Abstract
Purpose
α-Crystallins are molecular chaperones that protect various cells from apoptosis. This study used immunohistochemistry to examine the expression of αA-crystallin and αB-crystallin in human sebaceous carcinoma tissues.
Methods
Nine patients with sebaceous carcinoma of the eyelid underwent excision of the eyelid or orbital exenteration. Formalin-fixed, paraffin-embedded tissue sections were submitted for hematoxylin-eosin staining and immunohistochemistry with anti-αA- and αB-crystallin antibodies.
Results
In the noncancerous eyelid, α-crystallins were weakly and homogeneously expressed in the Meibomian gland lobules. In eyelids with sebaceous carcinoma, αA-crystallin and αB-crystallin were highly expressed in 4 cases, where the cytoplasmic immunoreactivity was heterogeneously detected in the tumor cells. Three and 2 cases were shown to express low levels of αA-crystallin and αB-crystallin, respectively. A statistically significant correlation was observed between expression levels of αA-crystallin and αB-crystallin in sebaceous carcinomas (p=0.012). All sebaceous carcinoma tissues contained mitotic tumor cells, which showed predominantly perinuclear immunoreactivity of αB-crystallin. The mitotic rate of tumor cells was 5.85±1.76 and 10.72±1.64 in high and moderate/low αB-crystallin-expressing cases, respectively The number of mitotic cells was significantly higher in moderate/low αB-crystallin-positive than in high-positive cases (p<0.01).
Conclusions
α-Crystallins are expressed in human sebaceous carcinomas including mitotic cells. Expression of αB-crystallin correlated with low number of mitotic tumor cells, suggesting that αB-crystallin may play a potential role in the regulation of tumor cell proliferation.
Keywords: α-Crystallin, Mitosis, Pagetoid change, Sebaceous carcinoma
INTRODUCTION
α-Crystallin, primarily found as a major protein in the mammalian lens, has 2 subunits: α.A and αB. Both α-crystallins are members of the small heat shock protein (sHSP) family. They act as molecular chaperones, interact to form an aggregate, and play a prominent role in the maintenance of lens transparency (1–3). Recent studies have demonstrated that α-crystallins are present not only in the lens, but also in other ocular tissues, including the retina and cornea (4). α-Crystallins have been shown to have an antiapoptotic function, blocking proapoptotic mitochondrial pathways and thereby inhibiting activation of downstream apoptotic events (5).
Although there are no published reports on the presence of αA-crystallin, the expression of αB-crystallin in tumor tissues varies. While αB-crystallin is expressed in renal cancers and retinoblastomas (6–8), it is absent in some types of thyroid cancers (9). Increased expression of αB-crystallin is related to more advanced tumors (10–12). In fact, αB-crystallin was shown to predict poorer prognosis in breast cancer (12). These varying degrees of αB-crystallin expression suggest that its protein structure and metabolic roles are uniquely expressed in tumors (8). α-Crystallins have been demonstrated to protect cancer cells from apoptotic signals (12, 13). These results indicate that expression of α-crystallins in tumor cells may regulate tumor growth.
Sebaceous carcinoma of the eyelid is a malignant solid tumor involving the Meibomian glands and the glands of Zeis. It is one of the most common eyelid malignancies of middle age. It can exhibit aggressive local behavior and can metastasize to regional lymph nodes and distant organs (14). Since mitotic activity is usually high in sebaceous carcinoma of the eyelid (15), it may be a good example in analysis of the relationship between mitosis and the targeted protein expression. This study used immunohistochemistry to examine the expression of αA-crystallins and αB-crystallins in human sebaceous carcinoma tissues.
MATERIALS AND METHODS
The Institutional Review Board of the University of Southern California approved our use of human specimens. All procedures conformed to the Declaration of Helsinki for research involving human subjects. We collected 9 cases with sebaceous carcinoma using the medical records of patients seen at the Doheny Eye Institute from January 1975 through August 2008. All eyeballs had been fixed in 4% paraformaldehyde soon after excision or orbital exenteration. Formalin-fixed, paraffin-embedded tissue sections were processed for routine hematoxylin-eosin (HE) staining and immunohistochemistry. The number of mitotic tumor cells was calculated in HE staining specimens under high magnification (objective lens: ×40) from 3 fields. The mitotic rate shows percentage of mitotic tumor cells in all tumor cells (%) in each case.
