Abstract
Three athletes who participated in a race in the tropical forest of the Caribbean island of Martinique were subsequently diagnosed with leptospirosis using polymerase chain reaction (PCR). We investigated an outbreak to evaluate possible risk factors, and to determine the appropriate public health recommendations. Of 230 athletes, we contacted 148 (64%) and 20 (13.5%) met our case definition. Five were hospitalized and none were fatal. Ten (91%) of the 11 ill athletes who were tested were confirmed by PCR or serology. Serogroup Pyrogenes was commonly found. Cutaneous cuts, reported by 14 (73.7%), was the only potential risk factor using univariate analysis. Sporting event participants in tropical areas should be made aware of specific warnings and recommendations concerning the risk of leptospirosis, especially after periods of heavy rainfall or flooding. Rapid diagnostic assays such as PCR are particularly appropriate in this setting for early diagnosis and for formulating public health recommendations.
Introduction
Leptospirosis, a bacterial zoonotic disease with worldwide prevalence, is an important emerging infectious disease with greater incidence in tropical areas where conditions for transmissions are favorable.1,2 Many wild and domestic animals serve as reservoirs for pathogenic Leptospira strains and contaminate the environment by shedding the organisms in their urine. Humans are usually infected through abraded skin or mucous membrane contact with water contaminated by the urine of animal reservoirs, and less frequently by direct contact with animals or their urine. The mean incubation period is 10 days, with a usual range of 2–20 days.1 Clinical manifestations are protean and the spectrum of symptoms range from subclinical or mild anicteric febrile illness to acute renal failure. Weil's disease and respiratory distress syndrome are associated with high mortality.1 Over the preceding decades, ecotourism and sporting events in tropical and sub-tropical areas have become more popular among travelers and athletes. These recreational activities in tropical areas have served to increase the risk of human exposure to these pathogens and consequently outbreaks of leptospirosis have been reported in these settings.3,4
For the period of June 1 to June 4, 2009, three adults were hospitalized in the teaching hospital of Fort de France, Martinique for febrile illnesses characterized by headache, myalgias, elevated liver enzyme levels, and leucocytosis associated with thrombocytopenia and; additionally, acute renal failure for one of the three. A diagnosis of leptospirosis was confirmed by specific amplification of pathogenic Leptospira spp. in blood samples from three patients. Those patients had participated in the “Tchimbe-Raid” race, held on the island of Martinique on May 16, 2009. The 230 participating athletes were from the two Caribbean islands of Martinique and Guadeloupe, as well as French Guyana and mainland France. Participants had the option to race in either an 80-km or an 30-km course (held on the second part of the 80-km route). This race was previously planned to be held on May 9, 2009 but was postponed because of heavy rainfall and flooding that occurred on May 4 and 5. The event was an endurance-length race involving two components: running and trekking. The majority of the racing event took place in the tropical forest and included crossing shallow rivers and using muddy trails. During the race, food and mineral water were regularly given to participants by the race organizers. After the identification of the three first cases, an advisory was issued to inform all participants about the risk of leptospirosis. An investigation was subsequently initiated to assess the number of ill athletes, to identify the risk factors, and to make the relevant public health recommendations. This work reports on the results of this investigation and discusses preventative measures for sporting participants in tropical environments.
Methods
Epidemiologic investigation.
Pursuant to the identification of the first three ill athletes the Regional Branch of the French Public Health Institute was informed on June 5 (20 days after the event) by the Infectious Disease unit and an investigation was initiated. The list of all participants in the event, including their telephone numbers and e-mail addresses, was obtained from the race organizers. An information letter was sent to all participants, in which they were informed of their potential exposure to leptospirosis during the race. Participants were advised to seek medical attention in the occurrence of fever associated with two or more of the following symptoms: chills, headache, muscles aches, joint aches, conjunctivitis, cough, diarrhea, or hemorrhaging. Accompanying the information letter that was sent to participants was a standardized questionnaire designed to determine the demographics, the symptoms, the duration of the illness, any previous antibiotic use, and any potential exposure during the period of 2 weeks before the race up to and during the competition. Information about patients' ethnicity was not reported because of the current strict medical ethics laws in France. With respect to any possible exposure, participants were queried regarding any muco-cutaneous exposure, river crossings, the swallowing of any river water, and consumption of coconut water that was distributed after the race. Participants who did not return their questionnaires were contacted by telephone. A “suspected case” of illness was defined as the onset of self-reported fever between May 16 and June 30 along with two or more of the above mentioned symptoms in a Tchimbe-Raid athlete.
