Abstract
We report a case of imported Plasmodium knowlesi malaria in a French tourist following a vacation in Thailand. This case shows, first, tourists may contract knowlesi malaria even only staying on the beach and second, the diagnosis remains difficult, even with polymerase chain reaction methods.
Plasmodium knowlesi was initially identified in 1931 in a Macaca fascicularis monkey originating in Singapore, and was shown to be capable of infecting humans one year later.1 The first naturally transmitted P. knowlesi infection in humans was reported in 1965 in a man after a visit to the Malaysian peninsula.2 No other reports were published on naturally acquired P. knowlesi infection in humans until 2004 when Sing and colleagues3 reported 100 human cases of P. knowlesi in Malaysian Borneo that were identified by polymerase chain reaction (PCR), having been first misdiagnosed as Plasmodium malariae on blood smears. Human P. knowlesi infection has since been observed in several Southeast Asian countries such as China,4 Thailand,5 Myanmar,6 Philippines,7 Singapore,8 and Indonesian Borneo.9 The geographical repartition of this zoonotic infection (the human-to-human cycle has not yet been documented) corresponds to the overlap of the distribution of the main natural hosts; the long-tailed (Macaca fascicularis) and the pig-tailed (Macaca nemestrina) macaques, and the competent vectors.10,11
Plasmodium knowlesi human infection has been also described in Western countries in travelers returning from Southeast Asia. In the six published cases, travelers had been infected in Malaysian Borneo (N = 3),9,12,13 in the Borneo peninsula (N = 1),14 in the Philippines (N = 1),15 and in Malaysia or in Vietnam (N = 1).16 We describe the first report of human P. knowlesi infection in a traveler returning from Thailand.
Case Report
On February 23, 2010, a 45-year-old man consulted the Infectious and Tropical Disease Unit of Cahors Hospital (France) with an 8-day history of fever, shivering, nausea, and anorexia. The patient had recently returned (February 6) from a 3-month holiday on the west coast of Thailand.
The clinical examination was reassuring with only pain in the right upper quadrant.
Blood tests showed thrombocytopenia (platelet count, 73 Giga/L), no anemia (hemoglobin, 130 g/L), and a normal white blood cell count. Some slight liver abnormalities were observed (serum alanine aminotranferase 75 IU/L, normal range (N) < 41; aspartate aminotransferase 58 IU/L, N < 38; gamma-glutamyltransferase 64 IU/L, N < 55; alkaline phosphatase 124 IU/L, N < 120). The lactic dehydrogenase was abnormal (787 IU/L, reference range < 620) and an inflammatory syndrome was also noted with C-reactive protein at 186 mg/L (N < 10). Blood sugar and creatinine were normal.
A thin blood smear revealed 0.8% Plasmodium parasitemia with a morphologic appearance suggestive of a mixed infection by both P. malariae and Plasmodium falciparum. Rapid diagnostic tests (RDTs) (immunochromatographic technique) were performed: the Core Malaria Pan/Pv/Pf test (Core Diagnostics, Birmingham, UK) was positive for Plasmodium vivax (specific P. vivax lactic dehydrogenase [pLDH]) and Pan-Plasmodium (pLDH common to the four human Plasmodium species). The BinaxNOW test (Inverness Medical, Sevres, France) was also positive for Pan-Plasmodium (aldolase common to the four human Plasmodium species). For these two RDTs the detection of the histidine-rich protein 2 (HRP2), a specific antigen of P. falciparum, was negative. Given the discrepancy between the thin blood smear and the RDTs, a blood sample was sent to the Parasitology-Mycology Unit of the Toulouse University Hospital (France).
A thin blood smear confirmed the first observation in Cahors Hospital (Figure 1) and the RDT Palutop+4 test (All Diag, Strasbourg, France) showed the same pattern as the previously used RDTs, namely, positive for P. vivax-LDH and Pan-pLDH and negative for the P. falciparum HRP2.
Figure 1.
