Abstract
Rift Valley fever virus (RVFV) is an emerging biodefense pathogen that poses significant threats to human and livestock health. To date, the interepidemic reservoirs of RVFV are not well defined. In a longitudinal survey of infectious diseases among African buffalo during 2000–2006, 550 buffalo were tested for antibodies against RVFV in 820 capture events in 302 georeferenced locations in Kruger National Park, South Africa. Overall, 115 buffalo (21%) were seropositive. Seroprevalence of RVFV was highest (32%) in the first study year, and decreased progressively in subsequent years, but had no detectable impact on survival. Nine (7%) of 126 resampled, initially seronegative animals seroconverted during periods outside any reported regional RVFV outbreaks. Seroconversions for RVFV were detected in significant temporal clusters during 2001–2003 and in 2004. These findings highlight the potential importance of wildlife as reservoirs for RVFV and interepidemic RVFV transmission in perpetuating regional RVFV transmission risk.
Introduction
Rift Valley fever virus (RVFV) causes intermittent epizootics and epidemics that result in massive losses of livestock and significant human morbidity and mortality within affected locations.1–3 Consequent animal export embargoes create significant economic hardship for affected local ranchers and pastoralist communities. Because of the ability of RVFV to infect many vector and animal species, and the likelihood of its regional persistence once introduced, it is essential to learn more about how RVFV is spread and persists within its transmission zones.
Rift Valley fever virus is transmitted to animals and humans through blood feeding of infected insect vectors, typically any of several locally abundant mosquito species.1 This virus can also be transmitted to humans by direct contact with, or aerosolization of, infected bodily fluids of viremic animals.1,3,4 Reported large outbreaks of RVFV in humans and domestic and wildlife species are often associated with heavy rainfall and flooding events, which enable an abrupt bloom of competent mosquito vectors that facilitate transmission.5,6 Floodwater Aedes species are known to infect their eggs transovarially, enabling persistence of RVFV in semi-arid areas during prolonged dry periods.7,8
During epizootics, many domestic and wildlife species become infected with RVFV, but during interepidemic periods (IEP), the dominant mechanisms for local maintenance or persistence of the virus remain uncertain. Most arthropod-borne viral infections persist in nature because of maintenance of the virus in an animal reservoir, but a predominant animal reservoir of RVFV has not been identified. Bats and vervet monkeys have been suggested as possible reservoirs, but no conclusive evidence has been obtained.9,10 Some models suggest that a long-term animal reservoir is not necessary for persistence of RVFV in nature, and instead persistence among mosquito species is sufficient.11 Nevertheless, domestic and wild ruminants are known to be amplifying hosts for RVFV, likely facilitating epizootics and epidemics in livestock and wildlife areas.3
Many wild animals have been shown to be seropositive for RVFV-neutralizing antibodies during IEP, including African buffalo, black rhino, lesser kudu, impala, African elephant, kongoni, and waterbuck.12 Of interest, the highest wild animal RVFV antibody prevalence (> 15%) was observed in black rhinos and among ruminants (kudu, impala, African buffalo, and waterbuck) and the highest hemagglutination-inhibition titers (≤ 1:1,280) were observed primarily in buffalo, including young animals born after known epizootics, i.e., during the IEP.12 African buffalo (Syncerus caffer) and Asian water buffalo (Bubalus bubalis) have been found to have high RVFV seroprevalence in other cross-sectional surveillance studies in Africa, and it has been suggested that they may serve as one of the amplifying hosts for RVFV.13,14 Published reports also document that buffalo experience significant morbidity (fetal loss) from wild-type RVFV infections, and RVFV has been isolated from a water buffalo (Bubalus bubalis) fetus.14,15
The objectives of this study were to identify whether low-level RVFV transmission is ongoing in African Buffalo (Syncerus caffer caffer) in Kruger National Park, South Africa, to examine the variation in buffalo RVFV seroprevalence over time, and to re-examine the potential contribution of infected buffalo to local RVFV transmission during and in between periods of known epizootics.
Populations, Materials, and Methods
Study population.