Laboratory investigation.
Samples were obtained from patients who met the suspected case definition and who underwent medical consultation. Samples were subsequently tested for microscopic agglutination using the microscopic agglutination test (MAT).5 The MAT was done using the following antigens: serogroups Australis (serovar Australis), Autumnalis (serovar Autumnalis), Bataviae (serovar Bataviae), Canicola (serovar Canicola), Ballum (serovar Castellonis), Cynopteri (serovar Cynopteri), Grippotyphosa (serovar Grippotyphosa), Sejroe (serovars Hardjo and Sejroe), Hebdomadis (serovar Hebdomadis), Icterohaemorrhagiae (serovar Copenhageni), Panama (serovar Panama), Pomona (serovar Pomona), Pyrogenes (serovar Pyrogenes), and Tarassovi (serovar Tarassovi). Sera were screened at a dilution of 1:50 and positive sera were titrated to endpoint using standard methods.5 Blood samples (EDTA plasma) obtained from patients during the first week of symptoms were also tested by a real-time polymerase chain reaction (PCR) assay. After initial concentration of bacteria, DNA was extracted using the QIAamp DNA Mini Kit (Qiagen SA, Courtaboeuf, France) according to the manufacturer's instructions. Real-time PCRs were performed on an iQTM5 real-time PCR detection system (Bio-Rad Laboratories, France) using the DNA-binding dye technique (SYBR Green). The primer set LFB1-F (5′ CATTCATGTTTCGAATCATTTCAAA 3′) and LFB1-R (5′ GGCCCAAGTTCCTTCTAAAAG 3′) used target DNA from pathogenic leptospires and amplify a 331-bp fragment.6 Diagnosis by culture, although it is a definitive diagnosis test allowing the recovery of leptospires from clinical specimen, is also challenging and requires several weeks of incubation to obtain results. Considering the low sensitivity of culture in our laboratory during the time the study took place, there was no attempt to culture leptospires and the PCR test was chosen instead. A suspected case was considered “laboratory-confirmed” if it tested positive with MAT or PCR. For the interpretation of MAT results, a titer superior to 100 against any of the pathogenic antigens was considered positive if a patient's origin was from a non-endemic area. For patients living in an endemic area, like the Caribbean region, a titer of 400 was preferred considering the eventuality of past exposure.
Statistical analysis.
Results of the investigation were entered into EpiInfo version 6.04b (CDC, Atlanta, GA); the dataset was then imported into Stata 9 (College Station, TX) for further analysis. Univariate logistic regression was used to explore if variables were related to confirmed or suspected cases of leptospirosis. The risk ratio (RR) for dichotomous variables was calculated. Suspected cases were compared with and without laboratory confirmation, with controls from the cohort of participants to identify risk factors for illness. A single factor has been identified with a P value < 0.05 by univariate analysis; it was therefore not possible to conduct a multivariable logistic regression.
Results
Epidemiologic investigation.
Of the 230 athletes competing in the Tchimbe-Raid race, 76 sent back a questionnaire and 72 were subsequently contacted by telephone. Of the 148 (64%) participants contacted, 20 (13.5%) athletes met the definition of a suspected case, so the attack rate was at least 8.7% (Figure 1). The median age of patients with a suspected case, with or without laboratory confirmation, was 41 years [28–69] and 18 (90%) were male. All cases were French and residents of the island of Martinique. The following data are related to 19 of the ill athletes. One participant was excluded from the analysis because of insufficient clinical data. No significant differences were found between ill athletes who met the suspected case definition and non-ill athletes by age or gender. Among the 19 athletes, 9 (47.4%) had participated in the 30-km course and 10 (52.6%) in the 80-km course. Nine (47.4%) athletes reported travel to other Caribbean islands during the 3 months before the race but none reported training in the forest or any river exposure activity during the previous 15 days. Of the 148 participants contacted, none reported taking doxycycline for prophylaxis of leptospirosis. The median incubation time from the start of the race until onset of illness for the 10 athletes with confirmed cases (13 days; range, 7–16) differed significantly from the median incubation period (9 days; range, 1–13) for the suspected cases without laboratory confirmation (P = 0.03). The peak onset of fever on May 26, 2009 corresponded to Day 10 after the race (Figure 2). No cases were detected after June 1 (Day 16). Sixteen of 20 (80%) suspected cases sought medical care, and five (25%) were hospitalized. One hospitalized patient presented with an icteric form characterized by hepatic and renal dysfunction. No other severe manifestations of leptospirosis or death were reported. The median duration of illness was 5 days [3–10]. The most common symptoms associated with fever were asthenia, headache, muscle aches, chills, joint aches, and diarrhea (Table 1).