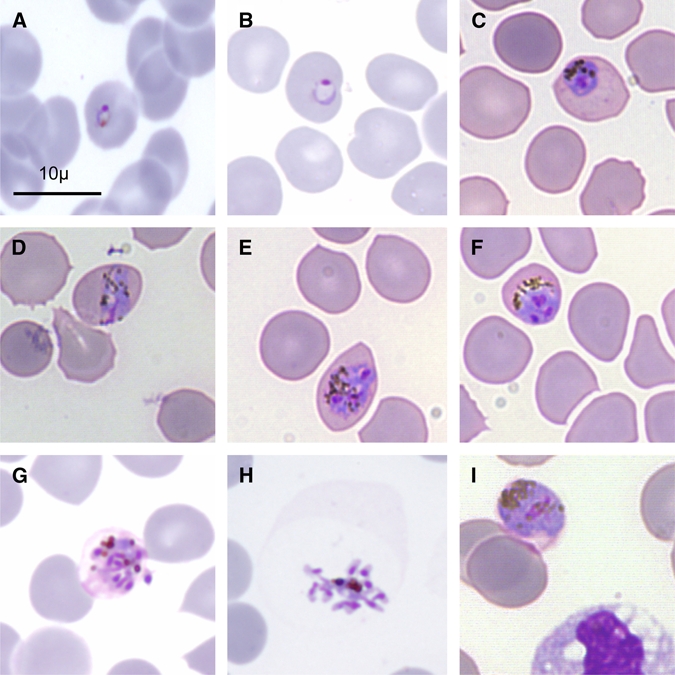
Microscopic morphology of Plasmodium knowlesi in a May-Grünwald Giemsa-stained thin blood smear. Infected erythrocytes were not enlarged with the presence of much hemozoin and lacked stippling. (A, B) Young trophozoites, (C) late trophozoite, (D) equatorial band form, (E, G) schizonts, (H) merozoites, (I) gametocyte.
As routinely carried out in the unit for the diagnosis of Plasmodium infections, a multiplex real-time PCR on a LightCycler apparatus (Roche Diagnostics, Meylan, France) was performed using SYBERgreen I chemistry (Roche Diagnostics). This technique is able to detect the DNA of both Plasmodium genus and P. falciparum as described by Fabre and others.17 The single amplification of the DNA of the Plasmodium genus confirmed the malaria attack but eliminated a P. falciparum infection. An additional set of three different real-time PCRs was used with the same technology to detect P. vivax, P. ovale, or P. malariae infection as described by the same authors17 (see Table 1 for the primers used). For this patient, only the P. vivax PCR was positive with the same melting temperature as the control (P. vivax DNA), confirming the specificity of amplification.
Table 1.
Description of primers
| Name | Specificity* | Gene target | bp† | Sequence | Reference |
|---|---|---|---|---|---|
| L1 L2 | Plasmodium spp. | Plastid-like large subunit ribosomal-RNA (LSU-rRNA) | 595 | 5′-GAC CTG CAT GAA AGA TG | Tan and others, 199718 |
| 5′-GTA TCG CTT TAA TAG GCG | |||||
| Pf1 Pf2 | P. falciparum | Mitochondrial Cox1 | 422 | 5′-GGA ATG TTA TTG CTA ACA C | Tham and others, 199919 |
| 5′-AAT GAA GAG CTG TGT ATC | |||||
| PLF MAR | P. malariae | Small subunit ribosomal RNA (SSUrRNA) | 269 | 5′-AGT GTG TAT CAA TCG AGT TT | Rubio and others, 199920 |
| 5′-GCC CTC CAA TTG CCT TCT | |||||
| PLF OVR | P. ovale | Small subunit ribosomal RNA (SSUrRNA) | 436 | 5′-AGT GTG TAT CAA TCG AGT TT | Rubio and others, 199920 |
| 5′-GCA TAA GGA ATG CAA AGA ACA G | |||||
| Pv1 Pv2 | P. vivax | Mitochondrial Cox1 | 871 | 5′-CAC CAT TAA GTA CAT CAC | Tham and others, 199919 |
| 5′-TGT TAA TAC AAC TCC AAT | |||||
| mtPk-FmtPk-R | P. knowlesi,P. vivax, P. inui,P. cynomolgi | Mitochondrial Cytb | 1,000 | 5′-AGG TAT TAT ATT CTT TAT ACA AAT ATT AAC | Jongwuties and others, 20045 |
| 5′-TCT TTT ATA ATG AAC AAG TGT AAA TAA TC |
Specificity known at the date of the publication.
Base pairs.
