Abstract
Dengue is endemic to Haiti but not recognized as an important illness in the autochthonous population. To evaluate the prevalence of antibodies to dengue virus (DENV), serum samples from infants and young children 7–36 months of age (n = 166) were assayed by plaque reduction neutralization assays to each DENV serotype. Dengue virus serotype 1 had infected 40% of this study population, followed by serotype 2 (12%), serotype 3 (11%), and serotype 4 (2%). Fifty-three percent of infants and young children less than 12 months of age had already experienced DENV infection, and the seroprevalence of antibody to DENV increased to 65% by 36 months. Heterotypic antibody responses were an important component of the total dengue immunity profile.
Introduction
Dengue is an acute, arthropod-borne, flavivviral disease that occurs in a global belt of tropical and subtropical countries. Illness is caused by one of four serotypes of dengue virus (DENV) and spread by Aedes species mosquitoes, most commonly Ae. aegypti. The disease is most commonly a febrile illness with headache, rash and musculoskeletal pain, but is remarkable because the severe manifestations, dengue hemorrhagic fever and dengue shock syndrome, typically occur in those who become infected either in the face of waning maternal antibody or after secondary infection in which immunity to the infecting strain has decreased to sub-neutralizing levels. A mechanism proposed for this association is antibody-dependent enhancement, a process of facilitated uptake of virions bound to non-neutralizing antibody by Fc receptor-bearing cells such as monocytes and dendritic cells, the primary targets for DENV replication.1 The consequent increase in DENV replication results in vascular permeability, hemorrhage, and/or vascular collapse. Differences among DENV strains and host physiologic conditions and the genetic background of the host also contribute to disease presentation.2–5
Port-au-Prince, the capital of Haiti, is remarkable for its poverty, crowded urban lifestyle, tropical climate, and poor sanitation, all of which would be predicted to lead to a high vector density. Infection with DENV has been repeatedly noted to cause febrile illness in travelers and military deployed in Haiti.6,7 However, the clinical impact of dengue on the autochthonous population is unclear.8 Dengue has been reported to be hyperendemic in school age children in Haiti on the basis of antibody screening, which showed high prevalence of antibodies to all four DENV serotypes.9
Remarkably, the most severe manifestations, dengue hemorrhagic fever and dengue shock syndrome, have not been described or recognized by pediatric practitioners in Haiti. A possible explanation is the pattern of less severe disease in persons of African descent that has been noted in Cuba and in Africa.10 Despite the absence of clinically recognized dengue, infants and young children in Haiti have a higher mortality rate for children less than five years of age than anywhere else in the Western Hemisphere (80/1,000 live births),11 and many of these deaths are accompanied by unexplained, febrile illness.12 The current study begins to define the epidemiology and potential impact of dengue in infants and young children in Haiti by looking at the imprint of prior infection by measuring the frequency and titer of neutralizing antibodies to each of the four DENV serotypes in infants and young children between the ages of 7 and 36 months.
Whereas dengue illness is rarely considered as a cause of febrile illness in Haiti, malaria is frequently considered in the differential diagnosis among adult and pediatric practitioners, although laboratory-confirmed cases are uncommon in this population. In this study, each sample was also tested for malaria antibodies to estimate the relative frequency of infection of this vector-borne illness that is more commonly considered in febrile patients.
Materials and Methods
Antibodies to DENV.
Venous blood samples were obtained During February–June 2007 at regularly scheduled visits from 166 infants and young children born to human immunodeficiency virus (HIV)–infected mothers at GHESKIO Center primarily to determine the HIV-status of the infant by continued detection or loss of maternal antibody to HIV-1. Serum was separated from blood samples after collection in Haiti and stored at –70°C from the time of acquisition until July 2007 when they were shipped overnight on dry ice to Vanderbilt University.
