Abstract
Electrospinning is a widely established polymer-processing technology that allows generation of fibers (in nanometer to micrometer size) that can be collected to form nonwoven structures. By choosing suitable process parameters and appropriate solvent systems, fiber size can be controlled. Since the technology allows the possibility of tailoring the mechanical properties and biological properties, there has been a significant effort to adapt the technology in tissue regeneration and drug delivery. This review focuses on recent developments in adapting this technology for tissue regeneration applications. In particular, different configurations of nozzles and collector plates are summarized from the view of cell seeding and distribution. Further developments in obtaining thick layers of tissues and thin layered membranes are discussed. Recent advances in porous structure spatial architecture parameters such as pore size, fiber size, fiber stiffness, and matrix turnover are summarized. In addition, possibility of developing simple three-dimensional models using electrosprayed fibers that can be utilized in routine cell culture studies is described.
Tissue Engineering
Tissue engineering or regeneration is a multidisciplinary study to restore, maintain, and enhance tissue and organ function.1 In tissue engineering, biodegradable scaffolds are used to support and guide cells to proliferate, organize, and produce their own extracellular matrix (ECM). Scaffolding material eventually disappears leaving only the necessary healthy tissue in a topologically required form.2,3 Assembly and maturation of ECM elements is important in determining the biomechanics and the quality of the tissue. For example, collagen provides tensile strength to the tissue, elastic fibers contribute to the elasticity of the tissue, while proteoglycans fills the extracellular space, creating a space for the tissue regulation of growth factors and other interactions.4 Delicate balance between different matrix elements is necessary to generate healthy tissue. The size and shape of collagen fibers in ECM relies on tissues and organs even in the same species. The shape is cord or tape with a width of 1–20 μm and the collagen fibrils (unit can be observed by electron microscopy) are cylindrical with a diameter ranging from 10 to over 500 nm where cell is attaching and hugging.5
Obtaining a biodegradable matrix conducive for cell colonization is a fundamental requirement for tissue regeneration. Like ECM, biomimic scaffold should allow cell attachment and migration, enables diffusion of vital cell nutrients, and retains cells. Further, chemical and mechanical properties of scaffold influence cell viability and proliferation.6 Appropriate pore size and porosity are essential to modulate cell seeding and diffusion. Biodegradability is essential because scaffolds are absorbed and distributed to the nearby tissue, which allows no surgical removal from body. The surface of scaffold is suitable for cell attachment and migration. The mechanical properties of scaffold are harmonious with the tissue at implant site.
Since manufacturing porous templates using pure components allow formation of matrices with required features in addition to large scale production, various methods7,8 to fabricate scaffolds using diverse biomaterials are investigated9 (Table 1). Several processes have been reported to fabricate scaffolds with suitable mechanical properties, appropriate pore size, and porosity. Solvent casting and particulate leaching allows the preparation of porous structures with regular porosity, but with a limited thickness.10 The polymer is dissolved into a suitable solvent. Then, the solution is cast into a mold filled with porogen particles. After the polymer solution has been cast, the solvent is allowed to fully evaporate. The composite structure in the mold is immersed in a liquid to dissolve the porogen. Once the porogen has been fully dissolved, a porous structure is obtained. Porogens, when dispersed in a polymer solution, are completely covered by the solution, limiting the interconnectivity of the pores within the scaffolds. Advances have been reported to fuse the porogens before the formation of a three-dimensional (3D) scaffold, and induce phase changes during leaching process to obtain improved scaffold properties.11
Table 1.
Fabrication Methods of Scaffold for Tissue Regeneration
| Method | Microscale fiber | Nanoscale fiber |
|---|---|---|
| Solvent casting/particular leaching | × | |
| Emulsification/freeze-drying | × | |
| Computer aid design/computer aid manufacturing | × | |
| Phase separation | × | |
| Nanofibers self-assembly | × | |
| Textile technology | × | × |
Freeze-drying requires no solid porogen like solvent casting and particulate leaching.12 Controlled rate freezing and lypohilization technique (CRFLT) is the method of choice while forming scaffolds from natural polymers such as chitosan and collagen, which dissolve in acidic water. CRFLT generates open pore structures, suitable for guiding cell ingrowth. Alternatively, a polymer is dissolved into a suitable solvent and water is added to the polymeric solution; the two liquids are mixed to form an emulsion. The emulsion is cast into a mold, frozen in liquid nitrogen (−196°C), and freeze-dried to remove the dispersed water and the solvent, thus leaving a porous polymeric structure. The drawbacks are relatively uneven pore size and mechanical stability. A major drawback in the scaffolds formed from CRFLT or salt leaching technique is that they do not have fibrous architecture present in the ECM.
Computer-assisted design and manufacturing (CAD/CAM) technologies are an alternative method that allows precise control of adding and bonding materials in layers and control the porosity and pore size.13 Such systems are referred by solid freeform fabrication, layered manufacturing, stereolithography, selective laser sintering, fused deposition modeling, laminated object manufacturing, inkjet-based systems, and 3D printing. In these processes, 3D structure are with various features are designed using CAD software. Integrating biomedical images via generation of computer-generated model has also been explored. The model is then implemented to a machine, which builds the desired object a layer at a time. Although objects can be formed with any geometric complexity or intricacy without the need for elaborate final assembly, limitations of CAD/CAM methods include forming structures using biocompatible materials with fiber size in nanoscale, similar to ECM.
Recently, scaffolds similar to the ECM architecture at the nanoscale have emerged as one major advances in scaffold fabrication techniques. Currently, three techniques have been reported in the synthesis of nanofibers14:
Phase separation involves five steps15,16: (i) dissolution of polymer, (ii) liquid–liquid phase separation process, (iii) polymer gelation (controls the porosity of nanoscale scaffolds at low temperature), (iv) extraction of solvent from the gel with water, and (v) freezing and freeze-drying under vacuum. Phase separation presents the drawbacks of uneven pore size.
Nanofiber self-assembly produces thinner fibrils,17 with diameters <10 nm and these come together into thicker fiber bundles tailored to peptide structure in nanoscale. However, nanofiber self-assembly is relatively costly.
Electrospinning technology has recently emerged as a novel technique for tissue regeneration because it is versatile and relatively economical to manufacture micro- and nanofibers similar to natural ECM.18,19 Electrospinning has been employed in industrial-scale textile technologies for the preparation of nonwoven meshes, which are similar to ECM in scale.20,21 Since the technology allows the possibility of tailoring the mechanical properties and biological properties, there has been a significant effort to adapt the technology in tissue regeneration.22 It is the focus of this review.
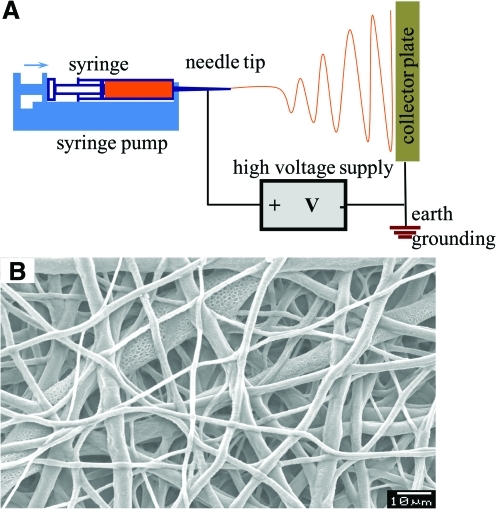
The electrospray apparatus (Fig. 1) consists of a syringe pump, syringe and needle tip, high-voltage power supply, earth grounding, and a collector. When a collector is spun to orient the fibers, it is referred to as electrospinning (electrospraying + spinning). When high voltage is applied in the system, the polymer solution flies from needle tip to collector.23,24 During the flight of the solution, the solvent evaporates and the polymer forms nonwoven fibers on the collector. Most soluble polymers with high molecular weight can be electrosprayed or electrospun.25 Nanofibers can be made of natural polymers, polymer blends, nanoparticle-permeated polymers, and ceramics. Different morphologies (beaded, smooth, core-shell, and porous fibers) have been reported. Since the technology allows the possibility of tailoring the mechanical properties and biological properties, there has been a significant effort to adapt the technology for use in tissue regeneration, drug delivery, and biomedical devices.24 One significant drawback of electrospinning technology tissue engineering is, however, inappropriate pore size for cells to grow inward into 3D scaffolds. In this article, we review the new developments in electrospinning technology relevant to tissue engineering. In particular, we address the formation of scaffolds with pore sizes suitable for uniform cell seeding, which facilitate (a) evaluation of single-cell behavior on fibers and (b) formation of thick tissues with uniform distribution of cells (cell-glued 3D scaffold).
FIG. 1.
Conventional electrospray setup. (A) Schematic showing conventional setup. (B) Micrograph of a polymeric structure obtained using conventional technique. Color images available online at www.liebertonline.com/ten.
History of Electrospinning
Electrospinning (electrostatic spinning) is a process of fabricating micro- and nanosized fibers with nonwoven structure, known for over 100 years.24,26 Electrospinning was first observed in 189727 and was first patented in the United States in 1902.28 In 1934 when Formhals patented a process and apparatus using electric charges to spin synthetic fibers, electrospinning became a valid technique to produce small-sized fibers.29 Formhals designed a movable thread-collecting device that allowed the collection of fibers in a stretched state. Using this apparatus, Formhals spun cellulose acetate in acetone/alcohol solution and collected aligned fibers. In 1939, Formhals also developed a new process by increasing the distance between collector sites to improve the problem of incomplete solvent evaporation.30 Researchers after Formhals focused on better understanding of the electrospinning process. In 1969, Taylor published about the jet formation process in which he examined how the droplet behavior of polymer solution at the edge of a capillary in an electric field.31 Taylor found that when the surface tension was balanced by electrostatic forces, the pendant droplet developed into a cone from the apex of which the fiber jet was emitted. This is one reason why electrospinning can generate much smaller fibers than the diameter of the capillary. Further, Taylor determined that 49.3 degrees with respect to the axis of the cone at the cone apex is the angle to balance the surface tension with the electrostatic forces by examining viscous fluids. After Taylor's work, interest shifted to exploring the effect of individual processing parameters on the structural properties of electrosprayed fibers. In 1971, Baumgarten investigated the relationship between parameters (solution viscosity, flow rate, applied voltage, etc.) and the properties of fibers (fiber diameter). Baumgarten found that fiber diameters initially decreased when electric field increased, reached the minimum, and started to increase with further increase in the applied electric field.32 In 1978, polyurethane meshes were first applied to tissue engineering for development of vascular prosthesis. In 1981, Larrondo and Manley also examined the effect of melt processing of the polymer on diameters of electrospun fibers.33,34 They found that melting temperature is inversely related to the fiber diameters, which were bigger than those fabricated from polymer solutions.
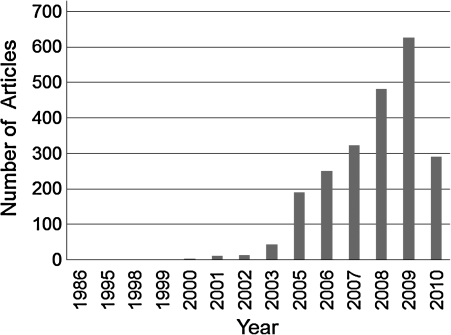
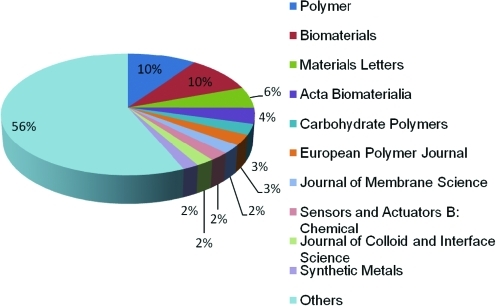
Since 1980s, electrospinning has gained attentions due to the advances in nanoscience and nanotechnology35 (Table 2). Over 200 universities and research institutes are studying electrospinning process for various fields, including tissue engineering.25 A search performed in Science Direct on March 1, 2010, indicates the dramatic increase in electrospinning and its application. In 2009 alone, >600 journal articles were published (Fig. 2) on various aspects of electrospinning process and fiber fabrication. Top 10 journals to publish the articles about electrospinning include Polymer, Biomaterials, Materials Letters, Acta Biomaterialia, Carbohydrate Polymers, European Polymer Journal, Journal of Membrane Science, Sensors and Actuators B: Chemical, Journal of Colloid and interface Science, and Synthetic Metals. These articles can be grouped into two categories. One category is research into various materials related and their fundamental characteristics, mainly published in journals such as Polymer, Materials Letters, and Carbohydrate Polymers. Major emphasis is placed on the relationship between the electrospinning parameters and distribution of fiber sizes. The other category is related to utilizing electrospinning technology in various applications, particularly biomedical applications and journals include Biomaterials and Acta Biomaterialia. An example is the possibility of forming electrosprayed fibers using biomaterials, modifying the fiber characteristics to suit the requirements of scaffold properties for tissue regeneration (Fig. 3), and evaluating the effect on cellular interactions. Only a brief summary into the developments related to the effect of various parameters on fiber size is provided while referring the readers to numerous recent reviews related to this topic (see Appendix for more details).