The results reported here were obtained as part of a larger program to study infectious disease dynamics among African buffalo in Kruger National Park on the northeastern border of South Africa. Description of area habitat and the details of animal capture, clinical assessment, specimen sampling, and tracking have been presented in detail elsewhere.16–18 During November 2000–July 2006, the estimated overall buffalo population in Kruger National Park was 23,000–25,500. Capture and survival data in the present study were part of an area-focused, annual disease survey in which sampling (n = 99–200/year) was performed in the central Satara (2001–2006) and the more southern Lower Sabie (2003–2006) regions of the park (Figure 1). Briefly, from a defined sub-region of Kruger National Park having a buffalo population of approximately 3,000, 593 buffalo were captured at 302 georeferenced locations by using aerial or ground darting techniques, working in close collaboration with veterinarians in Kruger National Park.
Figure 1.
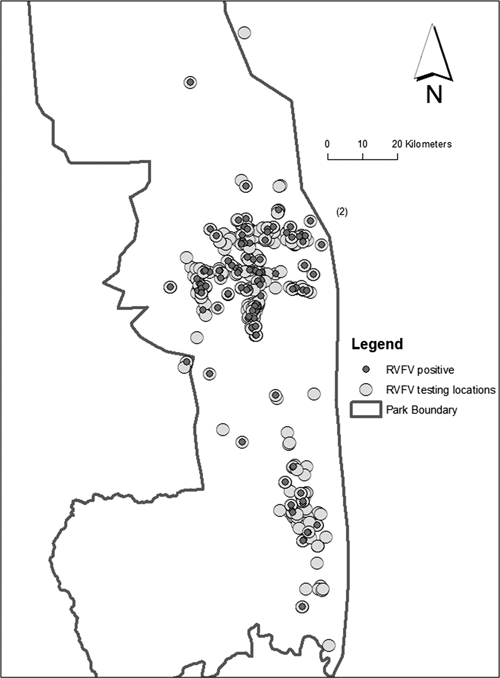
Kruger National Park in northeastern South Africa, showing the 820 capture locations of African buffalo (Syncerus caffer caffer) (light circles) during the November 2000–July 2006 study period. Capture locations of animals detected to be seropositive for antibody against Rift Valley fever virus (n = 115) are indicated by smaller dark circles.
Captured animals were tested for exposure to infections and uniquely branded and given subcutaneous insertion of microchip tags for later identification. A subset (n = 167) were fitted with either radio collars or global positioning system collars for tracking and weekly to monthly follow-up. Because male and female buffalo have horns, it was impossible for a radio collar to come off an animal without tearing through the belting. We identified mortality events by the belting still being intact and the remains of a carcass. We assumed that a radio collar had fallen off if the belting was severed and no carcass was present after an extensive search of the area.18 We estimated time since death (0–30 days) using the extent of carcass remains.18 During the study, there were 288 live repeat captures among the 593 buffalo studied and 55 documented deaths among the 167 tracked animals.
Ethical approval.
Institutional Animal Care and Use Committee approval was through University of California Berkeley #R217-0402 to Wayne M. Getz. This protocol was also reviewed and approved by the Animal Care and Use Committee for the South Africa National Parks.
Animal assessment.
Animals < 5 years of age were assigned to an age group on the basis of physical examination for incisor eruption pattern.19 Age of animals ≥ 5 years of age at the time of first examination was determined by visual comparison of horn development and body size to a calibrated photographic reference collection.17,20 Four age groups were considered: youngest = 1–3 years, mid-low = 3–5 years, mid-high = 5–8 years, highest = more than 8 years.
Testing for antibodies against RVFV was performed at the Agricultural Research Council, Onderstepoort Veterinary Institute in Onderstepoort, South Africa, by using a hemagglutination-inhibition (HAI) titration assay.21,22 In areas without circulation of other known phleboviruses (e.g., Toscana, Naples, Sicilian, Arumowot, and Punta Toro viruses),23–25 the HAI has shown high sensitivity and specificity (compared with plaque reduction neutralization testing) for detection of RVFV infection in animals, with performance equal to that of an IgG enzyme-linked immunosorbent assay.22 At the time of the study reported here, the HAI was the only assay used at the collaborating South African veterinary laboratory. The median positive titer was 1:160 and the range was 1:5 (6 animals) to 1:320 (31 animals). For the present analysis, an HAI titer ≥ 1:5 was considered positive. All nine seroconversions observed during the IEP resulted in HAI titers ≥ 1:10, suggesting recent transmission.
Climate and rainfall.
