Abstract
Some studies showed that children have a lower response to systemic use of pentavalent antimoniate than adults. We aimed to evaluate the response rate to Glucantime therapy in children and compare it with adults. One hundred and twelve patients with acute cutaneous leishmaniais (ACL) were divided into two equal groups of adults (> 15 yrs) and children (≤ 15 yrs). They received meglumine antimoniate; 20 mg/kg/day for 20 days, their improvement rate was evaluated 20 and 45 days after treatment. Perprotocol analysis showed a significantly lower response in the children group 20 and 45 days after initiation of the treatment (P = 0.0001, 95% confidence interval [CI] = 0.190 [0.079–0.456]/P = 0.0051, 95% CI = 0.317 [0.140–0.717], respectively). Moreover, after intention-to-treat analysis, the same results were seen in the younger group 20 and 45 days after treatment (P = 0.0003, 95% CI = 0.228 [0.098–0.528]/P = 0.0132, 95% CI = 0.382 [0.177–0.825], respectively). According to our results, systemic Glucantime has lower efficacy in treating ACL in children than adults.
Introduction
Cutaneous leishmaniasis (CL) is a chronic parasitic disease that is endemic in many regions of the world; 90% of all cases occur in only seven countries: Afghanistan, Algeria, Brazil, Iran, Peru, Saudi Arabia, and Syria.
Khorasan province North-East of Iran is one of the most prominent sites of CL.1 There are many treatment modalities for leishmaniasis including local and systemic methods. Pantavalanet antimoniates have been considered to be the first-line treatment of all forms of leishmaniasis, but according to our own experience and those in other literature a lower efficacy has been seen in children in comparison to adult patients.2 In addition, Cruz and others3 found a difference in pharmacokinetics of antimony in children treated with meglumine antimoniate (Glucantime) for leishmaniasis. Thus, knowing that Mashhad, the capital of Khorasan province, is one of the largest endemic regions for CL and children bear a substantial portion of the patients, we aimed to evaluate the efficacy of Glucantime in children and compare it to adults in our area where Leishmania tropica is endemic.4
Material and Methods
This study was a clinical trial that was performed during a 12-month period on CL-affected patients who visited the dermatology clinic of Qaem hospital and the CL outpatient clinic of Villa health center located in Ab-o-Bargh, one of the most well-known areas for CL in Mashhad. The parasitological diagnosis was confirmed by using Geimsa-stained direct smear; patients with a positive skin smear for CL were enrolled in the study and treatment was performed on an outpatient basis.
The exclusion criteria were as follows: patients with a lesion history of more than 3 months, allergy to antimoniate compounds, and a simultaneous use of any other therapeutic methods. Study goals were explained to all participants and an informed consent was obtained from patients or their parents in children cases. The study protocol was reviewed and approved by the Ethics Research Committee of Mashhad University of Medical Sciences.
To detect a 25% difference in efficacy between groups, with a 95% confidence interval (CI) and a beta error of 20%, 48 patients were required in each group. Because we considered a 15% expected loss in our patients' follow-up, we increased the total number of patients to 56 for each group resulting in a final sample size of 112 patients divided into two groups of adults (> 15 years of age) and children (≤ 15 years of age).2
Treatment and follow-up.
Intramuscular meglumine antimoniate (Glucantime, Specia, Paris, France) was injected for each patient with a dosage of 20 mg/kg/day for 20 days. The patients were strongly advised not to use any other therapeutic method during this period. The patients were asked to visit the clinic 20 days after initiating treatment and 45 days after termination of the treatment course for follow-up.
At each visit all patients were re-examined clinically by one expertise dermatologist to evaluate the response rate; it was determined by measuring the indurations as follows:
-
1.
Complete improvement: full re-epithelialization for ulcerative lesions and 100% decrease in induration size for papuloplaque or nodular ones (with or without a negative direct smear result).
-
2.
Significant improvement: decrease in the indurations size > 75%.
-
3.
Partial improvement: decrease in the indurations size between 50% and 75%.
