Abstract
One hundred fifty-two Trypanosoma cruzi seropositive women were submitted to a single hemoculture; 101 were pregnant, and 51 were not pregnant. Seven tubes from each individual were harvested with liver infusion tryptose (LIT) medium and observed monthly until the fifth month. Hemocultures were positive in 50% (76 of 152) of the women. Results showed that the positivity was 29.4% (15 of 51) among non-pregnant women and 60.4% (61 of 101) in pregnant women (P < 0.05). In relation to gestational age, there were significant differences in positivity, with a higher proportion of women with positive hemocultures (20 of 25) before 21 weeks and lower after 30 weeks (10 of 21; P = 0.02). We conclude that pregnancy enhances the parasitemia in Chagas disease, with a higher effect early in pregnancy.
Introduction
Chagas disease is still one of the main causes of disability in Latin America. Conservative estimates1,2 show that there are at least 8 million infected people. Control of triatomine vectors with insecticides and screening of blood donors are performed in most endemic countries, but the prophylaxis for vertical transmission is limited to early specific treatment of girls before fertile age. The incidence of vertical transmission varies from 1% to up to 12%, depending on the geographical area.3 The reason for this wide difference is not clearly known; however, parasitemia could be one of the factors involved.3
As known, there is an increase in parasitemia during pregnancy, observed by xenodiagnosis in Argentina4 and the northeast of Brazil.5 There are no studies employing hemoculture and comparing pregnant versus non-pregnant Chagasic women. The relationship with gestational age has not been precisely explored. Furthermore, geographical differences related with parasitemia in Chagas disease have been described.6
The states of Goias as well as Mato Grosso do Sul7 in Brazil hold a free screening program for all pregnant women living in these states. In this program, 19 markers are studied from filter-paper samples for the prevention of transmission of diseases such as hepatitis, syphilis, human immunodeficiency virus (HIV), human T-lymphotropic value (HTLV), and Chagas disease. For those pregnant women positive for any of the markers of infection, whole-blood samples are drawn for confirmation. The Laboratory for Research on Chagas Disease of the Federal University of Goias, where this work was performed, is in charge of diagnosis confirmation of those samples positive for antibodies to Trypanosoma cruzi.
To study T. cruzi parasitemia in pregnancy and to reach better understanding of the mechanisms involved during gestation, a group of pregnant and non-pregnant seropositive women were studied by hemoculture and compared. Furthermore, the issues of geographical differences, intensity of parasitemia, and gestational age were also explored.
Material and Methods
Study population.
After approval of the Ethical Committee (CEPMHA/HC/UFG Number 056/2006), all pregnant and non-pregnant women at the Laboratory for research on Chagas disease were invited to participate and after acceptance, signed an informed consent during the period of June 2006 to July 2007. Most of the pregnant women had been previously detected as seropositive by the laboratory of the State program for pregnant women. Goiás state has an estimated population of 5,647,035 people,8 and around 185,000 pregnant women were tested from 2004 up to the end of this study. A first screening is performed by enzyme-linked immunosorbent assay (ELISA) on filter paper. From those tested positive, a blood collection is requested, and an aliquot of serum is sent to the specialized laboratory where this work has been done. The laboratory confirmed 332 T. cruzi seropositive pregnant women in this same period, including those of the present study. After confirmed as seropositive, they were invited for a medical consultation at our outpatient clinic, where a new blood collection was performed. From the 332 pregnant women confirmed as seropositive, 153 came for a new blood collection and medical consult, and from those, 101 were invited to participate and included in the study. Fifty-one non-pregnant seropositive women were selected from our outpatient clinic at the same hospital, where 125 seropositive females of fertile age were confirmed during the study period (Figure 1). Only women more than 16 and less than 50 years old were invited to participate. For those pregnant, gestational age and last menstrual period were recorded. Geographical data were collected according to the state where the patients were born, which did not always correspond to where they were actually living (Table 1). No person included in this study had been specifically treated with benznidazole, the available drug for Chagas disease treatment in Brazil.
Figure 1.