Kruger National Park is a fenced wooded savannah that covers 19,485 km2 in the northeastern corner of South Africa and is bordered by Mozambique and Zimbabwe. The Kruger National Park habitat was effectively expanded in 2003 by removal of some fencing for the creation of the international Greater Limpopo Transfrontier Conservation Area. The area has wet–dry seasonality, and most rainfall occurs in the summer months.18 Average annual rainfall varies from 700 mm in the southwestern region of the park to approximately 400 mm in the northern plains. Soil composition is predominantly granitic in the western half of the park and basaltic clay in the eastern half.16 Park rainfall data were obtained from Scientific Services, Kruger National Park.
Statistical analysis.
Univariate and bivariate analysis of animal characteristics and outcomes were performed using SPSS version 16 (SPSS Inc., Chicago, IL). Mapping of the Kruger National Park study areas and capture events was performed by using ArcGIS version 9.2 (ESRI, Redlands, CA). Analysis for significant global spatial clustering of detection sites for seroconverted animals was tested by using a weighted Ripley's K statistic and the Point Pattern Analysis program.26,27 The weighted K-function analysis is used to determine if the rates or values at each point are clustered, dispersed, or random within the pattern of points. The weighted Ripley's K test determines whether seroconversion events were significantly more clustered or dispersed (i.e., outside the 95% confidence envelope) than observed clustering among all sampling sites relative to a pattern of complete spatial randomness (Supplemental Figure 1). Significant temporal clustering of seroconversions was identified by using Grimson's empty cells method and Clusterseer2 software (Terraseer, Ann Arbor, MI).28 Grimson's empty cells method examines the number of adjacent quarterly seroconversion events over the time series of the study to determine whether temporal clustering deviates significantly from a random Poisson distribution of events. Kaplan-Meier survival curves were used to examine survival duration on the basis of seropositivity. Cox proportional hazards regression was used to model survival time predicted by seropositivity while adjusting for important covariates such as age and sex. For all analyses, α-level for P values was set to 0.05, indicating statistical significance below this threshold.
Results
Five hundred fifty African buffalo were tested for antibodies against RVFV by HAI assay in 820 capture events. Overall, 115 (21%) of the buffaloes were seropositive for RVFV across the sampling locations in Kruger National Park (Figure 1). No significant global spatial clustering or annual local/focal clustering was detected for buffalo seropositive for RVFV tested among the georeferenced capture events in the study (Supplemental Figure 1).
Seroprevalence varied depending on the year of study. Prevalence of animals seropositive for antibodies against RVFV was highest (32%) in the first year of the study (2001). Prevalence then decreased progressively in subsequent years (30%, 18%, 19%, and 14% in 2002, 2003, 2004, and 2005, respectively) (Figure 2). Seropositivity among sampled male animals was 16% in any period; among females it was 24% (χ2 = 5.21, P = 0.022). Seroprevalence also varied by age, such that during each study year, the older animals were most likely to be seropositive (Figure 3). Interestingly, young animals were identified as seropositive in year 5 during a prolonged period without reported RVFV outbreaks in South Africa. Five animals, which initially were seropositive for RVFV, seroreverted during the study. These animals had titers of 1:5, 1:20, 1:40, 1:80, and 1:160, respectively, before converting to a negative status.
Figure 2.
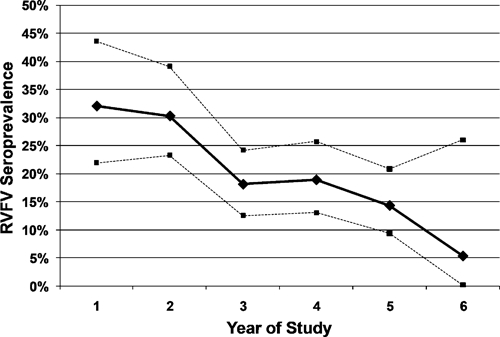
Overall prevalence of seropositivity for antibodies against Rift Valley fever virus (solid line indicates prevalence and dashed lines indicate upper and lower 95% confidence limits) among 550 African buffalo captured and serotested in Kruger National Park during the 2000–2006 study period. Numbers tested each year were as follows: year 1 = 78; year 2 = 142; year 3 = 177; year 4 = 159; year 5 = 161; year 6 = 19. Total number of tests indicates that 126 animals had repeated sampling during the study.
