Abstract
Diagnosis of Taenia solium cysticercosis is an important component in the control and elimination of cysticercosis and taeniasis. New detection assays using recombinant and synthetic antigens originating from the lentil lectin-purified glycoproteins (LLGPs) of T. solium cysticerci were developed in a QuickELISA™ format. We analyzed a panel of 474 serum samples composed of 108 serum samples from donors with two or more viable cysts, 252 serum samples from persons with other parasitic infections, and 114 serum samples from persons with no documented illnesses. The sensitivities and specificities of T24H QuickELISA™, GP50 QuickELISA™, and Ts18var1 QuickELISA™ were 96.3% and 99.2%, 93.5% and 98.6%, and 89.8% and 96.4%, respectively, for detecting cases with multiple, viable cysts. T24H QuickELISA™ performs best among the three assays, and has sensitivity and specificity values comparable to those of the LLGP enzyme-linked immunosorbent blot. The QuickELISA™ are simple, rapid quantitative methods for detecting antibodies specific for T. solium cysticerci antigens.
Introduction
Specific, sensitive, rapid, robust, and automated diagnostic tests with high throughput capability are essential for any systematic efforts leading to the control and elimination of infectious diseases. In the case of the disease complex of Taenia solium, cysticercosis and taeniasis, which the World Health Organization deemed eradicable1–3 and many consider the single most common cause of acquired epilepsy in the developing world,4 these assays are even more critical because disease surveillance and intervention progress monitoring depend on serosurveys of humans and pigs.
The serodiagnostic assay recognized by the World Health Organization and the Pan American Health Organization for cysticercosis and neurocysticercosis5 uses lentil lectin-purified glycoproteins (LLGP) in an enzyme-linked immunoelectrotransfer blot (EITB)5,6 format. Although this assay has high sensitivity and specificity, it requires sophisticated equipment and technical expertise for antigen purification. It is not a quantitative assay, and it is difficult to use in field studies. In addition, if cysticercosis eradication efforts succeed, the availability of cysticerci from naturally infected pigs that are used as a source of antigens for the EITB will be decreased.
In recent years, the seven native LLGP proteins have been characterized, cloned, and grouped into three families of diagnostic antigens: GP50, T24, and 8-kD.7–13 Recombinant and synthetic antigens from the GP50 and 8-KD families have been used in EITB and Falcon assay screening test–enzyme-linked immunosorbent assay (FAST-ELISA) formats for diagnosis of cysticerosis.14–16 However, these assay formats do not allow for the high throughput testing needed for large serosurveillance studies.
In an attempt to develop an automated diagnostic test for cysticercosis with a high sensitivity and specificity, we used QuickELISA™ as the assay format. QuickELISA™ is a quantitative assay and can be automated for high throughput in field studies. QuickELISA™, unlike conventional ELISA, does not use secondary enzyme-labeled conjugates but relies on using two antigen conjugates, antigen-streptavidin (SA) and antigen-horseradish peroxidase (HRP), for capture and detection of specific antibodies. The immunologic reaction takes place in solution, thus potentially increasing the accessibility for antigen to bind to antibody and eliminating any issues associated with the binding of antigen to polystyrene. In the present study, we report the assay performance of three serologic tests, T24H QuickELISA™, GP50 QuickELISA™M, and Ts18var1 QuickELISA™, by using baculovirus expressed recombinant proteins (rGP50 and rT24H) and a synthetic peptide (sTs18var1) as antigens. These assays were evaluated at laboratories at the Centers for Disease Control and Prevention (CDC) and in Peru.
Materials and Methods
Chemicals and reagents.
All chemicals were reagent or analytical quality. Unless specified otherwise, they were obtained from Mallinckrodt (St. Louis, MO). Ultrapure Tris, urea, and ammonium sulfate were obtained from MDP Biomedicals (Aurora, OH). Acrylamide and bis-acrylamide were obtained from Bio-Rad Laboratories (Hercules, CA).
Recombinant antigens.
The recombinant diagnostic antigens for cysticercosis have been described previously. The predicted extracellular domain of T24,13 rT24H, beginning at tyr104 and continuing through gly195 was cloned into the baculovirus transfer vector pAcGP67A (Becton Dickinson Biosciences, Sparks, MD) behind the GP67 signal sequence at the Xma I and Bgl II restriction enzyme sites. Recombinant baculovirus was used to infect Trichoplusia ni cells (Expression Systems, Woodland, CA) grown in ESF-921 serum-free medium (Expression Systems). Recombinant T24H was purified from culture supernatants by (NH4)2SO4 precipitation in the range of 60% to 80% saturation. The pellet was resuspended in 0.05 M Tris-HCl, pH 8.0, filtered, and desalted on a Superdex 30 sizing column (Amersham Biosciences, Piscataway, NJ). Fractions containing rT24H were pooled and separated again on a Superdex 75 sizing column (Amersham Biosciences). Fractions containing purified rT24H were pooled and the purified protein was quantified by absorbance at 280 nm by using the extinction coefficient calculated from the predicted protein sequence.
The mature rGP50 protein, representing amino acids 17 through 276 of the native protein, was expressed in Sf21 cells using a baculovirus expression system (Invitrogen, San Diego, CA), and the recombinant protein was purified from culture supernatant by (NH4)2 SO4 precipitation and anion exchange chromatography as described.12 The third cysticercosis diagnostic antigen, sTs18var1, one of the 8-kD antigens with 66 amino acids,8 was chemically synthesized by Anaspec (San Jose, CA).