Plaque neutralization assays were performed by using representative strains for DENV serotypes 1–4, type 1 (Puerto Rico/94), type 2 (000 8372), type 3 (Yogyakarta), and type 4, (rDEN4Δ30 derived from Dominica/814669/81), as described.13 Briefly, serum samples were serially diluted four-fold starting at a dilution of 1:10 and mixed with equal volumes of virus containing 50 plaque-forming units of the respective strain to give an effective starting dilution of 1:20. After a 60-minute incubation for neutralization, the serum–virus mixture was inoculated onto confluent cultures of Vero cells in 24-well tissue-culture plates. After incubation for five days in an atmosphere of 5% CO2 at 37°C with a methylcellulose overlay, plaques were detected with serotype-specific antibodies, visualized with immunoperoxidase, enumerated, and a serum reciprocal geometric mean titer that inhibited 60% of the plaques was calculated by regression analysis of the results of the serial dilutions. The assay was identical to that used in the Laboratory of Infectious Disease (National Institute of Allergy and Infectious Diseases [NIAID], National Institutes of Health, Bethesda, MD).14 Exchange of 20 serum samples from a DENV vaccine trial15 determined that our results for serotype 4 were comparable to those obtained at the National Institutes of Health. This is an important consideration because variability has been shown in the DENV neutralization assay.16
A 60% reduction in plaques at the initial 1:20 dilution qualified as a positive test result for a specific serotype. To discount possible confounding residual maternal antibody in the youngest children studied, minimal titers > 100 in 7-month-old children, > 50 in 8-month-old children, and > 25 in 9-month-old children were required to be counted as a primary infection. This assumption is based on the observation that with DENV and many other viruses the half-life of placentally transferred viral antibody approximates 30 days and would compensate for even a titer in the mother as high as 12,800 at delivery.17 For calculation of geometric mean titers, values < 20 were assigned a value of 10. The highest strain-specific titer was usually 10–100-fold higher than to other strains and was assumed to be a response to the infecting strain, although the titer was recognized to potentially be influenced by time since infection, height of strain-specific responses, and boosting by repeat infections.
Antibodies to malaria and HIV.
To define the prevalence of malaria, antibodies in the same 166 serum samples were assayed at the Laboratory of Malaria and Vector Research (National Institute of Allergy and Infectious Diseases, National Institutes of Health) for antibodies to apical membrane antigen-1 to Plasmodium falciparum malaria as described.18 This antigen is a marker of prior exposure to malaria,17 a commonly postulated cause of febrile illness in children in Haiti. All serum samples were screened by using rapid whole blood HIV antibody tests (Determine HIV 1/2; Abbott Laboratories, Abbott Park, IL or Capillus HIV 1 and 2; Trinity Biotech, Bray, Ireland).
Data analysis.
For purpose of analysis, the children were divided into six-month age blocks (7–12 months, 13–18 months, etc.). The frequency and magnitude of strain-specific antibody to DENV were the primary data for analysis.
Results
The GHESKIO Center, located in a densely populated, low-lying, urban area in Port-au-Prince, Haiti, serves primarily as a site for HIV counseling, testing, and treatment of local populations. Housing was marginal even before the earthquake of January 12, 2010; many of the GHESKIO patients lived in adjacent slums (Cite de L'Eternal and Cite de Dieu) that had no electricity or sanitation, inadequate nutrition, and frequent flooding. Voluntary HIV testing performed for more than 27,000 persons per year demonstrates a 15% prevalence of HIV infection in the population served. More than 8,000 persons are currently being treated with highly active antiretroviral therapy within the GHESKIO network. A special emphasis is placed on the prevention of mother-to-child transmission of HIV, and children born to HIV-infected mothers are carefully followed postpartum for evidence of HIV infection.19
Participants and study design.
The age range of the children was 7–36 months (mean age = 18.6 months, median age = 16.9 months, and intraquartile age range = 11.9–24.3 months). Of the 166 children, 76 were girls and 90 were boys. The children had recorded addresses throughout Port-au-Prince. Use of these samples, originally obtained to determine HIV status, for assays of DENV and malaria antibody and review of clinical histories was reviewed and approved by the GHESKIO, Vanderbilt University, and Cornell Institutional Review Boards under conditions in which the identity of the child remained anonymous.
Only two of the 166 patients were ultimately shown to have HIV. Thus, perinatally acquired HIV infection in the children did not substantially influence the results, although HIV infection is often a marker in a family in Haiti for poverty and sub-standard living conditions.
Seroprevalence of neutralizing antibodies to DENV.
A total of 108 (65%) of 166 infants and young children had serum antibody to at least one DENV serotype (Figure 1 and Table 1). Twenty-six (53%) of 49 infants tested who were 7–12 months of age had already been infected. However, the percentage infected did not increase asymptotically and had reached only 10 (65%) of 16 children who were 30–36 months of age. The pattern of acquisition raises the possibility that there had been lower levels of DENV transmission in the period 2–3 years prior to sampling, and that virtually all infections had occurred in the previous year.
Figure 1.
