Abstract
Helminth infections influence the clinical response to certain diseases and are associated with delayed healing time of patients with cutaneous leishmaniasis (CL) caused by Leishmania braziliensis. We conducted a randomized, double-blind, placebo-controlled clinical trial to examine the role of early versus deferred treatment of intestinal helminth infection on the clinical course of patients with CL treated with pentavalent antimony. (Clinicaltrials.gov number NCT00469495). A total of 90 patients were enrolled, 51.1% (N = 23) of control patients had persistent lesions at Day 90, compared with 62.2% (N = 28) in the treatment group (difference 11.1%, 95% confidence interval = −9.1–30.0%). There was no statistically significant difference in overall time to cure between groups, although there was a tendency for shorter cure times in the control group. This study shows that early introduction of antihelminthic therapy does not improve clinical outcome in patients co-infected with helminths and L. braziliensis.
Introduction
Leishmaniases are a group of diseases caused by an intracellular protozoan parasite and transmitted by the bite of the female sandfly. The principal species causing clinical disease in Bahia, Brazil is Leishmania braziliensis, which is characterized by one or more cutaneous ulcers most frequently located on the extremities, however 3% of subjects will develop mucosal disease.1 Standard treatment of L. braziliensis cutaneous leishmaniasis (CL) is 20 days of intravenous pentavalent antimony (Sbv) with response rates of 50–90%.2,3
A TH1-type immune response is crucial in controlling Leishmania infection, with release of interferon-gamma (IFN-γ) leading to activation of macrophages, and killing of the intracellular parasite. However, excessive TH1 cytokines have also been implicated in the pathogenesis of CL4,5 and several lines of evidence support this information: 1) parasites are absent or scarce in CL lesions6; 2) ulceration is preceded by a granulomatous vasculitis with lymphocytic infiltration7; 3) use of antimony in the early pre-ulcer phase of CL does not prevent lesion formation7; 4) pre-treatment elevations in IFN-γ and tumor necrosis factor-α (TNF-α) decrease with treatment8; 5) immune modulatory drugs combined with Sbv accelerate healing in cutaneous and mucosal leishmaniasis.9,10
Chronic infection with helminths is highly prevalent in rural areas in Brazil. Helminths modulate the immune response, altering the clinical presentation of immune-related diseases including asthma11 and Crohn's disease.12 This may be related to the induction of interleukin-10 (IL-10) that shifts the cellular response toward a Th2 profile or expands regulatory T-cells.13,14 In a recent study, we showed that CL in patients co-infected with helminths took longer to heal.15 In light of this result, we evaluated the effect of early antihelminthic therapy on the outcome of CL co-infected with helminths.
Materials and Methods
Patient selection.
Patients were recruited at the health post of Corte de Pedra, a referral center for the diagnosis and treatment of leishmaniasis in the state of Bahia, Brazil for over 30 years. This region has the highest prevalence of CL in the state of Bahia, and the clinic sees an average of 1,000 new cases per year. Leishmania braziliensis has been the unique causal agent in this area in the last 15 years.16 All consecutive patients who presented for evaluation and treatment from November 2006 until March 2007 were screened for study eligibility and if eligible, were offered participation in the trial. Cutaneous leishmaniasis was diagnosed by a typical ulcer and a positive intradermal antigen test. Selection was on the basis of the following inclusion criteria: 1) age between 13 and 50 years, 2) a maximum of three ulcers, 3) lesion diameter between 5 and 50 mm, and 4) a period of 15 to 60 days from the onset of the ulcer. Patients with a prior history of CL or Sbv use, patients who had been treated for helminths within 6 months, patients with evidence of mucosal or disseminated disease, pregnant or breastfeeding mothers, and patients with diabetes mellitus were excluded. After selection, a stool sample was examined and only patients with helminthic infection were included. A total of 90 patients were enrolled.
Intradermal skin test.
The volar surface of the left forearm was injected with 25 μg of Leishmania amazonensis (strain MHOM-BR-86BA-125) antigen in 0.1 mL of distilled water, and the largest diameter of induration was measured at 48–72 hours.17 The test was considered positive for induration greater than 5 mm.
Parasitological assays.