Table 2.
History of Electrospinning
| Year | Event |
|---|---|
| 1897 | Electrospinning phenomena |
| 1902 | U.S. patents with electrospinning |
| 1939 | Solving incomplete solvent evaporation |
| 1969 | Solution and process parameter study |
| 1971 | Usage of melt polymer |
| 1978 | Tissue engineering |
| 1981 | Era of nanotechnology |
FIG. 2.
Number of articles about electrospinning by Science Direct on March 1, 2010.
FIG. 3.
Distribution of journals publishing the article about electrospinning in 2009. Color images available online at www.liebertonline.com/teb.
Controlling Fiber Size in Electrospinning
Fiber characteristics depend on a number of parameters that can be broadly grouped into two categories: processing parameters and solution parameters.24 Processing parameters include applied voltage, polymer solution flow rate, and capillary-collector distance, and solution parameters include solvent volatability, molecular weight of the polymer, polymer concentration, solution viscosity, and solution conductivity. Along with process and solution parameters, environmental (also referred as ambient in some articles) parameters such as temperature and humidity also affect the fiber characteristics. Many of these parameters have been extensively discussed in relevance to biodegradable polymers.24,36 Only a brief summary is provided below.
When applied voltage is increased, the fiber diameter initially decreases and then increases. The fiber diameter also increases with increased flow rate of the polymer solution and with increased polymer concentration. However, fiber diameter decreases as distance between capillary and collector plate is increased or with increase in solution conductivity. In general, the process parameters affect fiber sizes but the exact relationship is unique to each polymer–solvent system. The processing environment also affects the morphology of fibers. Thus, under the optimized solvent and environmental conditions, the fiber size is controllable from 50 nm to 30 μm by manipulating the process parameters such as the distance between the nozzle and the collector plate.24,36
Materials for Electrospinning
Electrosprayed fibers can be fabricated using various materials (polymers, ceramics,37 and metals) used in many research fields such as energy and environmental engineering. However, for tissue regeneration the fibers are usually made of biocompatible materials (synthetic and natural polymers) (Table 3). Many of these materials are also biodegradable. Hence, scaffolds are chemically degradable under the physiological conditions, are nontoxic during in vivo and in vitro cell colonization, and have an ability to perform with an appropriate host response in a specific application. Both synthetic polymers and natural polymers like polycaprolactone (PCL),38,39 poly (glycolic acid), poly (lactic acid),40–46 amorphous 50:50 poly-lactide-co-glycolide, methacrylic terpolymers (such as methyl methacrylate),47 gelatin (denatured collagen),48 alginates,49 chitosan,50 glycosaminoglycans,51–57 fibrin,58 and silk59 have been explored in the formation of electrospun fibers.60,61 poly(glycolic acid) (PGA) was used to develop a synthetic absorbable suture. poly(lactic acid) (PLA) products, including tissue screws, tacks, and suture anchors, as well as systems for meniscus and cartilage repair, are commonly used. Synthetic polyesters degrade by hydrolysis62 and their degradation rates and mechanical properties can be altered via polymerization techniques63–65 and processing conditions.66–69 However, these polymers show poor regulation on cellular activity.70 Alternatively, block copolymers of poly(ethylene oxide) and poly(butylene terephthalate) have been developed. These materials are subjected to both hydrolysis (via ester bonds) and oxidation (via ether bonds). Although a number of other materials generated using polymer chemistry, synthetic polymers do not possess a surface chemistry that is familiar to cells. Despite significant efforts to improve these limitations via co-polymerization71 and grafting Arg-Gly-Asp peptides (necessary for cell adhesion),72,73 recreating all the biological responses needs significant investigation.
Table 3.
Materials for Electrospinning
| Synthetic polymer | Natural polymer | Metals/ceramics |
|---|---|---|
| Polycaprolactone | Gelatin | Cobalt acetate/poly(vinyl acetate) |
| Poly(d,l-lactic-co-glycolic acid) | Collagen | Magnesium titanate/poly(vinyl acetate) |
| Poly(ethylene-co-vinyl alcohol) | Fibrinogen | Nickel acetate/poly(vinyl acetate) |
| Polyl-lactide-co-ɛ-carprolactone) | Silk Fibroin | Palladium acetate/polycarbonate |
| Poly glycolic acid | Chitin | Zinc acetate/poly(vinyl acetate) |
| Polyurethane | Chitosan | Vanadium sol/poly(vinyl acetate) |
On the contrary, ECM components play a significant role in tissue remodeling under pathological conditions, and their role in diverse molecular mechanisms has been extensively studied in clinical samples.74,75 However, the problem in using natural polymers is processing into different forms without chemical modifications. For example, gelatin can be processed into 3D porous structure using electrospinning; however, it is not stable at physiological conditions. The 3D porous structure looses mechanical stability in few minutes without a stabilization reaction using a cross linker. Cross-linking reduces biological activity of gelatin in addition to introducing complexities related to toxicity of the cross-linker and calcification due to preferential chelation. In addition, Matrigel does not accurately reflect the diversity of proteins of most tissues; for example, Laminin-1 is not present at high quantities in most adult tissues.
Since each polymer system has a weakness in biological regulation or mechanical requirement, blending synthetic polymers with natural polymers has been explored.76–79 Using this concept, extensive work on generating scaffolds from blends of natural and synthetic polymers80–83 and other crystalline components has been performed.84 After selecting suitable solvents, different polymers have been blended to control the mechanical and biological nature of the porous scaffold.85–87 Electrosprayed fibers have been investigated as scaffolds to engineer various tissues such as skin, vascular grafts, bone, nerve, and tendon/ligaments. One could blend different polymers and control the biological nature of the formed structure.85–87 However, a major problem in electrospinning technology is the lack of generating structures with suitable and controllable microarchitecture24,88; for example, reduced pore size restricts cells and other particulates from infiltrating into the sub-layers accessing the 3D space.24,89–94 To overcome these barriers, significant effort is focused on altering the nozzle and collector configurations.
Nozzle Configuration and Collector Plates
Nozzle configuration
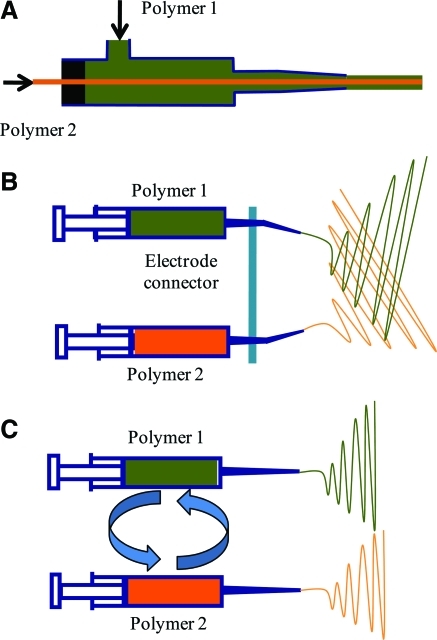
A variety of nozzle configurations have been explored to engineer the physical and chemical properties of electrosprayed fibers. The most common configuration is the single ejection system. In the system, polymer solution ejects from a single needle tip and flies from the needle tip to the collector plate when the high voltage is applied to the electrospraying system. Also, several dual ejection systems exist such as coaxial,95 sequential,96 and simultaneous96 configuration. In the coaxial system (Fig. 4A), two different polymer solutions flow through two different needles, with bigger needle circumscribing the smaller needle. The coaxial system forms a fiber containing a core of one polymer and shell of another polymer. The core-shell structure of the fiber allows the surface to be made of a bioreagulatory molecule while the mechanical property regulated by the inner core. Also, surface modification of the fiber can be performed. For example, one way to improve the mechanical property of natural polymers such as gelatin is by providing a core of a synthetic polymer such as PCL. Alternatively, inner core can be used to store a therapeutic component or a growth factor that can be released through the shell to the surface of the fiber at a controlled rate.97
FIG. 4.
Nozzle configuration: (A) Sequential, (B) Simultaneous, (C) Coaxial ejects to fabricate multilayered, mixed fibers, and core-shell structure of fibers, respectively. Color images available online at www.liebertonline.com/teb.
Another dual configuration is simultaneous system (Fig. 4B), where two separate polymer solutions are ejected from two different needle tips side by side but fly together to a collector plate at the same time. Thus, hybrid structures of the fibers can be fabricated, which allows tailorability of mechanical, chemical, and biological properties. Simultaneous deposition allows intermeshing of two polymers and controlling the microarchitecture at the nanoscale such as distribution of cell-adhesive domains. Also, a common electrode connector, typically made of aluminum foil, couples the tips of two syringes containing two different polymer solutions. The aluminum foil is very conductive, which supplies high voltage equally to both syringe tips.
The other system is sequential system (Fig. 4C), where two different polymer solutions are ejected from two different needle tips side by side but fly alternatively. The syringes containing two different polymeric solutions are exchanged in sequence. Fibers are layered one top of another. This allows formation of multilayer of fiber to improve the structural characteristic physically, chemically, and biologically. Sequential deposition allows controlling the microarchitecture at the nanoscale without intermeshing of two polymers. Since, different polymers are present in different layers, total mechanical property is determined by the interfacial strength. Segregated regions could help in differential regulation when layers of different cell types are necessary in the tissue. For example, while developing scaffolds for vascular grafts, using collagen and glycosaminoglycans has been explored. However, uniform distribution of both components may not be advantageous in the entire scaffolds. Glycosaminoglycans inhibit the proliferation of smooth muscle cells,98,99 whereas collagen supports smooth muscle cell growth.100 Hence using only lycosaminoglycan-based materials is preferred to abate smooth muscle cell hyperplasia, a clinically observed problem in vascular graft implants.50,101–104
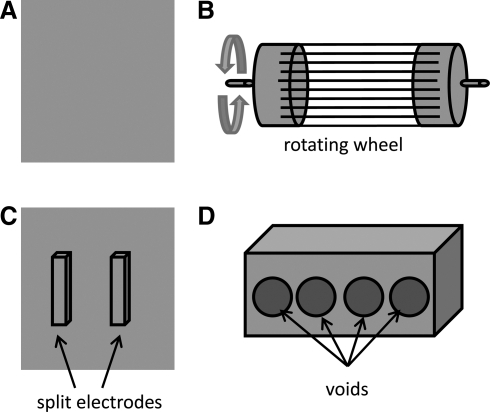
Collector plate configuration
Collector plates used to collect electrospun fibers have been modified in different configurations to alter the distribution of fibers. The general shape of a collector plate is a square block wrapped with aluminum foil (Fig. 5A). The fibers deposit on the collector plate105 as a random mass due to the bending instability of the highly charged jet. Collector plates shaped as rods and wheels are spun during the electrospinning process (Fig. 5B). The rotation speed of the collector plate determines the fiber alignment.106 Also, several split electrodes (Fig. 5C) have been used to fabricate aligned fibers using two conductive materials placed apart with a gap where aligned fibers are deposited.107
FIG. 5.
Collector plate: (A) Plane, (B) Rotating, and (C) Split collector plate, and (D) Innovative collector plate.
The reduced pore size is primarily due to multiple layers of fibers deposited to obtain thicker structures that withstand mechanical handling in subsequent steps. For example, fibers have to be peeled-off from the collector plate and transferred to the tissue culture condition, which could mechanically damage the structure. To obtain increased pore size in the scaffold, cooling the collector plate for crystallization of ice from the environment during the electrospinning process has also been reported recently.108 In this technique pore size is a function of the environmental humidity and the collector plate temperature. However, the effects of cooling on the properties of scaffolds have to be explored.
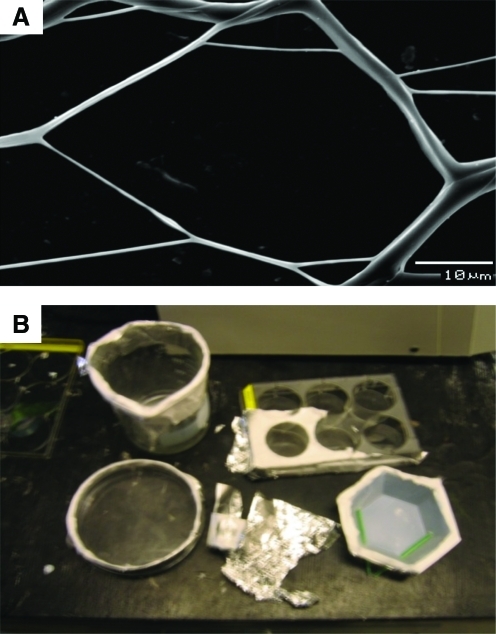
Introducing a void in the collector plate helps avoid the step of detaching the scaffold from the collector plate before use in cell colonization.109 Further, the aluminum rim could be used to handle the porous scaffold in subsequent steps, minimizing damage to the thin layers. Since very few layers can be deposited, pore size can be controlled based on the deposited volume of the polymer. A single collector plate with four holes in a wooden frame wrapped with aluminum foil is shown as an example (Fig. 5D). The rest of the electrospraying setup is not changed. The resulting fibrous structures from PCL and gelatin had large pore structures (Fig. 6A). The shape and size of the void collector plate can be changed based on the requirement (Fig. 6B) to obtain different structures. Thin layers up to 10 cm in length could be generated in various shapes such as circle, hexagon, and rectangle. Others have reported that when voids are introduced into the quartz collector plate, the insulating area changed the electric field configuration.110,111 These changes could be used to alter the orientation of fibers in the patterns, although further analyses are necessary in biocompatible polymeric systems.