-
4.
Slight improvement: decrease in the indurations size between 25% and 50%.
-
5.
No improvement: decrease in the indurations size < 25%.
The endpoint of the study for each patient was either complete healing of all skin lesions or withdrawal from the study. The participation of subjects through each stage of our study has been summarized in Figure 1.
Figure 1.
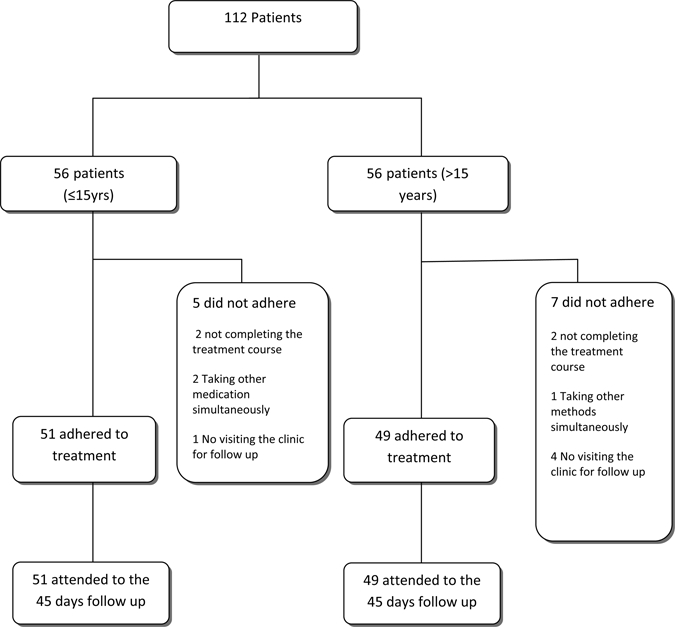
The flow of participants through each stage of our study.
Statistical analysis.
We used two approaches to compare treatment outcomes between the two groups. The first approach was an intention-to-treat (ITT) analysis that included all 112 patients enrolled at the beginning of the trial; this approach considered patients with irregular treatments and follow-ups as therapeutic failures. The second approach was more stringent and included solely the 100 patients with regular treatment and follow-ups. The coinciding use of these two statistical approaches are considered good compliers and better represented an explanatory approach that enabled interpretation of data in terms of effectively treated patients. Data is expressed as mean ± SD; treatment response rate and its 95% CI were compared between the two groups; the χ2 test was used to assay for significance (P < 0.05) between groups.
Results
During this study 12 (10.71%) patients were excluded for various reasons, such as taking other medications simultaneously, not completing the treatment course, not visiting the clinic for follow-up, or changing their home address and losing access for further follow-up.
The demographic characteristics of the patients have been summarized in Table 1. According to these data, the two groups were matched in sex, lesion's duration, type of lesions, and only varied in the lesion's location. The most common lesion sites were head and neck in children but upper and lower extremities in adults, which showed a significant difference between the two groups (P = 0.006). In addition, sex had no effect on the response rate in either group because the same response rate was observed in both males and females (P = 0.569). There was also no difference in response rate among different subgroups of children (< 5 yrs, 5–10 yrs, and 10–15 yrs) (P > 0.05).
Table 1.