The parasitological assay of feces included sedimentation, Baermann, and Katz–Kato methods for all three samples. Helminth burden was calculated and expressed in eggs/gram stool. On the basis of prior observational studies, the intestinal and systemic helminthiases endemic to the area are listed in Table 1.
Table 1.
Clinical characteristics, prevalence of helminths, and outcome of cutaneous leishmaniasis (CL) patients*
| Early treatment group (N = 45) | Control group (N = 45) | P value | |
|---|---|---|---|
| Male (%) | 34/45 (75.6%) | 32/45 (71.1%) | 0.64† |
| Age, mean in years ± SD | 25.7 ± 10.0 | 25.5 ± 9.8 | 0.93‡ |
| BMI, median in kg/m2 (IQR) | 21.3 (19.4–24.7) | 22.3 (20.7–24.0) | 0.67§ |
| No. (%) with 1 lesion | 34 (75.6%) | 34 (75.6%) | |
| No. (%) with 2 lesions | 7 (16.7%) | 8 (17.8%) | |
| No. (%) with 3 lesions | 4 (8.9%) | 3 (6.7%) | |
| Lesion size,¶ median (IQR), mm2 | 180 (70–400) | 198 (100–400) | 0.78§ |
| No (%) helminth | |||
| Mean egg count/gm stool (range) | |||
| Hookworm | 43/45 (95.6%) | 38/45 (84.4%) | 0.079† |
| 400 [24–1920] | 423 [24–2332] | ||
| Trichiura trichuris | 30/45 (66.7%) | 15/45 (33.3%) | 0.0016† |
| 324 [24–840] | 361 [24–2424] | ||
| Ascaris lumbricoides | 31/45 (68.9%) | 25/45 (55.6%) | 0.019† |
| 9786 [24–42,428] | 10,803 [24–48,404] | ||
| Strongyloides stercoralis | 2/45 (4.4%) | 3/45 (6.7%) | 1.00‖ |
| Schistosoma mansoni | 6/45 (13.3%) | 7/45 (15.6%) | 0.76† |
| 276 [24–768] | 124 [24–288] | ||
| Time to cure, median (IQR), days | 98 (64–138) | 88 (56–126) | 0.13** |
BMI = body mass index; IQR = interquartile range; SD = standard deviation.
χ2 test.
Student's t test.
Wilcoxon rank-sum test.
The lesion with the greatest area at presentation was considered to be the main lesion.
Fisher's exact test.
Log-rank test.
Patient evaluation.
A randomization table was used for group assignment and sealed envelopes were used for allocation concealment. Study design is shown in Figure 1. All patients were treated with intravenous Sbv (Glucantime) at 20 mg/kg/day for 20 days. The treatment group received albendazole (400 mg), ivermectin (200 μg/kg), and praziquantel (50 mg/kg) in an oral formulation at Days 0 and 30 and placebo at Day 60. The control group received placebo manufactured by Federal University of Bahia Pharmacy that was identical in form, color, and number to a treatment group at Days 0 and 30. These patients were also treated with the appropriate oral antihelminthic based on parasitological assay results on the 60-day visit; placebo tablets were provided so that the pill form and counts were identical to the treatment group. Patients were evaluated on initial presentation and at 30, 60, and 90 days after initiation of Sbv therapy. In the event that a patient did not return for follow-up at the specified time, visits were conducted in the patient's home within 15 days of the missed appointment. Patients in both groups provided stool samples at 0, 30, and 60 days for parasitological assays. Bidirectional measurements were taken of the patients' lesions at the initial visit and classified as either active or healed at the follow-up visit. Only lesions with complete re-epithelialization, without raised borders or eschars, were considered healed after evaluation by three clinicians who were unaware of the group assignment. Patients were also questioned about adverse events at all visits and events graded according to the National Institute of Allergy and Infectious Diseases (NIAID) Division of AIDS Toxicity Grading Scale.18 Written informed consent was obtained from all patients. This study was approved by the Ethics Committees of the Federal University of Bahia, Brazil and Weill Cornell Medical College.
Figure 1.