Because of the morphological aspect of the parasite on the smear that was hardly compatible with P. vivax, and given the possibility of infection with P. knowlesi during a stay in Southeast Asia, sequencing was done to confirm the malarial species. Amplification of a 1,000 bp fragment of the mitochondrial cytb gene was carried out as described by Jongwutiwes and others.5 The 5 μL of extracted DNA were resuspended in 45 μL of an PCR buffer containing 0.5 μM of each primer mtPk-F and mtPk-R (Table 1), 3.5 mM MgCl2, and 0.2U AmpliTaq Gold (Applied Biosystems, Courtaboeuf, France). The PCR amplification parameters were 96°C for 10 min, 40 cycles at 94°C for 30 s, 60°C for 1 min, and 72°C for 1 min, followed by a final extension for 10 min at 72°C. The cycle sequencing was then done with a BigDye Terminator Cycle Sequencing Kit (Applied Biosystems) according to the manufacturer's instructions and the products were analyzed on an ABI PRISM 3100 sequencer (Applied Biosystems). A basic local alignment search tool (BLAST) search of the GeneBank database with the sequence obtained (1,000 bp), showed 100% identity with P. knowlesi sequences (including sequences GenBank accession nos.: AB444108, EU880499, AF069621, AY580318)
The patient was treated with a 500 mg oral dose of chloroquine, daily for 5 days, which was well tolerated and efficient, and there has been no relapse to date. The patient recounted the details of his travel in Thailand. He stayed all of the 3 months on the West coast around the town of Rangon about 200 km from the well-known tourist site of Phuket. For the last month of his stay, he was on the island of Ko Payam (9°83′S; 98°45′E) where he lived in a bungalow directly on the beach. The area was surrounded by forest but he never slept in the jungle. In this place, the patient reported that there were many monkeys at liberty. He never took any malaria chemoprophylaxis and never used an anti-mosquito net.
Conclusion
In addition to being the first case of P. knowlesi malaria imported from Thailand into a Western country, this observation raises epidemiological issues. Usually human P. knowlesi infections occur during stays in forest areas3,5,9,12,13,16 as the four vector species suspected to be responsible for the transmission of P. knowlesi (Anopheles hackeri, Anopheles balabacensis, Anopheles cracens, Anopheles latens), are rather arboreal species.21–23 Indeed, these anopheles from the Leucosphyrus group, are found preferentially in the canopy of the rain forest as is the natural monkey host. In this case, as in that described from the Philippines,15 the subjects stayed on the seaside right next to the forest fringe and the malaria transmissions most likely happened directly on the beach. This particularity could be caused by a change in the behavior of mosquitoes linked to human pressure on the natural environment, including tourism. For example, in our case, many Macaca monkeys were present in the vicinity of the bungalow, and were considered as pets by tourists. These animals may constitute some bait for mosquitoes, which leave the forest canopy to colonize this new environment. Nevertheless, we cannot exclude that another vector species, with a more marked coastal tropism, was responsible of this atypical malaria transmission. In any case, in these popular and tourist areas of the West coast of Thailand, a scrupulous parasitological and entomological monitoring should be carried out.
Human P. knowlesi malaria can be severe or life-threatening. The associated symptoms are similar to those observed in severe malaria caused by P. falciparum and the biological parameters are almost the same, notably hyperparasitemia and marked hepatorenal dysfunction.24,25 However, most P. knowlesi infections seem benign with low parasitemia. Of the 94 P. knowlesi infections reported by Sing and colleagues3 in Malaysian Borneo, the median parasitemia was about 0.05% (2,641 parasites/μL). The mean parasitemia of the seven imported cases already described in Western countries, including ours, was 0.97% (0.003–2.9%). This could mean that people living in the endemic areas have a partial immunity to this parasite that may or may not be specific. Despite a higher level of parasitemia in infected travelers, none of them had clinical or biological signs of severity.