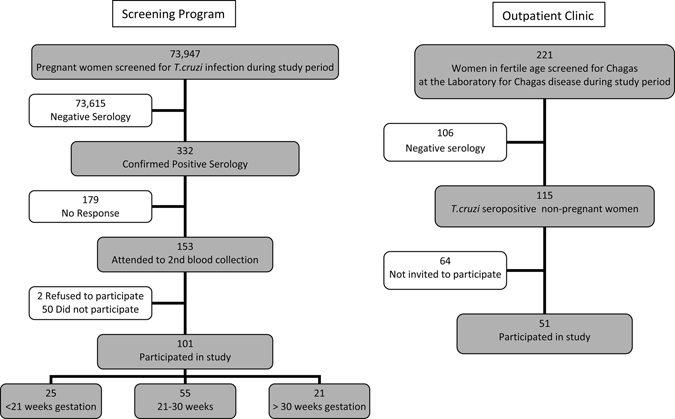
Flow chart showing the origin of patients selected for this study.
Table 1.
Distribution of characteristics in 152 pregnant and non-pregnant women diagnosed as seropositive for Chagas disease in Goiania, Brazil, from 2006 to 2007
| Pregnant (N = 101) | Non-pregnant (N = 51) | P value | |
|---|---|---|---|
| N (%) | N (%) | ||
| State of origin | |||
| Bahia | 58 (57.4) | 29 (56.9) | 0.95 |
| Goias | 35 (34.7) | 15 (29.4) | 0.52 |
| Other* | 8 (7.9) | 7 (13.7) | 0.26 |
| Mean age (SD) | 29.3 (0.6) | 34.1 (1.0) | < 0.01 |
| Age groups (years) | |||
| 17–20 | 7 (6.9) | 2 (3.9) | 0.46 |
| 21–30 | 52 (51.5) | 14 (27.5) | < 0.01 |
| 31–40 | 38 (37.6) | 23 (45.1) | 0.37 |
| 41–50 | 4 (4.0) | 12 (23.5) | < 0.01 |
| Clinical evaluation (%) | 77 (76.2) | 42 (82.3) | 0.39 |
| Megaesophagus (%) | 2 (2.6) | 2 (4.8) | 0.53 |
| Electrocardiogram | |||
| Normal (%) | 51 (66.2) | 24 (57.1) | 0.33 |
| CRBBB† (%) | 16 (20.8) | 8 (19.0) | 0.82 |
| Non-characteristic abnormalities‡ (%) | 16 (20.8) | 9 (21.4) | 0.93 |
| Cardiopathy (%) | 1 (1.3) | 0 (0.0) | 0.46 |
| Megacolon (%) | 1(1.3) | 1 (2.4) | 0.66 |
Other includes the states of Minas Gerais (two non-pregnant and four pregnant), Ceara (two non-pregnant and one pregnant), Paraiba (one non-pregnant and two pregnant), Pernambuco and Piaui (one non-pregnant each), and Tocantins (one pregnant).
CRBBB = complete right-bundle branch block.
Mainly primary ST–T changes on the electrocardiogram.
All infected patients, except for six, were from endemic regions. For the other six patients, because they were born in large cities, with no report of triatomine contact, congenital transmission was suspected.9 None reported receiving blood transfusions or organ transplantation.
The scope of this study was limited to parasitemia levels, and no attempts to perform exams and/or clinical examination were pursued for ethical reasons. Nevertheless, medical records were obtained from 77 of 101 pregnant and 42 of 51 non-pregnant women that had had a medical consult (Table 1).
Serology.
Serology was performed as described before6,10 by four different techniques: indirect immunofluorescence (Biomérieux conjugate, Biomérieux, Jacarepaguá, RJ, Brazil), indirect hemagglutination (Wiener Laboratorios, Rosario, Argentina), and two immunoenzimatic assays (an in-house ELISA [with Sigma conjugate, Sigma, Saint Louis, MI] and a kit of ELISA [EIE-Chagas, Biomanguinhos, Fiocruz, Rio de Janeiro, RJ, Brazil]). Only those cases confirmed as positive by at least three serological tests were included. In one case, all serological tests were negative; thus, this patient was excluded from the study. Each individual had two sets of sera: one from the original collection of blood sent by the laboratory of the State program, and another, for a secondary confirmation, from an aliquot of plasma of the blood collection for hemoculture.