FIG. 6.
Void collector plate. (A) Micrograph of a polymeric structure obtained using void collector plate (B). Various shapes, materials, and sizes of void collector plates. Color images available online at www.liebertonline.com/teb.
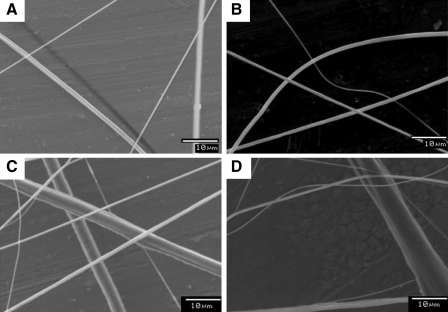
Several solvents for PCL were selected (see Appendix) and modified by methanol to increase the solvent volatility. In these cases, the fiber sizes were not affected by the void in the collector plate under the same solvent and process parameters. However, the pore sizes dramatically increased compared to those formed using block collector plates. The fiber size and shape factor are similar to the block collector plates under the same conditions. Further, both PCL fibers and gelatin fibers appeared intact in both the systems. Despite various handling steps involved during the processing of samples for scanning electron microscopy analysis, no mechanical damage to the fibers was observed (Fig. 7A). However, gelatin fibers appeared damaged mechanically when the aluminum frame was tailored (Fig. 7B). Presence of void in the collector plate does not affect sequential and simultaneous processes, described previously. In micrographs of both sequential and simultaneous systems (Fig. 7C, D), PCL and gelatin fibers existed together, which means that dual deposition can be performed, similar to other established techniques.
FIG. 7.
Evaluation of individual fiber characteristics. (A) Polymeric structure without mechanical damage, (B) with mechanical damage, (C) polymeric structure obtained by sequential, and (D) simultaneous injection. In (C) and (D), large fibers are made of polycaprolactone, and small fibers are made of gelatin.
One could use these sparsely distributed fibers in evaluating the various characteristics in nanoscale. For example, to understand the effect of hydration on the fibers, they were observed under light microscopy before and after hydration. Since distortions and characteristics can be observed in single fibers, this facilitates easy monitoring of fibers. Further, the effect of hydration on formed PCL and gelatin structures was investigated to assess the stability of gelatin. Upon hydration in phosphate-buffered saline (PBS), the PCL fibers remained the same, suggesting no significant effect. However, gelatin fibers completely dissolved in PBS. When composite structures were hydrated, the layers of PCL fibers still remained intact even one month of incubation. However, the fibers made of PCL/gelatin entangled and collapsed because of dissolution of gelatin in PBS. These observations suggest that similar technique can be utilized to evaluate the in situ stability of different components in single fibers explored in regenerating different tissues.112–114
Cellular Interactions
Importance of spatial architecture
Porous matrices offer a spatio-temporal configuration similar to in vivo conditions in addition to high surface to volume ratio required for various tissue regeneration strategies. Cellular adhesion, proliferation, and differentiation can be modified using specific molecules, such as growth factors. The presence of specific cellular binding sites in the porous structure greatly enhances cell adhesion. The proliferation and differentiation of cells may be controlled by incorporating signaling molecules into the porous matrix. Alterations in surface adhesion molecules have also been investigated.115–117 Many studies show the key role of integrins, a family of transmembrane receptors, in organizing cytoskeletal signaling complex within the focal adhesion118; for example, influence of FGF receptor-1 signal transduction is shown to be integrin-laminin pathway dependent.119 One of the integrins, αVβ3, is important during angiogenesis118 due to its exposed Arg-Gly-Asp binding domain for fibronectin, vitronectin, laminin, fibrinogen, and denatured collagen.120 Majority of the transmembrane signaling takes place via integrins in addition to easy migration due to upregulated integrin expression.121–123
Apart from chemical signaling, many in vivo studies show that the microarchitecture of the biomaterials is the primary determinant in the foreign body response.124 Many two-dimensional (2D) and 3D culture models exist and the cell response to various topographical features in 2D and 3D models such as grooves, ridges, stops, pores, wells, and nodes in micro- or nanoscale have been studied to various cells.125 Cellular responses to these topographies differ in cell attachment and migration to production of new tissue and differentiation. Further, 3D structures provide physical cues to guide cell colonization as well as chemical cues of cell-binding sites to support cell attachment and spreading. Few parameters relevant to electrospun fibers are summarized below.
Pore size
Pore size affects cell binding, migration, depth of cellular in-growth, cell morphology, and phenotypic expression.126 Many mature cell types, including endothelial cells, are unable to completely colonize scaffolds with the pore sizes >300 μm due to the difficulty in crossing large bridging distances.127–131 An optimum pore size range for supporting cell ingrowth for majority of the mature cell types (except osteoblasts and osteocytes) is in the range of 50–150 μm.127 Large pore size of fibrous scaffolds formed using subcooled collector plate allowed cell infiltration up to 50 μm after 10 day of cell culture, whereas that on conventional electrospun fibers were restricted to the top surface only. The depth of cell infiltration became 100% of its 100 μm thickness by 8 weeks, but the depth for conventional mesh was <30 μm [85]. Hence, for complete utilization of electrospraying technology, developing methods that allow better control of pore size is critical. Intuitively, the collector plate with voids can be used to control pore size between the fibers (Fig. 7) by controlling the deposit volume of the polymer as the reduced pore size is primarily due to the multiple layering of the deposited fibers. However, exploring these options has to be pursued.132
Fiber size
The fibers may be distributed randomly133–135 or form a highly organized system with regular repeating pore units.136 In electrospun fibers, the fiber thickness, length, width, and shape (circular rectangular, etc) are evaluated to understand their effect on cell colonization. For example, fibers of different sizes may offer different curvature effects to the cells. Many studies have been performed to understand the interaction between cells and scaffolds made of different fiber sizes. Some studies show better cell adhesion with nanosize fibers than microsize fibers,137 whereas some show the contrary effects.138 However, fiber diameter controls the pore size in conventional electrospun processes, leading to increased cell infiltration with larger diameters.139 Since increased fiber size increases pore size due to spatial restriction, observed differences in cell colonization could be attributed to multiple varying factors. To better understand the importance of fiber size, performing studies with constant pore size is necessary. Nevertheless, differentiation of stem cells from different origins has been explored on nanosize and microsize fibers made of biodegradable polymers.140–142 Two-week in vivo analyses demonstrated that the density of osteoprogenitor cells (MC3T1-E1 cell line) on fibers was lower than on smooth surfaces and the cell density increased with fiber diameter. Human mesenchymal stem cells showed better chondrogenesis on micro-sized fiber compared to nanosize fibers. Large pore size of fibers was helpful for mesenchymal stem cell differentiation, which is less sensitive to changes in the elastic moduli of fibers.141 Rat hippocampus-derived adult neural stem (or progenitor) cells showed improved proliferation and differentiation into various cells in the nervous system on nanofibrous scaffolds compared to microfibers.142 Further, significant reduction in viability of cells was also observed on microsize fibers compared to nanosize fibers. Overall, the fiber size may be tissue specific and could be determined by knowing the natural tissue matrix architecture.
Fiber alignment
The intension of using porous structures is to guide the cells during colonization. Hence aligning the fibers helps in aligning the colonization of cells. To understand this concept, influence of fiber alignment has also been studied. Aligned fibers have been shown to promote organized regeneration of periodontal tissue,143 myotubes,83 primary, and secondary neurons.132 After 3-day cell culture, rat periodontal ligament cells elongated along the aligned fibers.143 The cells on the aligned fibers proliferated and migrated more than on randomly distributed fibers. Human skeletal muscle cells also showed alignment along the fibers and better myotube formation than randomly oriented fibers.83 Schwan cells aligned along the PCL fibers and elongated along the orientation of the fibers, suggesting that the fibers guided the cell alignment and spreading.144 Similar alignment has also been shown in fibroblasts145 and endothelial cells.146 Thus, in tissues where ordered cell alignment is necessary, fibers can be oriented by spinning the collector plate or with different void spaces. Fiber alignment also helps in developing anisotropy in the mechanical properties of the structures,147 which may be necessary in certain tissues.
Topography of scaffold surface also influences spreading characteristics and activity of cells.148 The existence of grooves may inhibit cell movement to bend its cytoskeleton149 or reshape its actin filaments to adjust to the new topography.150 In electrospun nanofibers, surfaces on which cells are cultured could vary based on the orientation of fibers, although fibrous surface could be smooth. Hence, some studies have attributed the changes in cell growth to surface topography of fibers when describing the results of aligned fibers. For example, nanofibrous tapography is shown to affect neurite outgrowth of both primary motor and sensory neurons.132 Altering the surface of fibers are emerging to understand the effect of topography on cells.151 However, further studies with defined surface roughnesses are necessary to understand the effect of surface topography on electrospun fibers.
Fiber stiffness
Mechanical stimulus also plays a significant role in the morphogenesis of cells. Both the micro/nanoscale properties experienced by the cells and the bulk material properties that provide physical support for both the scaffold and the surrounding tissue are important during tissue regeneration. In skin wound healing studies, understanding the process of increased collagen packing has been extensively investigated152,153 and a variety of cell- or matrix-based continuum modeling has also been attempted.154,155 Some of the results suggest that the orientation of collagen fibers occurs by contact guidance during the collagen deposition by fibroblasts; fibroblasts hug the collagen fibers while depositing and induce tractional forces that lead to the development of contractile forces through which tissue heals. Based on this understanding, one of the approaches to minimize scarring is to increase the tensile strength via wound-dressing materials. 3D collagen sponges have been used alone156,157 or in conjunction with basic fibroblast growth factor158 or fibronectin and hyaluronic acid.159 These results showed that exogenous collagen increased wound tensile strength and increased degree of reepithelialization, that is, early dermal and epidermal wound healing. Further, hyaluronic acid and fibronectin may also be involved in faster wound healing via helping the migration of fibroblasts.160
At the microscale, cellular activity is shown to be influenced by the substrate stiffness in 3D porous structures.53,161,162 Cells show reduced spreading and disassembly of cytoskeletal actin even when soluble adhesive ligands are present in weak gels.163,164 This could be via the response of tractional forces between cells and materials; scaffold should be able to withstand cell contractile forces.162 Maximum tractional force generated by a cell could be as much as 10%–15% of substrate modulus.163 Similar to natural skin wound healing process, the rigidity of the scaffolds may affect the formation of ECM elements via the contact guidance.165 The stiffness of the matrix that fits cell spreading may be cell specific, with different cell types requiring different stiffnesses. These results have to be performed on electrospun fibers, which provide an ideal opportunity to understand the importance of contact guidance and matrix deposition. Formation of fibers and evaluation of mechanical properties at the nanoscale are beginning to emerge using electrospun fibers.166,167 As a next step, one has to form electrospun scaffolds with different stiffnesses and understand the effect on cell colonization.
Matrix turnover
Cell colonization also involves the deposition of ECM elements in response to different physical and chemical signals from surrounding 3D matrix. Assembly and maturation of these matrix elements in the tissue regeneration play a significant role in determining the biomechanics and the quality of the regenerated tissue. Many studies implicate an array of molecules in regulating the matrix synthesis and degradation process at transcriptional, translational, and post-translation levels. Matrix metalloproteinases, a degradative enzyme family with at least 20 members, mediate degradation of essentially all components of the ECM.168 Matrix turnover also influences cellular phenotypic characters, which in turn alters assembly of de novo synthesized matrix elements.
Unlike 2D architecture, the degradation of a 3D matrix can create more space for cell expansion, cell migration, and appropriate assembly of matrix elements. Scaffold degradation rate should be synchronized with the cell growth rate to ensure no space restriction due to slow degradation rate or the loss of structural support due to faster degradation. However, some synthetic materials can swell several times their dry weight.169 The molecular weight of a polymer will also change over the course of degradation, altering the mechanical properties. Electrospun fibers provide a significant opportunity to understand the dynamic changes in individual fibers. Some of these characteristics are summarized in a recent review on degradation of electrospun fibers.170 These analyses will help in designing next generation of scaffolds mimicking matrix architecture for regenerating complex tissues.
Mechanical stimulus is known to modulate the synthesis of all major ECM components and various growth factors. To an applied strain, substrate develops a stress, which is sensed by the adhering cells and they respond by changing their functions, including changes in gene expression, proliferation, and differentiation. Application of strain to fibroblasts cultured on polyurethane nanofibers showed increased collagen synthesis relative to no strain condition in aligned nanofibers.135 Further, on randomly distributed fibers, alignment of cells was observed along with increased collagen synthesis. Electrospun fibers provide unique opportunity to understand the direction of mechanical loading and their effect on various matrix components. Further research in this area will significantly help improve the possibility of generating thicker tissues.
