Abstract
Nitroimidazole antibiotics are the mainstay of treatment of invasive amebiasis; however, few comparative studies of applicable antibiotics are available. Evidence of sporadic clinical failure and rare reports of metronidazole resistance have led to the investigation of novel antiamebic therapeutics. The goal of this study was to examine drug efficacy in both in vitro and in vivo models of intestinal amebiasis. We studied six current and three novel drugs. Many drugs, including metronidazole, nitazoxanide, and nitazoxanide derivatives, were shown to be potently inhibitory in vitro. However, metronidazole remained the most effective in vivo, both in preventative and curative regimens, underscoring the value of animal models in evaluating future therapies.
Introduction
Intestinal amebiasis, the disease caused by the parasite Entamoeba histolytica, is a significant source of morbidity and mortality in developing countries and remains a threat to travelers.1,2 Estimates of the global burden range from 50 to 480 million cases per year.3 Treatment with antibiotics is generally highly effective; however, delays or failures in diagnosis or treatment lead to an estimated 40,000–100,000 deaths per year.
Oral metronidazole, a member of the 5-nitroimidazole family, is the standard therapy for treating intestinal amebiasis. Several related compounds, including tinidazole, are efficacious as well. Beyond nitroimidazoles, several alternative drug therapies have been used for invasive amebiasis, including emetine and dehydroemetine, which remain available in the United States for use by patients after failed metronidazole treatment. Chloroquine has also been used for extraintestinal abscess and has shown similar cure rates to metronidazole.4 More recently, nitazoxanide, a thiazolide derivative with a broad spectrum of activity against anaerobic bacteria and parasites, has also been shown to be an effective treatment in amebic diarrhea.5 After therapy with metronidazole, there can be a high rate of parasite persistence in the gut,6 which was recently observed in an outbreak in Japan.7 Hence, a treatment course with a luminal agent such as iodoquinol is recommended after metronidazole therapy.
There have been few comparisons of these drugs in humans. We have developed a mouse model of amebiasis whereby mice develop persistent infection with intestinal inflammation after injection of ameba. In this work, we use this model to examine the comparative efficacy of existing and novel drug compounds.
Materials and Methods
Ameba.
Laboratory strain (American Type Culture Collection, Manassas, VA) E. histolytica trophozoites HM1:IMSS were cultured axenically in complete TYI-S-33 medium for in vitro assays. E. histolytica trophozoites for in vivo experiments were derived from HM1:IMSS sequentially passaged through the mouse cecum and were cultivated xenically.
Antiamebic drugs.
For those drugs in clinical use (metronidazole, tinidazole, nitazoxanide, iodoquinol, emetine, and chloroquine), commercial pharmaceutical formulations were obtained from the University of Virginia or nearby pharmacies. Novel therapeutics (AMIX, VPC16a1006, and pyrvinium) were obtained as pure preparations. Drugs were dissolved in 100% dimethyl sulfoxide (DMSO) at 10× concentration and stored as stock solutions at −20°C. Stock solutions were diluted 1:10 in phosphate-buffered saline (PBS) immediately before use.
In vitro susceptibility assays.
In vitro drug susceptibility assays were performed using a variation on normal trophozoite culture. Briefly, 5 × 104 E. histolytica trophozoites were incubated for 72 hours at 37°C in 1 mL TYI-S-33. Antiamebic drugs were serially diluted in 5 μL DMSO. Negative controls for growth (time = 0 and 72 hours refrigerated cultures) and positive controls (culture medium plus DMSO) were included. After incubation, trophozoites were detached by chilling and counted on a hemocytometer. Raw counts were converted to a percentage growth scale, where the negative control equaled zero and the positive control equaled 100%. All cultures were performed in duplicate.
Mice and in vivo infection.