Hemoculture.
The technique of Chiari and others,11 modified by Luz and others,12 was performed. Briefly, 30 mL of heparinized blood were drawn and spun down in a refrigerated centrifuge (Janetzki model K-24 with fixed angle) for 10 min at 3,000 rpm. Plasma was transferred to a separate tube and spun down, and the pellet was harvested with 3 mL liver infusion tryptose (LIT medium) for the first culture. Six additional cultures were prepared as follows: the pellet of red blood cells was resuspended with equal parts of LIT medium and spun down again, and the washed pellet was distributed in six vials, each one with 3 mL LIT. All of the procedures were done in sterility at 4°C and processed less than 6 hours after blood was drawn.
Cultures were incubated at 26°C in a biochemical oxygen demand (BOD) incubator (FANEM, São Paulo, Brazil). Tubes were gently resuspended two times per a week, and an aliquot (20 µL) was drawn from each tube and checked monthly by microscopy for motile trypanosomes until the fifth month.
The intensity of parasitemia was evaluated by classifying it into three groups: non-detected (no positive tubes), medium (one to three positive tubes of seven), and high parasitemia (at least four positive tubes of seven).13 Another approach used was to record the time when each tube was found to be positive, assuming that the earlier that the parasites were detected, the higher the parasitemia.13
Statistical analysis.
We estimated that a sample size of 152 individuals would confer a power of 0.81 based on a reported prevalence of positive xenodiagnosis of 24% in non-pregnant individuals14 and assuming a prevalence of positive hemocultures of 50% in pregnant women. Comparison of the categorical variables was performed by the Pearson's χ2 or Fisher exact test. Continuous variables were compared using the Student t test. In all statistical tests, the α-error was set at 5%. We applied a logistic regression model to test the association of gestational age (categorized as less than 21 weeks, 21–30 weeks, and more than 30 weeks) and the probability of a positive hemoculture (outcome) adjusted for individual's age in years. We reported the adjusted odds ratio (OR) with respective 95% confidence intervals (CIs) as a measure of association.
Results
We evaluated 152 women—101 pregnant and 51 non-pregnant. The overall age range varied from 17 to 49 years. For the pregnant women, age range varied from 17 to 44 years old (mean = 29.3, standard deviation [SD] = 0.6), and for the control group, age range varied from 18 to 49 years old (mean = 34.1, SD = 1.0) (Table 1).
Parasites were detected in one or more cultures in 76 (50%) of 152 infected women (Table 2) in different periods of time from the first month of examination on (Figure 2). In three cases, parasites were detected only at the last examination (at 150 days). More than one-half (72.4%) of the cultures were first positive within the first 60 days of examination (Figure 2).
Table 2.
Positive and negative hemocultures in pregnant and non-pregnant women in Goiania, Brazil, from 2006 to 2007
| Positive hemoculture N (%) | Negative hemoculture N (%) | Total N (%) | |
|---|---|---|---|
| Pregnant | 61 (60.4) | 40 (39.6) | 101 (100) |
| Non-pregnant | 15 (29.4) | 36 (70.6) | 51 (100) |
| Total | 76 | 76 | 152 |
P < 0.01.
Figure 2.
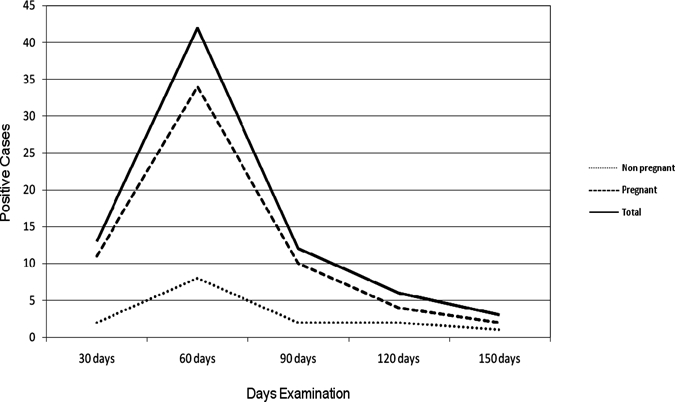
Results of a single hemoculture in 152 T. cruzi-infected women according to the time when the first tubes were positive (51 non-pregnant and 101 pregnant women in Goiania, Brazil, from 2006 to 2007).
