Abstract
A patient presenting with an atypical manifestation of cutaneous leishmaniasis after travel to Cyprus was successfully treated with miltefosine. The K26 typing revealed a hitherto undescribed strain of the Leishmania donovani/infantum complex as the causing agent.
Introduction
Cutaneous leishmaniasis (CL) is a re-emerging disease with an estimated 1.5 million cases annually worldwide. Established endemic areas for CL of the Old World (OWCL) are North Africa, the Mediterranean, the Middle East, the Indian subcontinent, and Central Asia. The species responsible for OWCL are mainly Leishmania major and Leishmania tropica and less frequently representatives of the Leishmania donovani/infantum complex.1
In contrast to mucocutaneous forms of the New World or visceral leishmaniasis, OWCL is mostly a benign and often self-healing disease. It is noteworthy that cases of mucosal leishmaniasis caused by species from Old World countries, including L. tropica, L. major, and L. infantum, and cases of visceral leishmaniasis caused by L. tropica have been described.2 Systemic treatment of OWCL is recommended for patients who present with big, multiple, or disseminated lesions and for those who have simple lesions involving cosmetically sensitive areas or joints, or with the presence of nodular lymphangitis.2
Several clinical trials have been carried out to establish strain-specific treatment options for CL, because different Leishmania strains vary in their sensitivities to drugs.2 However, for systemic treatment of CL caused by strains of the L. donovani/infantum complex there are no evidence-based data currently available, although clinical experience has shown that pentavalent antimonials and liposomal amphotericin B are effective in severe infections.3
Miltefosine is a new oral anti-leishmanial drug that interacts with Leishmania phospholipid synthesis. It is successfully used for the treatment of visceral leishmaniasis and is characterized by a favorable side-effect profile.4 However, data on the treatment of CL with miltefosine are limited,5–8 and the efficacy of miltefosine against CL caused by the L. donovani/infantum complex has been documented in only two patients.9,10
We report on a case of imported CL that was successfully treated with miltefosine, and on the follow-up investigation, which revealed a hitherto undescribed strain of the L. donovani/infantum complex.
Case Report
The patient was a 59-year-old Austrian woman with a 2-month history of a dense, non-tender, subcutaneous pasty swelling involving a 5 × 7 cm area on the right cheek (Figure 1). No epidermal involvement, ulcerations, or lymphadenopathy were observed. Previous treatment with systemic antibiotics showed no effect. She denied having fever, chills, night sweats, weight loss, or facial trauma. There was no history of immunodeficiency or immunosuppressive therapies. Laboratory tests including hematologic (red blood cell count, total and differential white blood cell count, platelet count) and biochemical parameters (aspartate and alanine aminotransferase, immunoglobulin M [IgM], IgG) were all normal.
Figure 1.
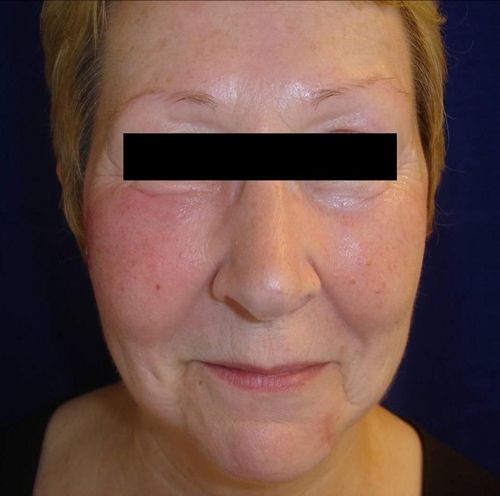
Atypical cutaneous leishmaniasis in a traveler returning from Cyprus: a subcutaneous swelling involving a 5 × 7 cm area of the right cheek without any epidermal involvement.