Demographic and cutaneous leishmaniasis characteristics of the studied population
| Characteristics | Children | Adult | P value |
|---|---|---|---|
| Number of patients | 56 | 56 | |
| Female | 27 (48.2%) | 24 (42.9%) | 0.569 |
| Duration of lesions (weeks) | 7.00 ± 6.13 | 5.13 ± 3.54 | 0.062 |
| Type of lesions | |||
| Papule | 13 (23.2%) | 9 (16.1%) | 0.063 |
| Plaque | 11 (19.6%) | 17 (30.4%) | |
| Nodule | 10 (17.9%) | 4 (7.1%) | |
| Ulcer | 4 (7.1%) | 10 (17.8%) | |
| Papulo-plaque | 12 (21.4%) | 9 (16.1%) | |
| Plaque and nodule | 1 (1.8%) | 3 (5.4%) | |
| Plaque and ulcer | 5 (8.95) | 4 (7.1%) | |
| Location of lesions | |||
| Head and neck | 38 (68.4%) | 18 (32.14) | 0.006 |
| Upper and lower limbs | 16 (28.8%) | 36 (64.28%) | |
| Trunk | 2 (3.6%) | 2 (3.6%) |
Finally and after per protocol analysis, comparison in response rate between the two groups of children and adults showed that children had a significantly lower response rate of 20 and 45 days after treatment (P = 0.0001, 95% CI = 0.190 [0.079–0.456]/P = 0.0051, 95% CI = 0.317 [0.140–0.717], respectively). Moreover, the same results were observed with ITT analysis after the same time durations (P = 0.0003, 95% CI = 0.228 [0.098–0.528]/P = 0.0132, 95% CI = 0.382 [0.177–0.825], respectively). The latter results have been summarized in Table 2.
Table 2.
Comparison of therapeutic failure rates for study groups, using the ITT approach and analysis of perprotocol after 20 and 45 days of treatment*
| Children | Adult | OR (95% CI) | χ2 | P value | ||
|---|---|---|---|---|---|---|
| Treatment | Perprotocol | 40/51 (78.4%) | 20/49 (40.8%) | 0.190 (0.079–0.456) | 14.733 | 0.0001 |
| After 20 days | ITT | 45/56 (80.4%) | 27/56 (48.2%) | 0.228 (0.098–0.528) | 12.600 | 0.0003 |
| Treatment | Perprotocol | 33/51 (64.7%) | 18/49 (36.7%) | 0.317 (0.140–0.717) | 7.824 | 0.0051 |
| After 45 days | ITT | 38/56 (67.9%) | 25/56 (44.6%) | 0.382 (0.177–0.825) | 6.132 | 0.0132 |
ITT = intention-to-treat; OR = odds ratio; CI = confidence interval; Perprotocol = analysis excluded patients who were lost to follow-up. Analysis for ITT included patients lost to follow-up throughout the study who were considered to have experienced treatment failure.
Serious post-treatment side effects were not seen in either group. However, three patients in the children group and four patients in the adult group showed erythema with mild pruritus and edema around the injection site.
Discussion
Treatment of CL, especially in children who represent 7–10% of leishmaniasis patients in endemic areas has always been an issue of great importance.5 Moreover, principals and methods that are being used for treating leishmaniasis in children are quite similar to those applied in adults.6–8
Pentavalent antimony (Sbv) drugs have been considered to be the first-line treatment of CL for over 50 years,3 and still have high efficacy in the treatment of Old World CL. The improvement rate in endemic regions has been reported to be 17% for CL with L. tropica up to 97% with undetermined species of leishmaniasis.9,10 In general, the average improvement in lesion size is considered as 60–80%.11 Resistance to Sbv for L. tropica has been reported in several endemic regions of Iran9,12; Faghihi and others13 reported 41.7% improvement in CL lesions in the central part of Iran, whereas in another study 34.5% improvement in lesion size was reported in the northeast of this country.14
On the other hand, there is some evidence indicating different response rates to this medication in children. In the study by Palacios and others2 the treatment failure in children with systemic Glucantime for CL caused by L. viania species showed a clinically significant lower response when compared with the same regimen dosage and duration in adults: < 5 years of age (25%), 5–14 years of age (75%), and 15 years of age or more (83%).2 Furthermore, in a recent clinical trial on CL children the therapeutic rate of cryotherapy with liquid nitrogen (−195°C) compared with intralesional Glucantime showed a higher response rate. (P = 0.012, CI = 3.33 [1.29–8.55]).14 Although the level of response to Sbv is multifactorial, it seems that a lower response in children may be mainly caused by age-dependent pharmacokinetic differences of antimony, a higher resistance to Sbv in endemic regions like Iran, and finally the pathogenicity of the species.