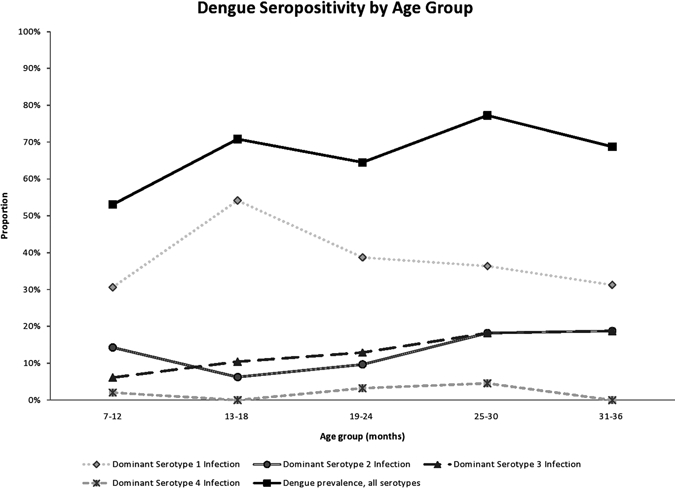
Antibody to dengue virus within each age group during the period sampled, Haiti. Each line represents the prevalence of the serotype indicated, and the top line represents the combined prevalence of antibody to any dengue serotype.
Table 1.
Serotype-specific antibody to dengue virus as a function of age, Haiti*
| Dengue virus serotype | Derivation and height of antibody | Age groups in months (no. in sample) | |||||
|---|---|---|---|---|---|---|---|
| 7–12 (49) | 13–18 (48) | 19–24 (31) | 25–30 (22) | 31–36 (16) | All age groups (166) | ||
| 1 | Dominant | 32% (15) | 54% (26) | 39% (12) | 36% (8) | 31% (5) | 40% (66) |
| GMT | 357 | 448 | 591 | 615 | 452 | 465 | |
| (95% CI) | (209–608) | (290–691) | (364–960) | (289–1,310) | (113–1,803) | (366–591) | |
| Cross-reactive | 12% (6) | 8% (4) | 19% (6) | 32% (7) | 19% (3) | 16% (26) | |
| 2 | Dominant | 15% (7) | 6% (3) | 10% (3) | 18% (4) | 19% (3) | 12% (20) |
| GMT | 257 | 607 | 1286 | 308 | 389 | 411 | |
| (95% CI) | (46–1,428) | (41–9,009) | (250–6,616) | (12–8,043) | (141–1,077) | (203–831) | |
| Cross-reactive | 20% (10) | 38% (18) | 35% (11) | 46% (10) | 43% (7) | 34% (56) | |
| 3 | Dominant | 6% (3) | 10% (5) | 13% (4) | 18% (4) | 19% (3) | 11% (19) |
| GMT | 467 | 467 | 815 | 909 | 1807 | 678 | |
| (95% CI) | (218–1,002) | (218–1,002) | (105–6,316) | (200–4,129) | (751–4,351) | (391–1,176) | |
| Cross-reactive | 31% (15) | 40% (19) | 42% (13) | 46% (10) | 25% (4) | 37% (61) | |
| 4 | Dominant | 2% (1) | 0 | 3% (1) | 0 | 5% (1) | 2% (3) |
| GMT | 33 | 125 | 280 | 133 | |||
| (95% CI) | NA | NA | NA | (4-4785) | |||
| Cross-reactive | 12% (6) | 15% (7) | 23% (7) | 22% (5) | 12% (2) | 16% (27) | |
GMT = geometric mean titer; CI = confidence interval; NA = not available.
Circulating DENV strains.
The dominant serotype-specific response within the composite of serotypes of each person was considered to represent the infecting serotype. By this criteria, three of the four serotypes were clearly shown to be circulating within this population; serotype 1 was the dominant serotype in 66 infants and young children (61%), serotype 2 in 20 infants and young children (19%), and serotype 3 in 19 infants and young children (18%). There were three instances in which antibodies to serotype 4 were the highest, which suggested that this strain, previously documented in Haiti,20 was also circulating although to a more limited extent (Table 1). Even in children 7–12 months of age there was evidence of dominant antibody responses to types 1, 2, or 3.
Evidence for a broad heterotypic response.
Heterotypic, or non-dominant, antibodies were commonly detected and contributed substantially to the overall DENV immunity (Table 1). Many DENV-infected children had measurable levels of neutralizing antibody to multiple serotypes (Figure 2). Of those infected, most had antibody against more than one serotype. Typical patterns of antibody responses are shown in Figure 3; dominant serotype titers were often > 1,000 with a median titer of 466 to type 1, 428 to type 2, and 839 to type 3. The median titer to type 4 was lower at a dilution of 1:125. In almost all cases, there is a dominant response with substantial diminution in the titer of the heterotypic response. The level of cross-reactivity seemed to be bidirectional and each strain was equally effective in generating cross-reactive antibodies.