Figure 3.
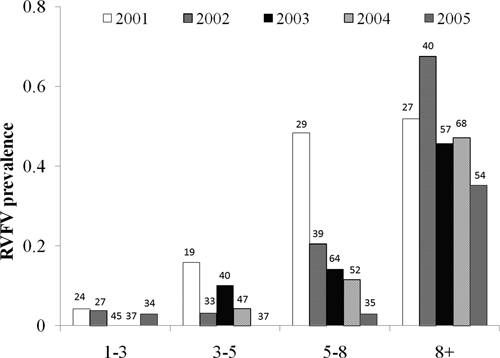
Seroprevalence of antibody against Rift Valley fever virus, by age category, among African buffalo in Kruger National Park during initial years 1–5 of the study (2001–2005). Bars indicate age-specific seroprevalence (in each year) for animal subgroups (defined as youngest group = 1–3 years of age; middle-low group = 3–5 years of age; middle-high group = 5–8 years of age; oldest group = more than 8 years of age) during that particular study year. Numbers above each bar indicate the number of animals that were tested in that age group in that study year.
Of 126 African buffalo that were initially seronegative (i.e., at risk for new RVFV infection) and had repeat blood sampling and RVFV testing, nine seroconverted during the study (Figure 4). Seroconversions were detected in significant temporal clusters during mid-2001–mid-2003 (seven of nine seroconversions) and during March–October 2004 (two of nine seroconversions) (Grimson's statistic E(E) = 16.362, P = 0.0008 for the observed data). As shown in Figure 5, observed seroconversions were detected after normal rainy seasons in Kruger National Park. No seroconversions were documented during or after the below-average rainfall during the rainy season in 2003, although sampling effort during this period was similar.
Figure 4.
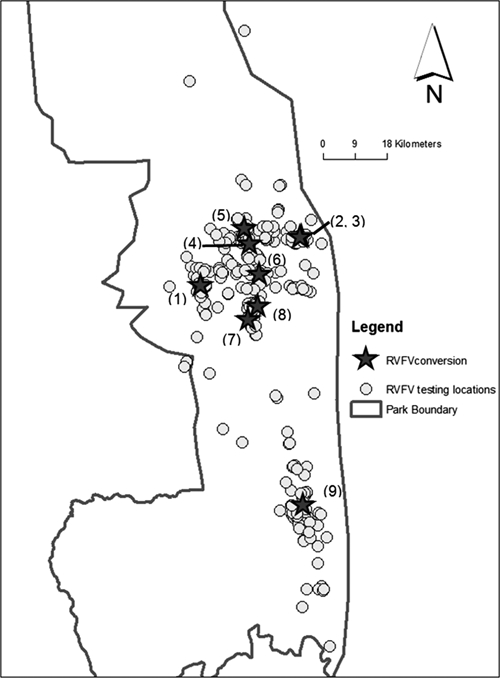
Capture locations (gray stars) of nine African buffalo found to have seroconverted for antibodies against Rift Valley fever virus (upon recapture and repeat serologic testing). Numbers next to starred locations indicate the order of detection of these seroconverting animals. Open circles indicate the distribution of all other capture events during November 2000–July 2006. Borders of Kruger National Park are indicated by the solid line.
Figure 5.
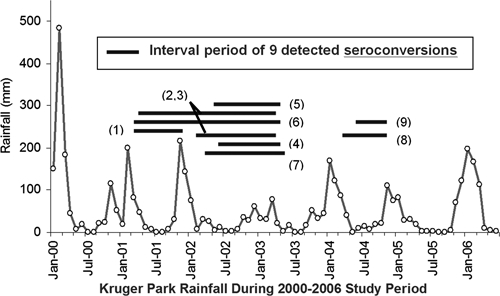
Time series of seroconversion events, relative to rainfall, in Kruger National Park. Circles indicate monthly rainfall for the study area during 2000–2006. Horizontal gray bars indicate the intervals (period between observations) for the nine seroconversion events to antibody against Rift Valley fever virus among 126 initially seronegative buffalo that were repeatedly sampled during the study. To indicate their location, numbers in parentheses correspond to sites indicated in Figure 4 and reflect the order of detection of seroconverted animals during the period of the study.