A case of cheilitis granulomatosa was suspected and a skin biopsy was performed, which revealed a granulomatous reaction pattern in the dermis with intracellular Leishmania amastigotes (Figure 2). We concluded from the patient's history that infection had occurred during a 2-week vacation in Polis, Cyprus, 8 months previously. A Leishmania genus-specific polymerase chain reaction (PCR)11 from the embedded tissue was positive and sequencing of the amplicon identified the L. donovani/infantum complex, the prominent taxon in the Mediterranean area.12 This universal Leishmania PCR amplifies a fragment (∼600 bp) of the small subunit ribosomal RNA (rRNA) gene using the R221 and R332 primer set.11 Further strain determination was carried out using the K26 PCR assay.13 The isolate showed a main 940-bp amplicon and two smaller ones at 870 and 370 bp (Figure 3).
Figure 2.
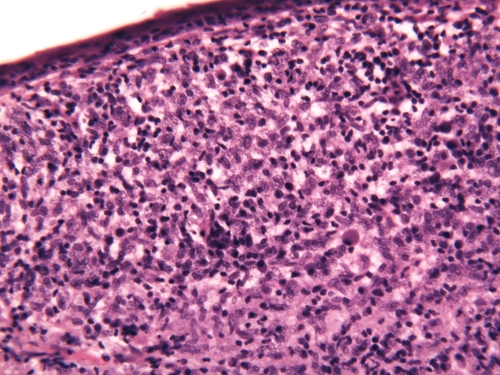
Histologic characteristics: The epidermis is thinned. Within the dermis, there is a nodular infiltrate of lymphocytes and enormous numbers of macrophages touching the epidermal junctional zone and reaching down to the superficial subcutis. The cytoplasm of macrophages (mainly within the papillary dermis) is filled with numerous Leishmania amastigotes. (Hematoxylin and eosin stained original magnification ×40).
Figure 3.
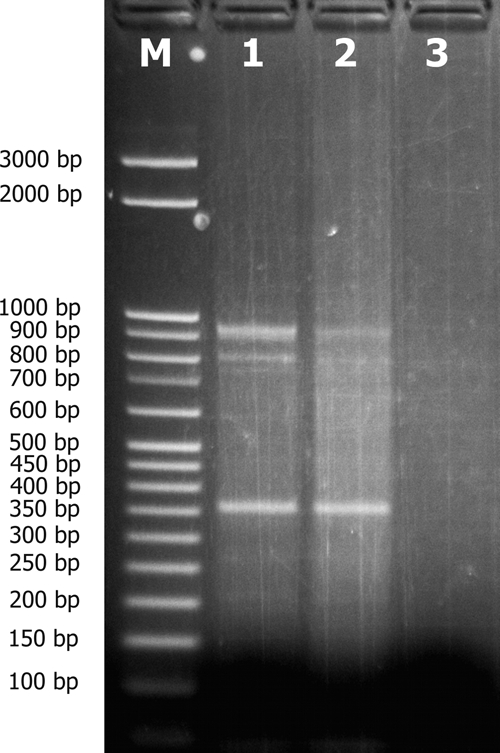
K26 polymerase chain reaction (PCR) assay used for strain discrimination within the Leishmania donovani/infantum complex shows a main 940-bp amplicon and two minor ones at 870 and 370 bp: Lane M, molecular weight markers; lanes 1 and 2, clinical samples (6 and 3 μL); lane 3, negative control.
Because the L. donovani/infantum complex is usually associated with visceral leishmaniasis, abdominal sonography was performed but showed no signs of visceral involvement in terms of hepatosplenomegaly. Serology for Leishmania was also negative. Mucosal involvement was ruled out by ear, nose, and throat examination.
Because of the size of the lesion in a cosmetically sensitive area, systemic treatment with miltefosine was started (50 mg by mouth three times daily for 28 days).Adverse drug reactions occurred in terms of nausea and vomiting after initiation of the therapy, and a mild transient elevation of liver enzymes. After 2 weeks the lesion diminished and then vanished completely over the following 3 months without functional or esthetic impairment. The patient was followed up for 2 years with regular clinical and laboratory examinations. No relapse occurred.