Because L. tropica is the predominant parasite species in our region and the duration and dosage of the administered drug in both groups has been the same (20 mg/kg/day for 20 days), the difference in response rates could partially be caused by host factors such as their immune response level, either innate or acquired, which may differ in adults and children.2,4
As noted before, the differences in pharmacokinetics of Glucantime could be the main cause of the different response rates in children versus adults. There are limited data for understanding pharmacodynamics and pharmacokinetics of Sbv in general, and for children in particular.15 Cruz and others3 compared the pharmacokinetics of antimony (Sbv) in adults and children (3–6 yrs) with CL who were treated with intramuscular Glucantime 20 mg/kg/day for 20 days; they concluded that children have a 42% lower area under the 24-h time-concentration curve (AUC [0–24]) in plasma compared with adults, also a 16% lower peak concentration of Glucantime and a 75% higher weight-adjusted total antimony clearance. In the study by Kearns and others16 it was proved that weight-adjusted glumeruler filtration rate in children is higher than adults; this is inconsistent with the higher total adjusted clearance rate of the Cruz and others study. Therefore, they concluded that drug exposure in children is significantly lower than adults with the same standard weight-adjusted regimen of Sbv; this is primarily caused by a higher Sbv clearance rate.3
Regarding the notion that in some regions like ours (Mashhad, Iran) CL is transferred through the anthroponotic route, a suboptimal response may lead to more resistance toward Sbv.3 In addition, because mainly children are affected in endemic regions and also their suboptimal response to Sbv is higher than adults; considering this in addition to the increasing resistance to Sbv in endemic regions, further investigation on the pharmacodynamics of Glucantime, especially in children of our region, is required to underscore the need for alternation in the currently available treatments for CL caused by L. tropica in this age group and the need to optimize treatment regimens with Sbv in children. This is important especially in those areas where children constitute a substantial proportion of the patient population and according to previous findings may show a lower response rate to the routine treatments in adults.
ACKNOWLEDGMENTS
We thank the Research Council of Mashhad University of Medical Sciences for their financial support in performing the study, and Ahmad Reza Taheri and Habib-o-Allah Esmaeili for their guidance and valuable comments in statistical analysis.
Footnotes
Authors' addresses: Pouran Layegh, Research Center for Skin Diseases and Cutaneous Leishmaniasis, Qaem Hospital, Mashhad University of Medical Sciences, Mashhad, Iran, E-mails: Layeghpo@mums.ac.ir or Pouran_layegh@yahoo.com. Sara Rahsepar, Research Center for Skin Diseases and Cutaneous Leishmaniasis, Qaem Hospital, Mashhad University of Medical Science, Mashhad, Iran, E-mail: rahsepars891@mums.ac.ir. Amir Ali Rahsepar, Qaem Hospital, Mashhad University of Medical Science, Mashhad, Iran, E-mail: rahseparaa841@mums.ac.ir.