Six-week-old CBA/J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Animals were maintained under specific pathogen-free conditions at the University of Virginia and were infected in accordance with Institutional Animal Care and Use Committee approved protocols.8 Briefly, animals were anesthetized, laparotomized, and intracecally injected with 2 × 106 E. histolytica trophozoites. Antiamebic drugs were administered in 100 μL of 10% DMSO or PBS/DMSO control. For initial drug treatment studies (drug regimen 1), drugs were administered by oral gavage or intraperitoneal injection to male CBA/J mice one time before surgery (day −1) and three times after surgery (days +1, +3, and +5), and infection status was assessed by sacrifice on day +7. For post-infection studies (drug regimen 2), mice were infected as described and assessed for established infection after > 7 days. Established infection was determined by In Vivo Imaging System (IVIS) luminescent visualization as described previously8 and/or laparotomy (visual inspection and cecal culture). Mice with confirmed infection were orally gavaged daily with drug for 3 consecutive days, and infection status was assessed after mice were euthanized on the fourth day. Amebic antigen in cecal contents was determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (TechLab, Blacksburg, VA). Additionally, cecal contents were used to inoculate complete TYI-S-33 medium to determine the presence of viable ameba.
Pathology and scoring of amebic colitis.
After infection and drug treatment, ceca were bisected longitudinally and preserved using Bouin's fixative. Tissue sections were paraffin-embedded, and 4-µm sections were stained with hematoxylin and eosin. Histopathological assessment of ameba score was determined in blinded fashion for each mouse as previously described.9
Statistics.
Statistical significance for means was determined using t test. Proportions were determined by χ2 analysis. Clinical scoring was analyzed using the Mann–Whitney U test.
Results
We first examined in vitro the effect of several drugs currently in use to treat amebiasis, including metronidazole, tinidazole, nitazoxanide, iodoquinol, emetine, and chloroquine (Figure 1). Axenic HM1:IMSS was cultured in the presence of dilutions of drug for 72 hours and counted manually. Metronidazole and tinidazole exhibited potent efficacy in vitro, with no observable growth even at a concentration of 0.005 μg/mL. Nitazoxanide, iodoquinol, and emetine exhibited growth only at the lower concentrations (0.005, 0.1, and 0.05 μg/mL, respectively). Chloroquine could not inhibit growth even at the highest concentration of 5 μg/mL.
Figure 1.
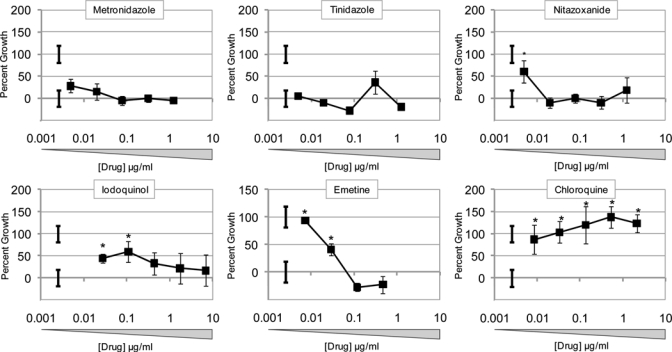
In vitro antiamebic efficacy of clinically relevant drugs. HM1:IMSS E. histolytica trophozoites (104/mL) were cultured in complete supplemented with drug for 72 hours. Cell numbers were counted on a hemocytometer and scored as the percentage growth relative to ameba grown in unsupplemented media. Data represent three counts of duplicated cultures ± standard deviation (SD). Isolated error bars indicate positive control (initial count) and negative control (no drug) ± SD. *P ≤ 0.05.
We then determined a dosing regimen for mice considering the in vitro potency as well as human dosing and likely achievable serum concentrations (Table 1). For most drugs, we used 10 mg/kg per day, because this has been commonly used for respective mouse studies. For iodoquinol, chloroquine, and emetine, we administered 55.9, 17.1, and 1.9 mg/kg per day, respectively, for proof of principle, because this was double the human dose. For initial experiments, groups of mice were administered the respective drug on day −1 relative to challenge and for three doses thereafter (drug regimen 1). Mice were then killed on day +7 and examined for evidence of infection. Culture positive rate, cecal parasite ELISA, and histologic ameba score showed similar findings, revealing potent efficacy (e.g., 0% culture positive rate) with metronidazole, tinidazole, and iodoquinol (Figure 2). In contrast, nitazoxanide, chloroquine, and emetine were statistically more efficacious than PBS, but a sizeable minority of mice (25%, 16%, and 16%, respectively) remained infected, with similar ameba scores to control mice. Most groups were not powered to discern small differences in efficacy between drug treatments; however, metronidazole was observed to be statistically superior to nitazoxanide in these experiments (P < 0.05). In addition, we examined parenteral metronidazole and emetine and found that both were efficacious versus PBS; again, 0% culture positivity was observed with metronidazole.