Serum samples.
Defined cysticercosis serum samples were obtained at the Instituto de Ciencias Neurologicas (Lima, Peru) from patients with clinical symptoms of neurocysticercosis. The definitive diagnosis of cysticercosis17 was confirmed by computed tomography or magnetic resonance imaging brain imaging and by serum antibody reactivity with the LLGP diagnostic antigens on EITB.6 Serum samples were sorted into four categories on the basis of imaging data for each patient. The following serum samples were tested at CDC. The category two or more viable cysts (n = 108) includes samples from patients who had multiple viable cysts or a racemose cyst regardless of any additional cysts, degenerating or calcified. The category single, viable cyst (n = 19) includes samples from patients with only one viable cyst. The categories degenerating cyst(s) (n = 66) and calcified cyst(s) (n = 114) include samples from patients with one or more of the described cysts and no other cysts. All serum samples were obtained in compliance with protocols reviewed and approved by the ethical review boards of all institutions involved, and specific permission was obtained for future use of stored samples. All patients involved in this study provided written consent.
To evaluate the specificities of the cysticercosis QuickELISA™, we assembled a panel of serum samples obtained from patients with other infections (Table 1). We included 28 serum samples from patients in Egypt with undefined infections for a total of 252 samples. This panel of other infection serum samples was combined with a panel of 114 normal human serum samples obtained from healthy residents of the United States and Egypt. Serum donors from Egypt were tested extensively by stool examination for intestinal parasites and all were negative.
Table 1.
Serum samples tested at CDC and in Peru for cysticercosis by using QuickELISA™*
| Sample | Description | Serum tested at CDC | Serum tested in Peru |
|---|---|---|---|
| No. | No. | ||
| Cysticercosis serum samples | 2 or more viable cysts | 108 | 150 |
| Single, viable cyst | 19 | 24 | |
| Degenerating cyst(s) only | 66 | 120 | |
| Calcified cyst(s) only | 114 | 155 | |
| Total no. | 307 | 449 | |
| Normal human serum | Resident of non-endemic region | 98† | 248‡ |
| Other | 16 | 0 | |
| Total no. | 114 | 248 | |
| Other infection serum samples | Ascaris lumbricoides | 2 | 0 |
| Echinococcus granulosus | 29 | 0 | |
| Echinococcus multilocularis | 1 | 0 | |
| Entamoeba histolytica | 7 | 0 | |
| Fasciola sp. | 15 | 0 | |
| Giardia lamblia | 3 | 0 | |
| Hymenolepis nana | 10 | 0 | |
| Hepatitis C | 6 | 0 | |
| Heterophyes heterophyes | 8 | 0 | |
| Plasmodium falciparum | 10 | 0 | |
| Schistosoma hematobium | 12 | 0 | |
| Schistosoma mansoni | 92 | 0 | |
| Taenia saginata | 11 | 0 | |
| Toxoplasma gondii | 10 | 0 | |
| Trichinella sp. | 8 | 0 | |
| Undefined infections | 28 | 0 | |
| Total no. | 252 | 0 |
CDC = Centers for Disease Control and Prevention.
These serum samples were collected from U.S. residents.
These serum samples were collected from residents of Iquitos, Peru, an area to which cysticercosis is not endemic.
For testing of the cysticercosis QuickELISA™ at Instituto Nacional de Ciencias Neurológicas in Lima, Peru, 250 serum samples from healthy persons were collected from Iquitos, Peru, an area to which cysticercosis is not endemic. All samples were tested by LLGP EITB, and two were excluded because of reactivity. A battery of 449 defined cysticercosis serum samples was tested; many were the same samples that were tested at CDC. These serum samples were divided into the following categories: two or more viable cysts (n = 150), single viable cyst (n = 24), degenerating cyst(s) (n = 120), and calcified cyst(s) (n = 155).
QuickELISA™.
QuickELISA™ is a proprietary technology developed by Immunetics, Inc. (Boston, MA) in which the antigen-antibody binding reaction occurs in solution and the target antibody is detected by an antigen conjugate.18 A QuickELISA™ was developed for each of the cysticercosis diagnostic antigens: rT24H, rGP50, and sTs18var1. Individual purified, recombinant, or synthetic antigens were covalently linked separately to either SA or HRP, which yielded two conjugates for each antigen, antigen-SA and antigen-HRP. Optimized concentrations of both conjugates are added to biotinylated–bovine serum albumin–coated wells of a 96-well microtiter plate containing buffer and 5 μL of serum sample. Bivalent antibody that recognizes the diagnostic antigen and binds one antigen labeled with HRP and one antigen labeled with SA is captured by the biotin-coated well and detected by the addition of the enzyme substrate.