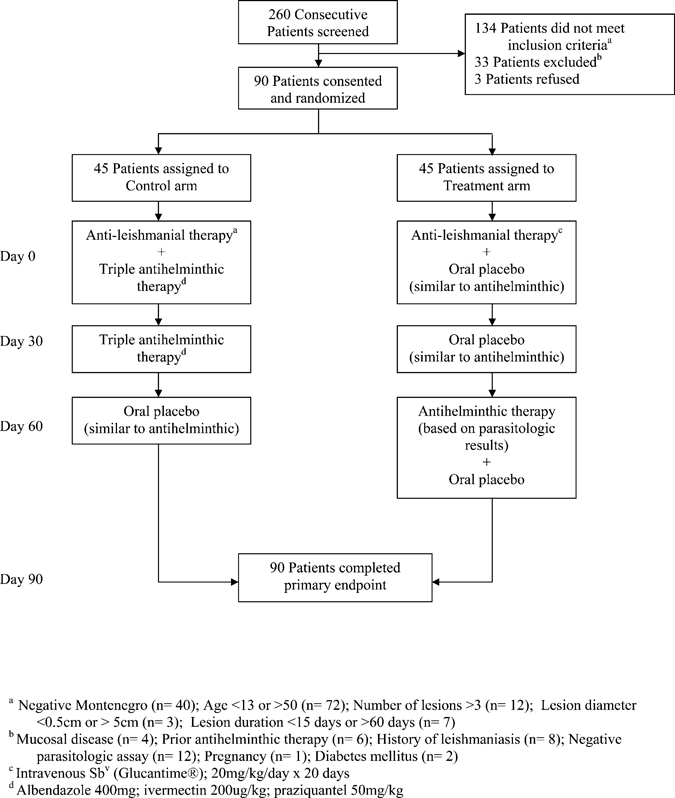
Diagramatic representation of the study design.
Statistical analysis.
Epi Info software (version 6.02, CDC, Atlanta, GA) was used for data entry. Statistical analysis was performed with the use of STATA (version 9.0, College Station, TX) and Statistical Analysis Software (version 9.1, SAS Corp., Cary, NC). Continuous variables were compared by Student's t test or Wilcoxon rank-sum test as appropriate. Categorical variables were compared by χ2 or Fisher's exact test as appropriate. The Kaplan–Meier method and log-rank test were used for the analysis of time to healing. Univariate and multivariate survival analysis was carried out with the use of Cox proportional hazards regression to evaluate the effect of covariates on time to healing. All analyses were based on the intention-to-treat principle. A P value of 0.05 was used to determine statistical significance. The sample size of 90 provided 80% power to detect a difference of 25% in the rate of cure between the groups with α = 0.05. The assumption that there would be a clinical difference in cure rate of 25% was based on a prior study showing response rates of 70% and 95% in helminth-positive versus helminth-negative subjects, respectively.15
Study endpoints.
The primary endpoint was defined as complete re-epithelialization at 90 days. Overall time to cure was evaluated as a secondary endpoint.
Results
Patient characteristics in this study roughly follow known demographic patterns for CL in this region. The majority of CL patients (73.3%) were male with ages ranging from 13 to 50 years (mean 25.6 ± 9.8 years). The majority presented with a single ulcer (75.6%) with a median duration of 30 days. There was no difference between treatment and control groups in relation to age, sex, number of lesions, skin test area, and main lesion area (Table 1).
The majority of patients (70%) harbored multiple helminth species, and hookworm was found in 87.8%. Prevalence by species can be found in Table 1. There was no difference in species or worm burden between the two groups on the initial parasitologic analysis (data not shown). The higher overall rates of Trichiura trichuris and Ascaris lumbricoides (P = 0.0016 and 0.019) in the control group based on the three combined assays is expected because of the increased sensitivity of multiple parasitologic analyses in detecting these organisms for the control group and the treatment received by the active group. Results from the Day 60 parasitological analysis revealed that 41/45 (91%) of the early treatment group were negative for any species as compared with 6/45 (13%) of the control group.
Comparison of values for hemoglobin, hematocrit, serum iron, transferring, and ferritin at Days 0 and 90 showed improvement in serum iron in the early treatment group with a median increase of 47 versus 21 μg/dL (P = 0.016). No significant improvements were observed in hemoglobin (P = 0.58), hematocrit (P = 0.77), transferrin (P = 0.55), or ferritin (P = 0.55) within the 90-day evaluation period (data not shown).