Table 1.
Clinical and experimental drug dosages
| Treatment | Lowest inhibitory dose (μg/mL) | Clinical dose for amebiasis (mg/kg per day) | Murine dosage (mg/kg) |
|---|---|---|---|
| Metronidazole | < 0.005 | 32.1 | 10.0 |
| Tinidazole | < 0.005 | 28.6 | 10.0 |
| Nitazoxanide | 0.02 | 14.3 | 10.0/20.0 |
| Iodoquinol | 0.435 | 27.9 | 55.7 |
| Chloroquine (base) | > 2.143 | 8.6 | 17.1 |
| Emetine | 0.058 | 0.09 | 1.9 |
| Pyrvinium pamoate | 0.117 | 7.5* | 15.0 |
| AMIX | < 0.005 | N/A | 10.0 |
| VPC16a1006 | 0.02 | N/A | 10.0 |
Antihelminthic dose in micrograms per kilogram.
Figure 2.
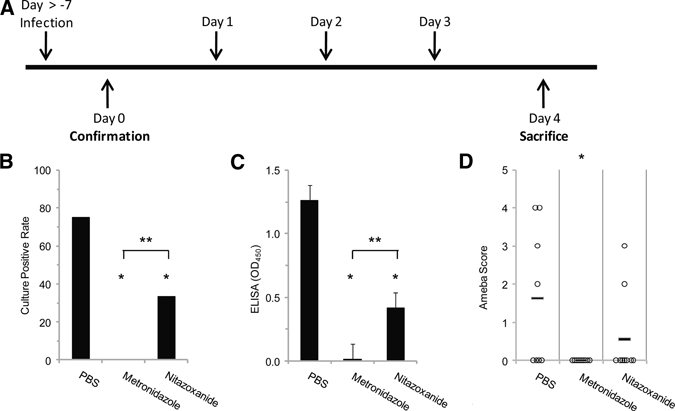
Efficacy of antiamebic drugs in vivo: regimen 1. (A) Drugs were administered by oral gavage or intraperitoneal injection before and after intracecal injection of E. histolytica trophozoites (days −1, +1, +3, and +5 relative to challenge). Mice were then killed for evaluation of infection by culture (B), cecal parasite antigen (C), and histology (D). Antigen data represent average OD value ± standard error of the mean (SEM). *P ≤ 0.05 between drug and PBS. **P ≤ 0.05 between metronidazole and PBS.
We then examined these drugs in the setting of established infection. Mice were challenged with E. histolytica, and infection was confirmed after 7 days by laparotomy or imaging. Mice were then administered three doses of either metronidazole (10 mg/kg per day) or nitazoxanide (20 mg/kg per day; drug regimen 2). Culture positive rate, cecal parasite ELISA, and histologic ameba score revealed that both drugs exhibited efficacy compared with PBS controls (Figure 3) but complete and superior efficacy with metronidazole versus nitazoxanide (P < 0.05).
Figure 3.