As shown in this report, the diagnosis of P. knowlesi malaria remains difficult. The parasite is very often mistakenly identified on a blood smear because the morphology of the blood-stage forms share similarities with P. malariae and P. falciparum such as “equatorial band forms” attributed to P. malariae, and a delicate ring forms, as seen with P. falciparum (Figure 1).24 Furthermore, results obtained with the RDTs are misleading and often lead to a diagnosis of P. vivax infection. Indeed, most of these RDTs use antibodies that in theory are specific for P. vivax LDH, when in fact, there is a cross-reaction with P. knowlesi LDH, and with most pLDHs of simian Plasmodium species.26,27 More generally, the P. knowlesi LDH is able to bind to all anti-pLDH antibodies of malarial species, namely those of P. falciparum and those of the genus Plasmodium.13,26 The cross-reaction with P. falciparum antibodies is of less consequence in so far as these antibodies were only used in the Diamed OptiMal RDT (Diamed, Paris, France). Moreover, our case report confirms that the anti-pan-Plasmodium aldolase antibodies found in the RDT BinaxNOW test (Inverness Medical), also bind P. knowlesi aldolase.13 On the contrary, anti-HRP2 antibodies, widely used for the identification of P. falciparum infections, do not cross-react with P. knowlesi or with other simian Plasmodia.13,26,27
In Western countries, most of the reference malaria parasitological units use PCR to diagnose low Plasmodium density malaria or to identify species. Nevertheless, as in the case with RDTs, identification of P. knowlesi infections by PCR can be difficult, especially with a P. vivax PCR. This is the difficulty we encountered in this clinical case. Indeed, we used a PCR targeting the mitochondrial genes (cox1) of P. vivax whose primer set were designed in 1999 (Table 1), long before the corresponding sequences of P. knowlesi were accessible in databases. When the mitochondrial cox3, cox1, and cytb genes of P. knowlesi (GenBank accession no. AB444108, 5,958 bp) are compared with the P. vivax sequence, 97% homology is observed. Thus, most of the primers, such as ours used to identify P. vivax, also amplify P. kowlesi DNA. These primers also amplify other simian genotypically close Plasmodium DNA, such as Plasmodium inui and Plasmodium cynomogolgi, which are also known to infect humans.28 For the small subunit ribosomal RNA (SSUrRNA) gene, another sequence usually used to design primers sets for specific PCR amplification of Plasmodium species, the situation is identical. A recent publication reports that primers described as being specific for the SSUrRNA gene of P. knowlesi can cross-hybridize with the P. vivax ortholog sequence.29 Indeed, the homology between the P. knowlesi SSUrRNA gene sequence (GenBank accession no. AY327554, 1,662 bp) and the P. vivax sequence, is 94%. In summary, all the primer sets designed to be specific for P. knowlesi, must be checked to rule out any cross-reaction that may be responsible for a misdiagnosis, especially if the primers were designed before the availability of database sequences of P. knowlesi.
In conclusion, in a reference laboratory, PCR is an indispensable tool to identify with certainty the species involved in malaria infections. The emergence of P. knowlesi, now known to be able to infect travelers only staying at the seaside in touristic countries of Southeast Asia, should be taken into account in diagnostic strategies. Nevertheless, whatever the powerful application of the techniques used, a careful microscopic examination of the blood sample associated with precise epidemiological data, can avoid a large proportion of diagnostic errors.
ACKNOWLEDGMENTS
We gratefully acknowledge Valérie Bans, Elodie Duthu, Séverine Gisquet, and Catherine Paris for technical assistance and John Woodley for the English revision of the manuscript.
Disclaimer: All authors have no conflicts of interest to declare and all have actively contributed to this study and review.
Footnotes
Authors' addresses: Antoine Berry, Xavier Iriart, Alexis Valentin, Sophie Cassaing, Sandie Menard, David Olagnier, and Judith Fillaux, Service de Parasitologie-Mycologie, Centre Hospitalier Universitaire de Toulouse, France; and UMR3 MD-UM-UPS, Université Paul Sabatier Toulouse III, France, E-mails: berry.a@chu-toulouse.fr, xabierlete@yahoo.fr, valentin.a@chu-toulouse.fr, cassaing.s@chu-toulouse.fr, sandie.menard@free.fr, david.olagnier@hotmail.fr, and fillaux.j@chu-toulouse.fr. Nathalie Wilhelm and Alain Le Coustumier, Service de Biologie Médicale CHG de Cahors, Cahors, France, E-mails: nathalie.wilhelm@ch-cahors.fr and alain.lecoustumier@ch-cahors.fr. Benoit Witkowski and Françoise Benoit-Vical, Laboratoire de Chimie de Coordination, UPR8241, Université de Toulouse III, Toulouse, France, E-mails: benwitkowski@yahoo.fr and Francoise.Vical@inserm.fr. Stephane Sire, Unité des Maladies Infectieuses et Tropicales, CHG de Cahors, Cahors, France, E-mail: stephane.sire@chu-cahors.fr. Jean-François Magnaval, Service de Parasitologie-Mycologie, Centre Hospitalier Universitaire de Toulouse, France, E-mail: magnaval@cict.fr.