To assess whether recovery from a past RVFV infection had an impact on long-term African buffalo survival, we examined outcomes in a cohort of 167 buffalo with known serostatus that were monitored monthly by radio collar. There were 52 documented deaths among the cohort, and survival analysis compared outcomes for 51 seropositive and 116 seronegative animals. Although analysis of crude mortality suggested that RVFV-seropositive animals were less likely to survive than seronegative animals, seropositive animals were also more likely to be older and female, and these characteristics were associated with differences in mortality risk.17 After multivariable adjustment of mortality hazard for age, sex, and age–sex interaction with the use of Cox proportional hazards technique, mortality rates were not significantly different between RVFV-seronegative and RVFV-seropositive buffalo (Figure 6). The multiply-adjusted hazard ratio for RVFV seropositivity was 1.09 (95% confidence interval = 0.57–2.08) for seronegative versus seropositive animals (χ2 = 0.074, degrees of freedom = 1, P = 0.78, not significant).
Figure 6.
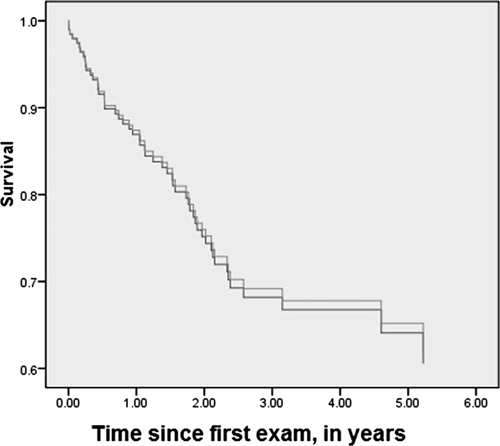
Kaplan-Meier survival plots of African buffalo in the Kruger Park study according to their serostatus for antibody against Rift Valley fever virus (RVFV). Lines indicate the cumulative age- and sex-adjusted percent survival (in years) of 51 animals seropositive for antibodies against RVFV (gray line) or 116 animals seronegative for antibodies against RVFV (dark line) that were serially monitored for survival during the period of the study.
Discussion
This longitudinal study suggests that low-level RVFV transmission occurred among African buffalo in Kruger National Park during 2000–2006. Although no South African epizootics were reported to the World Organisation for Animal Health during the study, we detected nine buffalo seroconversions during the program follow-up.29 Evidence of IEP transmission was also provided by detection of seropositivity among the youngest age group of animals (1–3 years of age) born during the study period. The apparent continuing low-level transmission to wild ruminants during periods of average rainfall suggests that such undetected transmission may be important to the maintenance of RVFV in natural habitats. Continuing local transmission could be an important factor in earlier and more rapid RVFV amplification and transmission to humans during recognized epizootic/epidemic periods.
Initial RVFV seroprevalence was high in our buffalo sample, consonant with rates detected in previous cross-sectional surveys of RVFV seroprevalence among wild ruminants in Kenya and Zimbabwe.12,13 Overall, in our Kruger National Park sample, seropositivity was highest in the first year of study (2000–2001), likely secondary to anomalous heavy rainfall that had occurred in 2000 (Figure 5). Transmission of RVFV is associated with excessive rainfall in high-risk areas because of the abrupt increase in mosquito numbers after flooding events.3,6,7 Thus, it is possible that many of the buffalo that we documented to be RVFV seropositive at the onset of the study had just recently seroconverted during this preceding heavy rainfall season. In addition, an RVFV outbreak was reported to have occurred in 1999 among wild fauna in South Africa, although its location was not described.29 Furthermore, two RVFV outbreaks were reported among cattle in nearby Zimbabwe during 2001. Those outbreaks resulted in 791 cases in cattle and 4 animal deaths.29
Among the study animals sampled, the observed RVFV seroprevalence waned over the study period. This finding could have been the result of several causes, including loss of immunity over time, insensitive testing (variable detection of HAI levels), or selective loss of seropositive individuals over the study period. Although the observed seroprevalence decreased in later years of the study, there were nine negative-to-positive seroconversions documented during the 2001–2004 interval. These conversions occurred after more typical November–April wet seasons had average rainfall, suggesting a connection between local seasonal weather patterns and RVFV transmission. This pattern appears more subtle than the large outbreaks of zoonotic RVFV disease seen after extreme rainfall events.3,6,7 It is possible that these lower levels of seroconversion may represent echo events of heavy RVFV transmission that occur during extremely heavy rainy seasons. These findings suggest the likelihood of continuing low-level transmission of RVFV to buffalo and other animals during non-flooding off years in suitable RVFV vector habitats.