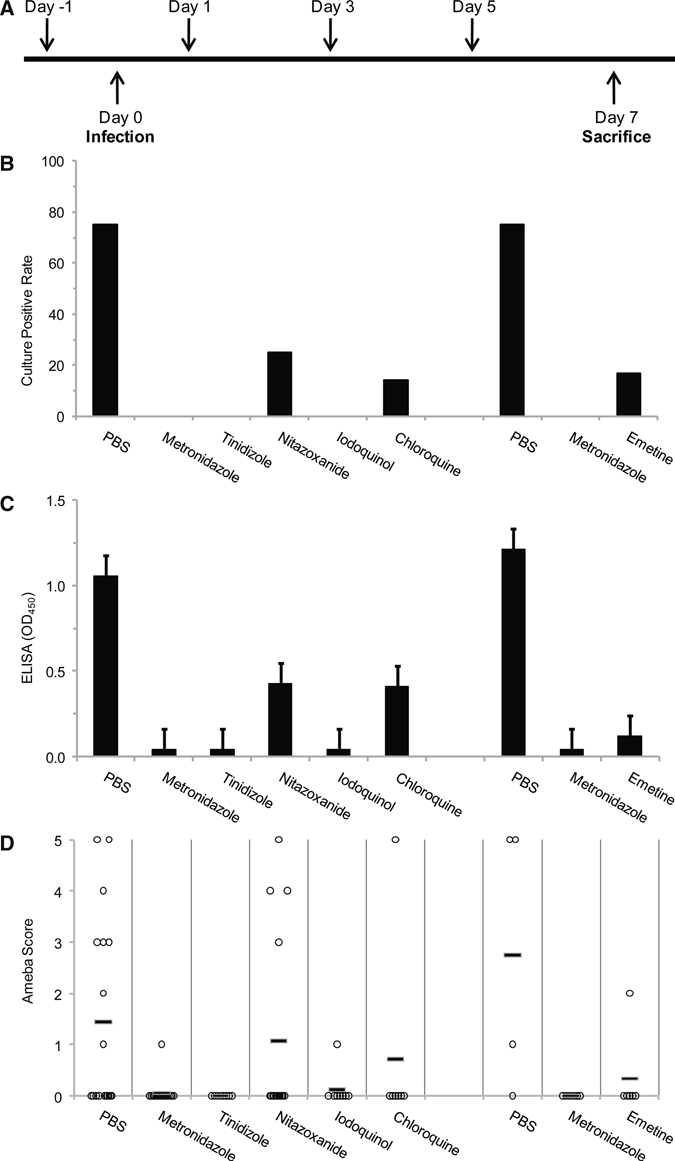
Efficacy of antiamebic drugs to cure established infection: regimen 2. (A) Drugs were administered by oral gavage or intraperitoneal injection for 3 consecutive days at least 7 days after infection was confirmed by laparotomy with visual inspection and culture or imaging. Mice were then killed for evaluation of infection by culture (B), cecal parasite antigen (C), and histology (D). Antigen data represent average optical density value ± SEM. *P ≤ 0.05.
Lastly, we examined novel drugs both in vitro and in vivo (Figure 4). AMIX and VPC16a1006 are hydrophilic nitazoxanide-related compounds10; pyrvinium is an antiparasitic medication that has been shown to possess antiamebic properties in vitro.11 AMIX and VPC16a1006 exhibited comparable potency with nitazoxanide in vitro. Pyrvinium had modest potency in vitro (lowest inhibitory dose of 0.05 μg/mL) but reasonable efficacy in vivo in regimen 1. AMIX showed some efficacy with drug regimen 1, but neither AMIX nor VPC16a1006 showed a measurable effect compared with control using drug regimen 2.
Figure 4.
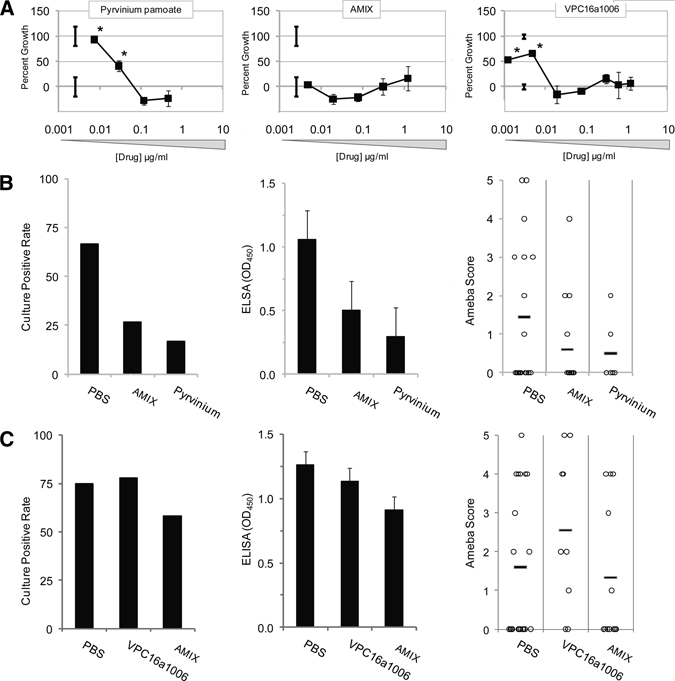
Assessment of novel antiamebic drugs. In vitro efficacy of novel drugs AMIX, VPC16a1006, and pyrvinium were examined (A). Drugs were then examined in vivo according to drug regimen 1 (B) and 2 (C) vide supra. *P ≤ 0.05.