Immunohistochemistry
The slides were deparaffinized using xylene (for 6 min), 100% ethanol, and 95% ethanol. The slides were rinsed with tap water for 1 minute and transferred to a container of 1× concentration of phosphate-buffered saline. These slides were incubated with 3% hydrogen peroxide for 10 minutes, then with normal goat serum for 30 minutes. Sections were then incubated with anti-αA-crystallin and αB-crystallin rabbit polyclonal antibody (1:100 Stressgen, Ann Arbor, Ml) at room temperature for 2 hours. Binding of the primary antibody was localized with the FITC-conjugated anti-rabbit secondary antibody (Jackson ImmunoResearchLaboratories, West Grove, PA) for 30 minutes. A negative control was incubated with FITC-conjugated rabbit immunoglobulin G without treatment of the primary antibody. Slides were examined using a Zeiss LSM510 (Zeiss, Thornwood, NY) confocal microscope.
Statistical analysis
To evaluate the correlation with αA-crystallin and αB-crystallin distribution, statistical analysis was performed using the chi-square trend test. Student t-test was applied for evaluation of the significant difference between mitotic rate and immunoreactivity of αB-crystallin. Pearson correlation coefficient test was applied for evaluation of correlation with α-crystallin expression and tumor differentiation. Accepted level of significance for all tests was p<0.05.
RESULTS
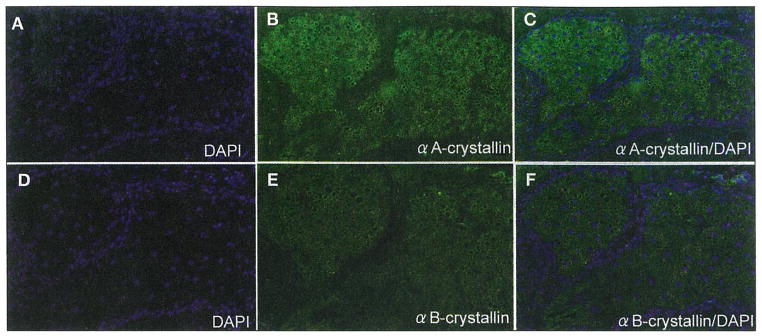
Histologically, normal sebaceous glands were present in the vicinity of tumor nests in six cases of sebaceous carcinoma examined in this study. In the noncancerous area, αA-crystallin was homogeneously expressed in the Meibomian gland lobules (Fig. 1, A–C) and glands of Zeis. Immunoreactivity for αB-crystallin was faintly detected in the sebaceous gland (Fig. 1, D–F). Immunoreactivity for αA-crystallin and αB-crystallin was strongly detected in the skeletal muscle and weakly detected in the squamous epithelium of the eyelid skin.
Fig. 1.
DAPI nuclear staining (A, C, D, F; blue) and immunoreactivity for αA-crystallin (B, C; green) and αB-crystallin (E, F; green) in normal human Meibomian glands. Normal glands weakly express αA-crystallin in the lobules (green; A–C). Immunoreactivity for αB-crystallin is barely detected in the glands (D–F)
Expression of α-crystallins in tumor cells of human sebaceous carcinoma
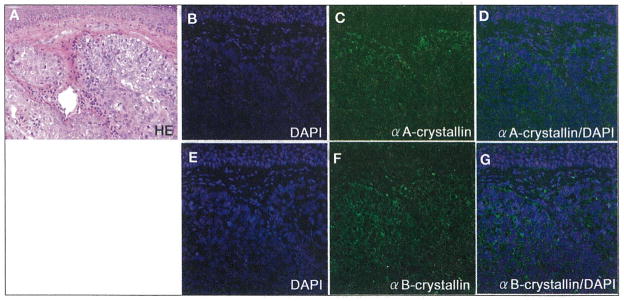
Table I summarizes the clinicopathologic profile revealed by this study. Expression of αA-crystallin showed a homogeneous pattern. αA-crystallin expression was low in 3 cases, and moderate in 2, while a high expression was seen in the remaining 4 cases. In Cases 2, 6, 7, and 9, αA-crystallin was highly expressed in tumor cells, where the immunoreactivity was detected in the cytoplasm of tumor cells (Fig. 2D). Expression of αB-crystallin was cytoplasmic, and showed a heterogeneous distribution. αB-crystallin expression was low in 2 cases, and moderate in 3, while a high expression was seen in the remaining 4 cases. Cases 1,2,6, and 7 represented high expression of αB-crystallin, where the cytoplasmic immunoreactivity was heterogeneously detected in the tumor cells (Fig. 2G). Low levels of αA-crystallin and αB-crystallin expressions were similar. High expression of both crystallins in the carcinoma was noted in 3 cases out of 9. A statistically significant correlation was observed between expression levels of αA-crystallin and αB-crystallin in sebaceous carcinomas (p<0.05). αA-crystallin (p=0.26) or αB-crystallin (p=0.23) expression did not correspond to degree of tumor differentiation. α-Crystallin immunoreactivity was not detected in inflammatory cells infiltrating the tumor stroma.