Adverse events were reported in 60% of patients in the treatment group and 60% of patients in the control group, with no statistically significant difference in the type or grade of events reported. Only grade 1 and 2 events were observed. Muscle pain (26%), headache (16%), leg pain (14%), weakness (14%), fever (13%), joint pain (12%), and dizziness (12%) were the most frequently reported symptoms.
At the 90-day study endpoint, 51.1% in the control group had persistent lesions, compared with 62.2% in the treatment group (mean difference 11.1%, 95% confidence interval [CI] = −9.1–30.0%). Overall failure rate in both groups was 57%. The median time to cure was 88 days in the control group versus 98 days in the treatment group (Table 1). By Kaplan–Meier analysis (Figure 2), patients who received early antihelminthic treatment took longer to heal their lesions than their non-treated counterparts, although this result was not statistically significant (P = 0.13).
Figure 2.
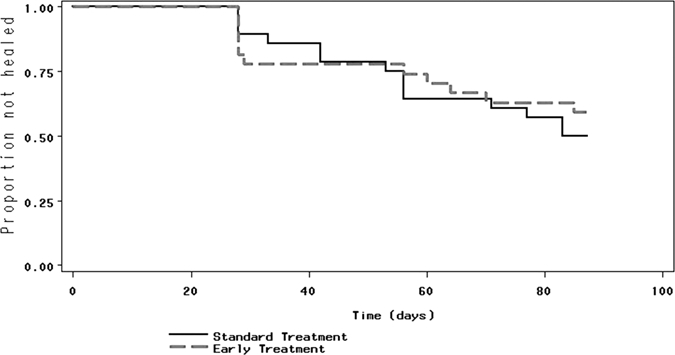
Lesion healing in patients with cutaneous leishmaniasis (CL), stratified by group assignment; P = 0.13 log-rank test.
Discussion
Helminth infections are widely prevalent in tropical areas and their benefits in attenuating inflammation and auto-immune diseases have been documented. Co-infection with Schistosoma mansoni limits the severity of asthma,11 and the ingestion of Trichuris suis eggs improved gastrointestinal manifestations of Crohn's disease.12 However, helminths may worsen the course of other infections. For instance, patients with S. mansoni are more prone to chronic Salmonella infections.19 In a recent study by our group, we showed that while CL patients co-infected with helminths presented with smaller initial lesion size, healing time was delayed in these patients, providing the rationale for the current study.15
Although no statistically significant difference was observed between groups, there was a tendency for delayed healing in the early treatment group. There are potential explanations for our negative findings. The study was powered to detect a 25% difference between treatment groups, therefore there exists a possibility that a smaller, but statistically significant effect exists. In the interest of protecting patients from delayed antihelminthic treatment, the control group received antihelminthics at Day 60, before the primary endpoint, which introduces a bias. However, Kaplan–Meier analysis performed with Day 60 as the primary endpoint did not alter the observed relationship between treatment assignment and outcome (data not shown). It is also possible that any favorable immunologic effects of antihelminthic therapy may take longer to manifest than 90 days. Given the tendency for delayed healing in the early treatment group, an alternative hypothesis is that introduction of antihelminthic treatment shifts the inflammatory response in such a way as to deter clearance of Leishmania organisms.
A major limitation of the study is the absence of parasitological confirmation of CL in any of the cases. In addition, the failure to completely and accurately characterize the helminthic infection of both groups by serial examination of three stool samples before initation of antihelminthic therapy was a limitation.
In addition to the primary outcome, several secondary outcomes were evaluated such as changes in hematocrit, hemoglobin, iron, transferring, and ferritin levels from the initial presentation to the primary endpoint. Improvements were seen in iron levels with early antihelminthic treatment within the 90-day time frame. No difference was observed in the reported adverse effects between groups, validating the safety of combined treatment with antimony and triple antihelminthic therapy. Triple therapy was shown to be effective with only 9% of treatment group patients having a positive stool sample at Day 60.
Cutaneous leishmaniasis and co-existing helminth infection is associated with poor response to therapy. However, introduction of early antihelminthic treatment in co-infected patients does not lead to improvement in overall cure rates or time to cure for CL and was associated with a tendency for delayed lesion healing.