Discussion
The most important finding of this work is the demonstration of a mouse model that can be used to compare drug efficacies in intestinal amebiasis. There have been limited comparisons in humans to understand relative drug efficacy. Here, we show that metronidazole and likely, tinidazole have superior efficacy in this model. These drugs are the mainstay of treatment of amebiasis; thus, although our findings are unsurprising, they are important for confirmation.
Although metronidazole therapy is very effective in resolving infections, issues of incomplete clearance against intestinal ameba and tolerability by patients remain. Additionally, metronidazole resistance has been documented in clinical isolates of Giardia intestinalis and Trichomonas vaginalis and has been artificially induced in a laboratory strain of E. histolytica,12,13 although resistance has not been seen clinically.14 Furthermore, transmission of metronidazole-refractory amebiasis has been reported.7 These data have spurred the development of novel antiamebic therapies; there have been many recent reports of novel potentially antiamebic compounds produced by de novo synthesis and screening of plant-derived compounds.15–22
Nitazoxanide is a drug recently approved in the United States for cryptosporidiosis. It has been reported to treat intestinal amebiasis both clinically and microbiologically,5 but no comparative trials with metronidazole have been performed. Our data would argue against its use over the nitroimidazoles. Because of its poor systemic bioavailability, nitazoxanide concentrates in the gut lumen. Because our model of amebiasis reveals significant tissue destruction and inflammation, we speculated that a drug with improved bioavailabiity may be superior. Therefore, we evaluated AMIX and VPC16a1006, which are nitazoxanide-related compounds with improved solubility and serum-binding characteristics that should enhance bioavailability. These drugs retained similar efficacy in vitro but exhibited unchanged or worse efficacy in vivo. To us, this suggests that the more bioavailable derivatives, because of improved absorption, may not achieve sufficiently high luminal concentrations as noted with nitazoxanide.
This in vitro to in vivo disconnect was a common finding for other drugs as well. Chloroquine, for example, was entirely ineffective in vitro, even at concentrations of 5 μg/mL, but it exhibited reasonable efficacy in vivo. The lack of in vitro effect has been shown before,23 and the mechanism of action in vivo is unclear.4
Emetine has long been used for severe amebiasis, both hepatic and intestinal, and dehydroemetine remains available in the United States through the Centers for Disease Control and Prevention. In our studies, we found emetine to be of moderate efficacy both in vitro and in vivo. It is only available as a parenteral agent, and we tested it against parenteral metronidazole, because the use of parenteral therapies for severe amebiasis is an area of surprisingly little data, essentially only one case series that showed utility with intravenous metronidazole.24 Our results again showed perfect efficacy with parenteral metronidazole; thus, our data further support this regimen in severe disease.
Iodoquinol has been widely used for amebiasis, typically after a treatment course with metronidazole, to prevent relapse. Additionally, a large study in India found it to be effective in symptomatic E. histolytica- and E. dispar-infected patients.25 Iodoquinol showed efficacy in our model, which was one of invasive disease; however, we studied it in the setting of regimen 1, which included a pre-challenge dose (where a luminal agent could presumably work). We also used a rather high dose, double that of humans, which would be a concern because of its known optic neuropathy side effects. Lastly, pyrvinium has been used as an antihelminthic for Enterobius infection but has broad activity against protozoan parasites, including Cryptosporidium parvum and Giardia intestinalis.11,26 In our model, it exhibited modest efficacy in regimen 1.
In summary, this mouse model of amebiasis is a tractable model to examine drug efficacy, particularly because in vitro and in vivo efficacies correlate rather poorly. For this reason, we would caution drug screening solely on the basis of in vitro efficacy. Our results support metronidizole as the first-line agent, and this model can be used to examine novel drugs in the future.
ACKNOWLEDGMENT
The authors thank David Sullivan for providing the pyrvinium.
Footnotes
Financial support: This work was supported by National Institutes of Health Grants AI071373 and AI075520 (to P.H.).