To estimate the numbers of parasites per individual studied, the number of positive tubes by hemoculture (seven tubes) was recorded (Table 3). In 17.1% (13 in 76 positive cases), a single tube showed parasites. In a single case, all cultures were positive, and in 15 cases, six tubes had parasites. Non-detected parasitemia was found in 76 (50.0%) cases. In 39 (25.7%) cases, one to three tubes were positive (i.e., medium parasitemia), and in 37 (24.3%) cases, four or more tubes were positive (i.e., high parasitemia) (Table 3).
Table 3.
Results of a single hemoculture in 152 T. cruzi-infected women according to the degree of parasitemia (number of positive tubes) distributed in non-pregnant and pregnant groups in Goiania, Brazil, from 2006 to 2007
| No. of positive tubes | Non-pregnant (N) | Pregnant (N) | Total N (%) | Parasitemia level |
|---|---|---|---|---|
| 0/7 | 36 | 40 | 76 (50.0) | Non-detected |
| 1/7 | 3* | 10 | 13 (8.6) | Medium |
| 2/7 | 5 | 13 | 18 (11.8) | Medium |
| 3/7 | 1 | 7 | 8 (5.3) | Medium |
| 4/7 | 1 | 7 | 8 (5.3) | High |
| 5/7 | 1 | 12 | 13 (8.6) | High |
| 6/7 | 4 | 11 | 15 (9.9) | High |
| 7/7 | 0 | 1 | 1 (0.7) | High |
| Total | 51 | 101 | 152 (100.0) |
In these three cases, the positive tube was found only at the 120- to 150-day examinations.
Another way to evaluate the intensity of parasitemia was to consider the timing in which each tube was positive for the first time.13 The majority (72.4%) was positive at 30- to 60-day examinations, and 27.6% (21 of 76) were positive at from 90 to 150 days.
The positivity stratified by age groups showed a higher positivity among younger women (in nine patients less than 21 years old, six were positive). In the 21- to 30-year-old women group, there was a 57.6% positivity. Women aged between 31 and 40 years and more than 40 years showed the lowest parasitemia, 41% and 43.8%, respectively (Table 4).
Table 4.
Results of a single hemoculture in 152 T. cruzi-infected women distributed by age group and non-pregnant and pregnant groups in Goiania, Brazil, from 2006 to 2007
| Age group (years) | Non-pregnant | Pregnant | All women | |||
|---|---|---|---|---|---|---|
| Positive N (%) | Total (N) | Positive N (%) | Total (N) | Positive N (%) | Total (N) | |
| 17–20 | 1 (50.0) | 2 | 5 (71.4) | 7 | 6 (66.7) | 9 |
| 21–30 | 3 (21.4) | 14 | 35 (67.3) | 52 | 38 (57.6) | 66 |
| 31–40 | 7 (30.4) | 23 | 18 (47.4) | 38 | 25 (41.0) | 61 |
| 41–50 | 4 (33.3) | 12 | 3 (75.0) | 4 | 7 (43.8) | 16 |
| Total | 15 (29.4) | 51 | 61 (60.4) | 101 | 76 (50.0) | 152 |
The distribution of positive hemocultures in relation to the state of origin was as follows. The majority of patients (57.2%) was from a neighbor state, Bahia, a highly endemic region where the triatomine control was obtained later15 than in the other states. The positivity in hemocultures for this group was 54.0% (47 of 87). Thirty percent (45 of 152) were born in the state of Goias, where this study was performed. In this group, cultures were positive in 22 of 45 women (48.9%). Furthermore, 14 women came from different states. Two of them were positive (14.3%).