Figure 1.
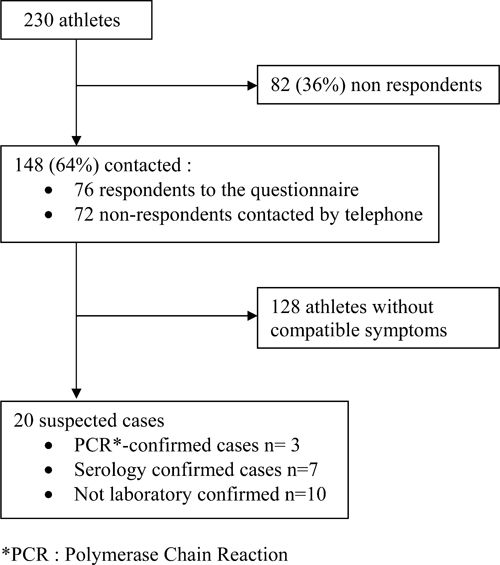
Flow diagram.
Figure 2.
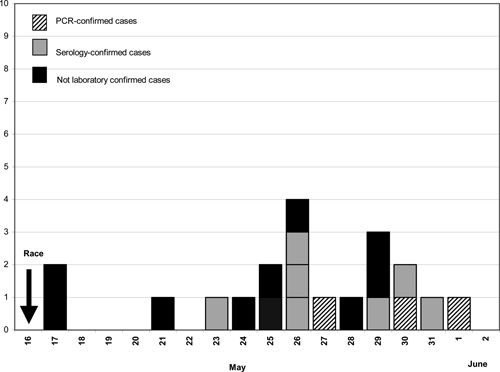
Date of fever onset for suspected and laboratory-confirmed cases of leptospirosis in Tchimbe-Raid athletes.
Table 1.
Self-reported clinical symptoms in 19 suspected cases* of leptospirosis after a race in the tropical forest of Martinique
| Symptom (number of respondents if not 19) | Number of athletes (%) |
|---|---|
| Fever | 19 (100) |
| Asthenia | 17 (89) |
| Headache | 16 (84) |
| Muscle aches | 15 (79) |
| Chills | 14 (74) |
| Joint aches | 12 (63) |
| Diarrhea (15) | 9 (60) |
| Nausea (14) | 6 (40) |
| Vomiting (15) | 5 (33) |
| Abdominal pain (15) | 5 (33) |
| Cough | 6 (31) |
| Red eyes | 2 (10.5) |
Race participants with onset of fever during the period from May 16 through June 30, 2009 plus the presence of two or more of the following symptoms or signs: chills, headache, muscles aches, joint aches, conjunctivitis, cough, diarrhea, and hemorrhage.
During the race, cuts and abrasions of the skin were reported by 14 (73.7%) of 19 ill-athletes. Among the 9 who described those cutaneous lesions, 5 had cuts on the legs, 1 had cuts on the hand, and 3 had lesions at both sites. All reported to have crossed more than 15 shallow rivers without being submerged and 2 of them drank water from those rivers. Six athletes reported the consumption of coconut water that was available at the arrival. Of those potential risk factors, only cutaneous cuts were associated with the outcome of leptospirosis using univariate analysis (RR, 3.2; 95% confidence interval [CI] = 1.1–8.4). Age, sex, consumption of coconut water, swallowing the river water, participating in the 80-km event, and duration of the race were not associated with illness.
Laboratory investigation.