Although the QuickELISA™ can easily be performed on the laboratory bench top, all data presented were generated by using an automated system (Triturus® EIA Analyzer; Grifols Inc., Miami, FL). For the QuickELISA™, biotinylated–bovine serum albumin–coated wells were washed with 300 μL of wash buffer (phosphate-buffered saline, 0.1% Tween-20) for 15 seconds. Fifty-five microliters of StabilZyme Select (SurModics, Inc., Eden Prairie, MN) was added to each well, followed by addition of 5 μL of serum and 60 μL of pre-mixed QuickELISA™ conjugates (1:1 v/v). The plate was incubated for 40 minutes at room temperature on a shaking platform, and then washed four times with 300 μL of wash buffer. One hundred twenty microliters of SureBlue™ Tetramethlybenzidine (TMB) Microwell Peroxidase Substrate (one component) (Kirkegaard and Perry Laboratories, Inc., Gaithersburg, MD) was added to each well and incubated for 5 minutes on a shaking platform at room temperature. The reaction was terminated by adding 120 μL of 450 nm Stop Reagent for TMB Microwell (BioFX Laboratories, Owing Mills, MD). For serum samples tested in Peru, one-component stable TMB-based substrate (BioFX Laboratories) was used for colorimetric development. Absorbance of each well at 450 nm (with a reference filter at 600 nm) was measured by using the Triturus system reader and converted to units/microliter on the basis of the standard curve by using Triturus software version 3.00b (Grifols Inc.).
Standard curve construction.
Two standard reference curves were used at CDC and in Peru. Briefly, at CDC, a reference standard positive serum pool was prepared from serum samples of five pigs in Peru with confirmed cysticercosis and high antibody activity against all cysticercosis diagnostic antigens when tested by LLGP EITB. This positive serum pool was arbitrarily assigned a value of 1,000 units/μL. A pool of serum samples from healthy pigs in the United States (Equitech-Bio, Kerrville, TX) was used and assigned a value of 0 units/μL. Proportional dilutions of the positive serum pool were made by using the normal pool. The unit value assignment was based on the dilution of the positive serum pool. The resulting dilutions comprised the primary standard curve. Standard curve units of 15, 10, 2.5, 1.0, 0.5, 0.25, 0.1 and 0.0 were used in the T24H and GP50 QuickELISA™. For the Ts18var1 QuickELISA™, standard curve units of 300, 50, 10, 5, 2.5, 1.0, 0.5, and 0.0 were used.
In Peru, a secondary standard curve was prepared as described above by using a different pool of positive serum samples from five pigs in Peru and the pool of serum samples from healthy pigs in the United States. The secondary standard curve prepared in Peru was calibrated against the CDC primary standard curve and each diluted standard was assigned the appropriate unit value on the basis of the primary standard curve.
Quality control.
Each 96-well plate included one well containing the normal human serum pool and one well containing a medium positive reference standard, which was within the linear range of the standard curve. For the T24H and GP50 QuickELISAs™, the medium positive reference sample was the 5 units/μL standard. For the Ts18var1 QuickELISA™, it was the 25 units/μL standard. These controls were used to determine inter-assay variability and validate results of each plate. The unit value of the negative control must be below the cutoff value used to identify a positive sample, and the unit value of the positive control must be within the established range of ±2 SD values of the mean value determined in inter- and intra- assays for the data on the plate to be considered valid.
All samples were tested individually in two runs. For most of the samples, results were similar and data from the first run was used. If the status of the tested sample changed from negative to positive or vice versa, a third assay was carried out and the concordant value was used.
Data analysis.
All assays were performed with a standard curve included in each 96-well plate. The Triturus software readily processes the digital data of raw absorbance value into a standard curve from which the concentration (units/microliter) of antibody activity of unknown samples can be derived.
The intra-assay and inter-assay variability was determined for the QuickELISAs™ run at CDC and in Peru. The assay variability was determined on the basis of testing of five replicates of the medium positive control for the CDC data and six replicates for the data from Peru. These replicates were included in 11 assays at CDC and 6 assays in Peru. An exception is that the intra- and inter-assay variability for the T24H QuickELISA™ is based on 14 runs, not 11. To determine intra-assay variability, the coefficient of variance (CV) was determined for each assay and the reported CV is the mean of the daily CVs. To determine inter-assay variability, a daily mean was determined and the between-run CV was calculated on the basis of the daily means.
We also used an alternative method of data analysis, one that does not require the inclusion of a standard curve. In this method, all data were analyzed by the ratio T/P, where T is the raw absorbance of the unknown test sample and P is the raw absorbance of a low positive sample included as a control in each assay. The 5 unit/μL standard was used as P for the T24H and GP50 QuickELISAs™. The 10 unit/μL standard was used for the Ts18var1 QuickELISA™.
Results
Evaluation of cysticercosis QuickELISA™.
Typical standard curve plots for each of the three QuickELISAs™ are shown in Figure 1. To determine the sensitivity and specificity of the recombinant and synthetic diagnostic antigens, we tested 673 defined serum samples (Table 1). Concentration of antibody activity in a test sample was derived from the standard curve by using a four-parameter equation. For numerous potential cutoff values, the J index19 was calculated by subtracting 1 from the sum of the sensitivity and specificity at that particular cutoff value. The cutoff value that resulted in the highest J index, and therefore maximized sensitivity and specificity, was selected for each diagnostic antigen. The maximum J index obtainable is 1.0 for an assay with 100% sensitivity and 100% specificity.
Figure 1.
