralizing antibody to PDGF-BB ameliorated cocaine-induced increase in cell permeability, thus implicating the role of PDGF-BB in cocaine-mediated disruption of BBB.
Cocaine-mediated induction of PDGF-BB in vivo
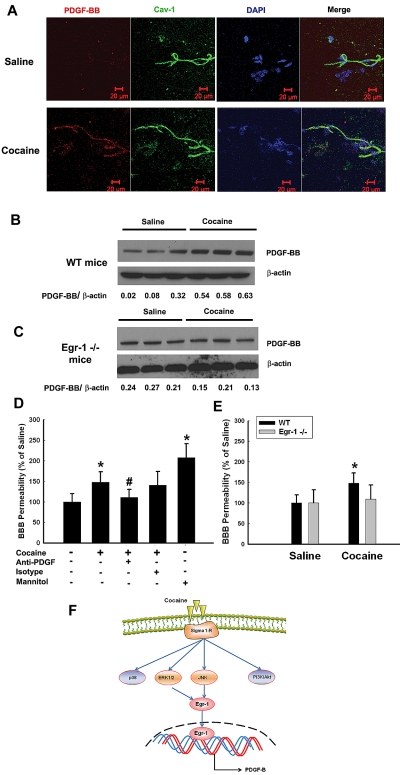
To validate cocaine-mediated induction of PDGF-BB in vivo, brain microvessels isolated from cocaine versus saline-treated mice were assessed for expression of PDGF-BB. Cocaine administration resulted in increased expression of PDGF-BB in isolated microvessels compared with saline injected mice as demonstrated by both immunostaining of freshly isolated brain microvessels (Figure 7A) and by Western blotting (Figure 7B) of the microvessel homogenates. Intriguingly, cocaine mediated up-regulation of PDGF-BB was ameliorated in Egr-1−/− mice compared with WT controls (Figure 7C).
Figure 7.
Cocaine-mediated induction of PDGF-BB in vivo. (A) Cocaine administration resulted in increased expression of PDGF-BB (red, bottom) in isolated microvesssels (Caveolin [Cav-1] positive, green) compared with saline-injected animals (top). PDGF-BB: red; Cav-1: green; nuclei: blue; n = 4 per group; Scale bar: 20 μm. (B) Western blot analysis of lysates from isolated microvessels from cocaine-administered mice demonstrated increased expression of PDGF-BB compared with saline-injected animals. (C) Egr-1−/− mice treated with cocaine failed to up-regulate PDGF-BB in the isolated microvessels. (D) Pretreatment of mice with PDGF-BB neutralizing antibody ameliorated cocaine-mediated increase in BBB permeability. Mannitol-treated group was used as a positive control. (E) Cocaine administration resulted in increased BBB permeability in WT but not in the Egr-1−/− mice. All the data are presented as mean ± SD, n = 6 per group. *P < .05 vs saline group; #P < .05 vs cocaine group. (F) Schematic of the signaling pathways involved in cocaine-mediated induction of PDGF-BB in HBMECs. Exposure of cocaine leads to σ receptor-mediated activation of ERK1/2, JNK, p38 MAPKs and PI3K/Akt signaling pathways. However, ERK1/2 and JNK MAPKs but not p38 MAPK or PI3K/Akt signaling result in the subsequent activation of the downstream transcription factor, Egr-1. Activation of Egr-1, in turn, leads to enhanced PDGF-BB expression.
Cocaine-mediated permeability in vivo involves PDGF-BB
To validate the role of PDGF-BB in impairment of barrier integrity, we examined the permeability of low-molecular-weight sodium fluorescein into the brain in cocaine-administered and untreated mice. In brief, mice injected with cocaine were administered either anti-PDGF-BB neutralizing antibody or the isotype control for 7 days, followed by tail vein injection of sodium fluorescein. As demonstrated in Figure 7D, and as expected, cocaine injection disrupted the BBB, and this effect was ameliorated by anti-PDGF-BB antibody, but not the isotype control, lending support to the suggestion that PDGF-BB played a critical role in BBB damage. Because Egr-1 binding was critical for cocaine-induced expression of PDGF-BB both in vitro and in vivo systems, we next examined the effect of Egr-1 deficiency on permeability of BBB. As shown in Figure 7E, cocaine administration failed to cause BBB damage in Egr-1−/− mice.