Figure 2.
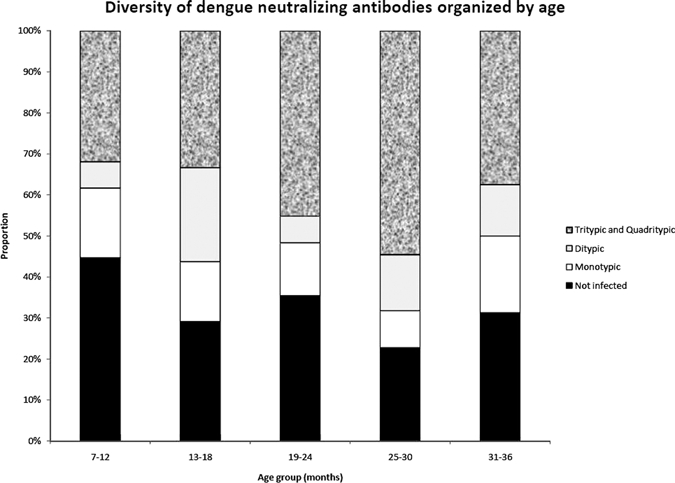
Breadth of heterotypicity of the antibody response to dengue virus, by age group, Haiti. Serum samples that did not demonstrate significant evidence of neutralizing antibody to any dengue virus serotype were counted as not infected, serum samples with antibodies against only one serotype were considered to be monotypic, antibodies against two serotypes as ditypic, antibodies against three serotypes of tritypic, and antibodies against all four serotypes as quadritypic.
Figure 3.
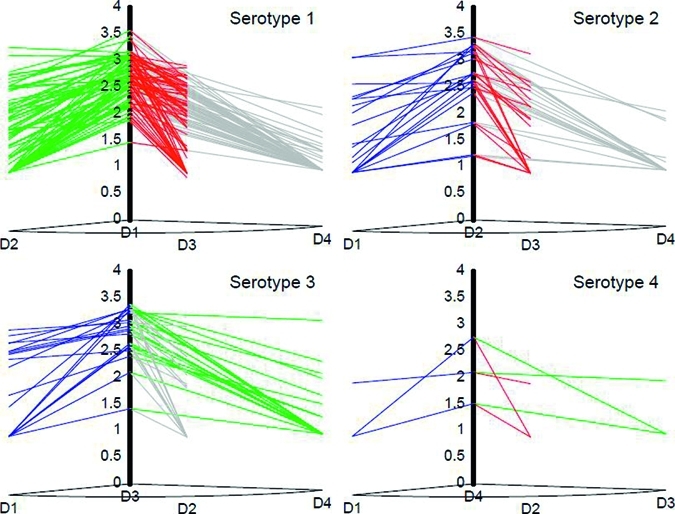
Relationship between serotypes in terms of cross-reactivity for antibodies to dengue virus, Haiti. Cross-reactivity is demonstrated by the sloping lines between dominant type and heterotypic titers.
Evidence for reinfection.
Arguments for reinfection based on a cross-sectional serostudy are weaker than they would be with prospective and serial samples but two observations argue against frequent reinfection in this hyperendemic setting. The first observation is that the geometric mean titer of the antibody to three most prominent serotypes showed no significant increase with age (Table 1). One might have predicted a continued and significant increase with repeated infections to different serotypes. The second observation is that only three children had comparable titers to two DENV serotypes, which might be interpreted as serial infections.
Malaria serosurvey.
Malaria antibodies to apical membrane antigen-1 were detected in only 2 of the 166 children tested. Although not a perfect measure of malaria exposure, this finding suggests that dengue has a far greater prevalence than P. falciparum malaria in the age group and during the seasons studied. This finding is corroborated by the rarity with which positive malaria smears are seen at GHESKIO. In spite of requesting 5–7 smears a week from the pediatric population, a positive malaria smear is reported less than once a month.
Discussion
A well-standardized and quantified serotype-specific DENV plaque neutralization assay was performed on carefully stored clinical samples from pediatric sera and demonstrated that DENV infection in the urban poor in Haiti is hyperendemic. Most children 7–12 months of age had already experienced a DENV infection with one of at least three types, DENV types 1, 2, and 3. Infection with a strain gave not only high strain-specific antibody but appears to induce cross-reactive neutralizing antibody to the one or more other DENV serotypes. There was no significant increase in geometric mean titer with age. This finding, coupled with the rare instances in which equivalent titers were seen against more than one strain, suggests that broad immunity may be induced by primary infection regardless of strain. If reinfection with a heterotypic virus occurred independently of initial infection, one might have expected to see multiple comparably high antibody titers. Thus, one possibility raised by this survey is that frequent early exposure to DENV with resultant broadly cross-neutralizing antibody creates an environment in which serious dengue is limited.