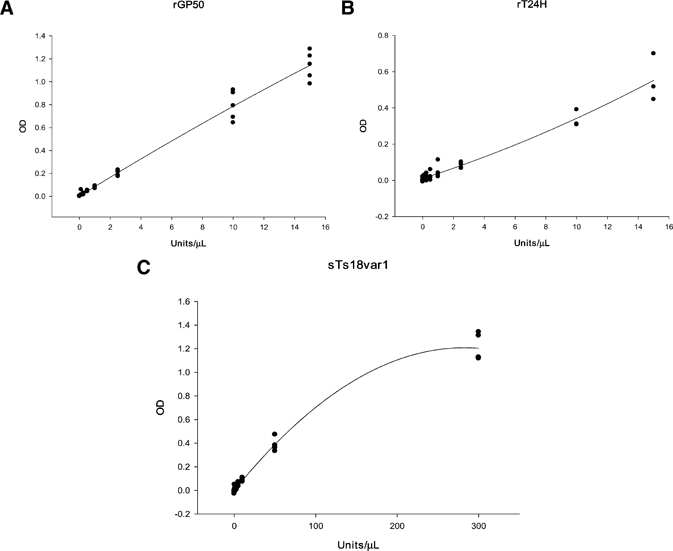
Typical standard curve plots for QuickELISA™. A, GP50 QuickELISA™. B, T24H QuickELISA™. C, Ts18var1 QuickELISA™. OD = optical density.
Of the 108 confirmed T. solium cysticerosis serum samples obtained from patients with two or more viable cysts, 104 were positive when tested with T24H, 101 with GP50, and 97 with Ts18var1 QuickELISA™. Of the 366 serum samples, which included normal human serum samples from Egypt and non-travelers from the United States and serum samples from persons with other helminth infections residing in countries to which cysticercosis was not endemic, 363 were negative when tested with T24H, 361 with GP50, and 353 with Ts18var1 QuickELISA™.
With a J index of 0.955, T24H QuickELISA™ had a sensitivity of 96.3% and a specificity of 99.2%, and GP50 QuickELISA™ had a J index of 0.922, a sensitivity of 93.5%, and a specificity of 98.6%. QuickELISA™ had a J index of 0.866, a sensitivity of 96.4%, and a specificity of 89.8% (Table 2). The antibody concentration cutoff values for T24H, GP50, and Ts18var1 QuickELISA™ were 0.5, 0.5, and 1.6 units/μL, respectively. These cutoff values were used for all assays performed, including the samples assayed in the Peru study. In addition, performances of the three QuickELISA™ are shown in detail in Table 3.
Table 2.
Sensitivity and specificity of the QuickELISAs™ for cysticercosis by using a standard curve for measurement of relative antibody concentration*
| Antigen | CDC data (evaluated by standard curve) | Peru data (evaluated by standard curve) | |||
|---|---|---|---|---|---|
| Cutoff value | Sensitivity† | Specificity‡ | Sensitivity† | Specificity§ | |
| T24H | 0.5 | 96.3% (104/108) | 99.2% (363/366) | 94.7% (142/150) | 96.0% (239/248) |
| GP50 | 0.5 | 93.5% (101/108) | 98.6% (361/366) | 92.7% (139/150) | 98.4% (244/248) |
| Ts18var1 | 1.6 | 89.8% (97/108) | 96.4% (353/366) | 88.7% (133/150) | 89.1% (221/248) |
CDC = Centers for Disease Control and Prevention.
Sensitivity for detecting cases with two or more viable cysts.
Based on the testing of 114 normal human serum samples from the United States and 252 parasitic infection serum samples from regions to which cysticercosis is not endemic.
Based on the testing of 248 normal human serum samples from Iquitos, Peru, an area to which cysticercosis is not endemic.
Table 3.
Performance of QuickELISA™ in detecting cysticercosis and non-cysticercosis cases by using a standard curve for measurement of relative antibody concentration*
| Positive results | Cysticercosis cases with two or more viable cysts (true positive) | Normal human serum samples and serum samples from other parasitic infections (false positive) | ||
|---|---|---|---|---|
| CDC (n = 108) | Peru (n = 150) | CDC (n = 366) | Peru (n = 248) | |
| All 3 QuickELISAs | 97 | 126 | 3 | 0 |
| Only T24H | 5 | 2 | 0 | 9 |
| Only GP50 | 2 | 0 | 0 | 0 |
| Only Ts18var1 | 0 | 2 | 8 | 23 |
| T24H and GP50 | 2 | 11 | 0 | 0 |
| GP50 and Ts18var1 | 0 | 2 | 2 | 4 |
| T24H and Ts18Var1 | 0 | 3 | 0 | 0 |
CDC = Centers for Disease Control and Prevention; n = total number of serum samples tested.
For serum samples obtained from persons with a single viable cyst, 47.4% (9 of 19) of serum samples were positive when tested with T24H QuickELISA™, 42.1% (8 of 19) were positive with GP50 QuickELISA™, and only 15.8% (3 of 19) were positive with Ts18var1 QuickELISA™. In serum samples from persons with degenerating cysts only, 50% (33 of 66), 48.5% (32 of 66), and 27.3% (18 of 66) were positive when tested with T24H, GP50, and Ts18var1 QuickELISA™, respectively. A panel of 114 serum samples obtained from persons with calcified cysts only was also included in this analysis. Positive antibody reactivity with T24H QuickELISA™ was detected in 52.6% (60 of 114) of these serum samples, in 43.9% (50 of 114) with GP50 QuickELISA™, and in 21.9% (25 of 114) with Ts18var1 QuickELISA™ (Table 4).