Authors' addresses: Stephen Becker, Paul Hoffman, and Eric R. Houpt, Division of Infectious Diseases and International Health, University of Virginia, Charlottesville, VA, E-mails: smb7yv@virginia.edu, psh2n@virginia.edu, and erh6k@virginia.edu.
References
- 1.Haque R, Huston CD, Hughes M, Houpt E, Petri WA., Jr Amebiasis. N Engl J Med. 2003;348:1565–1573. doi: 10.1056/NEJMra022710. [DOI] [PubMed] [Google Scholar]
- 2.Freedman DO, Weld LH, Kozarsky PE, Fisk T, Robins R, von Sonnenburg F, Keystone JS, Pandey P, Cetron MS. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med. 2006;354:119–130. doi: 10.1056/NEJMoa051331. [DOI] [PubMed] [Google Scholar]
- 3.Walsh JA. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Rev Infect Dis. 1986;8:228–238. doi: 10.1093/clinids/8.2.228. [DOI] [PubMed] [Google Scholar]
- 4.Cohen HG, Reynolds TB. Comparison of metronidazole and chloroquine for the treatment of amoebic liver abscess. A controlled trial. Gastroenterology. 1975;69:35–41. [PubMed] [Google Scholar]
- 5.Rossignol JF, Kabil SM, El-Gohary Y, Younis AM. Nitazoxanide in the treatment of amoebiasis. Trans R Soc Trop Med Hyg. 2007;101:1025–1031. doi: 10.1016/j.trstmh.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Irusen EM, Jackson TF, Simjee AE. Asymptomatic intestinal colonization by pathogenic Entamoeba histolytica in amebic liver abscess: prevalence, response to therapy, and pathogenic potential. Clin Infect Dis. 1992;14:889–893. doi: 10.1093/clinids/14.4.889. [DOI] [PubMed] [Google Scholar]
- 7.Fujishima T, Nishise S, Ichihara M, Kobayashi S, Takeuchi T. Difficulties in the treatment of intestinal amoebiasis in mentally disabled individuals at a Rehabilitation Institution for the Intellectually Impaired in Japan. Chemotherapy. 2010;56:348–352. doi: 10.1159/000320187. [DOI] [PubMed] [Google Scholar]
- 8.Asgharpour A, Gilchrist C, Baba D, Hamano S, Houpt E. Resistance to intestinal Entamoeba histolytica infection is conferred by innate immunity and Gr-1+ cells. Infect Immun. 2005;73:4522–4529. doi: 10.1128/IAI.73.8.4522-4529.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houpt ER, Glembocki DJ, Obrig TG, Moskaluk CA, Lockhart LA, Wright RL, Seaner RM, Keepers TR, Wilkins TD, Petri WA., Jr The mouse model of amebic colitis reveals mouse strain susceptibility to infection and exacerbation of disease by CD4+ T cells. J Immunol. 2002;169:4496–4503. doi: 10.4049/jimmunol.169.8.4496. [DOI] [PubMed] [Google Scholar]
- 10.Tchouaffi-Nana F, Ballard TE, Cary CH, Macdonald TL, Sifri CD, Hoffman PS. Nitazoxanide inhibits biofilm formation by Staphylococcus epidermidis by blocking accumulation on surfaces. Antimicrob Agents Chemother. 2010;54:2767–2774. doi: 10.1128/AAC.00901-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downey AS, Graczyk TK, Sullivan DJ. In vitro activity of pyrvinium pamoate against Entamoeba histolytica and Giardia intestinalis using radiolabelled thymidine incorporation and an SYBR Green I-based fluorescence assay. J Antimicrob Chemother. 2009;64:751–754. doi: 10.1093/jac/dkp296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samarawickrema NA, Brown DM, Upcroft JA, Thammapalerd N, Upcroft P. Involvement of superoxide dismutase and pyruvate:ferredoxin oxidoreductase in mechanisms of metronidazole resistance in Entamoeba histolytica. J Antimicrob Chemother. 1997;40:833–840. doi: 10.1093/jac/40.6.833. [DOI] [PubMed] [Google Scholar]