ACKNOWLEDGMENTS
We thank the patients at Corte de Pedra for their participation in this study; Leda Alcantara for performing the parasitologic assays; Ednaldo Lago and Maria Neuza Souza for their work in the endemic area; Elbe Silva for secretarial assistance; and the Fogarty International Center/Ellison Medical Foundation fellowship in global health and clinical research.
Disclaimer: The authors do not have commercial or other associations that might pose a conflict of interest.
Footnotes
Financial support: This study was supported by NIH/FIC grant D43 TW007127 and NIH/NIAID K24AI078884.
Authors' addresses: Tracey Newlove, Department of Dermatology, New York University, New York, NY, E-mail: tracey.newlove@nyumc.org. Luiz H. Guimarães, Serviço de Imunologia, Hospital Universitário Prof. Edgard Santos, Universidade Federal da Bahia, Salvador, Bahia, Brazil, E-mail: luizhenriquesg@yahoo.com.br. Daniel J. Morgan, Departments of Epidemiology and Preventive Medicine, University of Maryland, Baltimore, MD, E-mail: dmorgan@epi.umaryland.edu. Leda Alcântara, Faculdade de Farmácia, Universidade Federal da Bahia, Salvador, Bahia, Brazil, E-mail: alcantar@ufba.br. Marshall J. Glesby, Division of International Medicine and Infectious Diseases, Department of Medicine, Weill Medical College of Cornell University, New York, NY, E-mail: mag2005@med.cornell.edu. Edgar M. Carvalho, Serviço de Imunologia, Hospital Universitário Prof. Edgard Santos, Universidade Federal da Bahia, Salvador, Bahia, Brazil, E-mail: imuno@ufba.br. Paulo R. Machado, Serviço de Imunologia, Hospital Universitário Prof. Edgard Santos, Universidade Federal da Bahia, Salvador, Bahia, Brazil, E-mail: prlmachado@uol.com.br.
Reprint requests: Paulo R. Machado, Serviço de Imunologia, Complexo Hospitalar Universitário Prof. Edgard Santos, UFBA, Rua João das Botas s/n, Canela, 40110-160, BA, Brazil, E-mail: prlmachado@pq.cnpq.br/imuno@ufba.br.
References
- 1.Jones T, Johnson W, Barretto A, Lago E, Marsden P. Epidemiology of American cutaneous leishmaniasis due to Leishmania braziliensis. J Infect Dis. 1987;156:73–83. doi: 10.1093/infdis/156.1.73. [DOI] [PubMed] [Google Scholar]
- 2.Correia D, Macêdo V, Carvalho E, Barral A, Magalhães A, Abreu M, Orge M, Marsden P. Comparative study of meglumine antimonate, pentamidine isethionate and aminosidine sulfate in the treatment of primary skin lesions caused by Leishmania (Vianna) braziliensis. Revista da Sociedade Brasiliera de Medicina Tropical. 1996;29:447–453. doi: 10.1590/s0037-86821996000500007. [DOI] [PubMed] [Google Scholar]
- 3.Romero G, Guerra M, Paes M, Macedo V. Comparison of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis and L. (V.) guyanensis in Brazil: therapeutic response to meglumine antimoniate. Am J Trop Med Hyg. 2001;65:456–465. doi: 10.4269/ajtmh.2001.65.456. [DOI] [PubMed] [Google Scholar]
- 4.Bacellar O, Lessa H, Schriefer A, Machado P, Jesus A, Dutra WO, Gollob KJ, Carvalho EM. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70:6734–6740. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribeiro-de-Jesus A, Almeida R, Lessa H, Bacellar O, Carvalho E. Cytokine profile and pathology in human leishmaniasis. Braz J Med Biol Res. 1998;31:143–148. doi: 10.1590/s0100-879x1998000100020. [DOI] [PubMed] [Google Scholar]
- 6.Bittencourt A, Barral A. Evaluation of the histopathological classifications of American cutaneous and mucocutaneous leishmaniasis. Mem Inst Oswaldo Cruz. 1991;86:51–56. doi: 10.1590/s0074-02761991000100009. [DOI] [PubMed] [Google Scholar]