Discussion
Although antiviral therapies have had a profound impact on controlling systemic HIV-1 load, thus leading to increased longevity in AIDS patients, the inability of some of these drugs to cross the BBB actually results in a slow and smoldering infection/cell activation in the CNS. The brain thus becomes a sanctuary for virus-induced toxicity, leading to increased prevalence of HAND as these patients continue to live longer. Adding fuel to this problem is the increased use of illicit drug abuse that is also at issue in HIV-1–infected patients. Intriguingly, increased use of the psychostimulant cocaine in HIV-1–infected patients has been linked to increased HIV seroprevalence and disease progression.30 However, the detailed mechanism of its action remains elusive.
Cocaine has been shown to “open” up the BBB, thereby increasing emigration of infected/activated leukocytes from blood vessels into the brain through direct effect on HBMECs and also indirectly, through the paracrine effects on BBB via release of proinflammatory cytokines (tumor necrosis factor-α and inteferon-γ).2,31–33 Cocaine can also alter BBB permeability via down-regulation of tight junction protein-ZO-1 expression.34 Furthermore, exposure of brain endothelial cells to cocaine has been demonstrated to up-regulate the expression of endothelial adhesion molecules like intracellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin, thereby increasing leukocyte migration across the endothelial monolayers.31,32 Cocaine thus causes impairment of barrier permeability and endothelial transmigration of HIV-infected leukocytes.33 Although it is well known that cocaine abuse leads to changes in the limbic pathway resulting in addictive behavior, the aforementioned findings on the effect of cocaine on BBB, point to a yet another adjunctive role of this drug.
In the present study we demonstrated that exposure of HBMECs to cocaine resulted in induction of PDGF at both the transcriptional and translational levels, through the binding of cocaine to its cognate σ 1-R. Further dissection of the signaling pathways involved in cocaine-mediated induction of PDGF-BB by the use of pharmacologic and genetic approaches revealed activation of ERK, JNK, p38 MAPKs, and also PI3K/Akt pathway, a finding that is consistent previous reports on the effect of cocaine in microglia and neurons.18,25 Cocaine-mediated induction of PDGF-BB expression, however, involved ERK and JNK MAPKs but not p38 MAPK or PI3K/Akt pathways.
The transcription factor Egr-1 has emerged as a major regulatory transcription factor for several genes including growth factors such as PDGF-BB.35,36 Our findings demonstrated a time-dependent up-regulation of Egr-1 protein in HBMECs. Further dissection of Egr-1 regulation with the use of both the pharmacologic and genetic approaches revealed the activation of ERK and JNK MAPKs pathways upstream of Egr-1. In agreement with our findings, requirement of ERK and JNK activation for Egr-1 expression has also been reported in FGF-2 treated astrocytes.37
In an effort to determine a link between Egr-1 and PDGF-BB expression, we also demonstrated increased Egr-1 binding to the PDGF-B promoter in cocaine-exposed HBMECs, lending credence to the role of Egr-1 in PDGF-BB expression. Further support of Egr-1 involvement in cocaine-mediated PDGF-BB induction also was demonstrated by the use of both the siRNA and the genetic approaches. Our findings are in agreement with the report by Khachigian et al,26 who demonstrated interaction of Egr-1 with the PDGF-B promoter in arterial endothelial cells.
Our results also demonstrated that cocaine-induced PDGF-BB expression involved activation of ERK and JNK MAPKs cascade and downstream activation of Egr-1. The role of Egr-1 in PDGF-BB induction has been confirmed in previous studies.26 Moreover, MAPK-mediated transduction of its signal via activation of its downstream transcription factor Egr-1 has also been implicated in other cellular systems.37,38 Consistent with these findings, we also confirmed the role of Egr-1 translocation in cocaine-mediated induction of PDGF-BB expression.
