Abstract
Hepatitis E virus (HEV) in industrialized countries is zoonotically transmitted, and swine act as a major reservoir of HEV. Serum samples from 102 swine and plasma from 34 swine handlers in Vellore, India were tested by using a reverse transcription–polymerase chain reaction to detect and genotype HEV. We measured levels of IgG against HEV in swine handlers and in age and geographically matched controls from rural and urban populations in Vellore. HEV was amplified from two pigs and both viruses belonged to genotype 4. No HEV RNA was amplified from any swine handler, but 94.1% of swine handlers were positive for antibodies against HEV, a seroprevalence rate significantly higher than in rural and urban controls. The HEV genotype circulating in swine in India is different from that of humans, but the higher antibody levels in swine handlers support the possibility that zoonotic infections may occur.
Hepatitis E virus (HEV) is a hepatotropic virus that causes acute self-limiting hepatitis. This virus is the leading cause of outbreaks and sporadic acute hepatitis in industrialized countries. In developed countries, sporadic infections predominate and are usually considered to be imported through travel to disease-endemic countries. The virus is classified into four major genotypes (1, 2, 3, and 4) and has one serotype. All four virus genotypes infects humans. Generally genotypes 1 and 2 cause outbreaks and sporadic, water-borne infections, and genotypes 3 and 4 cause sporadic foodborne infections. Recently, more autochthonous infections are being reported from industrialized countries.1 This finding may be partially caused by zoonotic HEV infections. The first animal strain of HEV was isolated from pigs in the mid-western United States and its nucleotide sequence has been determined.2 Swine HEV has since been identified in all swine-producing countries worldwide. HEV sequences identified from swine belong to either genotype 3 or 4.3
Hepatitis E virus is considered enzootic worldwide; infected swine appear clinically normal but show microscopic evidence of hepatitis.4 Studies have showed experimental cross-species infection of primates by swine HEV and of pigs by human HEV.5 In a study in the United States, increased prevalence of antibodies against HEV was observed in pig handlers compared with controls.6 High genetic relatedness between human and swine HEV was observed in countries such as United States, Taiwan, Japan, China, and countries in Europe.2,7–10 These studies provide evidence for pigs as reservoirs of HEV infection in humans. However, in India, HEV infecting humans belongs to genotype 1 and swine are infected by genotype 4.11 Therefore, hepatitis E may not be a zoonotic disease, although high antibody (96.5%) prevalence rates have been demonstrated in pigs.12 The objective of our study was to detect and characterize HEV from swine and swine handlers in Vellore, southern India and to assess the possibility of human infection caused by swine HEV.
A total of 102 swine serum samples (60 males and 42 females) were obtained from slaughter houses in and near Vellore. Plasma samples were obtained from 34 swine handlers (20 males and 14 females) residing in Vellore who had a median (SD) age of 35 (12.8) years. Viral RNA was extracted by using the Trizol method (Invitrogen, Carlsbad, CA), and a reverse transcription–polymerase chain reaction was carried out by using India swine-specific genotype 4 primers and universal primers, respectively.11,13 Amplified products were sequenced, and phylogenetic analysis was performed to genotype HEV. An enzyme-linked immunosorbent assay (ELISA) was performed to detect IgG against HEV by using the Genelabs HEV IgG enzymeimmunoassay (EIA) (MP Bio Ltd., Singapore) and the Wantai HEV IgG EIA (Wantai Biological Pharmacy, Beijing, China) for swine handlers.14 Seroprevalence of antibodies against HEV in age and geographically matched controls were obtained from a previous study that used the Genelabs assay to compare HEV exposure in age-stratified urban and rural populations in Vellore.15 From the previous study, 200 samples, 100 each from urban and rural populations (91 males and 109 females) with a median (SD) age of 35 (2.5), were tested by using the Wantai assay to determine seroprevalence of antibodies against HEV. Data analysis was carried out by using SPSS version 16 (SPSS Inc., Chicago, IL). Groups were compared using by chi-square test/Fisher exact test for categorical data.