Of the 20 athletes who met the suspected case definition, serum was collected from 11 (55%) and 8 submitted both acute and convalescent-phase serum specimens. Ten (91%) of the 11 athletes with suspected cases who were tested had their cases confirmed by at least one of the following techniques: 7 were tested positive by MAT, 2 were tested positive by PCR and MAT, and 1 was tested positive by PCR only (Figure 2). For one inhabitant of Martinique, the MAT titer was of 1:200, suggesting an early phase of the disease or previous environmental exposure and the case was considered not laboratory confirmed in the absence of a second sample. Among the 19 athletes with complete information, no significant differences were found between biologically confirmed ill athletes and non-biologically confirmed ill athletes by age, gender, potential exposure, or clinical symptoms. Because there was no attempt to culture leptospires during the study period, we were not able to identify the infecting serovar. Instead, we used high rates of agglutination of the serum with one particular antigen by MAT to identify the presumptive serogroup.5 A significant seroreactivity was found for reference serogroups Pyrogenes (4 patients), Sejroe (1 patient), Tarassovi (1 patient), and Icterohaemorrhagiae (1 patient). Finally for 1 patient, the MAT titers from two samples could not differentiate between serogroup Sejroe (serovar Hardjo) and serogroup Icterohaemoragiae.
The main results of this study are reported in Table 2, together with epidemiological and laboratory data reported in the three other published outbreaks of leptospirosis among participants in endurance sporting events.4,7,8
Table 2.
Comparative epidemiological data from the four reported outbreaks of leptospirosis among participants to endurance sport events
| Location (ref) | Springfield, Illinois7 | Malaysian Borneo4 | Florida8 | Martinique (PS) |
|---|---|---|---|---|
| Date of the event | June 21, 1998 | August 21–September 1, 2000 | November 4–5, 2005 | May 16, 2009 |
| Race characteristics | Triathlon | Multisport race: jungle trekking, swimming and kayaking, spelunking, climbing, and mountain biking | Multisport race: paddling, cycling, trekking, and orienteering | Endurance-length jungle race |
| Number of participants | 876 | 304 | 200 | 230 |
| Contacted | 834 (95%) | 189 (62%) | 192 (96%) | 148 (64%) |
| Suspected cases | 98 (12%) | 80 (42%) | 44 (23%) | 20 (13.5%) |
| Laboratory-confirmed cases (% of cases tested) | 52 (61%) | 26 (68%) | 14 (45%) | 10 (91%) |
| Median age of suspected cases (years) | 35 | 34 (range, 21–50) | 37 (range, 19–66) | 41 (range, 28–69) |
| Men | 82% (of suspected cases) | 74% (of contacted cohort) | 66.7% (of all racers) | 90% (of suspected cases) |
| Median incubation time (days) | ||||
| Suspected cases | 14 (range, 1–34) | 15 (range, 1–24) | 12.8, (range, 2–32)* | 9 (range, 1–13)* |
| Laboratory-confirmed cases | 15 (range, 6–29) | NA | 13.5 (range, 2–22) | 13 (range, 7–16) |
| Number of hospitalizations (% of suspected cases) | 21 (40% of Laboratory-confirmed cases) | 29 (36%) | 3 (7%) | 5 (25%) |
| Fatality | 0 | 0 | 0 | 0 |
| Risk factors associated with illness | Swallowed lake water more than once (MA) | Swimming in the Segama river (MA) | Swallowing river or creek water | Cuts on the skin (UA) |
| Eating wet food (MA) | ||||
| Doxycycline prophylaxis | 0 | 20 (11%) | 0 | 0 |
| Commonly identified Leptospira serogroups | Grippotyphosa, Bratislava, Djasiman (serovars) | Australis | Australis | Pyrogenes |
| Leptospira organisms isolated | L. Kirschneri from 1 athlete (PCR amplification of target DNA) | L. weilii from 1 athlete (rrs and secY gene sequences) | Member of species L. noguchii (potential new serovar) | 0 |
| Isolation of pathogenic Leptospira species from environmental samples | 0 (lake water) | ND | 0 (water samples, soil samples) | ND |
| Precipitation before the race (type of climatic event) | Heavy rains | Heavy rains | Hurricane Wilma | Heavy rains |
PS = present study; MA = multivariable analysis; UA = univariable analysis; NA = not available; PCR = polymerase chain reaction; ND = not done.
Patients with suspected cases without laboratory confirmation.
Discussion
This outbreak was the first reported after a sporting event in the Caribbean islands and the ensuing investigation allowed identification of cutaneous cuts as a risk factor for infection. Data from this outbreak also suggest that rapid diagnostic assays such as PCR are particularly appropriate in this setting for early diagnosis, information of exposed participants, and epidemiological investigation.