Materials and Methods
For molecular analysis, DNA was extracted from the tissue using the QIAamp DNA Mini Kit (Qiagen GmbH, Hilden, Germany). The DNA extracted from 1 × 103 cells of L. donovani cultured in vitro was used as a positive control and double-distilled molecular-grade water (Sigma-Aldrich GmbH, Vienna, Austria) as a negative control. The amplicons were visualized on 2% agarose gel by staining with ethidium bromide and the amplified products were excised and purified using the Gel Band Purification Kit (GE Healthcare Bio-Sciences AB, Vienna, Austria). Amplicons were sequenced by direct sequencing using the BigDye sequencing kit and an automatic 310 ABI PRISM sequencer (PE Applied Biosystems, Weiterstadt, Germany) and were processed with the GeneDoc sequence editor.14 The sequences were compared with published sequences from the GenBank by basic local alignment search tool (BLAST) search. The K26 PCR assay for further strain discrimination was run with 3 and 6 μL samples of extracted DNA and with a negative control only (double-distilled molecular-grade water).13 Again, amplicons were visualized with ethidium bromide on a 2% agarose gel.
Discussion
Although today CL ranks among the 10 leading diseases in travelers presenting with skin disorders on return from endemic countries, imported CL is still frequently missed by physicians unfamiliar with the disease.15 Awareness of the possibility of leishmaniasis remains low and unusual clinical manifestations and relatively long intervals between return and lesion onset may further impede clinical suspicion. An additional problem is how to achieve a definitive diagnosis. In the present case, diagnosis of leishmaniasis was established from a positive histologic examination. However, bear in mind that the sensitivity of histopathologic examination in diagnosing CL may be only 14–18%15; thus, if CL is suspected, histologic examination should always be combined with modern molecular diagnostic techniques, mainly PCR, in skin specimens. The PCR analysis also allows characterization of the infecting parasite, which is essential for the further management of the case.1,2 Additional serologic testing can corroborate the diagnosis, but negative serology does not exclude CL.
The present case further shows that a relatively short stay (2 weeks) in an area non-endemic for human leishmaniasis may be sufficient for infection. Knowledge of the geographic distribution of leishmaniasis is also a prerequisite for diagnosis in travelers. However, in Europe autochthonous leishmaniasis is no longer limited to the Mediterranean region. It has spread northward, as shown by recent reports of cases of autochthonous visceral leishmaniasis in northern Italy and southern Germany.12 Thus, studies based on discriminatory taxonomy are needed for effective disease control. At present, the MON system, a multi-locus isoenzyme analysis (MLEE) based on 15 enzymes, is the reference method for Leishmania discrimination. After extensive use for more than 20 years, over 200 differing zymodeme types have now been classified. Notwithstanding these extensive investigations, the correlation between zymodeme group and clinical manifestation, natural course, treatment response, or geographic distribution remains low. Recent data based on molecular parasitologic investigations have revealed substantial discrepancies in the present taxonomy based on MLEE. Moreover, MLEE typing is frequently not sufficiently discriminatory to highlight parasite diversity in different endemic regions, decreasing its epidemiologic and clinical value.13,16 In addition, this technique requires isolation and culture of the parasites and is thus time-consuming.
In the past decade novel cost-effective PCR-based methods have been developed and can be carried out directly on clinical samples. Such methods allow rapid strain-specific diagnosis with a reported sensitivity of 98–100% and a specificity of 100% at the genus level.15 Harambulous and co-workers13 developed a molecular typing method for the L. donovani/infantum complex based on the amplification of the K26 antigen-coding gene. The size of the amplicon is specific and this technique is particularly useful for epidemiologic studies in the Mediterranean area. Evaluation of the genetic polymorphism in 112 strains from Africa, India, and several endemic European countries showed a clear correlation with the geographic origin of the strains.