Seroprevalence of RVFV, indicating past exposure to viral infection, was not associated with worsened survival during long-term follow-up of a closely monitored cohort of animals. Among humans, RVFV infection can be the cause of long-term neurologic sequelae and visual impairment caused by retinitis and anterior chamber eye inflammation.2,30 For buffalo survivors of RVFV infections, we were unable to detect a persistent health impact. Given the limited size of our available longitudinal sample, the confidence interval for the adjusted hazard ratio associated with RVFV seropositivity remains fairly broad, and it is possible that we missed a clinically significant effect caused by type II error in statistical inference. In addition, the RVFV testing performed yields evidence only of past RVFV infection, and we cannot comment on whether acute RVFV-related disease has a significant impact on buffalo survival. Previous analysis found no detectable delayed effects of the capture interventions of the study on buffalo survival.18
This study has several limitations. First, the RVFV study was a sub-study of a larger program that focused on infectious disease dynamics, particularly bovine tuberculosis (TB), among African buffalo. Our RVFV results may be biased because recaptures of African buffalo were intended to exclude those that were known to be TB positive from prior capture. Because TB-negative buffalo are more likely to be younger, recaptures were more likely to be younger. However, in years 2–6 of the study, buffalo > 8 years of age were adequately represented in serologic testing (> 30% of all animals sampled), such that the rates measured were unlikely to be markedly influenced by age bias.
Second, the method of RVFV testing in this study was HAI. Although HAI has been shown to have 100% sensitivity and specificity compared with plaque-reduction neutralization testing in sheep samples, no published studies have reported the test characteristics for buffalo.22 Five buffalo that initially tested positive by HAI (titers 1:5, 1:20, 1:40, 1:80, and 1:160, respectively) seroreverted during the course of the study, which may indicate a higher false-positive rate of HAI in buffalo than in sheep, or a loss of antibody levels, a loss of immunity, or both, in these buffalo over time.
In summary, in a prospective longitudinal African buffalo survey in Kruger National Park during 2000–2006, serologic testing for antibodies against RVFV showed a buffalo seroprevalence of 21% and 7% of retested individuals seroconverted, highlighting the potential importance of local interepidemic RVFV transmission among wildlife in perpetuating regional risk for RVFV transmission. Although there may be considerable variation of RVFV transmission across regional habitats and over time, it is evident that transmission continues on a regular basis in suitable locales. Better definition of the long-term patterns of transmission will provide better insights for planning for vector and population-based RVF prevention and control.
Supplementary Material
ACKNOWLEDGMENTS
We thank Craig Hay, Justin Bowers, Julie Wolhuter, Robert Dugtig, Stephan Ferreira, Kutani Bulunga, Augusta Mabunda, Fernando Muhlovo, Peter Buss, Markus Hofmeyr, Roy Bengis, and Simeon Muhlovu for help collecting field data; and Martin Haupt and Johan du Toit for providing technical and administrative support.
Disclaimers: The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the United States Geological Survey or the other institutions with which the authors are affiliated. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Note: Supplemental figure appears at www.ajtmh.org.
Footnotes
Financial support: This study was supported by National Science Foundation–National Institute of Health Ecology of Infectious Diseases program (grant DEB-0090323 to Wayne M. Getz) and the National Institutes of Health (grants U01AI45473-S1 to Charles H. King and KL2RR024990).
Authors' addresses: A. Desirée LaBeaud, Children's Hospital of Oakland Research Institute, Oakland, CA, E-mail: alabeaud@chori.org. Paul C. Cross, Northern Rocky Mountain Science Center, U.S. Geological Survey, Bozeman, MT, E-mail: pcross@usgs.gov. Wayne M. Getz, Department of Environmental Science Policy and Management, University of California, Berkeley, CA, E-mail: getz@nature.berkeley.edu. Allison Glinka, Cleveland Clinic Foundation JJ-604, Cleveland, OH, E-mail: glinkallison@gmail.com. Charles H. King, Center for Global Health and Diseases, Case Western Reserve University School of Medicine, Cleveland, OH, E-mail: chk@cwru.edu.