Growing thicker tissues
For successful regeneration of tissues, cells have to uniformly colonize the scaffold while generating their own ECM elements. If the formed structures have very small pore sizes compared to the human cells, then cells do not infiltrate into the layers below the surface. Hence, cell growth is restricted to surfaces only (Fig. 8A). In addition, many tissues in the body are of high thickness and to grow them requires optimum pore size for nutrient and metabolic waste transport, as described above. Although fibers formed by traditional collector plate have been utilized to form thicker tissues,96,171,172 thick fibrous layers with reduced pore size break cell–cell communication between different layers. As an alternative, scaffolds formed using electrospinning have been explored in generating thicker tissues using layer-by-layer assembly technique: place one layer of fibers, seed cells, and repeat the process. Although thicker scaffold seemed to make, cells grow only between the layers, not in the entire 3D scaffold. This leads to incomplete colonization of the porous structure, hindering cell–cell communication between adjacent layers (Fig. 8B). For instance, hybrid process incorporating direct polymer melt deposition and electrospinning process was developed to fabricate thick 3D scaffold and applied to produce highly functionalized ECM mimicked 3D scaffolds with an open porous network.171 However, further analyses of the cell-colonized structures showed that cell colonization was restricted to the surface of the top layer of 3D scaffold only. This is primarily attributed to the insufficient pore size for cell to infiltrate and colonize each other in 3D spaces. Alternative approach of cell seeding while forming fibers has problems with altering cell fate.172
FIG. 8.
Schematics of current drawback and breakthrough for tissue regeneration. (A) Schematics of current drawback on conventional structure of fibers. Cells are growing on only the surface of 3D scaffold of electrosprayed fibers due to the lack of polymeric structure of fibers. (B) The thick 3D scaffold of conventional fibers by using layer-by-layer assembly technique. The cell–matrix and cell–cell interaction was lack in entire 3D scaffold. (C) Advanced breakthrough but impractical process for cell seeding to overcome current drawback. Thoroughly distributed cells on 3D scaffold using simultaneous dual deposit system. However, cells exposure to toxic solvent, high shear stress, and suboptimal environment. 3D, three-dimensional. Color images available online at www.liebertonline.com/teb.
An approach proposed to circumvent the cell seeding problem is by depositing both the polymer as well as cells simultaneously.85 However, this approach has many issues to be evaluated. Cells are exposed to (a) toxic organic solvents used in the process, (b) high shear rates induced by the flow of fluids through narrow nozzles, and (c) suboptimal environmental conditions during manufacturing (Fig. 8C).
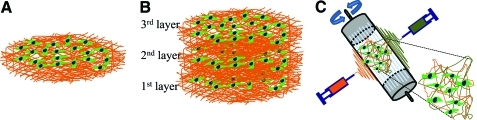
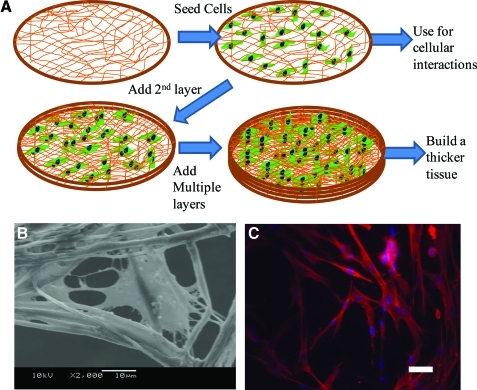
Thus, forming layers with thickness less than or similar to single cells and with large pores helps in uniform cell distribution. If thin layer of fibers are formed that is less than a cell thickness, then one could build thicker tissues using layer-by-layer concept. However, a primary issue is the fragility of the structures during sterilization, cell seeding, and handling. These requirements can be met by providing a supporting frame to protect the fibrous structures during various cell culture steps. Based on this concept, thin layers formed using void collector plate were tested by layer-by-layer assembly to generate thicker tissues: place one layer of thin fibers, seed cells, and repeat the process (Fig. 9A). Since cells adhere to adjacent layers similar to in vivo condition and generate matrix elements, no additional glue is essential to hold layers together. Recent report using PCL scaffolds and fibroblasts cultured on them in serum containing medium showed this possibility.173 During the initial period of cell culture, multiple layers could be separated by a tweezer although cells attached to the fibers at different heights (Fig. 9B). After 30-day cell culture, the layers merged into one stable 3D scaffold. The cells and random distribution of matrix elements acted as glue, attaching each layer. Structures remained stable even after removing the aluminum frame and the shape of the 3D scaffold was tailorable. One has to understand the changes in matrix composition and distribution in the scaffold. Nevertheless, the layer-by-layer approach has the advantage of defining heterogeneous environment at the microscale that could facilitate regeneration of complex tissues. For example, vascular structures could be generated by placing a layer of fibers containing unique angiogenic factors. Also, based on the understanding of the spatial architecture for each cell type, scaffolds can be designed to grow thicker tissues with properties similar to the natural tissues.
FIG. 9.
Cell culture in single and multilayer (A) Schematic procedure of cell culture. (B) SEM morphology of single cells on single fibers in a single thin layer. (C) Fluorescent micrographs of cells on fibers in three layers of fibers after 7-day cell culture. The samples were stained with Alexa phalloidin for cytoskeletal actin (red) and counterstained with DAPI for nuclei (blue) of cells. Scale bar corresponds to 50 μm. Color images available online at www.liebertonline.com/teb.
Performing cell cultures in 3D
Understanding cell–matrix interactions in cellular activity is critical to develop scaffolds with unique features necessary for tissue engineering and many other cellular interaction studies. The introduction of the Petri dish by Julius Richard Petri in 1887 provided an indispensable tool for growing bacteria in flat glass dishes. This simple 2D culture revolutionized the science of growing eukaryotic cells and permitted detailed dissection of interractable physiological systems into manageable units and well-defined studies. However, the 2D scaffold does not represent in vivo circumstance. Many cell types such as fibroblasts and mesenchymal, epithelial, and neural crest cells attached to 3D matrices show distinct adhesions from 2D culture.165,174 A possible consensus is that the 3D architecture could distribute binding sites differently from 2D architecture.174,175 3D focal adhesions appear distinct from 2D focal adhesions on a rigid 2D matrix and were termed as “3D matrix adhesions” to separate them from 2D counterparts. In addition to proteins present in focal adhesions on 2D matrices, cells may have cytoskeletal adaptor proteins on 3D matrix.174,176 Focal adhesion kinase in 3D matrix adhesion is poorly phosphorylated at its major tyrosine phosphorylation site for cell adhesion. Such differences in cell adhesion between 2D versus 3D causes different signal transduction and subsequent alteration cell morphology and rearrangement. However, cell responses to 3D scaffold have been limited due to technological barriers of forming 3D structures similar to ECM.177,178 Further, currently available 3D cultures are not adapted widely due to significant difficulties in 3D cultures relative to the 2D culture. Some of the difficulties include (i) attaining reliable and reproducible uniform cell distribution, (ii) routine monitoring under light microscopy nondestructively, and (iii) reducing variability in the outcome from laboratory to laboratory.
For adaptation in various laboratories, the developed technology should address the existing limitations of 3D cultures. Formed thin layers using the void collector plate facilitates easy monitoring under light microscopy while providing 3D environment (Fig. 9A). Thin layers can be inserted into tissue culture plastic-ware to study cell–matrix interaction, or cell–cell interaction in 3D space. Interactions can be routinely monitored in an inverted light microscope as the large pore structure of fibers allow the light sources of microscope to penetrate through the sample and to observe the cell morphology on the sample. This also eliminates the frequently encountered problems in 3D cultures such as uniform cell distribution and nutrient limitation due to increased thickness. Further, this is a modification to the existing electrospraying technology already utilized in polymer processing. There is a significant knowledge base in industrial-scale manufacturing using electrospraying technology. This helps in large-scale preparations from simple inexpensive polymers. Fibers of different sizes can be tested to mimic various in vivo microenvironments by controlling the process and solution parameters established in conventional electrospinning technology. Unlike traditional tissue culture plastic surface, where cells are restricted to spread in two-dimension, thin layers offer 3D environment. This could change cell shapes based on the distribution of cell adhesive domains on the fibers. Understanding these concepts is significant for the design of next generation of porous scaffolds.
Future Outlook
Given the versatility of existing electrospinning technology for fabricating biomimic micro- and nanofibers made of various biomaterials, it has significant potential for regeneration of different tissues. The advances in the design of the collector plate allows for the formation of thin layers of electrosprayed fibers with large pore size. Thin layer of fibers made of various bioamterials can be used to grow thicker tissues and study cellular interactions in 3D environment. This will provide an opportunity to study cellular activity such as cell attachment, cell duplication, and assembly of ECM in 3D. Further, challenging with signaling molecules could help in deciphering various disease mechanisms and developing surrogate models for diseases. Development of biologically inspired nano/microscale scaffolds mimicking the in vivo environment serve as permissive substrates for cell growth, differentiation, and biological function is key. These materials will be useful not only in furthering our understanding of cell biology, but also for advancing biotechnology, tissue engineering, regenerative biology, and medicine. Successful differentiation of stem cells from various sources is expected to significantly contribute to the development of functionally replaceable tissues.
Appendix
Table of Parameters of Electrospinning to Control Fiber Sizes
| Materials | Molecular weight (g/mol) | Concentration (w/v%) | Solvent | Flow rate (ml/h) | Syringe vol. (ml)/needle gauge | Distance (cm) | Strength of voltage (kV) | Diameter (nm) | Others |
|---|---|---|---|---|---|---|---|---|---|
| PCL179 | – | 20 | DCM/DMF (8:2) | 0.2–0.3 | –/23 | 7.6–14 | – | 250 | – |
| PCL180 | 80,000 | 8–12 | TFE | 1.5 | –/– | 15 | 12 | 100–500 | Calcium phosphate coating |
| PCL181 | 80,000 | – | Acetone DMF/CF | – | – | 5–50 | 5–20 | 250–2500 | – |
| PCL/C182 | – | – | HFP | 3 | –/18.5 | 10 | 20 | – | – |
| PCL-based SMPU183 | 180,000 | 3–12 | DMF | – | 5/– | 15 | 12–25 | 50–700 | – |
| PCL184 | 69,000 | 7–11 | DMF | 1.84 | 5/– | 15 | 615–4000 | Conical nozzle | |
| PCL185 | – | 3–10 | HFP | 3 | 5/18 | 10 | 20 | 582–637 | – |
| PCL186 | 60,000 | 16 | CF/MeOH (8:2) | 34 | –/– | 5 | 7 | 2026 | – |
| PCL187 | – | 8 | DMF/MeOH (7:3) | 2.5 | 10/– | 15 | 1400–11,900 | Three electrodes | |
| PCL188 | 40,000 | 0–15 | CTAB | 5 | 10/– | 30 | 20 | 180–220 | – |
| PCL189 | 80,000 | 0.14 | DMF/THF | – | –/– | 20 | 15 | 438–519 | – |
| PCL190 | 40,000 | 9 | Acetone | – | –/– | – | – | 691 | – |
| PCL191 | 65,000 | 12 | Acetone | 24 | –/– | 30 | 24 | – | Carbon dioxide |
| PCL192 | – | 15 | – | 5 | –/– | 20 | 20 | – | Ion intensity |
| PCL193 | 80,000 | 8 | CF/MeOH (7:3) | 0.5 | –/– | 10 | 0.8 (kV/cm) | 550–810 | Heparin |
| PCL/PLGA194 | – | – | – | 1–16 | –/– | – | – | 1500–2750 | – |
| PCL-PDMAEMA184 | 69,000 | 10.5–15 | DCM | 1.84 | 5/– | 15 | 15 | 390–850 | Conical nozzle |
| PCL/CL25A195 | 80,000 | – | DCM | 0.38 | 10/18 | 7 | 7.5–20 | 250–600 | – |
| PLCL/Gelatin196 | – | 2–3 | HFIP | 1–2 | –/22 | 15 | 15–18 | 200–1400 | – |
| PCL/PU197 | 2000 | 8 (w/w) | DMF,THF (7/3; w/w) | 0.3 | –/– | 15 | 14 | 350–560 | DDS |
| PCL/Gelatin198 | 10,000–20,000 | 1–10 | HFP | – | –/– | 15 | – | 640–880 | – |
| PCL-β-TCP199 | 80,000 | 12 | DCM | 0.6 | –/– | 7.5 | 5 | 200–2000 | – |
| PCL/Collagen200 | – | 5 | HFP | 3 | 5/18.5 | – | 20 | 275–334 | Different rotating rate |
| PCL/PEG201 | 80,000 | 10 | THF/DMF | 2.5 | 20/18 | 15 | 13 | – | Dual polymer |
| PCL/Collagen202 | 67,000 | 9 | HFP | 0.5 | –/20 | 20 | 20 | 541 ± 164 | Dual collector |
| PCL203PCLEEEP | 65,000 70,760 |
12 21.5 |
CF/MEOH(4:1) Acetone |
1.0 0.3 |
–/27 | – | 10 | 246–790 | – |
| PCL/PEO204 | 65,000 | 10–15 | CF | 1.2 | 5/22 | 12 | 15 | 1020 | – |
| PCL/Gelatin205 | 80,000 | 10 | TFE | 0.7 | 10/– | 21.5 | 10.5 | 300–600 | – |
PCL, polycaprolactone; PLGA, poly(lactic-co-glycolic acid); PDMAEMA, poly(2-dimethylamino)ethyl methacrylate; CL25A, Cloisite 25A, organically modified montmorilonite; PLCL, poly(l-lactide-co-caprolactone); PU, polyurethane; PCL-β-TCP, β-tricalcium phosphate conjugated PCL; PEO, polyethylene glycol; DCM, dichloromethane; DMF, dimethlyformamide; HFP, hexafluoropropylene; TFE, tetrafluoroethylene; MeOH, methanol; CF, chlroform; DDS, drug delivery system.