TABLE I.
CLINICOPATHOLOGICAL PROFILES IN HUMAN SEBACEOUS CARCINOMAS OF THE EYELID EXAMINED IN THIS STUDY
| No. | Sex | Age (M/F) | Side (L/R) | Size (mm) | Diff | Mitosis(%) | αA | αB |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 50 | L | 23×14 | Moderate | 6.1 | Moderate | High |
| 2 | F | 94 | L | 10×5 | Well | 3.3 | High | High |
| 3 | F | 48 | R | 15×6 | Well | 9.3 | Low | Low |
| 4 | M | 50 | L | 39×37 | Poor | 10.4 | Low | Low |
| 5 | M | 88 | L | 11×9 | Well | 13.2 | Low | Moderate |
| 6 | F | 90 | R | 10×7 | Moderate | 6.8 | High | High |
| 7 | F | 71 | R | 8×6 | Well | 7.2 | High | High |
| 8 | M | 97 | L | 49×45 | Poor | 11.4 | Moderate | Moderate |
| 9 | F | 49 | R | 40×35 | Well | 9.3 | High | Moderate |
M, male; F, female, L, left; R, right; Diff, differentiation; αA, αA-crystallin; αB; αB-crystallin
Fig. 2.
Hematoxylin-eosin (H–E) staining (A), DAPI nuclear staining (B, D, E, G; blue), and immunoreaction of αA-crystallin (C, D; green) and αB-crystallin (F, G; green) in human sebaceous gland carcinoma evaluated as a case showing high expression. H–E staining reveals sebaceous cell carcinoma, representing basophilic mass beneath the normal squamous epithelium of the eyelid (A). αA-crystallin is heterogeneously expressed in higher levels than normal squamous epithelium (B–D). αB-crystallin immunoreactivity is strongly detected in the cytoplasm of tumor cells (E–G).
Expression of α-crystallins in mitotic tumor cells
All sebaceous carcinoma tissues contained mitotic tumor cells (Fig. 3A). In α-crystallin-positive cases, mitotic cells showed perinuclear immunoreactivity of αA-crystallin and αB-crystallin, the latter of which was predominantly detected (Fig. 3). Mitotic rate of tumor cells was 5.85±1.76 and 10.72±1.64 in high and moderate/low αB-crystallin-positive cases, respectively. The number of mitotic cells was significantly higher in moderate/low αB-crystallin-positive than in high-positive cases (p<0.01). In contrast, the mitotic rate of tumor cells was 6.65±2.49 and 10.08±2.64 in high and moderate/low αA-crystallin-positive cases, respectively. No significant difference was seen between αA-crystallin expression and tumor cell mitoses (p=0.08).
Fig. 3.
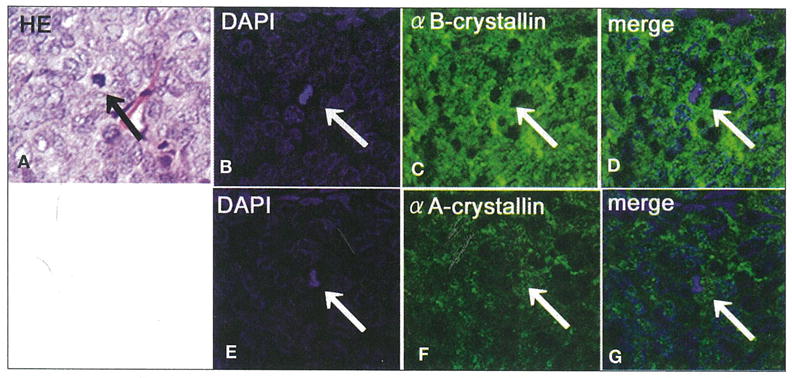
Hematoxylin-eosin (H–E) staining (A), DAPI nuclear staining (B, D, E, G; blue), and immunoreaction of αB-crystallin (C, D; green) and αA-crystallin (F, G; green) in mitotic cells of sebaceous gland carcinoma. H–E staining (A; arrow) and DAPI nuclear staining (B, D, E, G; arrow) show mitotic tumor cells in sebaceous cell carcinoma. The merged image shows the αB-crystallin to be highly expressed in perinuclear mitotic cells (B–D). The merged image represents αA-crystallin to be less expressed than αB-crystallin (E–G).