Leptospirosis was biologically confirmed for 10 (50%) of the 20 suspected cases. Although median incubation time for the suspected cases with or without laboratory confirmation was different, the absence of any clinical difference between biologically confirmed ill athletes and non-biologically confirmed ill athletes, combined with the low incidence of dengue during this period and the absence of any other reported arbovirus or malaria in Martinique, speak in favor of the diagnosis of leptospirosis.9 Real-time PCR-based diagnosis allowed early diagnosis for three of the participants and consequently early epidemiological investigation. However, for those three participants symptoms occurred late after the event (Figure 2), and at the time of investigation, no new patients were identified that could have been informed by public health recommendations before the occurrence of symptoms. At the present time, direct detection methods using PCR are the only evaluated techniques by which a positive diagnosis might be rapidly available during the early acute stage of the illness, before the appearance of immunoglobulin M (IgM) antibodies or culture results, and before occurrence of complications when treatment is likely to have the greatest benefit.1,10–12 Although only suggested by this study, the precocity of diagnosis has potential benefits for both the individual and the community in cases of a group exposure like a sporting event. Although PCR has been used to distinguish pathogenic from non-pathogenic serovars, definitive identification of the infecting serovar, which has significant epidemiological and public health value, still relies on culture rather than the microscopic agglutination test (MAT).1,13,14 Studies in Barbados and Thailand emphasized that the accuracy of the MAT in predicting the infecting serovar was poor in individual cases of leptospirosis.15,16 Paradoxical reactions and cross-reactions between serogroups may explain the difficulty to infer the identity of the infecting leptospiral serovar or serogroup. However, presumptive serogroup reactivity data could be used to gain a broad idea of the serogroups present at the population level.15 Although it remains the gold standard for the serological diagnosis of leptospirosis, early diagnosis is not possible with MAT because it relies on the detection of antibodies against leptospiral antigens, which do not become detectable until 1 week after the onset of symptoms. Over the last 2 years (2008–2009), MAT on serum samples (data from the National Reference Center of Leptospira, France) have shown that the most prevalent Leptospira serogroups in Martinique have been Icterohaemorrhagiae (34%), Sejroe (16%), Canicola (14%), and Pyrogenes (12%). In the current study the identification of several serogoups by MAT could be related to cross-reactions between serogroups or could be related to the multiple potential environmental sources of infection reported by the athletes (several rivers, muddy trails), along with the diversity of potential wild and domestic animal reservoirs in tropical areas.2 The potential enzootic sources of leptospirosis in the area where the race took place include numerous mammalian species including rodents, opossums, pigs, cattle, and dogs. Although participants were not specifically interrogated concerning animal species found along the race, some participants reported having seen pigs.
The first limitation of this study is the insufficient number of respondents (64%), which did not allow correctly estimating the attack rate. Other limitations of the study were the absence of Leptospira isolation in cultures and the lack of environmental investigation. Neither river or soil sampling, nor domestic or wild animal testing was performed. Following an outbreak of leptospirosis among triathlon participants in Illinois, a wide environmental investigation was performed, comprising sampling lake water and various domestic and wild animal testing.7 Nevertheless, despite epidemiologic evidence of widespread leptospiral contamination of the lake, the study did not identify any animal reservoir with the epidemic strain (Table 2). Moreover, the authors eventually raised concerns about the interpretation of both negative and positive samples when screening large bodies of water, concluding that such testing should not guide public health authorities. After another outbreak of leptospirosis among participants at an adventure race in Florida, environmental investigation again failed to isolate any Leptospira species despite numerous samples.8