Acknowledgment
Financial support was provided by the National Institutes of Health (1R21DK074858-01A2).
Disclosure Statement
No competing financial interests exist.
References
- 1.Langer R. Vacanti J. Tissue engineering. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Persidis A. Tissue engineering. Nat Biotechnol. 1999;17:508–510. doi: 10.1038/8700. [DOI] [PubMed] [Google Scholar]
- 3.Vyavahare N. Ogle M. Schoen F.J. Zand R. Gloeckner D.C. Sacks M., et al. Mechanisms of bioprosthetic heart valve failure: fatigue causes collagen denaturation and glycosaminoglycan loss. J Biomed Mater Res. 1999;46:44. doi: 10.1002/(sici)1097-4636(199907)46:1<44::aid-jbm5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 4.Kim B.-S. Mooney D.J. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998;16:224. doi: 10.1016/s0167-7799(98)01191-3. [DOI] [PubMed] [Google Scholar]
- 5.Ushiki T. Collagen fibers, reticular fibers and elastic fibers. a comprehensive understanding from a morphological viewpoint. Arch Histol Cytol. 2002;65:109. doi: 10.1679/aohc.65.109. [DOI] [PubMed] [Google Scholar]
- 6.Thomson R.C. Wake M.C. Yaszemski M.J. Mikos A.G. Biodegradable polymer scaffolds to regenerate organs. Adv Polym Sci. 1995;122:245. doi: 10.1163/156856295x00805. [DOI] [PubMed] [Google Scholar]
- 7.Hollister S.J. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 8.Choi N.W. Cabodi M. Held B. Gleghorn J.P. Bonassar L.J. Stroock A.D. Microfluidic scaffolds for tissue engineering. Nat Mater. 2007;6:908. doi: 10.1038/nmat2022. [DOI] [PubMed] [Google Scholar]
- 9.Hubbell J.A. Biomaterials in tissue engineering. Nat Biotechnol. 1995;13:565. doi: 10.1038/nbt0695-565. [DOI] [PubMed] [Google Scholar]
- 10.Pamula E. Dobrzynski P. Bero M. Paluszkiewicz C. Hydrolytic degradation of porous scaffolds for tissue engineering from terpolymer of l-lactide, [epsilon]-caprolactone and glycolide. J Mol Struct. 2005;744–747:557. [Google Scholar]
- 11.Ozkan S. Kalyon D.M. Yu X. McKelvey C.A. Lowinger M. Multifunctional protein-encapsulated polycaprolactone scaffolds: fabrication and in vitro assessment for tissue engineering. Biomaterials. 2009;30:4336. doi: 10.1016/j.biomaterials.2009.04.050. [DOI] [PubMed] [Google Scholar]
- 12.Liu X. Won Y. Ma P.X. Porogen-induced surface modification of nano-fibrous poly(l-lactic acid) scaffolds for tissue engineering. Biomaterials. 2006;27:3980. doi: 10.1016/j.biomaterials.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Sun W. Lal P. Recent development on computer aided tissue engineering—a review. Comput Methods Programs Biomed. 2002;67:85. doi: 10.1016/s0169-2607(01)00116-x. [DOI] [PubMed] [Google Scholar]
- 14.Vasita R. Katti D.S. Nanofibers and their applications in tissue engineering. Int J Nanomed. 2006;1:15. doi: 10.2147/nano.2006.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X. Smith L.A. Hu J. Ma P.X. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials. 2009;30:2252. doi: 10.1016/j.biomaterials.2008.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiyun Zhang P.X.M. Synthetic nano-fibrillar extracellular matrices with predesigned macroporous architectures. J Biomed Mater Res. 2000;52:430. doi: 10.1002/1097-4636(200011)52:2<430::aid-jbm25>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S. Zhao X. Design of molecular biological materials using peptide motifs. J Mater Chem. 2004;14:2082. [Google Scholar]
- 18.Li D. Xia Y. Electrospinning of nanofibers: reinventing the wheel? Adv Mater. 2004;16:1151. [Google Scholar]
- 19.Shin H. Jo S. Mikos A.G. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 20.Blaker J.J. Nazhat S.N. Boccaccini A.R. Development and characterisation of silver-doped bioactive glass-coated sutures for tissue engineering and wound healing applications. Biomaterials. 2004;25:1319. doi: 10.1016/j.biomaterials.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Zhao P. Jiang H. Pan H. Zhu K. Chen W. Biodegradable fibrous scaffolds composed of gelatin coated poly(ε-caprolactone) prepared by coaxial electrospinning. J Biomed Mater Res A. 2007;83A:372. doi: 10.1002/jbm.a.31242. [DOI] [PubMed] [Google Scholar]
- 22.Kumbar S.G. James R. Nukavarapu S.P. Laurencin C.T. Electrospun nanofiber scaffolds: engineering soft tissues. Biomed Mater. 2008;3:034002. doi: 10.1088/1748-6041/3/3/034002. [DOI] [PubMed] [Google Scholar]
- 23.Anthony L. Andrady D.S.E. Electrospinning in a controlled gaseous environment description/claims. US Patent. 2008:0063741.
- 24.Sill T.J. von Recum H.A. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29:1989. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Ramakrishna S. Fujihara K. Teo W.-E. Yong T. Ma Z. Ramaseshan R. Electrospun nanofibers: solving global issues. Mater Today. 2006;9:40. [Google Scholar]
- 26.Bhardwaj N. Kundu S.C. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv. 2010;28:325. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Zeleny J. The electrical discharge from liquid points, and a hydrostatic method of measuring the electric intensity at their surfaces. Phys Rev. 1914;3:69. [Google Scholar]
- 28.Morton W.J. Method of dispersing fluids. US Patent 705691. 1902
- 29.Formhals A. Process and apparatus for preparing artificial threads. US Patent 504. 1934
- 30.Formhals A. Method and apparatus for spinning. US Patent. 1939
- 31.Taylor G. Electrically driven jets. Proc R Soc of Lond A Math Phys Sci. 1969;313:453. [Google Scholar]
- 32.Baumgarten P.K. Electrostatic spinning of acrylic microfibers. J Colloid Interface Sci. 1971;36:71. [Google Scholar]
- 33.Larrondo L. Manley R.S.J. Electrostatic fiber spinning from polymer melts. I. Experimental observations on fiber formation and properties. J Polym Sci Polym Phys Ed. 1981;19:909. [Google Scholar]
- 34.Larrondo L. Manley R.S.J. Electrostatic fiber spinning from polymer melts. II. Examination of the flow field in an electrically driven jet. J Polym Sci Polym Phys Ed. 1981;19:921. [Google Scholar]
- 35.Huang Z.-M. Zhang Y.Z. Kotaki M. Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites Sci Technol. 2003;63:2223. [Google Scholar]
- 36.Bhardwaj N. Kundu S.C. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv. 2010;28:325. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Chronakis I.S. Novel nanocomposites and nanoceramics based on polymer nanofibers using electrospinning process—a review. J Mater Process Technol. 2005;167:283. [Google Scholar]
- 38.Hutmacher D.W. Schantz T. Zein I. Ng K.W. Teoh S.H. Tan K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J Biomed Mater Res. 2001;55:203. doi: 10.1002/1097-4636(200105)55:2<203::aid-jbm1007>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 39.Lowry K.J. Hamson K.R. Bear L. Peng Y.B. Calaluce R. Evans M.L., et al. Polycaprolactone/glass bioabsorbable implant in a rabbit humerus fracture model. J Biomed Mater Res. 1997;36:536. doi: 10.1002/(sici)1097-4636(19970915)36:4<536::aid-jbm12>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 40.Lavik E. Teng Y.D. Snyder E. Langer R. Seeding neural stem cells on scaffolds of PGA, PLA, and their copolymers. Methods Mol Biol. 2002;198:89. doi: 10.1385/1-59259-186-8:89. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura T. Hitomi S. Watanabe S. Shimizu Y. Jamshidi K. Hyon S.H., et al. Bioabsorption of polylactides with different molecular properties. J Biomed Mater Res. 1989;23:1115. doi: 10.1002/jbm.820231003. [DOI] [PubMed] [Google Scholar]
- 42.Mooney D.J. Mazzoni C.L. Breuer C. McNamara K. Hern D. Vacanti J.P., et al. Stabilized polyglycolic acid fibre-based tubes for tissue engineering. Biomaterials. 1996;17:115. doi: 10.1016/0142-9612(96)85756-5. [DOI] [PubMed] [Google Scholar]
- 43.Gao J. Niklason L. Langer R. Surface hydrolysis of poly(glycolic acid) meshes increases the seeding density of vascular smooth muscle cells. J Biomed Mater Res. 1998;42:417. doi: 10.1002/(sici)1097-4636(19981205)42:3<417::aid-jbm11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 44.Marra K.G. Szem J.W. Kumta P.N. DiMilla P.A. Weiss L.E. In vitro analysis of biodegradable polymer blend/hydroxyapatite composites for bone tissue engineering. J Biomed Mater Res. 1999;47:324. doi: 10.1002/(sici)1097-4636(19991205)47:3<324::aid-jbm6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 45.Engelberg I. Kohn J. Physico-mechanical properties of degradable polymers used in medical applications: a comparative study. Biomaterials. 1991;12:292. doi: 10.1016/0142-9612(91)90037-b. [DOI] [PubMed] [Google Scholar]
- 46.Ozawa T. Mickle D.A. Weisel R.D. Koyama N. Wong H. Ozawa S., et al. Histologic changes of nonbiodegradable and biodegradable biomaterials used to repair right ventricular heart defects in rats. J Thorac Cardiovasc Surg. 2002;124:1157. doi: 10.1067/mtc.2002.127449. [DOI] [PubMed] [Google Scholar]
- 47.Heath D.E. Cooper S.L. Interaction of endothelial cells with methacrylic terpolymer biomaterials. J Biomed Mater Res B Appl Biomater. 2010;92:289. doi: 10.1002/jbm.b.31514. [DOI] [PubMed] [Google Scholar]
- 48.Mao J.S. Liu H.F. Yin Y.J. Yao K.D. The properties of chitosan-gelatin membranes and scaffolds modified with hyaluronic acid by different methods. Biomaterials. 2003;24:1621. doi: 10.1016/s0142-9612(02)00549-5. [DOI] [PubMed] [Google Scholar]
- 49.Dar A. Shachar M. Leor J. Cohen S. Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol Bioeng. 2002;80:305. doi: 10.1002/bit.10372. [DOI] [PubMed] [Google Scholar]
- 50.Madihally S.V. Matthew H.W. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20:1133. doi: 10.1016/s0142-9612(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 51.Rothenburger M. Vischer P. Volker W. Glasmacher B. Berendes E. Scheld H.H., et al. In vitro modelling of tissue using isolated vascular cells on a synthetic collagen matrix as a substitute for heart valves. Thorac Cardiovasc Surg. 2001;49:204. doi: 10.1055/s-2001-16108. [DOI] [PubMed] [Google Scholar]
- 52.Yannas I.V. Models of organ regeneration processes induced by templates. Ann NY Acad Sci. 1997;831:280. doi: 10.1111/j.1749-6632.1997.tb52203.x. [DOI] [PubMed] [Google Scholar]
- 53.Zaleskas J.M. Kinner B. Freyman T.M. Yannas I.V. Gibson L.J. Spector M. Growth factor regulation of smooth muscle actin expression and contraction of human articular chondrocytes and meniscal cells in a collagen-GAG matrix. Exp Cell Res. 2001;270:21. doi: 10.1006/excr.2001.5325. [DOI] [PubMed] [Google Scholar]
- 54.Spilker M.H. Asano K. Yannas I.V. Spector M. Contraction of collagen-glycosaminoglycan matrices by peripheral nerve cells in vitro. Biomaterials. 2001;22:1085. doi: 10.1016/s0142-9612(00)00345-8. [DOI] [PubMed] [Google Scholar]
- 55.Kessler P.D. Byrne B.J. Myoblast cell grafting into heart muscle: cellular biology and potential applications. Annu Rev Physiol. 1999;61:219. doi: 10.1146/annurev.physiol.61.1.219. [DOI] [PubMed] [Google Scholar]
- 56.Taylor P.M. Allen S.P. Dreger S.A. Yacoub M.H. Human cardiac valve interstitial cells in collagen sponge: a biological three-dimensional matrix for tissue engineering. J Heart Valve Dis. 2002;11:298. discussion 306–307. [PubMed] [Google Scholar]
- 57.Pieper J.S. Hafmans T. van Wachem P.B. van Luyn M.J. Brouwer L.A. Veerkamp J.H., et al. Loading of collagen-heparan sulfate matrices with bFGF promotes angiogenesis and tissue generation in rats. J Biomed Mater Res. 2002;62:185. doi: 10.1002/jbm.10267. [DOI] [PubMed] [Google Scholar]
- 58.Bensaid W. Triffitt J.T. Blanchat C. Oudina K. Sedel L., et al. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24:2497. doi: 10.1016/s0142-9612(02)00618-x. [DOI] [PubMed] [Google Scholar]