Contrary to the absence of the diagnosis of dengue infection in infants in Haiti, the age range of 7–12 months is considered a special risk period for infants in southeast Asia; severe dengue disease is reported to increase as maternal antibody wanes to a sub-neutralizing titer.7 Notably, the genetic phenotype and nutritional status of host and differing virulence of DENV subtypes contribute to disease phenotype.21 In particular, severe dengue illness appears to occur less frequently in people of African descent.10,22 Possibly, Haitians are genetically or in some way protected from severe disease.
Alternatively, it may be that clinical dengue is present but under-diagnosed in the population in Haiti. There is a high rate of infant mortality that correlates with the period of rapid acquisition to antibody to DENV. Poor access to care, lack of awareness of dengue, and difficulty in visualizing dermatologic manifestations in darker-skinned persons are all factors that could contribute to a lack of recognition of clinical dengue. Our data significantly extend observations made in the Carrefour District of Port-au-Prince that school age children have had extensive experience with dengue, as shown by endpoint measurement of antibody titers and by demonstrating DENV exposure in infancy.7
A nationwide survey of children 0–4 years of age with fever for antibodies to dengue and positive malaria smears was conducted in June 2007 (Enquête Nationale sur la Prévalence du Paludisme et de la Fièvre Dengue, unpublished data). In that survey, 5.9% of children 0–4 years of age had IgM against dengue, and 1.3% had positive malaria smears in the Ouest Department in which Port-au-Prince is located. This survey may under-represent DENV infections as a cause of febrile illness because only those patients with fever longer than five days would be expected to be detected by the IgM-based enzyme-linked immunosorbent assay used in the survey. Further confounding the diagnosis of DENV infection by IgM is that a recent study in Leogane, a coastal town south of Port-au-Prince, showed that 36% of randomly selected adults had IgM against DENV.23 These three studies each demonstrate evidence of frequent infection with DENV in the population of Haiti. However, a correlation with clinical disease is unclear.
The observation that approximately 50% of urban children in Haiti experience infection with DENV in the first year of life distinguishes Haiti as having the highest recorded DENV infection rate in infancy. In contrast, neutralizing antibody to any DENV serotype was found in 12% of two-year-old children in Thailand.24 Seroprevalence to any DENV serotype in two-year-old children olds varied from 22% to 40%, as determined by an enzyme-linked immunosorbent inhibition assay over a four-year period, in Nicaragua.25 The high seroprevalence in Haiti would be consistent with the observation that DENV is a marker for the high degree of urban poverty and disruption of social services that characterized Port-au-Prince even before the recent earthquake.26
The relative importance of dengue and malaria cannot be fully assessed from this study but the infrequent detection of malaria antibodies and the relative rarity of positive malaria smears at the GHESKIO clinic suggest malaria is not a dominant cause of febrile illness. The incidence of malaria in Haiti is reported to be decreasing.27
A retrospective review of patient charts was not revealing except to indicate the need for a systematic, prospective study. Obstacles encountered included incomplete clinical records during a period of transition from paper to electronic records, the presentation of patients only after prolonged periods of fever, and the absence of diagnostic tests for dengue used by this clinic. A model for assessing the impact of dengue has recently been implemented in Nicaragua in children more than two years of age.28,29
Such careful prospective, denominator-based, clinical studies are necessary to determine the extent of severe clinical disease associated with acquisition of dengue in infants and young children in Haiti. Studies will need to be coupled with active case surveillance, molecular-based diagnosis, detailed study of the vector, risk factors in the environment, and serial serologic determinations.
Given the rapid acquisition of dengue in the first year of life, it is of interest that approximately one-third of the population were still seronegative at three years of age. Their protection from infection could be explained by circulation patterns of DENV but also may be caused by protected water supplies or the use of bed nets or screens. A recent study in Haiti surprisingly showed a 50% reduction in IgM against dengue in a community that used bed nets in spite of the diurnal biting patterns of Ae. aegypti.30
This study was a cross-sectional prevalence study of a sample set obtained for a different purpose, determination of HIV status. Urban poor comprise most of the population in Port-au-Prince. Mothers in Haiti who are HIV infected often fall within that strata, and there is no reason to postulate that exposure of their families to DENV will be markedly different than in families without HIV infection. Plotting of population dwelling places shows a broad distribution of acquisition of antibodies to DENV throughout Port-au-Prince and no unusual clustering of the DENV-seropositive persons.