When the patients were allocated to the pregnant and non-pregnant groups (Table 2), a higher proportion of positive hemocultures was observed for the pregnant group. Sixty percent of all pregnant women had at least one positive tube (61 of 101) compared with 29.4% (15 of 51) in the non-pregnant group (P < 0.01). In addition, considering the positivity obtained, a higher proportion of pregnant women had high parasitemia by the criterion of number of positive tubes. This number was consistently higher (two times or more) in the pregnant group. The main difference was found in those with five of seven positive tubes; 11.9% (12 of 101) in the pregnant group were positive compared with 2% (1 of 51) in the non-pregnant group (Table 3).
In relation to the time that cultures were positive for the first time, there were no differences among pregnant and non-pregnant women (Figure 2).
When results were stratified by age group, the positivity was higher in the pregnant group (from 47.4% to 75% compared with 21.4% to 50% in the non-pregnant group), with higher positivity among younger patients in both groups (Table 4).
When the results from pregnant women were stratified by gestational age (Table 5), the proportion of positive hemocultures observed among those tested before 21 weeks of gestation, between 21 and 30 weeks, and after 30 weeks were, respectively, 80.0%, 56.4%, and 47.6% (P = 0.04). There was no difference in the mean age for those three age groups (P = 0.93). This result was confirmed by the logistic regression models. In an univariate model, women of gestational age of 21–30 weeks were less likely to have a positive hemoculture compared with women of gestational age < 21 weeks, and women of gestational age > 30 weeks were less likely to have a positive hemoculture than those in the 21–30 weeks group (OR = 0.49; P = 0.025; 95% CI = 0.26–0.91). When the model was adjusted for individual's age, the results were similar (OR = 0.44; P = 0.017; 95% CI = 0.23–0.86).The parasitemia in the group before 21 weeks of gestation was also higher than in the other two groups, 11 of 20 (55.0%) with four or more positive tubes compared with 16 of 31 (51.6%) and 4 of 10 (40.0%) in the other two groups; however, this difference was not statistically significant.
Table 5.
Results of a single hemoculture in 101 T. cruzi-infected pregnant women stratified by gestational age in Goiania, Brazil, from 2006 to 2007
| Gestational age | N | Mean maternal age (SD) | Positive hemocultures (%) | Cases with more than or equal to three of seven tubes (percent of cases with high parasitemia) |
|---|---|---|---|---|
| < 21 weeks | 25 | 29.6 (1.4) | 20 (80.0) | 11/20 (55.0) |
| 21–30 weeks | 55 | 29.2 (0.75) | 31 (56.4) | 16/31 (51.6) |
| 31 or more weeks | 21 | 28.9 (1.2) | 10 (47.6) | 4/10 (40.0) |
| Total | 101 | 29.3 (0.6) | 61 (60.4) | 31/61 (50.8) |
SD = standard deviation.
Discussion
We observed in this study that hemocultures were positive two times more frequently during gestation compared with the non-pregnant group. To our knowledge, few reports on this subject have been published before. In those studies, xenodiagnosis was used, and it reported similar results (i.e., an increase in parasitemia during pregnancy).4,5 There are no reports in the literature with other methods for measuring parasitemia (i.e., hemoculture or polymerase chain reaction [PCR]) comparing pregnant versus non-pregnant groups. A single study reports on hemoculture only in pregnant women using 2.0 mL of blood and obtaining positivity in 28 of 81 (34.6%), which is a high value considering the low volume of blood used.16 The figures obtained in this study compare with those published for xenodiagnosis. Storni and Bolsi4 found 33 of 50 (66%) pregnant women with a positive xenodiagnosis compared with 12 of 40 (30%) in the non-pregnant group, similar to our results (60.4% and 29.4%). Menezes and other5 studied 119 pregnant women and compared the parasitemia of the same women after delivery. The positivity was 70% during pregnancy versus 41% after delivery.