- 59.McClure M.J. Sell S.A. Ayres C.E. Simpson D.G. Bowlin G.L. Electrospinning-aligned and random polydioxanone-polycaprolactone-silk fibroin-blended scaffolds: geometry for a vascular matrix. Biomed Mater. 2009;4:055010. doi: 10.1088/1748-6041/4/5/055010. [DOI] [PubMed] [Google Scholar]
- 60.Lee K.Y. Jeong L. Kang Y.O. Lee S.J. Park W.H. Electrospinning of polysaccharides for regenerative medicine. Adv Drug Deliv Rev. 2009;61:1020. doi: 10.1016/j.addr.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 61.Jang J.H. Castano O. Kim H.W. Electrospun materials as potential platforms for bone tissue engineering. Adv Drug Deliv Rev. 2009;61:1065. doi: 10.1016/j.addr.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 62.Kulkarni R.K. Moore E.G. Hegyeli A.F. Leonard F. Biodegradable poly(lactic acid) polymers. J Biomed Mater Res. 1971;5:169. doi: 10.1002/jbm.820050305. [DOI] [PubMed] [Google Scholar]
- 63.Saito N. Okada T. Toba S. Miyamoto S. Takaoka K. New synthetic absorbable polymers as BMP carriers: plastic properties of poly-D,L-lactic acid-polyethylene glycol block copolymers. J Biomed Mater Res. 1999;47:104. doi: 10.1002/(sici)1097-4636(199910)47:1<104::aid-jbm15>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 64.Park I.K. Yang J. Jeong H.J. Bom H.S. Harada I. Akaike T., et al. Galactosylated chitosan as a synthetic extracellular matrix for hepatocytes attachment. Biomaterials. 2003;24:2331. doi: 10.1016/s0142-9612(03)00108-x. [DOI] [PubMed] [Google Scholar]
- 65.Ho K.L. Witte M.N. Bird E.T. 8-ply small intestinal submucosa tension-free sling: spectrum of postoperative inflammation. J Urol. 2004;171:268. doi: 10.1097/01.ju.0000098680.60020.32. [DOI] [PubMed] [Google Scholar]
- 66.Pistner H. Bendix D.R. Muhling J. Reuther J.F. Poly(L-lactide): a long-term degradation study in vivo. Part III. Analytical characterization. Biomaterials. 1993;14:291. doi: 10.1016/0142-9612(93)90121-h. [DOI] [PubMed] [Google Scholar]
- 67.Kranz H. Ubrich N. Maincent P. Bodmeier R. Physicomechanical properties of biodegradable poly(D,L-lactide) and poly(D,L-lactide-co-glycolide) films in the dry and wet states. J Pharm Sci. 2000;89:1558. doi: 10.1002/1520-6017(200012)89:12<1558::aid-jps6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 68.Yoon J.J. Park T.G. Degradation behaviors of biodegradable macroporous scaffolds prepared by gas foaming of effervescent salts. J Biomed Mater Res. 2001;55:401. doi: 10.1002/1097-4636(20010605)55:3<401::aid-jbm1029>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 69.Lu L. Peter S.J. Lyman M.D. Lai H.L. Leite S.M. Tamada J.A., et al. In vitro and in vivo degradation of porous poly(DL-lactic-co-glycolic acid) foams. Biomaterials. 2000;21:1837. doi: 10.1016/s0142-9612(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 70.Massia S.P. Hubbell J.A. Covalently attached GRGD on polymer surfaces promotes biospecific adhesion of mammalian cells. Ann NY Acad Sci. 1990;589:261. doi: 10.1111/j.1749-6632.1990.tb24251.x. [DOI] [PubMed] [Google Scholar]
- 71.Park A. Wu B. Griffith L.G. Integration of surface modification and 3D fabrication techniques to prepare patterned poly(L-lactide) substrates allowing regionally selective cell adhesion. J Biomater Sci Polym Ed. 1998;9:89. doi: 10.1163/156856298x00451. [DOI] [PubMed] [Google Scholar]
- 72.Eid K. Chen E. Griffith L. Glowacki J. Effect of RGD coating on osteocompatibility of PLGA-polymer disks in a rat tibial wound. J Biomed Mater Res. 2001;57:224. doi: 10.1002/1097-4636(200111)57:2<224::aid-jbm1162>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 73.Fussell G.W. Cooper S.L. Synthesis and characterization of acrylic terpolymers with RGD peptides for biomedical applications. Biomaterials. 2004;25:2971. doi: 10.1016/j.biomaterials.2003.09.062. [DOI] [PubMed] [Google Scholar]
- 74.Lindahl U. Kusche-Gullberg M. Kjellen L. Regulated diversity of heparan sulfate. J Biol Chem. 1998;273:24979. doi: 10.1074/jbc.273.39.24979. [DOI] [PubMed] [Google Scholar]
- 75.Babu M. Diegelmann R. Oliver N. Fibronectin is overproduced by keloid fibroblasts during abnormal wound healing. Mol Cell Biol. 1989;9:1642. doi: 10.1128/mcb.9.4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu H. Ko Y.-G. Kawazoe N. Chen G. Cartilage tissue engineering using funnel-like collagen sponges prepared with embossing ice particulate templates. Biomaterials. 2010;31:5825. doi: 10.1016/j.biomaterials.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 77.Zhang X. Thomas V. Xu Y. Bellis S.L. Vohra Y.K. An in vitro regenerated functional human endothelium on a nanofibrous electrospun scaffold. Biomaterials. 2010;31:4376. doi: 10.1016/j.biomaterials.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 78.Ghasemi-Mobarakeh L. Prabhakaran M.P. Morshed M. Nasr-Esfahani M.-H. Ramakrishna S. Electrospun poly([var epsilon]-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials. 2008;29:4532. doi: 10.1016/j.biomaterials.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y.Z. Feng Y. Huang Z.M. Ramakrishna S. Lim C.T. Fabrication of porous electrospun nanofibres. Nanotechnology. 2006;17:901. [Google Scholar]
- 80.Linh N.T. Min Y.K. Song H.Y. Lee B.T. Fabrication of polyvinyl alcohol/gelatin nanofiber composites and evaluation of their material properties. J Biomed Mater Res B Appl Biomater. 2010;95:184. doi: 10.1002/jbm.b.31701. [DOI] [PubMed] [Google Scholar]
- 81.Tigli R.S. Kazaroglu N.M. Mav I.S.B. Gumusderel I.O.M. Cellular behavior on epidermal growth factor (EGF)-immobilized PCL/gelatin nanofibrous scaffolds. J Biomater Sci Polym Ed. 2011;22:207–223. doi: 10.1163/092050609X12591500475424. [DOI] [PubMed] [Google Scholar]
- 82.Lee H. Kim G. Biocomposites electrospun with poly(epsilon-caprolactone) and silk fibroin powder for biomedical applications. J Biomater Sci Polym Ed. 2010;21:1687. doi: 10.1163/092050609X12548956645680. [DOI] [PubMed] [Google Scholar]
- 83.Choi J.S. Lee S.J. Christ G.J. Atala A. Yoo J.J. The influence of electrospun aligned poly(epsilon-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials. 2008;29:2899. doi: 10.1016/j.biomaterials.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 84.Sheikh F.A. Barakat N.A. Kanjwal M.A. Nirmala R. Lee J.H. Kim H., et al. Electrospun titanium dioxide nanofibers containing hydroxyapatite and silver nanoparticles as future implant materials. J Mater Sci Mater Med. 2010;21:2551. doi: 10.1007/s10856-010-4102-9. [DOI] [PubMed] [Google Scholar]
- 85.Stankus J.J. Freytes D.O. Badylak S.F. Wagner W.R. Hybrid nanofibrous scaffolds from electrospinning of a synthetic biodegradable elastomer and urinary bladder matrix. J Biomater Sci Polym Ed. 2008;19:635. doi: 10.1163/156856208784089599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gupta D. Venugopal J. Mitra S. Giri Dev V.R. Ramakrishna S. Nanostructured biocomposite substrates by electrospinning and electrospraying for the mineralization of osteoblasts. Biomaterials. 2009;30:2085. doi: 10.1016/j.biomaterials.2008.12.079. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Y. Venugopal J.R. El-Turki A. Ramakrishna S. Su B. Lim C.T. Electrospun biomimetic nanocomposite nanofibers of hydroxyapatite/chitosan for bone tissue engineering. Biomaterials. 2008;29:4314. doi: 10.1016/j.biomaterials.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 88.Nisbet D.R. Forsythe J.S. Shen W. Finkelstein D.I. Horne M.K. Review paper: a review of the cellular response on electrospun nanofibers for tissue engineering. J Biomater Appl. 2009;24:7. doi: 10.1177/0885328208099086. [DOI] [PubMed] [Google Scholar]
- 89.Lowery J.L. Datta N. Rutledge G.C. Effect of fiber diameter, pore size and seeding method on growth of human dermal fibroblasts in electrospun poly([var epsilon]-caprolactone) fibrous mats. Biomaterials. 2010;31:491. doi: 10.1016/j.biomaterials.2009.09.072. [DOI] [PubMed] [Google Scholar]
- 90.Nerurkar N.L. Baker B.M. Sen S. Wible E.E. Elliott D.M. Mauck R.L. Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus. Nat Mater. 2009;8:986. doi: 10.1038/nmat2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krogman K.C. Lowery J.L. Zacharia N.S. Rutledge G.C. Hammond P.T. Spraying asymmetry into functional membranes layer-by-layer. Nat Mater. 2009;8:512. doi: 10.1038/nmat2430. [DOI] [PubMed] [Google Scholar]
- 92.Xia Y. Nanomaterials at work in biomedical research. Nat Mater. 2008;7:758. doi: 10.1038/nmat2277. [DOI] [PubMed] [Google Scholar]
- 93.Li X. Xie J. Lipner J. Yuan X. Thomopoulos S. Xia Y. Nanofiber scaffolds with gradations in mineral content for mimicking the tendon-to-bone insertion site. Nano Lett. 2009;9:2763. doi: 10.1021/nl901582f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nisbet D.R. Forsythe J.S. Shen W. Finkelstein D.I. Horne M.K. Review paper: a review of the cellular response on electrospun nanofibers for tissue engineering. J Biomater Appl. 2009;24:7. doi: 10.1177/0885328208099086. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y. Huang Z.-M. Xu X. Lim C.T. Ramakrishna S. Preparation of core–shell structured pcl-r-gelatin bi-component nanofibers by coaxial electrospinning. Chem Mater. 2004;16:3406. [Google Scholar]
- 96.Kidoaki S. Kwon I.K. Matsuda T. Mesoscopic spatial designs of nano- and microfiber meshes for tissue-engineering matrix and scaffold based on newly devised multilayering and mixing electrospinning techniques. Biomaterials. 2005;26:37. doi: 10.1016/j.biomaterials.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 97.Jia X. Zhao C. Li P. Zhang H. Huang Y. Li H., et al. Sustained release of VEGF by coaxial electrospun dextran/PLGA fibrous membranes in vascular tissue engineering. J Biomater Sci Polym Ed. 2010 doi: 10.1163/092050610X528534. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 98.Lindner V. Olson N.E. Clowes A.W. Reidy M.A. Inhibition of smooth muscle cell proliferation in injured rat arteries. Interaction of heparin with basic fibroblast growth factor. J Clin Invest. 1992;90:2044. doi: 10.1172/JCI116085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nugent M.A. Nugent H.M. Iozzo R.V. Sanchack K. Edelman E.R. Perlecan is required to inhibit thrombosis after deep vascular injury and contributes to endothelial cell-mediated inhibition of intimal hyperplasia. Proc Natl Acad Sci USA. 2000;97:6722. doi: 10.1073/pnas.97.12.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yamamoto M. Yamamoto K. Noumura T. Type I collagen promotes modulation of cultured rabbit arterial smooth muscle cells from a contractile to a synthetic phenotype. Exp Cell Res. 1993;204:121. doi: 10.1006/excr.1993.1016. [DOI] [PubMed] [Google Scholar]
- 101.Madihally S.V. Flake A.W. Matthew H.W. Maintenance of CD34 expression during proliferation of CD34+ cord blood cells on glycosaminoglycan surfaces. Stem Cells. 1999;17:295. doi: 10.1002/stem.170295. [DOI] [PubMed] [Google Scholar]
- 102.Denuziere A. Ferrier D. Domard A. Interactions between chitosan and glycosaminoglycans (chondroitin sulfate and hyaluronic acid): physicochemical and biological studies. Ann Pharm Fr. 2000;58:47. [PubMed] [Google Scholar]
- 103.Madihally S.V. A bioreactor system for non-differentiative expansion of CD34+ Cord Blood Cells [PhD Dissertation] Wayne State University; Detroit, MI: 1998. [Google Scholar]
- 104.Chupa J.M. Foster A.M. Sumner S.R. Madihally S.V. Matthew H.W. Vascular cell responses to polysaccharide materials: in vitro and in vivo evaluations. Biomaterials. 2000;21:2315. doi: 10.1016/s0142-9612(00)00158-7. [DOI] [PubMed] [Google Scholar]