References
- 1.Davies FG. The historical and recent impact of Rift Valley fever in Africa. Am J Trop Med Hyg. 2010;83:73–74. doi: 10.4269/ajtmh.2010.83s2a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laughlin LW, Meegan JM, Strausbaugh LJ. Epidemic Rift Valley fever in Egypt: observations of the spectrum of human illness. Trans R Soc Trop Med Hyg. 1979;73:630–633. doi: 10.1016/0035-9203(79)90006-3. [DOI] [PubMed] [Google Scholar]
- 3.Murithi RM, Munyua P, Ithondeka PM, Macharia JM, Hightower A, Luman ET, Breiman RF, Njenga MK. Rift Valley fever in Kenya: history of epizootics and identification of vulnerable districts. Epidemiol Infect. 2011;139:372–380. doi: 10.1017/S0950268810001020. [DOI] [PubMed] [Google Scholar]
- 4.Peters CJ, Spertzel R, Patrick W. In: Biological Threats and Terrorism: Assessing the Science and Response Capabilities. Knobler SL, Mahmoud AAF, Pray LA, editors. Washington: National Academy Press; 2002. pp. 66–77. (Aerosol technology and biological weapons). [PubMed] [Google Scholar]
- 5.Davies FG, Highton RB. Possible vectors of Rift Valley fever in Kenya. Trans R Soc Trop Med Hyg. 1980;74:815–816. doi: 10.1016/0035-9203(80)90213-8. [DOI] [PubMed] [Google Scholar]
- 6.Davies FG, Linthicum KJ, James AD. Rainfall and epizootic Rift Valley fever. Bull World Health Organ. 1985;63:941–943. [PMC free article] [PubMed] [Google Scholar]
- 7.Linthicum KJ, Bailey CL, Davies FG, Kairo A, Logan TM. The horizontal distribution of Aedes pupae and their subsequent adults within a flooded dambo in Kenya: implications for Rift Valley fever virus control. J Am Mosq Control Assoc. 1988;4:551–554. [PubMed] [Google Scholar]
- 8.Linthicum KJ, Davies FG, Kairo A, Bailey CL. Rift Valley fever virus (family Bunyaviridae, genus Phlebovirus). Isolations from diptera collected during an inter-epizootic period in Kenya. J Hyg (Lond) 1985;95:197–209. doi: 10.1017/s0022172400062434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies FG, Onyango E. Rift Valley Fever: the role of the vervet monkey as a reservoir or maintenance host for this virus. Trans R Soc Trop Med Hyg. 1978;72:213–214. doi: 10.1016/0035-9203(78)90074-3. [DOI] [PubMed] [Google Scholar]
- 10.Oelofsen MJ, Van der Ryst E. Could bats act as reservoir hosts for Rift Valley fever virus? Onderstepoort J Vet Res. 1999;66:51–54. [PubMed] [Google Scholar]
- 11.Favier C, Chalvet-Monfray K, Sabatier P, Lancelot R, Fontenille D, Dubois MA. Rift Valley fever in West Africa: the role of space in endemicity. Trop Med Int Health. 2006;11:1878–1888. doi: 10.1111/j.1365-3156.2006.01746.x. [DOI] [PubMed] [Google Scholar]
- 12.Evans A, Gakuya F, Paweska JT, Rostal M, Akoolo L, Van Vuren PJ, Manyibe T, Macharia JM, Ksiazek TG, Feikin DR, Breiman RF, Kariuki Njenga M. Prevalence of antibodies against Rift Valley fever virus in Kenyan wildlife. Epidemiol Infect. 2008;136:1261–1269. doi: 10.1017/S0950268807009806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson EC, Rowe LW. The prevalence of antibody to the viruses of bovine virus diarrhoea, bovine herpes virus 1, Rift Valley fever, ephemeral fever and bluetongue and to Leptospira spp. in free-ranging wildlife in Zimbabwe. Epidemiol Infect. 1998;121:441–449. doi: 10.1017/s0950268898001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoogstraal H, Meegan JM, Khalil GM, Adham FK. The Rift Valley fever epizootic in Egypt 1977–78. 2. Ecological and entomological studies. Trans R Soc Trop Med Hyg. 1979;73:624–629. doi: 10.1016/0035-9203(79)90005-1. [DOI] [PubMed] [Google Scholar]