Pregnant and non-pregnant women were comparable by age group; however, among those pregnant, we observed a higher proportion of women between 21 and 30 years old, which reflects the age period when pregnancy is most frequent. Among those in the non-pregnant group, a higher proportion of older women was observed. However, we believe that these differences did not bias our results, because there was an even increase in the proportion of positive hemocultures in all age groups among those pregnant. In relation to the state that they were born, groups were also comparable, because there were similar proportions of pregnant and non-pregnant women from Bahia, Goias, and other states. Even if not all patients underwent a detailed clinical study, for those that did, differences were not detected (Table 1).
The relationship between parasitemia and age with Chagas disease, also observed in this study, is a well-known fact.6,17 Younger infected people have higher parasitemia, probably because a shorter period of time has elapsed since the infection and the laboratory exam.
An unexpected high correlation was found with gestational age. This is the first time, to our knowledge, that such a relationship was detected among pregnant women. Bittencourt18 reported the incidence of congenital transmission in different periods of gestation, mostly among fetuses, and Menezes and others5 showed an increase of parasitemia at the third trimester, which was not statistically different. These differences could not be attributed to age group, because they were similar. The increase of parasitemia in the first one-half of pregnancy may help us to understand the pathogeny of the congenital transmission, which may take place at this period.
It was possible to detect differences in parasitemia among those women with positive hemocultures. It is believed that the initial number of parasites is low when a single tube is found positive, especially when this finding is found on the last examination at 150 days. Conversely, if all seven tubes show parasites at the first examination (30 days), the initial level of parasitemia was higher. Obviously, at least one parasite should be present in each of the seven tubes and able to grow. We used both approaches. The number of positive tubes did show an even distribution between 10.5% of all positive cultures (three and four positive tubes of seven) and 23.6% (two positives of seven). There were no striking differences among the number of positive hemocultures with different numbers of positive tubes in the entire positive group or when separated into pregnant and non-pregnant groups. This shows an even distribution, which probably reflects different degrees of parasitemia in both groups. The other approach, looking for the timing by which first cultures became positive, had similar results.
Nevertheless, patients could be classified in three major groups: those with non-detected parasitemia (i.e., negative hemocultures), which were one-half of the group, those with medium parasitemia, defined arbitrarily as having up to three positive tubes, and those with high parasitemia, with more than three of seven tubes positive. In other studies, patients were assigned in those same groups by seriated xenodiagnosis17 or hemoculture19; the results observed were similar to the non-pregnant group.
The proportion observed in the non-pregnant group (29.4% positives) is also similar to a previous report by us using a single examination, with a positivity on xenodiagnosis of 24%.14 Castro and others19 reported higher values (40% with a single hemoculture) with the same technique used in this work.
All patients, with the exception of seven (three that were pregnant), included in this study were born in Central Brazil. In relation to the type of T. cruzi present in this region, it has been shown that it corresponds mainly to TcII (previously known as zymodeme 2),20 according to the new nomenclature,21 which was isolated in all chronic phase patients in previous investigations in Central Brazil. This region has been recognized to harbor a higher prevalence of megaesophagus and severe cardiopathy and a lower prevalence of congenital transmission22 compared with other regions of South America. TcII has been thought to be responsible for these geographical differences. For these reasons, we believe that the material is rather homogeneous in relation to the type of T. cruzi and therefore, that different Tc types would not be likely involved in the individuals studied. For the same reason, these findings may not be valid for other regions where TcI or TcV/TcVI predominates, such as north of the Amazon River or other countries of the South Cone.
Therefore, we conclude that T. cruzi seropositive pregnant women had higher parasitemia than non-pregnant women, without a particular profile of positivity. Another finding, unexpected, was that parasitemia was higher in the first one-half of pregnancy. The mechanisms underlining the increase in parasitemia during pregnancy are not clearly understood. Immunological factors, such as a decreased production of interferon gamma, have been suggested.16 The fact, shown in this investigation, that this increase may start early in pregnancy could focus other studies, both experimental and human. This could also focus future research on vertical transmission of T. cruzi, another subject which is currently debated. We hope that these findings could help to provide a better understanding of the relationship between pregnancy and T. cruzi infection and the mechanisms underlying congenital transmission.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Secretary of Health of Goias as well as the non-profit organization Associação de Pais e Amigos dos Excepcionais for sending samples and pregnant infected women to our laboratory, Dr. Daniela V. Luquetti for the review of the English version, and Dr. Bernardo H. L. Goulart for the statistical analysis.