- 105.Frenot A. Chronakis I.S. Polymer nanofibers assembled by electrospinning. Curr Opin Colloid Interface Sci. 2003;8:64. [Google Scholar]
- 106.Xu S. Zhang J. He A. Li J. Zhang H. Han C.C. Electrospinning of native cellulose from nonvolatile solvent system. Polymer. 2008;49:2911. [Google Scholar]
- 107.Wang H. Tang H. He J. Wang Q. Fabrication of aligned ferrite nanofibers by magnetic-field-assisted electrospinning coupled with oxygen plasma treatment. Mater Res Bull. 2009;44:1676. [Google Scholar]
- 108.Leong M.F. Chan W.Y. Chian K.S. Rasheed M.Z. Anderson J.M. Fabrication and in vitro and in vivo cell infiltration study of a bilayered cryogenic electrospun poly(D,L-lactide) scaffold. J Biomed Mater Res A. 2010;94:1141. doi: 10.1002/jbm.a.32795. [DOI] [PubMed] [Google Scholar]
- 109.Hong J.K. Madihally S.V. Three-dimensional scaffold of electrosprayed fibers with large pore size for tissue regeneration. Acta Biomater. 2010;6:4734. doi: 10.1016/j.actbio.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li D. Wang Y. Xia Y. Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Lett. 2003;3:1167. [Google Scholar]
- 111.Li D. Ouyang G. McCann J.T. Xia Y. Collecting electrospun nanofibers with patterned electrodes. Nano Lett. 2005;5:913. doi: 10.1021/nl0504235. [DOI] [PubMed] [Google Scholar]
- 112.Tuzlakoglu K. Santos M.I. Neves N.M. Reis R.L. Design of nano- and micro-fiber combined scaffolds by electrospinning of collagen onto starch-based fiber meshes: a man-made equivalent of natural extracellular matrix. Tissue Eng Part A 2010. 2010;17:463–73. doi: 10.1089/ten.TEA.2010.0178. [DOI] [PubMed] [Google Scholar]
- 113.Kim H.M. Chae W.P. Chang K.W. Chun S. Kim S. Jeong Y., et al. Composite nanofiber mats consisting of hydroxyapatite and titania for biomedical applications. J Biomed Mater Res B Appl Biomater. 2010;94:380. doi: 10.1002/jbm.b.31664. [DOI] [PubMed] [Google Scholar]
- 114.McCullen S.D. Miller P.R. Gittard S.D. Gorga R.E. Pourdeyhimi B. Narayan R.J., et al. In situ collagen polymerization of layered cell-seeded electrospun scaffolds for bone tissue engineering applications. Tissue Eng Part C Methods. 2010;16:1095. doi: 10.1089/ten.tec.2009.0753. [DOI] [PubMed] [Google Scholar]
- 115.Velazquez O.C. Snyder R. Liu Z.J. Fairman R.M. Herlyn M. Fibroblast-dependent differentiation of human microvascular endothelial cells into capillary-like 3-dimensional networks. FASEB J. 2002;16:1316. doi: 10.1096/fj.01-1011fje. [DOI] [PubMed] [Google Scholar]
- 116.Satake S. Kuzuya M. Ramos M.A. Kanda S. Iguchi A. Angiogenic stimuli are essential for survival of vascular endothelial cells in three-dimensional collagen lattice. Biochem Biophys Res Commun. 1998;244:642. doi: 10.1006/bbrc.1998.8313. [DOI] [PubMed] [Google Scholar]
- 117.Tille J.C. Pepper M.S. Mesenchymal cells potentiate vascular endothelial growth factor-induced angiogenesis in vitro. Exp Cell Res. 2002;280:179. doi: 10.1006/excr.2002.5635. [DOI] [PubMed] [Google Scholar]
- 118.Ruoslahti E. Engvall E. Integrins and vascular extracellular matrix assembly. J Clin Invest. 1997;99:1149. doi: 10.1172/JCI119269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kanda S. Tomasini-Johansson B. Klint P. Dixelius J. Rubin K. Claesson-Welsh L. Signaling via fibroblast growth factor receptor-1 is dependent on extracellular matrix in capillary endothelial cell differentiation. Exp Cell Res. 1999;248:203. doi: 10.1006/excr.1999.4400. [DOI] [PubMed] [Google Scholar]
- 120.Eliceiri B.P. Cheresh D.A. The role of alphav integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Investig. 1999;103:1227. doi: 10.1172/JCI6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Urbich C. Dernbach E. Reissner A. Vasa M. Zeiher A.M. Dimmeler S. Shear stress-induced endothelial cell migration involves integrin signaling via the fibronectin receptor subunits {alpha}5 and {beta}1. Arterioscler Thromb Vasc Biol. 2002;22:69. doi: 10.1161/hq0102.101518. [DOI] [PubMed] [Google Scholar]
- 122.Jalali S. del Pozo M.A. Chen K.-D. Miao H. Li Y.-S. Schwartz M.A., et al. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. PNAS. 2001;98:1042. doi: 10.1073/pnas.031562998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhu C. Bao G. Wang N. Cell mechanics: mechanical response, cell adhesion, and molecular deformation. Annu Rev Biomed Eng. 2000;2:189. doi: 10.1146/annurev.bioeng.2.1.189. [DOI] [PubMed] [Google Scholar]
- 124.Sieminski A.L. Gooch K.J. Biomaterial-microvasculature interactions. Biomaterials. 2000;21:2232. doi: 10.1016/s0142-9612(00)00149-6. [DOI] [PubMed] [Google Scholar]
- 125.Huang Y. Siewe M. Madihally S.V. Effect of spatial architecture on cellular colonization. Biotechnol Bioeng. 2006;93:64. doi: 10.1002/bit.20703. [DOI] [PubMed] [Google Scholar]
- 126.O'Brien F.J. Harley B.A. Yannas I.V. Gibson L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials. 2004;25:1077. doi: 10.1016/s0142-9612(03)00630-6. [DOI] [PubMed] [Google Scholar]
- 127.Yannas I.V. Lee E. Orgill D.P. Skrabut E.M. Murphy G.F. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci USA. 1989;86:933. doi: 10.1073/pnas.86.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zeltinger J. Sherwood J.K. Graham D.A. Mueller R. Griffith L.G. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001;7:557. doi: 10.1089/107632701753213183. [DOI] [PubMed] [Google Scholar]
- 129.Salem A.K. Stevens R. Pearson R.G. Davies M.C. Tendler S.J. Roberts C.J., et al. Interactions of 3T3 fibroblasts and endothelial cells with defined pore features. J Biomed Mater Res. 2002;61:212. doi: 10.1002/jbm.10195. [DOI] [PubMed] [Google Scholar]
- 130.Ng K.W. Khor H.L. Hutmacher D.W. In vitro characterization of natural and synthetic dermal matrices cultured with human dermal fibroblasts. Biomaterials. 2004;25:2807. doi: 10.1016/j.biomaterials.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 131.Wang Y.C. Ho C.C. Micropatterning of proteins and mammalian cells on biomaterials. FASEB J. 2004;18:525. doi: 10.1096/fj.03-0490fje. [DOI] [PubMed] [Google Scholar]
- 132.Corey J.M. Gertz C.C. Wang B.S. Birrell L.K. Johnson S.L. Martin D.C., et al. The design of electrospun PLLA nanofiber scaffolds compatible with serum-free growth of primary motor and sensory neurons. Acta Biomater. 2008;4:863. doi: 10.1016/j.actbio.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pham Q.P. Sharma U. Mikos A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 134.Li W.J. Laurencin C.T. Caterson E.J. Tuan R.S. Ko F.K. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 135.Lee C.H. Shin H.J. Cho I.H. Kang Y.M. Kim I.A. Park K.D., et al. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials. 2005;26:1261. doi: 10.1016/j.biomaterials.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 136.Sachlos E. Czernuszka J.T. Making tissue engineering scaffolds work. review: the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur Cells Mater J. 2003;5:29. doi: 10.22203/ecm.v005a03. [DOI] [PubMed] [Google Scholar]
- 137.Chen M. Patra P.K. Warner S.B. Bhowmick S. Role of fiber diameter in adhesion and proliferation of NIH 3T3 fibroblast on electrospun polycaprolactone scaffolds. Tissue Eng. 2007;13:579. doi: 10.1089/ten.2006.0205. [DOI] [PubMed] [Google Scholar]
- 138.Badami A.S. Kreke M.R. Thompson M.S. Riffle J.S. Goldstein A.S. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials. 2006;27:596. doi: 10.1016/j.biomaterials.2005.05.084. [DOI] [PubMed] [Google Scholar]
- 139.Balguid A. Mol A. van Marion M.H. Bank R.A. Bouten C.V. Baaijens F.P. Tailoring fiber diameter in electrospun poly(epsilon-caprolactone) scaffolds for optimal cellular infiltration in cardiovascular tissue engineering. Tissue Eng Part A. 2009;15:437. doi: 10.1089/ten.tea.2007.0294. [DOI] [PubMed] [Google Scholar]
- 140.He L. Liao S. Quan D. Ma K. Chan C. Ramakrishna S., et al. Synergistic effects of electrospun PLLA fiber dimension and pattern on neonatal mouse cerebellum C17.2 stem cells. Acta Biomater. 2010;6:2960. doi: 10.1016/j.actbio.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 141.Shanmugasundaram S. Arinzeh T. Microscale versus nanoscale scaffold architecture for mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2011;17:831–39. doi: 10.1089/ten.TEA.2010.0409. [DOI] [PubMed] [Google Scholar]
- 142.Christopherson G.T. Song H. Mao H.Q. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials. 2009;30:556. doi: 10.1016/j.biomaterials.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 143.Shang S. Yang F. Cheng X. Walboomers X.F. Jansen J.A. The effect of electrospun fibre alignment on the behaviour of rat periodontal ligament cells. Eur Cell Mater. 2010;19:180. doi: 10.22203/ecm.v019a18. [DOI] [PubMed] [Google Scholar]
- 144.Chew S.Y. Mi R. Hoke A. Leong K.W. The effect of the alignment of electrospun fibrous scaffolds on Schwann cell maturation. Biomaterials. 2008;29:653. doi: 10.1016/j.biomaterials.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhong S. Teo W.E. Zhu X. Beuerman R.W. Ramakrishna S. Yung L.Y. An aligned nanofibrous collagen scaffold by electrospinning and its effects on in vitro fibroblast culture. J Biomed Mater Res A. 2006;79:456. doi: 10.1002/jbm.a.30870. [DOI] [PubMed] [Google Scholar]
- 146.Ma Z. He W. Yong T. Ramakrishna S. Grafting of gelatin on electrospun poly(caprolactone) nanofibers to improve endothelial cell spreading and proliferation and to control cell Orientation. Tissue Eng. 2005;11:1149. doi: 10.1089/ten.2005.11.1149. [DOI] [PubMed] [Google Scholar]
- 147.Courtney T. Sacks M.S. Stankus J. Guan J. Wagner W.R. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials. 2006;27:3631. doi: 10.1016/j.biomaterials.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 148.Ranucci C.S. Kumar A. Batra S.P. Moghe P.V. Control of hepatocyte function on collagen foams: sizing matrix pores toward selective induction of 2-D and 3-D cellular morphogenesis. Biomaterials. 2000;21:783. doi: 10.1016/s0142-9612(99)00238-0. [DOI] [PubMed] [Google Scholar]
- 149.Clarke K.M. Lantz G.C. Salisbury S.K. Badylak S.F. Hiles M.C. Voytik S.L. Intestine submucosa and polypropylene mesh for abdominal wall repair in dogs. J Surg Res. 1996;60:107. doi: 10.1006/jsre.1996.0018. [DOI] [PubMed] [Google Scholar]
- 150.Walboomers X.F. Croes H.J. Ginsel L.A. Jansen J.A. Contact guidance of rat fibroblasts on various implant materials. J Biomed Mater Res. 1999;47:204. doi: 10.1002/(sici)1097-4636(199911)47:2<204::aid-jbm10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 151.Truong Y.B. Glattauer V. Lang G. Hands K. Kyratzis I.L. Werkmeister J.A., et al. A comparison of the effects of fibre alignment of smooth and textured fibres in electrospun membranes on fibroblast cell adhesion. Biomed Mater. 2010;5:25005. doi: 10.1088/1748-6041/5/2/025005. [DOI] [PubMed] [Google Scholar]
- 152.Trelstad R.L. Hayashi K. Tendon collagen fibrillogenesis: intracellular subassemblies and cell surface changes associated with fibril growth. Dev Biol. 1979;71:228. doi: 10.1016/0012-1606(79)90166-0. [DOI] [PubMed] [Google Scholar]
- 153.Hsieh P. Chen L.B. Behavior of cells seeded in isolated fibronectin matrices. J Cell Biol. 1983;96:1208. doi: 10.1083/jcb.96.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tranquillo R.T. Murray J.D. Continuum model of fibroblast-driven wound contraction: inflammation-mediation. J Theor Biol. 1992;158:135. doi: 10.1016/s0022-5193(05)80715-5. [DOI] [PubMed] [Google Scholar]