Functional implication of cocaine-mediated induction of PDGF-BB both in vitro and in vivo models of BBB also was assessed. Taking into account that PDGF-BB is a known agent involved in disruption of BBB and that cocaine-mediated decrease in ZO-1 expression involves PDGF-BB, we were interested to compare the kinetics of ZO-1 down-regulation independently by cocaine and PDGF-BB alone. Interestingly, PDGF-mediated down-regulation of ZO-1 preceded that of cocaine, leading us to speculate that cocaine exerted its effect indirectly via induction of PDGF-BB. This was also confirmed by treating cells with PDGF-B neutralizing antibody, which resulted in reversal of cocaine-mediated down-regulation of ZO-1 expression. These findings are in agreement with a recent study implicating the role of PDGF family members in BBB damage.5
In summary, our findings demonstrated detailed molecular pathway of cocaine-mediated induction of PDGF-BB in HBMECs involving activation of ERK and JNK MAPKs and downstream transcription factor Egr-1 pathways, resulting in increased PDGF-BB expression, and ultimately culminating into BBB damage, as summarized in Figure 7F. These findings have implications for HIV-1–infected cocaine abusers that are known to have increased risk of stroke and neuroinflammation.
Acknowledgments
We thank Dr Guoku Hu for designing PDGF-B chain promoter primers for the ChIP assays. The technical support of Yaman Lu is highly appreciated.
This work was supported by grants MH-068212, DA020392, DA023397, DA024442 (to S.B.), and DA030285 (to H.Y.) from the National Institutes of Health in addition to Nebraska Tobacco Settlement Biomedical Research.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.H.Y. designed and performed research and wrote the manuscript; M.D. performed research; and S.B. planned and designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shilpa J. Buch, PhD, Department of Pharmacology and Experimental Neuroscience, 985880 Nebraska Medical Center (DRC 8011), University of Nebraska Medical Center, Omaha, NE 68198-5880; e-mail: sbuch@unmc.edu.
References
- 1.Fiala M, Eshleman AJ, Cashman J, et al. Cocaine increases human immunodeficiency virus type 1 neuroinvasion through remodeling brain microvascular endothelial cells. J Neurovirol. 2005;11(3):281–291. doi: 10.1080/13550280590952835. [DOI] [PubMed] [Google Scholar]
- 2.Fiala M, Gan XH, Zhang L, et al. Cocaine enhances monocyte migration across the blood-brain barrier. Cocaine's connection to AIDS dementia and vasculitis? Adv Exp Med Biol. 1998;437:199–205. doi: 10.1007/978-1-4615-5347-2_22. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Looney D, Taub D, et al. Cocaine opens the blood-brain barrier to HIV-1 invasion. J Neurovirol. 1998;4(6):619–626. doi: 10.3109/13550289809114228. [DOI] [PubMed] [Google Scholar]
- 4.Potula R, Dhillion N, Sui Y, et al. Association of platelet-derived growth factor-B chain with simian human immunodeficiency virus encephalitis. Am J Pathol. 2004;165(3):815–824. doi: 10.1016/S0002-9440(10)63344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su EJ, Fredriksson L, Geyer M, et al. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med. 2008;14(7):731–737. doi: 10.1038/nm1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao R, Brakenhielm E, Li X, et al. Angiogenesis stimulated by PDGF-CC, a novel member in the PDGF family, involves activation of PDGFR-alphaalpha and -alphabeta receptors. FASEB J. 2002;16(12):1575–1583. doi: 10.1096/fj.02-0319com. [DOI] [PubMed] [Google Scholar]