References
- 1.Garnham PC. Malaria Parasites and Other Haemosporidia. Oxford, UK: Blackwell Scientific Publications; 1966. [Google Scholar]
- 2.Chin W, Contacos PG, Coatney GR, Kimball HR. A naturally acquited quotidian-type malaria in man transferable to monkeys. Science. 1965;149:865. doi: 10.1126/science.149.3686.865. [DOI] [PubMed] [Google Scholar]
- 3.Singh B, Kim Sung L, Matusop A, Radhakrishnan A, Shamsul SS, Cox-Singh J, Thomas A, Conway DJ. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004;363:1017–1024. doi: 10.1016/S0140-6736(04)15836-4. [DOI] [PubMed] [Google Scholar]
- 4.Zhu HM, Li J, Zheng H. Human natural infection of Plasmodium knowlesi. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2006;24:70–71. [PubMed] [Google Scholar]
- 5.Jongwutiwes S, Putaporntip C, Iwasaki T, Sata T, Kanbara H. Naturally acquired Plasmodium knowlesi malaria in humans, Thailand. Emerg Infect Dis. 2004;10:2211–2213. doi: 10.3201/eid1012.040293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang N, Chang Q, Sun X, Lu H, Yin J, Zhang Z, Wahlgren M, Chen Q. Co-infections with Plasmodium knowlesi and other malaria parasites, Myanmar. Emerg Infect Dis. 2010;16:1476–1478. doi: 10.3201/eid1609.100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luchavez J, Espino F, Curameng P, Espina R, Bell D, Chiodini P, Nolder D, Sutherland C, Lee KS, Singh B. Human infections with Plasmodium knowlesi, the Philippines. Emerg Infect Dis. 2008;14:811–813. doi: 10.3201/eid1405.071407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng OT, Ooi EE, Lee CC, Lee PJ, Ng LC, Pei SW, Tu TM, Loh JP, Leo YS. Naturally acquired human Plasmodium knowlesi infection, Singapore. Emerg Infect Dis. 2008;14:814–816. doi: 10.3201/eid1405.070863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figtree M, Lee R, Bain L, Kennedy T, Mackertich S, Urban M, Cheng Q, Hudson BJ. Plasmodium knowlesi in human, Indonesian Borneo. Emerg Infect Dis. 2010;16:672–674. doi: 10.3201/eid1604.091624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox-Singh J, Singh B. Knowlesi malaria: newly emergent and of public health importance? Trends Parasitol. 2008;24:406–410. doi: 10.1016/j.pt.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sallum MA, Peyton EL, Wilkerson RC. Six new species of the Anopheles leucosphyrus group, reinterpretation of An. elegans and vector implications. Med Vet Entomol. 2005;19:158–199. doi: 10.1111/j.0269-283X.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- 12.Bronner U, Divis PC, Farnert A, Singh B. Swedish traveller with Plasmodium knowlesi malaria after visiting Malaysian Borneo. Malar J. 2009;8:15. doi: 10.1186/1475-2875-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Hellemond JJ, Rutten M, Koelewijn R, Zeeman AM, Verweij JJ, Wismans PJ, Kocken CH, van Genderen PJ. Human Plasmodium knowlesi infection detected by rapid diagnostic tests for malaria. Emerg Infect Dis. 2009;15:1478–1480. doi: 10.3201/eid1509.090358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantele A, Marti H, Felger I, Muller D, Jokiranta TS. Monkey malaria in a European traveler returning from Malaysia. Emerg Infect Dis. 2008;14:1434–1436. doi: 10.3201/eid1409.080170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC). Simian malaria in a U.S. traveler–New York MMWR Morb Mortal Wkly Rep. 2008;58:229–232. [PubMed] [Google Scholar]