- 155.Dallon J.C. Sherratt J.A. Maini P.K. Mathematical modelling of extracellular matrix dynamics using discrete cells: fiber orientation and tissue regeneration. J Theor Biol. 1999;199:449. doi: 10.1006/jtbi.1999.0971. [DOI] [PubMed] [Google Scholar]
- 156.Dunn M.G. Doillon C.J. Berg R.A. Olson R.M. Silver F.H. Wound healing using a collagen matrix: effect of DC electrical stimulation. J Biomed Mater Res. 1988;22:191. doi: 10.1002/jbm.820221310. [DOI] [PubMed] [Google Scholar]
- 157.Inoue M. Ono I. Tateshita T. Kuroyanagi Y. Shioya N. Effect of a collagen matrix containing epidermal growth factor on wound contraction. Wound Repair Regen. 1998;6:213. doi: 10.1046/j.1524-475x.1998.60307.x. [DOI] [PubMed] [Google Scholar]
- 158.Ono I. Tateshita T. Inoue M. Effects of a collagen matrix containing basic fibroblast growth factor on wound contraction. J Biomed Mater Res. 1999;48:621. doi: 10.1002/(sici)1097-4636(1999)48:5<621::aid-jbm5>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 159.Doillon C.J. Silver F.H. Olson R.M. Kamath C.Y. Berg R.A. Fibroblast and epidermal cell-type I collagen interactions: cell culture and human studies. Scan Microsc. 1988;2:985. [PubMed] [Google Scholar]
- 160.Doillon C.J. Silver F.H. Collagen-based wound dressing: effects of hyaluronic acid and fibronectin on wound healing. Biomaterials. 1986;7:3. doi: 10.1016/0142-9612(86)90080-3. [DOI] [PubMed] [Google Scholar]
- 161.Lee C.R. Grodzinsky A.J. Spector M. The effects of cross-linking of collagen-glycosaminoglycan scaffolds on compressive stiffness, chondrocyte-mediated contraction, proliferation and biosynthesis. Biomaterials. 2001;22:3145. doi: 10.1016/s0142-9612(01)00067-9. [DOI] [PubMed] [Google Scholar]
- 162.Sieminski A.L. Hebbel R.P. Gooch K.J. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp Cell Res. 2004;297:574. doi: 10.1016/j.yexcr.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 163.Lo C.M. Wang H.B. Dembo M. Wang Y.L. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pelham R.J., Jr. Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA. 1997;94:13661. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wozniak M.A. Modzelewska K. Kwong L. Keely P.J. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 166.Carlisle C.R. Coulais C. Guthold M. The mechanical stress-strain properties of single electrospun collagen type I nanofibers. Acta Biomater. 2010;6:2997. doi: 10.1016/j.actbio.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ionescu L.C. Lee G.C. Sennett B.J. Burdick J.A. Mauck R.L. An anisotropic nanofiber/microsphere composite with controlled release of biomolecules for fibrous tissue engineering. Biomaterials. 2010;31:4113. doi: 10.1016/j.biomaterials.2010.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nelson A.R. Fingleton B. Rothenberg M.L. Matrisian L.M. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 169.Smeds K.A. Grinstaff M.W. Photocrosslinkable polysaccharides for in situ hydrogel formation. J Biomed Mater Res. 2001;54:115. doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 170.Dong Y. Liao S. Ngiam M. Chan C.K. Ramakrishna S. Degradation behaviors of electrospun resorbable polyester nanofibers. Tissue Eng Part B Rev. 2009;15:333. doi: 10.1089/ten.TEB.2008.0619. [DOI] [PubMed] [Google Scholar]
- 171.Park S.H. Kim T.G. Kim H.C. Yang D.-Y. Park T.G. Development of dual scale scaffolds via direct polymer melt deposition and electrospinning for applications in tissue regeneration. Acta Biomater. 2008;4:1198. doi: 10.1016/j.actbio.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 172.Stankus J.J. Soletti L. Fujimoto K. Hong Y. Vorp D.A. Wagner W.R. Fabrication of cell microintegrated blood vessel constructs through electrohydrodynamic atomization. Biomaterials. 2007;28:2738. doi: 10.1016/j.biomaterials.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hong J.K. Madihally S.V. Three-dimensional scaffold of electrosprayed fibers with large pore size for tissue regeneration. Acta Biomater. 2010;6:4734. doi: 10.1016/j.actbio.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sato H. Takahashi M. Ise H. Yamada A. Hirose S. Tagawa Y., et al. Collagen synthesis is required for ascorbic acid-enhanced differentiation of mouse embryonic stem cells into cardiomyocytes. Biochem Biophys Res Commun. 2006;342:107. doi: 10.1016/j.bbrc.2006.01.116. [DOI] [PubMed] [Google Scholar]
- 175.Chang Z. Meyer K. Rapraeger A.C. Friedl A. Differential ability of heparan sulfate proteoglycans to assemble the fibroblast growth factor receptor complex in situ. FASEB J. 2000;14:137. doi: 10.1096/fasebj.14.1.137. [DOI] [PubMed] [Google Scholar]
- 176.Cukierman E. Pankov R. Stevens D.R. Yamada K.M. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 177.Stevens M.M. George J.H. Exploring and engineering the cell surface interface. Science. 2005;310:1135. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 178.Derda R. Laromaine A. Mammoto A. Tang S.K. Mammoto T. Ingber D.E., et al. Paper-supported 3D cell culture for tissue-based bioassays. Proc Natl Acad Sci USA. 2009;106:18457. doi: 10.1073/pnas.0910666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xie J. Willerth S.M. Li X. Macewan M.R. Rader A. Sakiyama-Elbert S.E., et al. The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials. 2009;30:354. doi: 10.1016/j.biomaterials.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yang F. Wolke J.G.C. Jansen J.A. Biomimetic calcium phosphate coating on electrospun poly([var epsilon]-caprolactone) scaffolds for bone tissue engineering. Chem Eng J. 2008;137:154. [Google Scholar]
- 181.Wong S.-C. Baji A. Leng S. Effect of fiber diameter on tensile properties of electrospun poly([var epsilon]-caprolactone) Polymer. 2008;49:4713. [Google Scholar]
- 182.Tillman B.W. Yazdani S.K. Lee S.J. Geary R.L. Atala A. Yoo J.J. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials. 2009;30:583. doi: 10.1016/j.biomaterials.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 183.Zhuo H. Hu J. Chen S. Electrospun polyurethane nanofibres having shape memory effect. Mater Lett. 2008;62:2074. [Google Scholar]
- 184.Paneva D. Bougard F. Manolova N. Dubois P. Rashkov I. Novel electrospun poly([epsilon]-caprolactone)-based bicomponent nanofibers possessing surface enriched in tertiary amino groups. Eur Polym J. 2008;44:566. [Google Scholar]
- 185.Lee S.J. Oh S.H. Liu J. Soker S. Atala A. Yoo J.J. The use of thermal treatments to enhance the mechanical properties of electrospun poly([var epsilon]-caprolactone) scaffolds. Biomaterials. 2008;29:1422. doi: 10.1016/j.biomaterials.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 186.Chew S.Y. Mi R. Hoke A. Leong K.W. The effect of the alignment of electrospun fibrous scaffolds on Schwann cell maturation. Biomaterials. 2008;29:653. doi: 10.1016/j.biomaterials.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wu Y. Carnell L.A. Clark R.L. Control of electrospun mat width through the use of parallel auxiliary electrodes. Polymer. 2007;48:5653. [Google Scholar]
- 188.Van Royen P. Schacht E. Ruys L. Van Vaeck L. Characterisation of the surface composition in electrospun nanowebs with static secondary ion mass spectrometry (S-SIMS) Talanta. 2007;71:1464. doi: 10.1016/j.talanta.2006.06.042. [DOI] [PubMed] [Google Scholar]
- 189.Li W.-J. Mauck R.L. Cooper J.A. Yuan X. Tuan R.S. Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J Biomech. 2007;40:1686. doi: 10.1016/j.jbiomech.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kang X. Xie Y. Powell H.M. James Lee L. Belury M.A. Lannutti J.J., et al. Adipogenesis of murine embryonic stem cells in a three-dimensional culture system using electrospun polymer scaffolds. Biomaterials. 2007;28:450. doi: 10.1016/j.biomaterials.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 191.Ayodeji O. Graham E. Kniss D. Lannutti J. Tomasko D. Carbon dioxide impregnation of electrospun polycaprolactone fibers. J Supercrit Fluids. 2007;41:173. [Google Scholar]
- 192.Van Royen P. dos Santos A.M. Schacht E. Ruys L. Van Vaeck L. Characterisation of electrospun nanowebs with static secondary ion mass spectrometry (S-SIMS) Appl Surf Sci. 2006;252:6992. [Google Scholar]
- 193.Luong-Van E. Grøndahl L. Chua K.N. Leong K.W. Nurcombe V. Cool S.M. Controlled release of heparin from poly([epsilon]-caprolactone) electrospun fibers. Biomaterials. 2006;27:2042. doi: 10.1016/j.biomaterials.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 194.Puppi D. Detta N. Piras A.M. Chiellini F. Chiellini E. Electrospun polymeric meshes for application in tissue engineering. Biomed Pharmacother. 2008;62:489. [Google Scholar]
- 195.Marras S.I. Kladi K.P. Tsivintzelis I. Zuburtikudis I. Panayiotou C. Biodegradable polymer nanocomposites: the role of nanoclays on the thermomechanical characteristics and the electrospun fibrous structure. Acta Biomater. 2008;4:756. doi: 10.1016/j.actbio.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 196.Lee J. Tae G. Kim Y.H. Park I.S. Kim S.-H. Kim S.H. The effect of gelatin incorporation into electrospun poly(l-lactide-co-[var epsilon]-caprolactone) fibers on mechanical properties and cytocompatibility. Biomaterials. 2008;29:1872. doi: 10.1016/j.biomaterials.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 197.Jeon H.J. Kim J.S. Kim T.G. Kim J.H. Yu W.-R. Youk J.H. Preparation of poly([var epsilon]-caprolactone)-based polyurethane nanofibers containing silver nanoparticles. Appl Surf Sci. 2008;254:5886. [Google Scholar]
- 198.Heydarkhan-Hagvall S. Schenke-Layland K. Dhanasopon A.P. Rofail F. Smith H. Wu B.M., et al. Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials. 2008;29:2907. doi: 10.1016/j.biomaterials.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Erisken C. Kalyon D.M. Wang H. Functionally graded electrospun polycaprolactone and [beta]-tricalcium phosphate nanocomposites for tissue engineering applications. Biomaterials. 2008;29:4065. doi: 10.1016/j.biomaterials.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 200.Choi J.S. Lee S.J. Christ G.J. Atala A. Yoo J.J. The influence of electrospun aligned poly([var epsilon]-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials. 2008;29:2899. doi: 10.1016/j.biomaterials.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 201.Baker B.M. Gee A.O. Metter R.B. Nathan A.S. Marklein R.A. Burdick J.A., et al. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials. 2008;29:2348. doi: 10.1016/j.biomaterials.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Schnell E. Klinkhammer K. Balzer S. Brook G. Klee D. Dalton P., et al. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-[epsilon]-caprolactone and a collagen/poly-[epsilon]-caprolactone blend. Biomaterials. 2007;28:3012. doi: 10.1016/j.biomaterials.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 203.Lin K. Chua K.-N. Christopherson G.T. Lim S. Mao H.-Q. Reducing electrospun nanofiber diameter and variability using cationic amphiphiles. Polymer. 2007;48:6384. [Google Scholar]
- 204.Kim T.G. Lee D.S. Park T.G. Controlled protein release from electrospun biodegradable fiber mesh composed of poly([var epsilon]-caprolactone) and poly(ethylene oxide) Int J Pharm. 2007;338:276. doi: 10.1016/j.ijpharm.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 205.Chong E.J. Phan T.T. Lim I.J. Zhang Y.Z. Bay B.H. Ramakrishna S., et al. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007;3:321. doi: 10.1016/j.actbio.2007.01.002. [DOI] [PubMed] [Google Scholar]