- 7.Machado P, Araújo C, daSilva A, Almeida RP, D'Oliveira A, Jr, Bittencourt A, Carvalho EM. Failure of early treatment of cutaneous leishmaniasis in preventing the development of an ulcer. Clin Infect Dis. 2002;34:e69–e73. doi: 10.1086/340526. [DOI] [PubMed] [Google Scholar]
- 8.DaCruz A, Oliveira MD, Luca PD, Mendonca S, Coutinho S. Tumor necrosis factor-alpha in human American tegumentary leishmaniasis. Mem Inst Oswaldo Cruz. 1996;91:225–229. doi: 10.1590/s0074-02761996000200019. [DOI] [PubMed] [Google Scholar]
- 9.Almeida R, D'Oliveira A, Jr, Machado P, Bacellar O, Ko AI, de Jesus A, Mobashery N, Brito Santos J, Carvalho EM. Randomized, double-blind study of stibogluconate plus human granulocyte macrophage colony-stimulating factor versus stibogluconate alone in the treatment of cutaneous leishmaniasis. J Infect Dis. 1999;180:1735–1737. doi: 10.1086/315082. [DOI] [PubMed] [Google Scholar]
- 10.Lessa H, Machado P, Lima F, Cruz A, Bacellar O, Guerreiro J, Carvalho EM. Successful treatment of refractory mucosal leishmaniasis with pentoxifylline plus antimony. Am J Trop Med Hyg. 2001;65:87–89. doi: 10.4269/ajtmh.2001.65.87. [DOI] [PubMed] [Google Scholar]
- 11.Medeiros JM, Figueiredo JP, Almeida MC, Matos MA, Araújo MI, Cruz AA, Atta AM, Rego MA, de Jesus AR, Taketomi EA, Carvalho EM. Schistosoma mansoni infection is associated with a reduced course of asthma. J Allergy Clin Immunol. 2003;111:947–951. doi: 10.1067/mai.2003.1381. [DOI] [PubMed] [Google Scholar]
- 12.Summers RW, Elliott DE, Qadir K, Urban JF, Thompson R, Weinstock JV. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2003;98:2034–2041. doi: 10.1111/j.1572-0241.2003.07660.x. [DOI] [PubMed] [Google Scholar]
- 13.Maizels RM. Infections and allergy–helminths, hygiene and host immune regulation. Curr Opin Immunol. 2005;17:656–661. doi: 10.1016/j.coi.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Araujo M, Hoppe B, Medeiros M, Jr, Alcântara L, Almeida MC, Schriefer A, Oliveira R, Kruschewsky R, Figueiredo J, Cruz A, Carvalho E. Impaired T helper 2 response to aeroallergen in helminth-infected patients with asthma. J Infect Dis. 2004;190:1797–1803. doi: 10.1086/425017. [DOI] [PubMed] [Google Scholar]
- 15.O'Neal SE, Guimarães LH, Machado PR, Alcântara L, Morgan DJ, Passos S, Glesby MJ, Carvalho EM. Influence of helminth infections on the clinical course of and immune response to Leishmania braziliensis cutaneous leishmaniasis. J Infect Dis. 2007;195:142–148. doi: 10.1086/509808. [DOI] [PubMed] [Google Scholar]
- 16.Schriefer A, Goes-Neto A, Guimaraes LH, Carvalho LP, Almeida RP, Machado PR, Lessa HA, de Jesus AR, Riley LW, Carvalho EM. Multiclonal Leishmania braziliensis population structure and its clinical implication in a region of endemicity for American tegumentary leishmaniasis. Infect Immun. 2004;72:508–514. doi: 10.1128/IAI.72.1.508-514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reed S, Badaró R, Masur H, Carvalho E, Lorenco R, Lisboa A, Teixeira R, Johnson W, Jr, Jones T. Selection of a skin test antigen for American visceral leishmaniasis. Am J Trop Med Hyg. 1986;35:79–85. doi: 10.4269/ajtmh.1986.35.79. [DOI] [PubMed] [Google Scholar]
- 18.NIAID Division of AIDS Toxicity Grading Scale Guidance, Compliance & Regulatory Information (Biologics) 2007. http://www.fda.gov/cber/guidelines.htm Available at. Accessed September 14.
- 19.Rocha H, Kirk J, Hearey C., Jr Prolonged Salmonella bacteremia in patients with Schistosoma mansoni infection. Arch Intern Med. 1971;128:254–257. doi: 10.1001/archinte.1971.00310200090009. [DOI] [PubMed] [Google Scholar]