In Cyprus, L. donovani/infantum complex MON-1 is re-emerging as canine leishmaniasis with seroprevalence rates reaching 15%.17 Significantly, in a recent canine survey one of 62 isolates was identified as a MON-98 strain.17 In K26 typing, MON-1 strains from Cyprus show a single amplicon at 626 bp. Data on K26 typing regarding the Cyprian MON-98 strain are unavailable.13 However, reports of human infections are rare, which may partly be explained by the local population of the non-man-biting vector sub-species Phlebotomus tobbi.17 Remarkably, in 2006, five cases of leishmaniasis caused by a non-MON-1 strain of the L. donovani/infantum complex were diagnosed in permanent residents of Cyprus.18 The isolates were typed as MON-37 with a 700-bp amplicon in the K26 assay, representing the first autochthonous European cases caused by the etiologic agent of anthroponotic leishmaniasis in East Africa and in the Indian subcontinent.
The isolate identified in our patient showed a main 940-bp amplicon and two smaller amplification products with 870 and 370 bp. Interestingly, the combination of a 940-bp and an 870-bp product has as yet been identified only in Greece, whereas a 370-bp amplicon product has been observed solely in strains originating from Spain.13
Sequence analysis of the predominant 940-bp amplicon proved similarity to Greek strains, for the smaller amplicons no reference data were available. All MON-98 strains from Greece generated only the 940-bp amplicons.13 The strain identified in our patient appears to be a hitherto undescribed member of the L. donovani/infantum complex, with a high similarity to strains endemic in Greece, underlining the emergence and spread of the disease. To our knowledge, this is the first case of leishmaniasis in a traveler returning from Cyprus.
ACKNOWLEDGMENTS
We are grateful to Marcellus Fischer (German Armed Forces Hospital and Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany) for helpful discussions and critical reading of the manuscript. We thank Peter Petzelbauer (Skin and Endothelium Research Division, Department of Dermatology, Medical University Vienna) for the histological description.
Footnotes
Authors' addresses: Wolfgang Poeppl, Tom Pustelnik, and Gerhard Mooseder, Department of Dermatology, Military Hospital Vienna, Vienna, Austria, E-mails: wolfgang.poeppl@meduniwien.ac.at, tom@hautarztpustelnik.at, and gmoos@gmx.at. Julia Walochnik and Herbert Auer, Institute of Specific Prophylaxis and Tropical Medicine, Department of Medical Parasitology, Medical University of Vienna, Vienna, Austria, E-mails: julia.walochnik@meduniwien.ac.at and herbert.auer@meduniwien.ac.at.
References
- 1.Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez U, Pinart M, Reveiz L, Alvar J. Interventions for Old World cutaneous leishmaniasis. Cochrane Database Syst Rev. 2008:CD005067. doi: 10.1002/14651858.CD005067.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Blum J, Desjeux P, Schwartz E, Beck B, Hatz C. Treatment of cutaneous leishmaniasis among travellers. J Antimicrob Chemother. 2004;53:158–166. doi: 10.1093/jac/dkh058. [DOI] [PubMed] [Google Scholar]
- 4.Mondal S, Bhattacharya P, Ali N. Current diagnosis and treatment of visceral leishmaniasis. Expert Rev Anti Infect Ther. 2010;8:919–944. doi: 10.1586/eri.10.78. [DOI] [PubMed] [Google Scholar]
- 5.Calvopina M, Gomez EA, Sindermann H, Cooper PJ, Hashiguchi Y. Relapse of new world diffuse cutaneous leishmaniasis caused by Leishmania (Leishmania) mexicana after miltefosine treatment. Am J Trop Med Hyg. 2006;75:1074–1077. [PubMed] [Google Scholar]