- 7.Koyama N, Watanabe S, Tezuka M, Morisaki N, Saito Y, Yoshida S. Migratory and proliferative effect of platelet-derived growth factor in rabbit retinal endothelial cells: evidence of an autocrine pathway of platelet-derived growth factor. J Cell Physiol. 1994;158(1):1–6. doi: 10.1002/jcp.1041580102. [DOI] [PubMed] [Google Scholar]
- 8.Sato N, Beitz JG, Kato J, et al. Platelet-derived growth factor indirectly stimulates angiogenesis in vitro. Am J Pathol. 1993;142(4):1119–1130. [PMC free article] [PubMed] [Google Scholar]
- 9.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22(10):1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown RL, Breeden MP, Greenhalgh DG. PDGF and TGF-alpha act synergistically to improve wound healing in the genetically diabetic mouse. J Surg Res. 1994;56(6):562–570. doi: 10.1006/jsre.1994.1090. [DOI] [PubMed] [Google Scholar]
- 11.Lepistö J, Kujari H, Niinikoski J, Laato M. Effects of heterodimeric isoform of platelet-derived growth factor PDGF-AB on wound healing in the rat. Eur Surg Res. 1994;26(5):267–272. doi: 10.1159/000129345. [DOI] [PubMed] [Google Scholar]
- 12.Graham S, Leonidou A, Lester M, Heliotis M, Mantalaris A, Tsiridis E. Investigating the role of PDGF as a potential drug therapy in bone formation and fracture healing. Expert Opin Investig Drugs. 2009;18(11):1633–1654. doi: 10.1517/13543780903241607. [DOI] [PubMed] [Google Scholar]
- 13.Stürzl M, Roth WK, Brockmeyer NH, Zietz C, Speiser B, Hofschneider PH. Expression of platelet-derived growth factor and its receptor in AIDS-related Kaposi sarcoma in vivo suggests paracrine and autocrine mechanisms of tumor maintenance. Proc Natl Acad Sci U S A. 1992;89(15):7046–7050. doi: 10.1073/pnas.89.15.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao Y, Cao R, Hedlund EM. R Regulation of tumor angiogenesis and metastasis by FGF and PDGF signaling pathways. J Mol Med. 2008;86(7):785–789. doi: 10.1007/s00109-008-0337-z. [DOI] [PubMed] [Google Scholar]
- 15.Claesson-Welsh L. Mechanism of action of platelet-derived growth factor. Int J Biochem Cell Biol. 1996;28(4):373–385. doi: 10.1016/1357-2725(95)00156-5. [DOI] [PubMed] [Google Scholar]
- 16.Hede SM, Hansson I, Afink GB, et al. GFAP promoter driven transgenic expression of PDGFB in the mouse brain leads to glioblastoma in a Trp53 null background. Glia. 2009;57(11):1143–1153. doi: 10.1002/glia.20837. [DOI] [PubMed] [Google Scholar]
- 17.Hellberg C, Ostman A, Heldin CH. PDGF and vessel maturation. Recent Results Cancer Res. 2010;180:103–114. doi: 10.1007/978-3-540-78281-0_7. [DOI] [PubMed] [Google Scholar]
- 18.Yao H, Yang Y, Kim KJ, et al. Molecular mechanisms involving sigma receptor-mediated induction of MCP-1: implication for increased monocyte transmigration. Blood. 2010;115(23):4951–4962. doi: 10.1182/blood-2010-01-266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao H, Peng F, Dhillon N, et al. Involvement of TRPC channels in CCL2-mediated neuroprotection against tat toxicity. J Neurosci. 2009;29(6):1657–1669. doi: 10.1523/JNEUROSCI.2781-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang W, Rha GB, Chen L, et al. Inhibition of telomerase activity alters tight junction protein expression and induces transendothelial migration of HIV-1-infected cells. Am J Physiol Heart Circ Physiol. 2010;298(4):H1136–1145. doi: 10.1152/ajpheart.01126.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Swartz KR, Toborek M. Vessel microport technique for applications in cerebrovascular research. J Neurosci Res. 2009;87(7):1718–1727. doi: 10.1002/jnr.21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalasinsky KS, Bosy TZ, Schmunk GA, et al. Regional distribution of cocaine in postmortem brain of chronic human cocaine users. J Forensic Sci. 2000;45(5):1041–1048. [PubMed] [Google Scholar]