Of 102 swine serum samples tested, 2 samples were positive for HEV RNA. They were characterized as genotype 4 (Genbank accession nos. GQ494002 and GQ494003) and clustered with other swine HEV from India (Figure 1). Of 34 plasma samples from swine handlers tested, none were positive for HEV RNA. However, the Wantai assay showed that 94.1% were positive for IgG against HEV. Prevalence in swine handlers was higher than in rural (59%) and urban (73%) adults, respectively (P < 0.001). Seroprevalence rates determined by using the Genelabs HEV IgG EIA showed a similar pattern, but lower prevalence rates (Figure 2). The prevalence of 67.6% in swine handlers using the Genelabs assay was also significantly higher (P < 0.001) than in rural (20.5%) and urban (35.5%) adults. Seroprevalence rates for rural and urban adults determined by using the Genelabs assay have been reported.15 In comparison with controls, the swine handlers had higher exposure (odds ratio = 8.2 , 95 % confidence interval = 1.91–35.42 and odds ratio = 5.34, 95% confidence interval = 2.55–11.18) when determined by using the Wantai and Genelabs kits, respectively.
Figure 1.
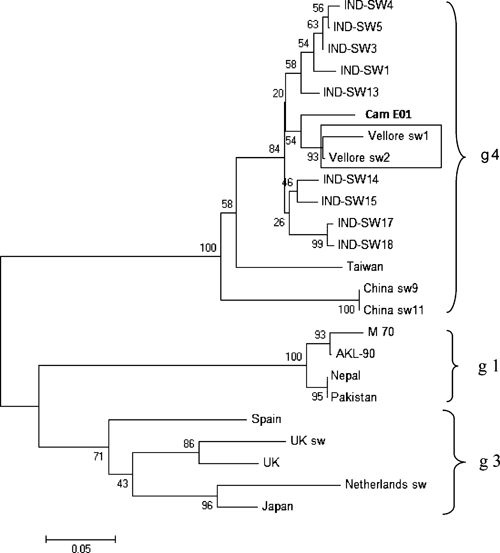
Phylogenetic tree constructed on the basis of partial nucleotide sequences of the open reading frame 2 region of the hepatitis E virus (HEV). Vellore swine strains are indicated in the box and the genotype 4 HEV strain (Cam E01) from a patient in the United Kingdom in indicated in bold. Other strains indicated represent genotypes 1, 3, and 4.
Figure 2.
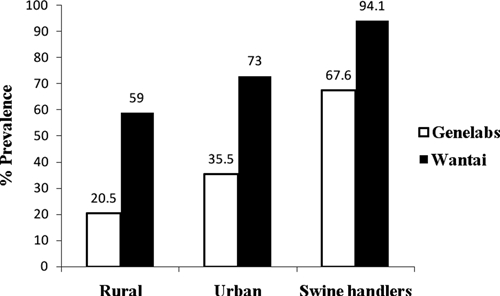
Percentage prevalence of IgG against hepatitis E virus (HEV) in 34 swine handlers and 100 rural and 100 urban age and geographically matched controls evaluated by two IgG anti-HEV enzyme immunoassays.
Our results partially support the finding that genotype 4 HEV circulates in swine but not in humans in southern India, which is consistent with results from studies in western India and northern India.11,12 We have no direct evidence of ongoing infection in swine handlers but the numbers tested were small. Although human and swine HEV belong to different genotypes in India, there is antigenic cross-reactivity between the genotypes. The high prevalence and levels of IgG against HEV in swine handlers, which were greater than seen in age- and geographically matched population controls, provide indirect evidence for possible infection with swine HEV in swine handlers. It is also possible that in India, HEV endemicity and high HEV exposure with human strains could provide cross-protection against disease, but increased exposure to swine HEV in handlers could result in a boosting of the antibody response. This hypothesis could also explain the differences in disease susceptibility to swine HEV in developing countries and industrialized countries.
It is also important to note that HEV isolated from swine in this study clustered closely with a previously reported zoonotically transmitted genotype 4 strain isolated from a patient in the United Kingdom (Figure 1), who had traveled in India before onset of the disease.16 These data provide insight into possible non–feco-oral transmission modes of HEV in India and call for increased surveillance for genotype 4 HEV in humans.
Footnotes
Authors' addresses: Rosario Vivek and Gagandeep Kang, Wellcome Trust Research Laboratory, Department of Gastrointestinal Sciences, Christian Medical College, Vellore 632004, India, E-mails: vivekm@cmcvellore.ac.in and gkang@cmcvellore.ac.in.