- 13.Wassmann C, Hellberg A, Tannich E, Bruchhaus I. Metronidazole resistance in the protozoan parasite Entamoeba histolytica is associated with increased expression of iron-containing superoxide dismutase and peroxiredoxin and decreased expression of ferredoxin 1 and flavin reductase. J Biol Chem. 1999;274:26051–26056. doi: 10.1074/jbc.274.37.26051. [DOI] [PubMed] [Google Scholar]
- 14.Bansal D, Sehgal R, Chawla Y, Mahajan RC, Malla N. In vitro activity of antiamoebic drugs against clinical isolates of Entamoeba histolytica and Entamoeba dispar. Ann Clin Microbiol Antimicrob. 2004;3:27–31. doi: 10.1186/1476-0711-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seifert K, Duchene M, Wernsdorfer WH, Kollaritsch H, Scheiner O, Wiedermann G, Hottkowitz T, Eibl H. Effects of miltefosine and other alkylphosphocholines on human intestinal parasite Entamoeba histolytica. Antimicrob Agents Chemother. 2001;45:1505–1510. doi: 10.1128/AAC.45.5.1505-1510.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navarrete-Vazquez G, Cedillo R, Hernandez-Campos A, Yepez L, Hernadez-Luis F, Valdez J, Morales R, Cortes R, Hernandez M, Castillo R. Synthesis and antiparasitic activity of 2-(trifluoromethyl)-benzimidazole derivatives. Bioorg Med Chem Lett. 2001;11:187–190. doi: 10.1016/s0960-894x(00)00619-3. [DOI] [PubMed] [Google Scholar]
- 17.Makioka A, Kumagai M, Kobayashi S, Takeuchi T. Possible role of calcium ions, calcium channels and calmodulin in excystation and metacystic development of Entamoeba invadens. Parasitol Res. 2002;88:837–843. doi: 10.1007/s00436-002-0676-6. [DOI] [PubMed] [Google Scholar]
- 18.Ranque S, Gazin P, Delmont J. Schistosomiasis and tourism in the Dogon country, Mali. Med Trop (Mars) 2004;64:31–32. [PubMed] [Google Scholar]
- 19.Abid M, Azam A. 1-N-substituted thiocarbamoyl-3-phenyl-2-pyrazolines: synthesis and in vitro antiamoebic activities. Eur J Med Chem. 2005;40:935–942. doi: 10.1016/j.ejmech.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Sawangjaroen N, Phongpaichit S, Subhadhirasakul S, Visutthi M, Srisuwan N, Thammapalerd N. The anti-amoebic activity of some medicinal plants used by AIDS patients in southern Thailand. Parasitol Res. 2006;98:588–592. doi: 10.1007/s00436-005-0119-2. [DOI] [PubMed] [Google Scholar]
- 21.Tasanor O, Brem B, Leitsch D, Binder M, Duchene M, Greger H, Wernsdorfer WH. Development of a pharmacodynamic screening model with Entamoeba histolytica. Wien Klin Wochenschr. 2007;119:88–95. doi: 10.1007/s00508-007-0874-4. [DOI] [PubMed] [Google Scholar]
- 22.Bhat AR, Athar F, Azam A. New derivatives of 3,5-substituted-1,4,2-dioxazoles: synthesis and activity against Entamoeba histolytica. Eur J Med Chem. 2009;44:926–936. doi: 10.1016/j.ejmech.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Neal RA. Experimental amoebiasis and the development of anti-amoebic compounds. Parasitology. 1983;86:175–191. doi: 10.1017/s0031182000057279. [DOI] [PubMed] [Google Scholar]
- 24.Kimura M, Nakamura T, Nawa Y. Experience with intravenous metronidazole to treat moderate-to-severe amebiasis in Japan. Am J Trop Med Hyg. 2007;77:381–385. [PubMed] [Google Scholar]
- 25.Kaur J, Mathur TN. Comparative drug trials in symptomatic and asymptomatic non-dysenteric amoebic colitis. Indian J Med Res. 1972;60:1547–1553. [PubMed] [Google Scholar]
- 26.Downey AS, Chong CR, Graczyk TK, Sullivan DJ. Efficacy of pyrvinium pamoate against Cryptosporidium parvum infection in vitro and in a neonatal mouse model. Antimicrob Agents Chemother. 2008;52:3106–3112. doi: 10.1128/AAC.00207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