Table 4.
Sensitivity of QuickELISA™ for detecting cases of cysticercosis that exhibit a single viable cyst, calcified cysts only, or degenerating cysts only by using a standard curve for the measurement of relative antibody concentration*
| Antigen | CDC data | Peru data | ||||
|---|---|---|---|---|---|---|
| Single, viable cyst | Degenerating cyst(s) only | Calcified cyst(s) only | Single, viable cyst | Degenerating cyst(s) only | Calcified cyst(s) only | |
| T24H | 47.4% (9/19) | 50.0% (33/66) | 52.6% (60/114) | 66.7% (16/24) | 52.5% (63/120) | 59.4% (92/155) |
| GP50 | 42.1% (8/19) | 48.5% (32/66) | 43.9% (50/114) | 41.7% (10/24) | 31.7% (38/120) | 43.9% (68/155) |
| Ts18var1 | 15.8% (3/19) | 27.3% (18/66) | 21.9% (25/114) | 20.8% (5/24) | 40.0% (48/120) | 33.5% (52/155) |
CDC = Centers for Disease Control and Prevention.
Performance of cysticercosis QuickELISA™ in Peru.
In addition to the testing in the CDC laboratory, the recombinant and synthetic antigen QuickELISAs™ were further evaluated by the Cysticercosis Working Group in Peru where large surveys and surveillance programs are being conducted. A total of 697 serum samples from Peru were tested with the three QuickELISAs™; 449 serum samples from confirmed cases of T. solium cysticerosis and 248 normal human serum samples obtained from Iquitos, Peru, an area to which cysticercosis is not endemic. Of the 150 serum samples obtained from patients with two or more viable cysts, 142 were positive when tested with T24H QuickELISA™, 139 with GP50 QuickELISA™, and 133 with Ts18var1 QuickELISA™. Of the 248 normal human serum samples from Peru, 239 were negative when tested with T24H, 244 with GP50, and 221 with Ts18var1 QuickELISA™. Thus, T24H QuickELISA™ had a sensitivity of 94.7% and a specificity of 96.0% for detection of cysticercosis, and GP50 and Ts18var1 QuickELISA™ had sensitivities of 94.7% and 88.7% and specificities of 98.4% and 89.1%, respectively (Table 2).
For serum samples obtained from persons with a single cyst, 66.7% (16 of 24) of serum samples were positive when tested with T24H QuickELISA™, 41.7% (10 of 24) were positive with GP50 QuickELISA™, and only 20.8% (5 of 24) were positive with Ts18var1 QuickELISA™. In serum samples obtained from persons with degenerating cysts only, 52.5% (63 of 120), 31.7% (38 of 120), and 40.0% (48 of 120) were positive when tested with T24H, GP50, and Ts18var1 QuickELISA™, respectively (Table 4). For serum samples obtained from persons with calcified cysts only, positive antibody reactivity with T24H QuickELISA™ was detected in 59.4% (92 of 155), with GP50 in 43.9% (68 of 155), and with Ts18var1 in 33.5% (52 of 155).
Intra-assay and inter-assay variability.
The intra-assay variability was 4.9%, 5.2%, and 5.7% for T24H, GP50, and Ts18var1 QuickELISA™, respectively, at CDC. In Peru, the intra-assay variability was 4.0%, 3.1%, and 4.1% for the corresponding QuickELISA™. The inter-assay variability was 7.6%, 8.2%, and 6.0% for the T24H, GP50, and Ts18var1 QuickELISA™, respectively, at CDC. In Peru, the inter-assay variability was 3.6%, 3.8%, and 3.4% for the corresponding QuickELISA™.
Evaluation of data by using T/P.
To simplify QuickELISA™ and lower the cost of testing, a T/P ratio was used for evaluating the sensitivity and specificity of the three QuickELISAs™. The antibody reactivity with the diagnostic antigens was derived from the division of the raw absorbance of the test sample (T) by the raw absorbance of the medium range positive sample (P) obtained in the same test run. Similar to the data evaluation with a standard curve, various J indexes were calculated and the cutoff value with the highest J index was selected for each diagnostic antigen. The T/P cutoff values for T24H, GP50, and Ts18var1 QuickELISA™ were 0.20, 0.15, and 0.24, respectively. These cutoff values were used for all assays, including the study in Peru. For serum samples tested at CDC, T24H QuickELISA™ had a maximum J index of 0.917, a sensitivity of 92.6% (100 of 108), and a specificity of 97.8% (358 of 366). A J index of 0.876 indicated that GP50 QuickELISA™ had a sensitivity of 89.8% (97 of 108) and a specificity of 97.5% (357 of 366) (Table 4). When the QuickELISAs™ were evaluated by T/P ratio with the serum samples from Peru and using serum samples with two or more viable cysts, the sensitivities were 82.7% (124 of 150), 92.7% (139 of 150), and 60.7% (91 of 150) for T24H, GP50, and Ts18var1 QuickELISAs™, respectively. When testing was conducted with normal human serum samples, the specificities were 98.8% (245 of 248), 98.8% (245 of 248), and 94.3% (234 of 248) for T24H, GP50 and Ts18var1 QuickELISAs™, respectively (Table 5).