The short window of five months in which samples were obtained collected the young age of the patients prevent us from extrapolating the endemicity of DENV strains over time. Rainfall generally occurs each month in Port-au-Prince, and average rainfall typically exceeds 100 mm during April–June and August–October.31 No information has been reported about the seasonality of dengue in Haiti. However, there is no question that substantial concurrent circulation of types 1, 2, and 3 occurred in the year before the samples were obtained, as shown by their respective antibody seroprevalence in children 7–12 months of age.
We have inferred that the highest serotype-specific titer represents the infecting strain. As shown in Figure 3, almost all infected children had one strain to which their antibody titer was 10–100-fold higher than to other strains. Alternative explanations would include that the highest titer represented the most recent infection because antibody will decay slightly over time or that secondary infection led to a boosted or anamnestic response in antibody to the secondary strain. Other limitations include the fact that strains used for neutralization may not have been a perfect antigenic match for DENV strains in Haiti. Further confirming DENV circulation, we were able to amplify DENV types 1 and 3 by using standard primers from adult serum samples obtained at the same time.
This study defines broad heterotypic immunity to wild-type infection in a hyperendemic setting that left most of this young population with neutralizing antibody to the three most commonly circulating strains. The frequent immune response to primary infection in children less than one year of age indicates that dengue vaccination might have to be given in infancy, perhaps before the age of six months, to protect this vulnerable population. Encouragingly from our data, these young infants are capable of mounting a vigorous humoral response. An intervention becomes important only if there is a more substantial clinical impact of hyperendemic dengue that has to date been appreciated. It can be speculated that limiting infection in early childhood might lead to more severe disease if the broad natural immunity demonstrated is lost.
ACKNOWLEDGMENTS
We thank the technicians and administrative staff at the GHESKIO clinic for their assistance, Dr. Kathryn Hanley (New Mexico State University) for providing extremely helpful comments on a late draft of the manuscript, and Jason Moore (Dartmouth Department of Biostatistics) for conceptual contributions to presentation of data.
Footnotes
Financial support: The study was supported by an Infectious Disease Society of America summer fellowship to Meghan Rioth, a supplement to the Caribbean, Central, and South America network for HIV epidemiology (CCASAnet), and the Vanderbilt Emphasis Program.
Authors' addresses: Meghan Rioth, Vanderbilt University, Nashville, TN, E-mail: meghan.rioth@vanderbilt.edu. Carole Anne Beauharnais, Francine Noel, and Jean W. Pape, GHESKIO Centers, Port-au-Prince, E-mails: caroleanne26@hotmail.com, fsevere@gheskio.org, and jwpape@gheskio.org. Mine R. Ikizler, Pediatric Infectious Diseases, Nashville, TN, E-mail: mine.ikizler@vanderbilt.edu. Sapna Mehta, E-mail: mehta_sapna@hotmail.com. Yuwei Zhu, Department of Biostatistics, Vanderbilt University, Nashville, TN, E-mail: yuwei.zhu@vanderbilt.edu. Carole A. Long, Malaria Immunology Section, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, E-mail: clong@niaid.nih.gov. Peter F. Wright, Dartmouth Medical School, Lebanon, NH, E-mail: peter.f.wright@dartmouth.edu.