The amount of rainfall in Martinique in May 2009 was nearly three times higher than that observed over the past 60 years for this specific month. On a site located at the end of the race, 114 mm of rain fell between 04 and 05 May 2009, this being 4 to 5 times more than the average values observed for the same period during the previous 3 years.17 Outbreaks of leptospirosis are typically associated with heavy rainfall and periods of flooding. The largest outbreak of leptospirosis reported in the United States occurred among triathlon participants and community residents in Springfield, Illinois, after heavy rains.7 Heavy rainfall was also reported before the two massive outbreaks of leptospirosis, which involved participants in multisport races in Malaysian Borneo and Florida.4,8 Furthermore, in the Caribbean and Latin America, epidemics of leptospirosis have been reported after periods of flooding, heavy rainfall, and hurricanes.3,18–20 Considering the higher risk of leptospirosis in tropical countries, especially after heavy rainfall or periods of flooding, travelers and participants to outdoor events with inevitable exposure to potentially contaminated water or soil should be informed as to personal prevention measures and chemoprophylaxis.21 As illustrated by the triathlon in Illinois, the multisport races in Malaysian Borneo and Florida, this current study in Martinique, and several other outbreaks of leptospirosis associated with water; the first steps of prevention in athletes should be to avoid swimming in rivers, swallowing lake or river water, and prevent dermal cuts.3,4,7,8,22,23 In addition, several studies suggest that doxycycline could be used either as chemoprophylaxis or as post-exposure prophylaxis and post-exposure empirical treatment.21,24–27 Both a study of U.S. Army soldiers who participated in a 3-week training exercise in the jungles of Panama and another study in residents of a rural area of the Andaman Islands found preventive efficacy of doxycycline, administered 200 mg once weekly.24,25 Administration of doxycycline, 100 mg daily, represent an alternative chemoprophylaxis, in areas where malaria and leptospirosis are endemic, as suggested by the preventive efficacy of such treatment during the race in Borneo.4 The Caribbean islands of Martinique and Guadeloupe are not an endemic area for malaria and none of the participants in our study reported taking such chemoprophylaxis. The use of post-exposure chemoprophylaxis has been studied in Brazil for rural residents with high-risk exposure to pathogenic leptospires and should be evaluated for asymptomatic athletes after the identification of cases of leptospirosis.26,28 In following, this study would not recommend empiric self-treatment of febrile travelers and rather advise them to seek medical attention as a first course, considering the broad spectrum of tropical diseases causing acute febrile illness, such as malaria and arbovirus.29
Sporting events and ecotourism in tropical areas are increasing and travelers are exposed to a wide range of pathogens, some of them exclusively tropical but also pathogens of worldwide distribution such as leptospirosis, whose incidence is even higher following periods of heavy rainfall and flooding. Such travelers should be made aware of specific warnings and recommendations concerning the recognized route of transmission of these bacteria, and information regarding chemoprophylaxis should be disseminated when the risk of transmission is high and unavoidable.
ACKNOWLEDGMENTS
We thank Marie Barrau and Claudine Suivant for their contribution in the gathering of data. We also thank Ric Yetman for editorial assistance.
Footnotes
Authors' addresses: Patrick Hochedez and Sylvie Abel, Service de Maladies Infectieuses et Tropicales, Centre Hospitalier Universitaire de Fort de France, Fort de France, Martinique, France, E-mails: patrick.hochedez@chu-fortdefrance.fr and sylvie.abel@chu-fortdefrance.fr. Jacques Rosine and Philippe Quénel, Cellule de l'Institut de Veille Sanitaire en Région, Fort-de-France, Martinique, France, E-mails: Jacques.ROSINE@ars.sante.fr and Philippe.QUENEL@ars.sante.fr. Rafaelle Théodose, Service de Bactériologie, Centre Hospitalier Universitaire de Fort de France, Fort de France, Martinique, France, E-mail: rafaelle.theodose@chu-fortdefrance.fr. Pascale Bourhy and Mathieu Picardeau, Unité de Biologie des Spirochètes, Institut Pasteur, Paris, France, E-mails: pbourhy@pasteur.fr and mpicard@pasteur.fr. André Cabié, Clinical Research Center of French West Indies and French Guiana, INSERM CIE 802/Université Antilles-Guyane JE 2503, Centre Hospitalier Universitaire de Fort de France, Fort de France, Martinique, France, E-mail: andre.cabie@chu-fortdefrance.fr.