Table 5.
Sensitivity and specificity of the QuickELISA™ for cysticercosis using the ratio T/P*
| Antigen | CDC data (evaluated by T/P) | Peru data (evaluated by T/P) | |||
|---|---|---|---|---|---|
| Cutoff value | Sensitivity† | Specificity‡ | Sensitivity† | Specificity§ | |
| T24H | 0.20 | 92.6% (100/108) | 97.8% (358/366) | 82.7% (124/150) | 98.8% (245/248) |
| GP50 | 0.15 | 89.8% (97/108) | 97.5% (357/366) | 92.7% (139/150) | 98.8% (245/248) |
| Ts18var1 | 0.24 | 84.3% (91/108) | 93.4% (342/366) | 60.7% (91/150) | 94.3% (234/248) |
For T/P, T is the raw absorbance of the unknown test sample and P is the raw absorbance of a low positive sample included as a control in each assay. CDC = Centers for Disease Control and Prevention.
Sensitivity for detecting cases with two or more viable cysts.
Based on the testing of 114 normal human serum samples from the United States and 252 parasitic infection serum samples from regions to which cysticercosis is not endemic.
Based on the testing of 248 normal human serum samples from Iquitos, Peru an area to which cysticercosis is not endemic.
Discussion
The T24H QuickELISA™ outperformed the other two assays tested. With data generated at CDC by using a standard curve, we determined that the sensitivity and specificity of T24H QuickELISA™M are 96% and 99% for serum samples from persons infected with two or more viable cysts. These values are comparable to the reported sensitivity of 98% and specificity of 100% for the EITB in persons with multiple, viable cysts.6,20 The slightly higher sensitivity of EITB may be the result of having multiple diagnostic antigens present versus just one antigen as in QuickELISA™ or may reflect the fact that different batteries of defined serum samples were tested in each assay. A separate study found the sensitivity and specificity of EITB to be 94% and 100%, respectively.20 GP50 and Ts18var1 QuickELISAs™ are comparable to the FAST-ELISAs for GP50 and Ts18var1, which have sensitivities of 94.7% and 90.4% and specificities of 93.8% and 90.3%, respectively.15 The performance of Ts18var1 QuickELISA™ does not match that of sTs18var1 EITB, which has a sensitivity of 97% and a specificity of 100%.16 All of these studies reported sensitivity data for persons with two or more viable cysts.
A combination of T24H and GP50 QuickELISAs™ increases the sensitivity for detection of cysticercosis from 96.3% to 98.1% in the CDC data set and from 94.7% to 96.0% in the Peru data set. However, there is a corresponding decrease in specificity, from 99.2% to 98.6% and from 96.0% to 94.8% in the CDC and Peru data sets, respectively. Thus, the additional cost of performing GP50 QuickELISA™ and the decrease in specificity does not warrant the small increase in sensitivity.
The clinical presentation of cysticercosis varies.21,22 In Peru, of the persons have neurocysticercosis; the number of infecting cysts ranges from one to hundreds and the viability of cysts ranges from viable to degenerating to calcified.23,24 The humoral antibody response detected at any one time is dependent upon the number of infecting cysts and therefore the antigen load, the physiologic state of the cysts, and the half-life of the antibody responses.4 Sensitivity determinations for cysticercosis antibody detection assays are generally based on serum samples from persons with two or more viable cysts as detected by brain imaging. It is well recognized that the sensitivity for single, viable cysts is greatly decreased.16,25 With the T24H QuickELISA™, the CDC laboratory detected 47% of the 19 cases with a single, viable cyst, and the laboratory in Peru detected 67% of 24 cases. Combining the CDC and Peru data yielded sensitivities of 57% for detecting cases with a single viable cyst, 51% for detecting cases with degenerating cyst(s), and 56% for detecting cases with calcified cyst(s). It is likely that these low numbers reflect a limited exposure of cysticerci antigen to the immune system. In these persons, a diagnostic sensitivity of 50–60% may be the best that can be obtained with the current technologies.
The performance of the QuickELISAs™M was slightly lower when using a T/P ratio than that obtained when using a standard curve. The T24H QuickELISA™ had a sensitivity and specificity of 92.6% and 97.8% using a T/P ratio versus 96.3% and 99.2% using the standard curve. However, there are several advantages associated with the use of a T/P ratio for data analysis. First, 10% more samples can be assayed on each 96-well plate. Second, data curve fit software is not required for data analysis. Third, assay procedures are simpler and reagent costs are lower. In this study, the cutoff value that resulted in the highest J index and therefore maximized sensitivity and specificity was selected for each diagnostic antigen when using standard curve or T/P ratio for analysis. However, a different cutoff value can be chosen to maximize the sensitivity or specificity depending upon the objectives of the study. An example is using a cutoff value that maximizes sensitivity for monitoring cysticercosis infection in a control or elimination program, where the T/P ratio may be used for data analysis without compromising assay performance.