References
- 1.Halstead SB. Dengue. Curr Opin Infect Dis. 2002;15:471–476. doi: 10.1097/00001432-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Guzman MG, Kouri G. Dengue haemorrhagic fever integral hypothesis: confirming observations, 1987–2007. Trans R Soc Trop Med Hyg. 2008;102:522–523. doi: 10.1016/j.trstmh.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Rico-Hesse R. Dengue virus virulence and transmission determinants. Curr Top Microbiol Immunol. 2010;338:45–55. doi: 10.1007/978-3-642-02215-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanakaratne N, Wahala WM, Messer WB, Tissera HA, Shahani A, Abeysinghe N, de-Silva AM, Gunasekera M. Severe dengue epidemics in Sri Lanka, 2003–2006. Emerg Infect Dis. 2009;15:192–199. doi: 10.3201/eid1502.080926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guzman MG, Sierra B, Kouri G, Farrar J, Simmons C. In: Frontiers in Dengue Virus Research. Hanley KA, Weaver SC, editors. Norfolk, United Kingdom: Caister Academic Press; 2010. pp. 79–102. (Host and virus determinants of susceptibility and dengue disease severity). [Google Scholar]
- 6.Rossi CA, Drabick JJ, Gambel JM, Sun W, Lewis TE, Henchal EA. Laboratory diagnosis of acute dengue fever during the United Nations Mission in Haiti, 1995–1996. Am J Trop Med Hyg. 1998;59:275–278. doi: 10.4269/ajtmh.1998.59.275. [DOI] [PubMed] [Google Scholar]
- 7.Trofa AF, DeFraites RF, Smoak BL, Kanesa-thasan N, King AD, Burrous JM, MacArthy PO, Rossi C, Hoke CH., Jr Dengue fever in US military personnel in Haiti. JAMA. 1997;277:1546–1548. [PubMed] [Google Scholar]
- 8.Ventura AK, Ehrenkranz NJ. Endemic dengue virus infection in Hispaniola. I. Haiti. J Infect Dis. 1976;134:436–441. doi: 10.1093/infdis/134.5.436. [DOI] [PubMed] [Google Scholar]
- 9.Halstead SB, Streit TG, Lafontant JG, Putvatana R, Russell K, Sun W, Kanesa-Thasan N, Hayes CG, Watts DM. Haiti: absence of dengue hemorrhagic fever despite hyperendemic dengue virus transmission. Am J Trop Med Hyg. 2001;65:180–183. doi: 10.4269/ajtmh.2001.65.180. [DOI] [PubMed] [Google Scholar]
- 10.de la C Sierra B, Kourí G, Guzmán MG. Race: a risk factor for dengue hemorrhagic fever. Arch Virol. 2007;152:533–542. doi: 10.1007/s00705-006-0869-x. [DOI] [PubMed] [Google Scholar]
- 11.WHO Statistical Information System (WHOSIS) Probability of Dying Aged < 5 Years per 1000 Live Births (Under-Five Mortality Rate) 2006. http://www.who.int/whosis/indicators/2007MortChild/en/index.html Available at. Accessed April 1, 2010.
- 12.Noel F, Wright PF, Bois G, Deschamps MM, de Matteis P, Cassangnol R, Thimothee M, Celestin K, Vaz L, Bradshaw JA, Brignoli E, Zhu Y, Johnson WD, Fitzgerald D, Pape JW. Contribution of bacterial sepsis to morbidity in infants born to HIV-infected Haitian mothers. J Acquir Immune Defic Syndr. 2006;43:313–319. doi: 10.1097/01.qai.0000242463.73817.c6. [DOI] [PubMed] [Google Scholar]
- 13.Russell PK, Nisalak A, Sukhavachana P, Vivona S. A plaque reduction test for dengue virus neutralizing antibodies. J Immunol. 1967;99:285–290. [PubMed] [Google Scholar]
- 14.Durbin AP, Karron RA, Sun W, Vaughn DW, Reynolds MJ, Perreault JR, Thumar B, Men R, Lai CJ, Elkins WR, Chanock RM, Murphy BR, Whitehead SS. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3′-untranslated region. Am J Trop Med Hyg. 2001;65:405–413. doi: 10.4269/ajtmh.2001.65.405. [DOI] [PubMed] [Google Scholar]
- 15.Wright PF, Durbin AP, Whitehead SS, Ikizler MR, Henderson S, Blaney JE, Thumar B, Ankrah S, Rock MT, McKinney BA, Murphy BR, Schmidt AC. Phase 1 trial of the dengue virus type 4 vaccine candidate rDEN4{Delta}30-4995 in healthy adult volunteers. Am J Trop Med Hyg. 2009;81:834–841. doi: 10.4269/ajtmh.2009.09-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas SJ, Nisalak A, Anderson KB, Libraty DH, Kalayanarooj S, Vaughn DW, Putnak R, Gibbons RV, Jarman R, Endy TP. Dengue plaque reduction neutralization test (PRNT) in primary and secondary dengue virus infections: how alterations in assay conditions impact performance. Am J Trop Med Hyg. 2009;81:825–833. doi: 10.4269/ajtmh.2009.08-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polley SD, Mwangi T, Kocken CH, Thomas AW, Dutta S, Lanar DE, Remarque E, Ross A, Williams TN, Mwambingu G, Lowe B, Conway DJ, Marsh K. Human antibodies to recombinant protein constructs of Plasmodium falciparum apical membrane antigen 1 (AMA1) and their associations with protection from malaria. Vaccine. 