References
- 1.Dalton HR, Bendall R, Ijaz S, Banks M. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis. 2008;8:698–709. doi: 10.1016/S1473-3099(08)70255-X. [DOI] [PubMed] [Google Scholar]
- 2.Meng XJ, Purcell RH, Halbur PG, Lehman JR, Webb DM, Tsareva TS, Haynes JS, Thacker BJ, Emerson SU. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci USA. 1997;94:9860–9865. doi: 10.1073/pnas.94.18.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng XJ. Swine hepatitis E virus: cross-species infection and risk in xenotransplantation. Curr Top Microbiol Immunol. 2003;278:185–216. doi: 10.1007/978-3-642-55541-1_7. [DOI] [PubMed] [Google Scholar]
- 4.Halbur PG, Kasorndorkbua C, Gilbert C, Guenette D, Potters MB, Purcell RH, Emerson SU, Toth TE, Meng XJ. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J Clin Microbiol. 2001;39:918–923. doi: 10.1128/JCM.39.3.918-923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng XJ, Halbur PG, Shapiro MS, Govindarajan S, Bruna JD, Mushahwar IK, Purcell RH, Emerson SU. Genetic and experimental evidence for cross-species infection by swine hepatitis E virus. J Virol. 1998;72:9714–9721. doi: 10.1128/jvi.72.12.9714-9721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng XJ, Wiseman B, Elvinger F, Guenette DK, Toth TE, Engle RE, Emerson SU, Purcell RH. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J Clin Microbiol. 2002;40:117–122. doi: 10.1128/JCM.40.1.117-122.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsieh SY, Meng XJ, Wu YH, Liu ST, Tam AW, Lin DY, Liaw YF. Identity of a novel swine hepatitis E virus in Taiwan forming a monophyletic group with Taiwan isolates of human hepatitis E virus. J Clin Microbiol. 1999;37:3828–3834. doi: 10.1128/jcm.37.12.3828-3834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishizawa T, Takahashi M, Mizuo H, Miyajima H, Gotanda Y, Okamoto H. Characterization of Japanese swine and human hepatitis E virus isolates of genotype IV with 99% identity over the entire genome. J Gen Virol. 2003;84:1245–1251. doi: 10.1099/vir.0.19052-0. [DOI] [PubMed] [Google Scholar]
- 9.Wang YC, Zhang HY, Xia NS, Peng G, Lan HY, Zhuang H, Zhu YH, Li SW, Tian KG, Gu WJ, Lin JX, Wu X, Li HM, Harrison TJ. Prevalence, isolation, and partial sequence analysis of hepatitis E virus from domestic animals in China. J Med Virol. 2002;67:516–521. doi: 10.1002/jmv.10131. [DOI] [PubMed] [Google Scholar]
- 10.Pina S, Buti M, Cotrina M, Piella J, Girones R. HEV identified in serum from humans with acute hepatitis and in sewage of animal origin in Spain. J Hepatol. 2000;33:826–833. doi: 10.1016/s0168-8278(00)80316-5. [DOI] [PubMed] [Google Scholar]
- 11.Arankalle VA, Chobe LP, Joshi MV, Chadha MS, Kundu B, Walimbe AM. Human and swine hepatitis E viruses from western India belong to different genotypes. J Hepatol. 2002;36:417–425. doi: 10.1016/s0168-8278(01)00297-5. [DOI] [PubMed] [Google Scholar]
- 12.Shukla P, Chauhan UK, Naik S, Anderson D, Aggarwal R. Hepatitis E virus infection among animals in northern India: an unlikely source of human disease. J Viral Hepat. 2007;14:310–317. doi: 10.1111/j.1365-2893.2006.00815.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhai L, Dai X, Meng J. Hepatitis E virus genotyping based on full-length genome and partial genomic regions. Virus Res. 2006;120:57–69. doi: 10.1016/j.virusres.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Bendall R, Ellis V, Ijaz S, Ali R, Dalton H. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J Med Virol. 2010;82:799–805. doi: 10.1002/jmv.21656. [DOI] [PubMed] [Google Scholar]
- 15.Vivek R, Chandy GM, Brown DW, Kang G. Seroprevalence of IgG antibodies to hepatitis E in urban and rural southern India. Trans R Soc Trop Med Hyg. 2010;104:307–308. doi: 10.1016/j.trstmh.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Rolfe KJ, Curran MD, Mangrolia N, Gelson W, Alexander GJ, L'Estrange M, Vivek R, Tedder R, Ijaz S. First case of genotype 4 human hepatitis E virus infection acquired in India. J Clin Virol. 2010;48:58–61. doi: 10.1016/j.jcv.2010.02.004. [DOI] [PubMed] [Google Scholar]