Footnotes
Authors' addresses: Liliane da Rocha Siriano, Alejandro O. Luquetti, and Neusa Leal Marra, Laboratório de Pesquisa da doença de Chagas, Hospital das Clínicas, Universidade Federal de Goiás, Goiânia, Brazil, E-mails: liliane.siriano@yahoo.com.br, aluquetti@hotmail.com, and nmarra@cardiol.br. Juliana Boaventura Avelar and Ana Maria de Castro, Departamento de Microbiologia, Imunologia, Parasitologia e Patologia, Instituto de Patologia Tropical e Saúde Pública, Universidade Federal de Goiás, Goiânia, Brazil, E-mail: jubavelar@hotmail.com and amaria.ana@gmail.com.
References
- 1.Schofield CJ, Jannin J, Salvatella R. The future of Chagas disease control. Trends Parasitol. 2006;22:583–588. doi: 10.1016/j.pt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Control of Chagas Disease. Second Report of the WHO Expert Committee. Geneva, Switzerland: World Health Organization; 2002. WHO Technical Report Series 905. [Google Scholar]
- 3.Carlier Y, Torrico F. Congenital infection with Trypanosoma cruzi: from mechanisms of transmission to strategies for diagnosis and control. Rev Soc Bras Med Trop. 2003;36:767–771. doi: 10.1590/s0037-86822003000600024. [DOI] [PubMed] [Google Scholar]
- 4.Storni P, Bolsi FL. Embarazo y parasitismo por Trypanosoma cruzi. Medicina (B Aires) 1979;39:193–197. [PubMed] [Google Scholar]
- 5.Menezes CAS, Bittencourt AL, Mota E, Sherlock I, Ferreira J. Avaliação da parasitemia em mulheres portadoras de infecção pelo Trypanosoma cruzi durante e após a gestação. Rev Soc Bras Med Trop. 1992;25:109–113. doi: 10.1590/s0037-86821992000200004. [DOI] [PubMed] [Google Scholar]
- 6.Luquetti AO, Rassi A. In: Trypanosoma cruzi e Doença de Chagas. 2nd ed. Brener Z, Andrade Z, Barral-Neto M, editors. Rio de Janeiro, Brazil: Guanabara Koogan; 2000. pp. 344–378. (Diagnóstico Laboratorial da Infecção pelo Trypanosoma cruzi). [Google Scholar]
- 7.Botelho CAO, Tomaz CAB, da Cunha RV, Botelho LO, Assis DM, Pinho DLM. Prevalência dos agravos triados no programa de proteção a gestante do estado de Mato Grosso do Sul de 2004 a 2007. Rev Patol Trop. 2008;37:341–353. [Google Scholar]
- 8.Instituto Brasileiro de Geografia e Estatística Contagem da População 2007. 2009. http://www.ibge.gov.br/home/estatistica/populacao/contagem2007/contagem_final/tabela1_1_26.pdf Available at. Accessed June 2, 2009.