- 23.Stephens BG, Jentzen JM, Karch S, Mash DC, Wetli CV. Criteria for the interpretation of cocaine levels in human biologic samples and their relation to the cause of death. Am J Forensic Med Pathol. 2004;25(1):1–10. doi: 10.1097/01.paf.0000118960.58334.a9. [DOI] [PubMed] [Google Scholar]
- 24.Van Dyke C, Barash PG, Jatlow P, Byck R. Cocaine: plasma concentrations after intranasal application in man. Science. 1976;191(4229):859–861. doi: 10.1126/science.56036. [DOI] [PubMed] [Google Scholar]
- 25.Yao H, Allen JE, Zhu X, Callen S, Buch S. Cocaine and human immunodeficiency virus type 1 gp120 mediate neurotoxicity through overlapping signaling pathways. J Neurovirol. 2009;15(2):164–175. doi: 10.1080/13550280902755375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khachigian LM, Lindner V, Williams AJ, Collins T. Egr-1-induced endothelial gene expression: a common theme in vascular injury. Science. 1996;271(5254):1427–1431. doi: 10.1126/science.271.5254.1427. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi T, Su TP. Sigma-1 receptors (sigma(1) binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. J Pharmacol Exp Ther. 2003;306(2):718–725. doi: 10.1124/jpet.103.051284. [DOI] [PubMed] [Google Scholar]
- 28.Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009;124(2):195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131(3):596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 30.Goodkin K, Shapshak P, Metsch LR, et al. Cocaine abuse and HIV-1 infection: epidemiology and neuropathogenesis. J Neuroimmunol. 1998;83(1–2):88–101. doi: 10.1016/s0165-5728(97)00225-7. [DOI] [PubMed] [Google Scholar]
- 31.Gan X, Zhang L, Berger O, et al. Cocaine enhances brain endothelial adhesion molecules and leukocyte migration. Clin Immunol. 1999;91(1):68–76. doi: 10.1006/clim.1998.4683. [DOI] [PubMed] [Google Scholar]
- 32.Gan X, Zhang L, Newton T, et al. Cocaine infusion increases interferon-gamma and decreases interleukin-10 in cocaine-dependent subjects. Clin Immunol Immunopathol. 1998;89(2):181–190. doi: 10.1006/clin.1998.4607. [DOI] [PubMed] [Google Scholar]
- 33.Nair MPN, Mahajan S, Hou J, Sweet AM, Schwartz SA. The stress hormone, cortisol, synergizes with HIV-1 gp-120 to induce apoptosis of normal human peripheral blood mononuclear cells. Cell Mol Biol (Noisy-le-grand) 2000;46(7):1227–1238. [PubMed] [Google Scholar]
- 34.Dhillon NK, Peng F, Bokhari S, et al. Cocaine-mediated alteration in tight junction protein expression and modulation of CCL2/CCR2 axis across the blood-brain barrier: implications for HIV-dementia. J Neuroimmune Pharmacol. 2008;3(1):52–56. doi: 10.1007/s11481-007-9091-1. [DOI] [PubMed] [Google Scholar]
- 35.James AB, Conway AM, Morris BJ. Genomic profiling of the neuronal target genes of the plasticity-related transcription factor— Zif268. J Neurochem. 2005;95(3):796–810. doi: 10.1111/j.1471-4159.2005.03400.x. [DOI] [PubMed] [Google Scholar]
- 36.Silverman ES, Collins T. Pathways of Egr-1-mediated gene transcription in vascular biology. Am J Pathol. 1999;154(3):665–670. doi: 10.1016/S0002-9440(10)65312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin SY, Song H, Kim CG, et al. Egr-1 is necessary for fibroblast growth factor-2-induced transcriptional activation of the glial cell line-derived neurotrophic factor in murine astrocytes. J Biol Chem. 2009;284(44):30583–30593. doi: 10.1074/jbc.M109.010678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng F, Yao H, Bai X, et al. Platelet-derived growth factor-mediated induction of the synaptic plasticity gene Arc/Arg3.1. J Biol Chem. 2010;285(28):21615–21624. doi: 10.1074/jbc.M110.107003. [DOI] [PMC free article] [PubMed] [Google Scholar]