References
- 1.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 3.From the Centers for Disease Control and Prevention Outbreak of leptospirosis among white-water rafters–Costa Rica, 1996. JAMA. 1997;278:808–809. [PubMed] [Google Scholar]
- 4.Sejvar J, Bancroft E, Winthrop K, Bettinger J, Bajani M, Bragg S, Shutt K, Kaiser R, Marano N, Popovic T, Tappero J, Ashford D, Mascola L, Vugia D, Perkins B, Rosenstein N. Leptospirosis in “Eco-Challenge” athletes, Malaysian Borneo, 2000. Emerg Infect Dis. 2003;9:702–707. doi: 10.3201/eid0906.020751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postic D, Merien F, Perolat P, Baranton G. Diagnostic Biologique Leptospirose-Borreliose de Lyme. Paris, France: Institut Pasteur; 2000. [Google Scholar]
- 6.Merien F, Portnoi D, Bourhy P, Charavay F, Berlioz-Arthaud A, Baranton G. A rapid and quantitative method for the detection of Leptospira species in human leptospirosis. FEMS Microbiol Lett. 2005;249:139–147. doi: 10.1016/j.femsle.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Morgan J, Bornstein SL, Karpati AM, Bruce M, Bolin CA, Austin CC, Woods CW, Lingappa J, Langkop C, Davis B, Graham DR, Proctor M, Ashford DA, Bajani M, Bragg SL, Shutt K, Perkins BA, Tappero JW. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin Infect Dis. 2002;34:1593–1599. doi: 10.1086/340615. [DOI] [PubMed] [Google Scholar]
- 8.Stern EJ, Galloway R, Shadomy SV, Wannemuehler K, Atrubin D, Blackmore C, Wofford T, Wilkins PP, Ari MD, Harris L, Clark TA. Outbreak of leptospirosis among adventure race participants in Florida. Clin Infect Dis. 2005;50:843–849. doi: 10.1086/650578. [DOI] [PubMed] [Google Scholar]
- 9.CIRE Antilles Guyane. Surveillance épidémiologique mensuelle de la dengue: Mai 2009. 2009. http://www.invs.sante.fr/surveillance/dengue/points_martinique/2009/PEM_Martinique_2009-05_Dengue.pdf Available at. Accessed June 8, 2009.
- 10.Merien F, Baranton G, Perolat P. Comparison of polymerase chain reaction with microagglutination test and culture for diagnosis of leptospirosis. J Infect Dis. 1995;172:281–285. doi: 10.1093/infdis/172.1.281. [DOI] [PubMed] [Google Scholar]
- 11.Brown PD, Gravekamp C, Carrington DG, van de Kemp H, Hartskeerl RA, Edwards CN, Everard CO, Terpstra WJ, Levett PN. Evaluation of the polymerase chain reaction for early diagnosis of leptospirosis. J Med Microbiol. 1995;43:110–114. doi: 10.1099/00222615-43-2-110. [DOI] [PubMed] [Google Scholar]
- 12.Levett PN, Morey RE, Galloway RL, Turner DE, Steigerwalt AG, Mayer LW. Detection of pathogenic leptospires by real-time quantitative PCR. J Med Microbiol. 2005;54:45–49. doi: 10.1099/jmm.0.45860-0. [DOI] [PubMed] [Google Scholar]
- 13.Murgia R, Riquelme N, Baranton G, Cinco M. Oligonucleotides specific for pathogenic and saprophytic leptospira occurring in water. FEMS Microbiol Lett. 1997;148:27–34. doi: 10.1111/j.1574-6968.1997.tb10262.x. [DOI] [PubMed] [Google Scholar]
- 14.Woo TH, Smythe LD, Symonds ML, Norris MA, Dohnt MF, Patel BK. Rapid distinction between Leptospira interrogans and Leptospira biflexa by PCR amplification of 23S ribosomal DNA. FEMS Microbiol Lett. 1997;150:9–18. doi: 10.1111/j.1574-6968.1997.tb10343.x. [DOI] [PubMed] [Google Scholar]
- 15.Levett PN. Usefulness of serologic analysis as a predictor of the infecting serovar in patients with severe leptospirosis. Clin Infect Dis. 2003;36:447–452. doi: 10.1086/346208. [DOI] [PubMed] [Google Scholar]
- 16.Smythe LD, Wuthiekanun V, Chierakul W, Suputtamongkol Y, Tiengrim S, Dohnt MF, Symonds ML, Slack AT, Apiwattanaporn A, Chueasuwanchai S, Day NP, Peacock SJ. The microscopic agglutination test (MAT) is an unreliable predictor of infecting Leptospira serovar in Thailand. Am J Trop Med Hyg. 2009;81:695–697. doi: 10.4269/ajtmh.2009.09-0252. [DOI] [PubMed] [Google Scholar]
- 17.Meteo France Annual Climatic Bulletin 2009. http://www.meteo.fr/temps/domtom/antilles/pack-public/alaune/bca_2009_martinique.pdf Available at. Accessed January 25, 2010.