- 9.Luquetti AO, Ferreira AW, Oliveira RA, Tavares SBN, Rassi A, Dias JCP, Prata A. Transmisión congenita del Trypanosoma cruzi en Brasil: estimativa de prevalencia baseada en resultados preliminares de la encuesta nacional serológica en niños menores de cinco años, así como otras fuentes. Rev Soc Bras Med Trop. 2005;38((Suppl 2)):24–26. [PubMed] [Google Scholar]
- 10.Zicker F, Smith PG, Luquetti AO, Oliveira OS. Mass screening for Trypanosoma cruzi infections using the immunofluorescence, ELISA and haemagglutination tests on serum samples and on blood eluates from filter-paper. Bull World Health Organ. 1990;68:465–471. [PMC free article] [PubMed] [Google Scholar]
- 11.Chiari E, Dias JCP, Lana M, Chiari CA. Hemocultures for the parasitological diagnosis of human chronic Chagas' disease. Rev Soc Bras Med Trop. 1989;22:19–23. doi: 10.1590/s0037-86821989000100004. [DOI] [PubMed] [Google Scholar]
- 12.Luz ZMP, Coutinho MC, Cançado JR, Krettli AU. Hemocultura: técnica sensível na detecção do Trypanosoma cruzi em pacientes chagásicos na fase crônica da doença de Chagas. Rev Soc Bras Med Trop. 1994;27:143–148. doi: 10.1590/s0037-86821994000300004. [DOI] [PubMed] [Google Scholar]
- 13.Castro AM, Luquetti AO, Rassi A, Rassi GG, Chiari E, Galvão LMC. Blood culture and polymerase chain reaction for the diagnosis of the chronic phase of human infection with Trypanosoma cruzi. Parasitol Res. 2002;88:894–900. doi: 10.1007/s00436-002-0679-3. [DOI] [PubMed] [Google Scholar]
- 14.Luquetti AO, Gonçalves AV, Carneiro LB, Santos JF, Medeiros LB, Gomes DVA, Machado MN, Campos DE, Rassi A. Tentativa de correlação do xenodiagnóstico com o nível de parasitemia na fase crônica da doença de Chagas. Rev Soc Bras Med Trop. 1994;27((Suppl 2)):93–94. [Google Scholar]
- 15.Dias JCP. In: El control de la enfermedad de Chagas en los países del Cono Sur de América. Silveira AC, editor. Uberaba, Brazil: Faculdade de Medicina do Triangulo Mineiro; 2002. pp. 145–250. (O controle da doença de Chagas no Brasil). [Google Scholar]
- 16.Hermann E, Truyens C, Alonso-Vega C, Rodrigues P, Berthe A, Torrico F, Carlier Y. Congenital transmission of Trypanosoma cruzi is associated with maternal enhanced parasitemia and decreased production of interferon gamma in response to parasite antigens. J Infect Dis. 2004;189:1274–1281. doi: 10.1086/382511. [DOI] [PubMed] [Google Scholar]
- 17.Castro CN. Influencia da parasitemia no quadro clínico da doença de Chagas. Rev Patol Trop. 1980;9:73–136. [Google Scholar]
- 18.Bittencourt AL. Possible risk factors for vertical transmission of Chagas disease. Rev Inst Med Trop Sao Paulo. 1992;34:403–408. doi: 10.1590/s0036-46651992000500006. [DOI] [PubMed] [Google Scholar]
- 19.Castro AM, Luquetti AO, Rassi A, Chiari E, Galvão LMC. Detection of parasitemia profiles by blood culture after treatment of human chronic Trypanosoma cruzi infection. Parasitol Res. 2006;99:379–383. doi: 10.1007/s00436-006-0172-5. [DOI] [PubMed] [Google Scholar]
- 20.Luquetti AO, Miles MA, Rassi A, de Rezende JM, de Souza AA, Povoa MM, Rodrigues I. Trypanosoma cruzi: zymodemes associated with acute and chronic Chagas' disease in central Brazil. Trans R Soc Trop Med Hyg. 1986;80:462–470. doi: 10.1016/0035-9203(86)90347-0. [DOI] [PubMed] [Google Scholar]
- 21.Zingales B, Andrade SA, Briones MRS, Campbell DA, Chiari E, Fernandes O, Guhl F, Lages-Silva E, Macedo AM, Machado CR, Miles MA, Romanha AJ, Sturm NR, Tibayrenc M, Schijman AG. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- 22.Rassi A, Amato Neto V, Rassi GG, Amato VS, Rassi Junior A, Luquetti AO, Rassi SG. A retrospective search for maternal transmission of Chagas infection from patients in the chronic phase. Rev Soc Bras Med Trop. 2004;37:485–489. doi: 10.1590/s0037-86822004000600011. [DOI] [PubMed] [Google Scholar]