- 16.Ta TT, Salas A, Ali-Tammam M, Martinez Mdel C, Lanza M, Arroyo E, Rubio JM. First case of detection of Plasmodium knowlesi in Spain by real-time PCR in a traveller from Southeast Asia. Malar J. 2010;9:219. doi: 10.1186/1475-2875-9-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabre R, Berry A, Morassin B, Magnaval JF. Comparative assessment of conventional PCR with multiplex real-time PCR using SYBR Green I detection for the molecular diagnosis of imported malaria. Parasitology. 2004;128:15–21. doi: 10.1017/s0031182003004219. [DOI] [PubMed] [Google Scholar]
- 18.Tan TM, Nelson JS, Ng HC, Ting RC, Kara UA. Direct PCR amplification and sequence analysis of extrachromosomal Plasmodium DNA from dried blood spots. Acta Trop. 1997;14:105–114. doi: 10.1016/s0001-706x(97)00080-6. [DOI] [PubMed] [Google Scholar]
- 19.Tham JM, Lee SH, Tan TM, Ting RC, Kara UA. Detection and species determination of malaria parasites by PCR: comparison with microscopy and with ParaSight-F and ICT malaria Pf tests in a clinical environment. J Clin Microbiol. 1999;37:1269–1273. doi: 10.1128/jcm.37.5.1269-1273.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubio JM, Benito A, Berzosa PJ, Roche J, Puente S, Subirats M, Lopez-Velez R, Garcia L, Alvar J. Usefulness of seminested multiplex PCR in surveillance of imported malaria in Spain. J Clin Microbiol. 1999;37:3260–3264. doi: 10.1128/jcm.37.10.3260-3264.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baird JK. Malaria zoonoses. Travel Med Infect Dis. 2009;7:269–277. doi: 10.1016/j.tmaid.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Tan CH, Vythilingam I, Matusop A, Chan ST, Singh B. Bionomics of Anopheles latens in Kapit, Sarawak, Malaysian Borneo in relation to the transmission of zoonotic simian malaria parasite Plasmodium knowlesi. Malar J. 2008;7:52. doi: 10.1186/1475-2875-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vythilingam I, Noorazian YM, Huat TC, Jiram AI, Yusri YM, Azahari AH, Norparina I, Noorrain A, Lokmanhakim S. Plasmodium knowlesi in humans, macaques and mosquitoes in peninsular Malaysia. Parasit Vectors. 2008;1:26. doi: 10.1186/1756-3305-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox-Singh J, Davis TM, Lee KS, Shamsul SS, Matusop A, Ratnam S, Rahman HA, Conway DJ, Singh B. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis. 2008;46:165–171. doi: 10.1086/524888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox-Singh J, Hiu J, Lucas SB, Divis PC, Zulkarnaen M, Chandran P, Wong KT, Adem P, Zaki SR, Singh B, Krishna S. Severe malaria—a case of fatal Plasmodium knowlesi infection with post-mortem findings: a case report. Malar J. 2010;9:10. doi: 10.1186/1475-2875-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCutchan TF, Piper RC, Makler MT. Use of malaria rapid diagnostic test to identify Plasmodium knowlesi infection. Emerg Infect Dis. 2008;14:1750–1752. doi: 10.3201/eid1411.080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawai S, Hirai M, Haruki K, Tanabe K, Chigusa Y. Cross-reactivity in rapid diagnostic tests between human malaria and zoonotic simian malaria parasite Plasmodium knowlesi infections. Parasitol Int. 2009;58:300–302. doi: 10.1016/j.parint.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Coatney GR. The simian malarias: zoonoses, anthroponoses, or both? Am J Trop Med Hyg. 1971;20:795–803. doi: 10.4269/ajtmh.1971.20.795. [DOI] [PubMed] [Google Scholar]
- 29.Imwong M, Tanomsing N, Pukrittayakamee S, Day NP, White NJ, Snounou G. Spurious amplification of a Plasmodium vivax small-subunit RNA gene by use of primers currently used to detect P. knowlesi. J Clin Microbiol. 2009;47:4173–4175. doi: 10.1128/JCM.00811-09. [DOI] [PMC free article] [PubMed] [Google Scholar]