- 18.Trevejo RT, Rigau-Perez JG, Ashford DA, McClure EM, Jarquin-Gonzalez C, Amador JJ, de los Reyes JO, Gonzalez A, Zaki SR, Shieh WJ, McLean RG, Nasci RS, Weyant RS, Bolin CA, Bragg SL, Perkins BA, Spiegel RA. Epidemic leptospirosis associated with pulmonary hemorrhage-Nicaragua, 1995. J Infect Dis. 1998;178:1457–1463. doi: 10.1086/314424. [DOI] [PubMed] [Google Scholar]
- 19.Sanders EJ, Rigau-Perez JG, Smits HL, Deseda CC, Vorndam VA, Aye T, Spiegel RA, Weyant RS, Bragg SL. Increase of leptospirosis in dengue-negative patients after a hurricane in Puerto Rico in 1996 [correction of 1966] Am J Trop Med Hyg. 1999;61:399–404. doi: 10.4269/ajtmh.1999.61.399. [DOI] [PubMed] [Google Scholar]
- 20.Storck CH, Postic D, Lamaury I, Perez JM. Changes in epidemiology of leptospirosis in 2003–2004, a two El Nino Southern Oscillation period, Guadeloupe archipelago, French West Indies. Epidemiol Infect. 2008;136:1407–1415. doi: 10.1017/S0950268807000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haake DA, Dundoo M, Cader R, Kubak BM, Hartskeerl RA, Sejvar JJ, Ashford DA. Leptospirosis, water sports, and chemoprophylaxis. Clin Infect Dis. 2002;34:e40–e43. doi: 10.1086/339942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cacciapuoti B, Ciceroni L, Maffei C, Di Stanislao F, Strusi P, Calegari L, Lupidi R, Scalise G, Cagnoni G, Renga G. A waterborne outbreak of leptospirosis. Am J Epidemiol. 1987;126:535–545. doi: 10.1093/oxfordjournals.aje.a114686. [DOI] [PubMed] [Google Scholar]
- 23.Corwin A, Ryan A, Bloys W, Thomas R, Deniega B, Watts D. A waterborne outbreak of leptospirosis among United States military personnel in Okinawa, Japan. Int J Epidemiol. 1990;19:743–748. doi: 10.1093/ije/19.3.743. [DOI] [PubMed] [Google Scholar]
- 24.Takafuji ET, Kirkpatrick JW, Miller RN, Karwacki JJ, Kelley PW, Gray MR, McNeill KM, Timboe HL, Kane RE, Sanchez JL. An efficacy trial of doxycycline chemoprophylaxis against leptospirosis. N Engl J Med. 1984;310:497–500. doi: 10.1056/NEJM198402233100805. [DOI] [PubMed] [Google Scholar]
- 25.Sehgal SC, Sugunan AP, Murhekar MV, Sharma S, Vijayachari P. Randomized controlled trial of doxycycline prophylaxis against leptospirosis in an endemic area. Int J Antimicrob Agents. 2000;13:249–255. doi: 10.1016/s0924-8579(99)00134-x. [DOI] [PubMed] [Google Scholar]
- 26.Gonsalez CR, Casseb J, Monteiro FG, Paula-Neto JB, Fernandez RB, Silva MV, Camargo ED, Mairinque JM, Tavares LC. Use of doxycycline for leptospirosis after high-risk exposure in Sao Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 1998;40:59–61. doi: 10.1590/s0036-46651998000100012. [DOI] [PubMed] [Google Scholar]
- 27.McClain JB, Ballou WR, Harrison SM, Steinweg DL. Doxycycline therapy for leptospirosis. Ann Intern Med. 1984;100:696–698. doi: 10.7326/0003-4819-100-5-696. [DOI] [PubMed] [Google Scholar]
- 28.CDC Update: outbreak of acute febrile illness among athletes participating in Eco-Challenge-Sabah 2000–Borneo, Malaysia, 2000. MMWR Morb Mortal Wkly Rep. 2001;50:21–24. [PubMed] [Google Scholar]
- 29.Freedman DO, Weld LH, Kozarsky PE, Fisk T, Robins R, von Sonnenburg F, Keystone JS, Pandey P, Cetron MS. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med. 2006;354:119–130. doi: 10.1056/NEJMoa051331. [DOI] [PubMed] [Google Scholar]