The need for simpler diagnostics for cysticercosis in humans and pigs was recognized by the World Health Organization International Task Force for Disease Eradication.1,3 The cysticercosis QuickELISA™ described may fulfill that need. This assay can be performed as a manual, benchtop assay or as a fully automated assay as described in this report. It performed equally well when used without automation. This assay is quick; 10 serum samples can be tested on the benchtop in 60 minutes or 320 serum samples can be tested by using a Triturus® EIA Analyzer in 150 minutes. For benchtop testing, the equipment needed are a refrigerator, a plate shaker, and a microwell plate reader. Based on the low intra-assay and inter-assay variability, we recommend testing serum samples in single rather than duplicate or triplicate to maximize assay throughput but do suggest retesting of serum samples with values near the cutoff for positivity. The use of recombinant and synthetic antigens as diagnostic antigens in QuickELISA™ increases test specificity, eliminates the need for purification of antigens from parasitic material, and subsequently decreases the manufacturing cost of detection assays. In addition, the QuickELISA™ format detects bivalent or multivalent antibodies from any species that binds to the antigens, thus simplifying and expediting serosurveillance procedures in cysticercosis control and elimination programs because antibodies of human and porcine origin can be detected by the same assay.
ACKNOWLEDGMENTS
We thank Patricia A Paredes, Milagrytos P Montoya, Yesenia C Berrios, Maria Silva-Ibanez, and John Noh for technical assistance.
Footnotes
Financial support: This study was supported by the Bill & Melinda Gates Foundation (grant no. 23981) and National Institutes of Health SIBR grants 1R43AI64988-01 and 2R44A1064988-02.
Authors' addresses: Yeuk-Mui Lee, Sukwan Handali, Kathy Hancock, Sowmya Pattabhi, and Christina M. Scheel, Division of Parasitic Diseases and Malaria, Centers for Diseases Control and Prevention, Atlanta, GA, E-mails: ylee@cdc.gov, SHandali@cdc.gov, KHancock@cdc.gov, spattabhi@idri.org, and CScheel@cdc.gov. Victor A. Kovalenko and Andrew Levin, Immunetics, Inc., Boston, MA, E-mails: vdiagnosticnno@maine.rr.com and alevin@Immunetics.com. Silvia Rodriguez and Hector H. Garcia, Department of Microbiology, Universidad Peruana Cayetano Heredia, Lima, Peru and Cysticercosis Unit, Instituto de Ciencias Neurologicas, Lima, Peru, E-mails: silvia@peruresearch.com and hgarcia@jhsph.edu. Sehching Lin, Scientific Resource Program, Coordinating Center for Infectious Diseases, Center for Diseases Control and Prevention, Atlanta, GA, E-mail: SLin@cdc.gov. Armando E. Gonzalez, School of Veterinarian Medicine, Universidad de San Marcos, Lima, Peru, E-mail: agonzale@jhsph.edu. Robert H. Gilman, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, E-mail: rgilman@jhsph.edu. Victor C. W. Tsang, Division of Parasitic Diseases and Malaria, Centers for Diseases Control and Prevention, Atlanta, GA, and Department of Biology, Georgia State University, Atlanta, GA, E-mail: vcwtsang@mindspring.com.
References
- 1.Centers for Disease Control and Prevention Recommendations of the International Task Force for Disease Eradication. MMWR Morb Mortal Wkly Rep. 1993;42:1–25. [Google Scholar]
- 2.Schantz PM, Cruz M, Sarti E, Pawlowski Z. Potential eradicability of taeniasis and cysticercosis. Bull Pan Am Health Organ. 1993;27:397–403. [PubMed] [Google Scholar]
- 3.Schantz PM, Tsang VC. The US Centers for Disease Control and Prevention (CDC) and research and control of cysticercosis. Acta Trop. 2003;87:161–163. doi: 10.1016/s0001-706x(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 4.Garcia HH, Gilman RH, Catacora M, Verastegui M, Gonzalez AE, Tsang VC. Serologic evolution of neurocysticercosis patients after antiparasitic therapy. Cysticercosis Working Group in Peru. J Infect Dis. 1997;175:486–489. doi: 10.1093/infdis/175.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan American Health Organization . PAHO/WHO Informal Consultation on the Taeniasis/Cysticercosis Complex. Brasilia, Brazil: Pan American Health Organization; 1995. [Google Scholar]
- 6.Tsang VC, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium) J Infect Dis. 1989;159:50–59. doi: 10.1093/infdis/159.1.50. [DOI] [PubMed] [Google Scholar]
- 7.Greene RM, Wilkins PP, Tsang VC. Diagnostic glycoproteins of Taenia solium cysts share homologous 14- and 18-kDa subunits. Mol Biochem Parasitol. 1999;99:257–261. doi: 10.1016/s0166-6851(99)00004-3. [DOI] [PubMed] [Google Scholar]