- 6.Mohebali M, Fotouhi A, Hooshmand B, Zarei Z, Akhoundi B, Rahnema A, Razaghian AR, Kabir MJ, Nadim A. Comparison of miltefosine and meglumine antimoniate for the treatment of zoonotic cutaneous leishmaniasis (ZCL) by a randomized clinical trial in Iran. Acta Trop. 2007;103:33–40. doi: 10.1016/j.actatropica.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Soto J, Toledo JT. Oral miltefosine to treat new world cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:7. doi: 10.1016/S1473-3099(06)70665-X. [DOI] [PubMed] [Google Scholar]
- 8.Tappe D, Muller A, Stich A. Resolution of cutaneous old world and new world leishmaniasis after oral miltefosine treatment. Am J Trop Med Hyg. 2010;82:1–3. doi: 10.4269/ajtmh.2010.09-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faber WR, Wonders J, Jensema AJ, Chocholova E, Kager PA. Cutaneous leishmaniasis with lymphadenopathy due to Leishmania donovani. Clin Exp Dermatol. 2009;34:e196–e198. doi: 10.1111/j.1365-2230.2008.02996.x. [DOI] [PubMed] [Google Scholar]
- 10.Neub A, Krahl D, Stich A, Amon U. Cutaneous infection with Leishmania infantum in an infant treated successfully with miltefosine. J Dtsch Dermatol Ges. 2008;6:1061–1064. doi: 10.1111/j.1610-0387.2008.06779.x. [DOI] [PubMed] [Google Scholar]
- 11.van Eys GJ, Schoone GJ, Kroon NC, Ebeling SB. Sequence analysis of small subunit ribosomal RNA genes and its use for detection and identification of Leishmania parasites. Mol Biochem Parasitol. 1992;51:133–142. doi: 10.1016/0166-6851(92)90208-2. [DOI] [PubMed] [Google Scholar]
- 12.Dujardin JC, Campino L, Canavate C, Dedet JP, Gradoni L, Soteriadou K, Mazeris A, Ozbel Y, Boelaert M. Spread of vector-borne diseases and neglect of leishmaniasis, Europe. Emerg Infect Dis. 2008;14:1013–1018. doi: 10.3201/eid1407.071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haralambous C, Antoniou M, Pratlong F, Dedet JP, Soteriadou K. Development of a molecular assay specific for the Leishmania donovani complex that discriminates L. donovani/Leishmania infantum zymodemes: a useful tool for typing MON-1. Diagn Microbiol Infect Dis. 2008;60:33–42. doi: 10.1016/j.diagmicrobio.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Nicholas KB, Nicholas HB, Jr., Deerfield DW., II GeneDoc: analysis and visualization of genetic variation. Embnet News. 1997;4:1–4. [Google Scholar]
- 15.Antinori S, Gianelli E, Calattini S, Longhi E, Gramiccia M, Corbellino M. Cutaneous leishmaniasis: an increasing threat for travellers. Clin Microbiol Infect. 2005;11:343–346. doi: 10.1111/j.1469-0691.2004.01046.x. [DOI] [PubMed] [Google Scholar]
- 16.Alam MZ, Haralambous C, Kuhls K, Gouzelou E, Sgouras D, Soteriadou K, Schnur L, Pratlong F, Schonian G. The paraphyletic composition of Leishmania donovani zymodeme MON-37 revealed by multilocus microsatellite typing. Microbes Infect. 2009;11:707–715. doi: 10.1016/j.micinf.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Mazeris A, Soteriadou K, Dedet JP, Haralambous C, Tsatsaris A, Moschandreas J, Messaritakis I, Christodoulou V, Papadopoulos B, Ivovic V, Pratlong F, Loucaides F, Antoniou M. Leishmaniases and the Cyprus paradox. Am J Trop Med Hyg. 2010;82:441–448. doi: 10.4269/ajtmh.2010.09-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antoniou M, Haralambous C, Mazeris A, Pratlong F, Dedet JP, Soteriadou K. Leishmania donovani leishmaniasis in Cyprus. Lancet Infect Dis. 2008;8:6–7. doi: 10.1016/S1473-3099(07)70297-9. [DOI] [PubMed] [Google Scholar]