2004;23:718–728. doi: 10.1016/j.vaccine.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 18.Miura K, Zhou H, Diouf A, Moretz SE, Fay MP, Miller LH, Martin LB, Pierce MA, Ellis RD, Mullen GE, Long CA. Anti-apical-membrane-antigen-1 antibody is more effective than anti-42-kilodalton-merozoite-surface-protein-1 antibody in inhibiting Plasmodium falciparum growth, as determined by the in vitro growth inhibition assay. Clin Vaccine Immunol. 2009;16:963–968. doi: 10.1128/CVI.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simmons CP, Chau TN, Thuy TT, Tuan NM, Hoang DM, Thien NT, Lien le B, Quy NT, Hieu NT, Hien TT, McElnea C, Young P, Whitehead S, Hung NT, Farrar J. Maternal antibody and viral factors in the pathogenesis of dengue virus in infants. J Infect Dis. 2007;196:416–424. doi: 10.1086/519170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noel F, Mehta S, Zhu Y, Rouzier Pde M, Marcelin A, Shi JR, Nolte C, Severe L, Deschamps MM, Fitzgerald DW, Johnson WD, Wright PF, Pape JW. Improving outcomes in infants of HIV-infected women in a developing country setting. PLoS ONE. 2008;3:e3723. doi: 10.1371/journal.pone.0003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chau TN, Quyen NT, Thuy TT, Tuan NM, Hoang DM, Dung NT, Lien le B, Quy NT, Hieu NT, Hieu LT, Hien TT, Hung NT, Farrar J, Simmons CP. Dengue in Vietnamese infants–results of infection-enhancement assays correlate with age-related disease epidemiology, and cellular immune responses correlate with disease severity. J Infect Dis. 2008;198:516–524. doi: 10.1086/590117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaturvedi U, Nagar R, Shrivastava R. Dengue and dengue haemorrhagic fever: implications of host genetics. FEMS Immunol Med Microbiol. 2006;47:155–166. doi: 10.1111/j.1574-695X.2006.00058.x. [DOI] [PubMed] [Google Scholar]
- 23.Lenhart A, Orelus N, Maskill R, Alexander N, Streit T, McCall PJ. Insecticide-treated bednets to control dengue vectors: preliminary evidence from a controlled trial in Haiti. Trop Med Int Health. 2008;13:56–67. doi: 10.1111/j.1365-3156.2007.01966.x. [DOI] [PubMed] [Google Scholar]
- 24.Pengsaa K, Limkittikul K, Luxemburger C, Yoksan S, Chambonneau L, Ariyasriwatana C, Lapphra K, Chanthavanich P, Lang J, Sabchareon A. Age-specific prevalence of dengue antibodies in Bangkok infants and children. Pediatr Infect Dis J. 2008;27:461–463. doi: 10.1097/INF.0b013e3181646d45. [DOI] [PubMed] [Google Scholar]
- 25.Balmaseda A, Standish K, Mercado JC, Matute JC, Tellez Y, Saborío S, Hammond SN, Nuñez A, Avilés W, Henn MR, Holmes EC, Gordon A, Coloma J, Kuan G, Harris E. Trends in patterns of dengue transmission over 4 years in a pediatric cohort study in Nicaragua. J Infect Dis. 2010;201:5–14. doi: 10.1086/648592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anonymous Asia: dengue fever, a man-made disease. The Economist. 1998;347((8066)):538. [Google Scholar]
- 27.Bonnlander H, Rossignol AM, Rossignol PA. Malaria in central Haiti: a hospital-based retrospective study, 1982–1986 and 1988–1991. Bull Pan Am Health Organ. 1994;28:9–16. [PubMed] [Google Scholar]
- 28.Balmaseda A, Standish K, Mercado JC, Matute JC, Tellez Y, Saborío S, Hammond SN, Nuñez A, Avilés W, Henn MR, Holmes EC, Gordon A, Coloma J, Kuan G, Harris E. Trends in patterns of dengue transmission over 4 years in a pediatric cohort study in Nicaragua. J Infect Dis. 2010;201:5–14. doi: 10.1086/648592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuan G, Gordon A, Avilés W, Ortega O, Hammond SN, Elizondo D, Nuñez A, Coloma J, Balmaseda A, Harris E. The Nicaraguan pediatric dengue cohort study: study design, methods, use of information technology, and extension to other infectious diseases. Am J Epidemiol. 2009;170:120–129. doi: 10.1093/aje/kwp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lenhart A, Orelus N, Maskill R, Alexander N, Streit T, McCall PJ. Insecticide-treated bednets to control dengue vectors: preliminary evidence from a controlled trial in Haiti. Trop Med Int Health. 2008;13:56–67. doi: 10.1111/j.1365-3156.2007.01966.x. [DOI] [PubMed] [Google Scholar]
- 31.Climate Information for Haiti. http://www.climatetemp.info/haiti/ Available at.