- 15.Arthur RR, El-Sharkawy S, Cope SE. Recurrence of Rift Valley fever in Egypt. Lancet. 1993;342:1149–1150. doi: 10.1016/0140-6736(93)92128-g. [DOI] [PubMed] [Google Scholar]
- 16.Caron A, Cross PC, Du Toit JT. Ecological implications of bovine tuberculosis in African buffalo herds. Ecol Appl. 2003;13:1338–1345. [Google Scholar]
- 17.Cross PC, Heisey DM, Bowers JA, Hay CT, Wolhuter J, Buss P, Hofmeyr M, Michel AL, Bengis RG, Bird TL, Du Toit JT, Getz WM. Disease, predation and demography: assessing the impacts of bovine tuberculosis on African buffalo by monitoring at individual and population levels. J Appl Ecol. 2009;46:467–475. [Google Scholar]
- 18.Oosthuizen WC, Cross PC, Bowers JA, Hay CT, Ebinger MR, Buss P, Hofmeyr M, Cameron EZ. Effects of chemical immobilization on survival of African buffalo in the Kruger National Park. Wildlife Management. 2009;73:149–153. [Google Scholar]
- 19.Grimsdell JJ. Age determination of the African buffalo, Syncerus caffer, Sparrman. East Afr J Wildlife. 1973;11:31–53. [Google Scholar]
- 20.Pienaar U. Observations of developmental biology, growth and some aspects of the population ecology of African buffalo in the Kruger National Park. Koedoe. 1969;12:29–52. [Google Scholar]
- 21.Olaleye OD, Tomori O, Schmitz H. Rift Valley fever in Nigeria: infections in domestic animals. Rev Sci Tech. 1996;15:937–946. doi: 10.20506/rst.15.3.966. [DOI] [PubMed] [Google Scholar]
- 22.Scott RM, Feinsod FM, Allam IH, Ksiazek TG, Peters CJ, Botros BA, Darwish MA. Serological tests for detecting Rift Valley fever viral antibodies in sheep from the Nile Delta. J Clin Microbiol. 1986;24:612–614. doi: 10.1128/jcm.24.4.612-614.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tesh RB, Peralta PH, Shope RE, Chaniotis BN, Johnson KM. Antigenic relationships among phlebotomus fever group arboviruses and their implication for the epidemiology of sandfly fever. Am J Trop Med Hyg. 1975;24:135–144. doi: 10.4269/ajtmh.1975.24.135. [DOI] [PubMed] [Google Scholar]
- 24.Tesh RB, Peters CJ, Meegan JM. Studies on the antigenic relationship among phleboviruses. Am J Trop Med Hyg. 1982;31:149–155. doi: 10.4269/ajtmh.1982.31.149. [DOI] [PubMed] [Google Scholar]
- 25.Collao X, Palacios G, de Ory F, Sanbonmatsu S, Perez-Ruiz M, Navarro JM, Molina R, Hutchison SK, Lipkin IW, Tenorio A, Sanchez-Seco MP. Granada virus: a natural phlebovirus reassortant of the sandfly fever Naples serocomplex with low seroprevalence in humans. Am J Trop Med Hyg. 2010;83:760–765. doi: 10.4269/ajtmh.2010.09-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen D, Getis A. Point Pattern Analysis. San Diego, CA: Department of Geography, San Diego State University; 1998. [Google Scholar]
- 27.Getis A. Interaction modeling using second-order analysis. Environ Plan A. 1984;16:173–183. [Google Scholar]
- 28.Grimson RC. Disease clusters, exact distributions of maxima, and P-values. Stat Med. 1993;12:1773–1794. doi: 10.1002/sim.4780121906. [DOI] [PubMed] [Google Scholar]
- 29.World Organisation for Animal Health Handistatus II. 2010. http://www.oie.int/hs2/report.asp Available at. Accessed March 29, 2010.
- 30.Al-Hazmi A, Al-Rajhi AA, Abboud EB, Ayoola EA, Al-Hazmi M, Saadi R, Ahmed N. Ocular complications of Rift Valley fever outbreak in Saudi Arabia. Ophthalmology. 2005;112:313–318. doi: 10.1016/j.ophtha.2004.09.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