References
- 1.Talari SA, Talaei R, Shajari G, Vakili Z, Taghaviardakani A. Childhood cutaneous leishmaniasis: report of 117 cases from Iran. Korean J Parasitol. 2006;44:355–360. doi: 10.3347/kjp.2006.44.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palacios R, Osorio LE, Grajalew LF, Ochoa MT. Treatment failure in children in a randomized clinical trial with 10 and 20 days of meglumine antimonate for cutaneous leishmaniasis due to Leishmania viannia species. Am J Trop Med Hyg. 2001;64:187–193. doi: 10.4269/ajtmh.2001.64.187. [DOI] [PubMed] [Google Scholar]
- 3.Cruz A, Rainey PM, Herwaldt BL, Stagni G, Palacios R, Trujillo R, Saravia NG. Pharmacokinetics of antimony in children treated for leishmaniasis with meglumine antimoniate. J Infect Dis. 2007;195:602–608. doi: 10.1086/510860. [DOI] [PubMed] [Google Scholar]
- 4.Shahbazi F, Shahabi S, Kazemi B, Mohebali M, Abadi AR, Zare Z. Evaluation of PCR assay in diagnosis and identification of cutaneous leishmaniasis: a comparison with the parasitological methods. Parasitol Res. 2008;103:1159–1162. doi: 10.1007/s00436-008-1111-4. [DOI] [PubMed] [Google Scholar]
- 5.Sharifi I, Fekri AR, Aflatonian MR, Nadim A, Nikian Y, Kamesipour A. Cutaneous leishmaniasis in primary school children in the south-eastern Iranian city of Bam, 1994–95. Bull World Health Organ. 1998;76:289–293. [PMC free article] [PubMed] [Google Scholar]
- 6.Kafetzis DA. An overview of paediatric leishmaniasis. J Postgrad Med. 2003;49:31–38. doi: 10.4103/0022-3859.930. [DOI] [PubMed] [Google Scholar]
- 7.Minodier P, Noel G, Blanc P, Uters M, Retornaz K, Garnier JM. Management of cutaneous leishmaniasis in adults and children. Med Trop. 2005;65:487–495. [PubMed] [Google Scholar]
- 8.Stavropoulos PG, Papakonstantinou AM, Petropoulou H, Kontochristopoulos G, Katsambas A. Cryotherapy as an adjunct to systemic antimonials for cutaneous leishmaniasis in children. J Eur Acad Dermatol Venereol. 2006;20:765–766. doi: 10.1111/j.1468-3083.2006.01542.x. [DOI] [PubMed] [Google Scholar]
- 9.Firooz A, Khamesipour A, Ghoorchi MH, Nassiri-Kashani M, Eskandari SE, Khatami A, Hooshmand B, Gorouhi F, Rashighi-Firoozabadi M, Dowlati Y. Imiquimod in combination with meglumine antimoniate for cutaneous leishmaniasis: a randomized assessor-blind controlled trial. Arch Dermatol. 2006;142:1575–1579. doi: 10.1001/archderm.142.12.1575. [DOI] [PubMed] [Google Scholar]
- 10.Tallab TM, Bahamdam KA, Mirdad S, Johargi H, Mourad MM, Ibrahim K, el Sherbini AH, Karkashan E, Khare AK, Jamal A. Cutaneous leishmaniasis: schedules for intralesional treatment with sodium stibogluconate. Int J Dermatol. 1996;35:594–597. doi: 10.1111/j.1365-4362.1996.tb03669.x. [DOI] [PubMed] [Google Scholar]
- 11.Esfandiarpour I, Alavi A. Evaluating the efficacy of allopurinol and meglumine antimoniate (Glucantime) in the treatment of cutaneous leishmaniasis. Int J Dermatol. 2002;41:521–524. doi: 10.1046/j.1365-4362.2002.01526.x. [DOI] [PubMed] [Google Scholar]
- 12.Hadighi R, Mohebali M, Boucher P, Hajjaran H, Khamesipour A, Ouellette M. Unresponsiveness to Glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS Med. 2006;3:e162. doi: 10.1371/journal.pmed.0030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faghihi G, Tavakoli-kia R. Treatment of cutaneous leishmaniasis with either topical paromomycin or intralesional meglumine antimoniate. Clin Exp Dermatol. 2003;28:13–16. doi: 10.1046/j.1365-2230.2003.01169.x. [DOI] [PubMed] [Google Scholar]
- 14.Layegh P, Pezeshkpoor F, Soruri AH, Naviafar P, Moghiman T. Efficacy of cryotherapy versus intralesional meglumine antimoniate (Glucantime) for treatment of cutaneous leishmaniasis in children. Am J Trop Med Hyg. 2009;80:172–175. [PubMed] [Google Scholar]
- 15.Chulay JD, Fleckenstein L, Smith DH. Pharmacokinetics of antimony during treatment of visceral leishmaniasis with sodium stibogluconate or meglumine antimoniate. Trans R Soc Trop Med Hyg. 1988;82:69–72. [PubMed] [Google Scholar]
- 16.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology–drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]