- 8.Greene RM, Hancock K, Wilkins PP, Tsang VC. Taenia solium: molecular cloning and serologic evaluation of 14- and 18-kDa related, diagnostic antigens. J Parasitol. 2000;86:1001–1007. doi: 10.1645/0022-3395(2000)086[1001:TSMCAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Sako Y, Nakao M, Ikejima T, Piao XZ, Nakaya K, Ito A. Molecular characterization and diagnostic value of Taenia solium low-molecular-weight antigen genes. J Clin Microbiol. 2000;38:4439–4444. doi: 10.1128/jcm.38.12.4439-4444.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obregon-Henao A, Gil DL, Gomez DI, Sanzon F, Teale JM, Restrepo BI. The role of N-linked carbohydrates in the antigenicity of Taenia solium metacestode glycoproteins of 12, 16 and 18 kD. Mol Biochem Parasitol. 2001;114:209–215. doi: 10.1016/s0166-6851(01)00256-0. [DOI] [PubMed] [Google Scholar]
- 11.Hancock K, Khan A, Williams FB, Yushak ML, Pattabhi S, Tsang VC. Characterization of the 8-kilodalton antigens of Taenia solium metacestodes and evaluation of their use in an enzyme-linked immunosorbent assay for serodiagnosis. J Clin Microbiol. 2003;41:2577–2586. doi: 10.1128/JCM.41.6.2577-2586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hancock K, Pattabhi S, Greene RM, Yushak ML, Williams F, Khan A, Priest JW, Levine MZ, Tsang VC. Characterization and cloning of GP50, a Taenia solium antigen diagnostic for cysticercosis. Mol Biochem Parasitol. 2004;133:115–124. doi: 10.1016/j.molbiopara.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Hancock K, Pattabhi S, Whitfield FW, Yushak ML, Lane WS, Garcia HH, Gonzalez AE, Gilman RH, Tsang VC. Characterization and cloning of T24, a Taenia solium antigen diagnostic for cysticercosis. Mol Biochem Parasitol. 2006;147:109–117. doi: 10.1016/j.molbiopara.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Handali S, Gonzalez AE, Hancock K, Garcia HH, Roberts JM, Gilman RH, Tsang VC. Porcine antibody responses to Taenia solium antigens rGp50 and sTs18var1. Am J Trop Med Hyg. 2004;71:322–326. [PubMed] [Google Scholar]
- 15.Bueno EC, Scheel CM, Vaz AJ, Machado LR, Livramento JA, Takayanagui OM, Tsang VC, Hancock K. Application of synthetic 8-kD and recombinant GP50 antigens in the diagnosis of neurocysticercosis by enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 2005;72:278–283. [PubMed] [Google Scholar]
- 16.Scheel CM, Khan A, Hancock K, Garcia HH, Gonzalez AE, Gilman RH, Tsang VC. Serodiagnosis of neurocysticercosis using synthetic 8-kD proteins: comparison of assay formats. Am J Trop Med Hyg. 2005;73:771–776. [PubMed] [Google Scholar]
- 17.Del Brutto OH, Rajshekhar V, White AC, Jr, Tsang VC, Nash TE, Takayanagui OM, Schantz PM, Evans CA, Flisser A, Correa D, Botero D, Allan JC, Sarti E, Gonzalez AE, Gilman RH, Garcia HH. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001;57:177–183. doi: 10.1212/wnl.57.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Handali S, Pattabhi S, Lee YM, Silva-Ibanez M, Kovalenko VA, Levin AE, Gonzalez AE, Roberts JM, Garcia HH, Gilman RH, Hancock K, Tsang VC. Development andevaluation of porcine cysticercosis QuickELISA in Triturus EIA analyzer. J ImmunoassayImmunochem. 2010;1:60–70. doi: 10.1080/15321810903405068. [DOI] [PubMed] [Google Scholar]
- 19.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Diaz JF, Verastegui M, Gilman RH, Tsang VC, Pilcher JB, Gallo C, Garcia HH, Torres P, Montenegro T, Miranda E. Immunodiagnosis of human cysticercosis (Taenia solium): a field comparison of an antibody-enzyme-linked immunosorbent assay (ELISA), an antigen-ELISA, and an enzyme-linked immunoelectrotransfer blot (EITB) assay in Peru. The Cysticercosis Working Group in Peru (CWG) Am J Trop Med Hyg. 1992;46:610–615. doi: 10.4269/ajtmh.1992.46.610. [DOI] [PubMed] [Google Scholar]
- 21.Carpio A. Neurocysticercosis: an update. Lancet Infect Dis. 2002;2:751–762. doi: 10.1016/s1473-3099(02)00454-1. [DOI] [PubMed] [Google Scholar]
- 22.Garcia HH, Gonzalez AE, Evans CA, Gilman RH. Taenia solium cysticercosis. Lancet. 2003;362:547–556. doi: 10.1016/S0140-6736(03)14117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montano SM, Villaran MV, Yiquimiche L, Figueroa JJ, Rodriguez S, Bautista CT, Gonzalez AE, Tsang VC, Gilman RH, Garica HH. Neurocysticercosis: association between seizures, serology, and brain CT in rural Peru. Neurology. 2005;65:229–233. doi: 10.1212/01.wnl.0000168828.83461.09. [DOI] [PubMed] [Google Scholar]
- 24.Marconi VC, Garcia HH, Katz JT. Neurocysticercosis. Curr Infect Dis Rep. 2006;8:293–300. doi: 10.1007/s11908-006-0074-9. [DOI] [PubMed] [Google Scholar]
- 25.Wilson M, Bryan RT, Fried JA, Ware DA, Schantz PM, Pilcher JB, Tsang VC. Clinical evaluation of the cysticercosis enzyme-linked immunoelectrotransfer blot in patients with neurocysticercosis. J Infect Dis. 1991;164:1007–1009. doi: 10.1093/infdis/164.5.1007. [DOI] [PubMed] [Google Scholar]