DISCUSSION
Previous reports have shown that αB-crystallin is expressed in various malignant solid tumors. We showed that not only αB-crystallin, but also αA-crystallin, was expressed in sebaceous carcinoma cells. In addition, we showed a significant distribution between the expression of both crystallins in tumors. Mao et al (5) demonstrated that both αA-crystallin and αB-crystallin exert an anti-apoptotic function by blocking proapoptotic mitochondrial pathways, thereby inhibiting the activation of downstream apoptotic events. On the other hand, Khurana et al (13) demonstrated that αA-crystallin upregulation protected photoreceptors from apoptotic signals associated with experimental ocular inflammation. These results suggest that αA-crystallin has a potential role in tumor growth as well. Uehara and Ohba (16) confirmed that cytotoxic and helper T-lymphocytes infiltrated the stroma of sebaceous carcinoma tissues. Therefore, α-crystallin allows tumor cells to acquire immune privilege and escape from apoptotic signals in sebaceous carcinoma.
We showed that 3 and 2 sebaceous carcinoma cases examined represented low expression of αA-crystallin and αB-crystallin, respectively. This phenomenon may be explained by at least 2 presumed mechanisms. Mineva et al (9) demonstrated that αB-crystallin was markedly down-regulated in malignant anaplastic thyroid carcinomas in contrast to their benign lesions. Although αB-crystallin may prevent the cell death of the tumor in its early stages, a very specific αB-crystallin gene silencing occurs later that correlates with downregulation of αB-crystallin and subsequent chromosomal damage (9). The other possibility is that sebaceous carcinoma cells showing low expression of α-crystallins may use different sHSPs, such as HSP27, to escape from apoptotic signals like various systemic cancers (17).
In the present study, we found that immunoreactivity for α-crystallins was detected not only in the cytoplasm of non-mitotic tumor cells, but in perinuclei of mitotic cells. α-Crystallins regulate cell cycle and cell growth (18). αA-crystallin expression in vivo protects against cell death during mitosis in the epithelial cells (19). αB-crystallin helps maintain the genomic stability and cell proliferation in epithelial cells (18). We also demonstrated that αB-crystallin immunoreactivity correlated with low mitotic activity. These important facts suggest that αB-crystallin may regulate tumor cell replication at an early stage. Those cases that are αB-crystallin-negative, however, may be later stages and may represent carcinomas of a more aggressive nature than those positive cases.
The present study has demonstrated that α-crystallins were expressed in sebaceous carcinoma cells, suggesting that α-crystallin may be a novel therapeutic target. Therapies that attempt to balance the level of α-crystallins by inhibiting the signal transduction pathway in tumor cells (12) would be effective at alleviating tumor growth. Other possible solutions to stop α-crystallin activity include using antisense or nucleotide-based therapies to sensitize the tumor to apoptotic inducers and anticancer drugs (17). Therefore, peptide/RNA aptamers or chemical chaperones that bind to specific structures on α-crystallin may reduce the tumors’ activities. Development and understanding of the functions of α-crystallins may contribute to application of a novel therapy for patients with sebaceous carcinoma in the future.
Acknowledgments
Supported by NIH grants EY015714 and EY03040 and a grant from Research to Prevent Blindness.
Footnotes
The authors have no proprietary interest in any product mentioned.
References
- 1.Fujii N, Hisano T, Fujii N. Study of subunit interactions of alpha A- and alpha B-crystallins and the effects of gamma-irradiation on their interactions by surface plasmon resonance. Biochim Biophys Acta Epub. 2008 doi: 10.1016/j.bbapap.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka N, Tanaka R, Tokuhara M, Kunugi S, Lee YF, Hamada D. Amyloid fibril formation and chaperone-like activity of peptides from alphaA-crystallin. Biochemistry. 2008;47:2961–7. doi: 10.1021/bi701823g. [DOI] [PubMed] [Google Scholar]
- 3.Klemenz R, Andres AC, Frohli E, Schafer R, Aoyama A. Expression of the murine small heat shock proteins hsp 25 and alpha B crystallin in the absence of stress. J Cell Biol. 1993;120:639–45. doi: 10.1083/jcb.120.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao NA, Saraswathy S, Wu GS, Katselis GS, Wawrousek EF, Bhat S. Elevated retina-specific expression of the small heat shock protein, alphaA-crystallin is associated with photoreceptor protection in experimental uveitis. Invest Ophthalmol Vis Sci. 2008;49:1161–71. doi: 10.1167/iovs.07-1259. [DOI] [PubMed] [Google Scholar]
- 5.Mao YW, Liu JP, Xiang H, Li DW. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11:512–26. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- 6.Kase S, Parikh JG, Rao NA. Expression of heat shock protein 27 and alpha-crystallins in human retinoblastoma after chemoreduction. Br J Ophthalmol. 2009;93:541–4. doi: 10.1136/bjo.2008.145508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinder SE, Balsitis M, Ellis IO, Landon M, Mayer RJ, Lowe J. The expression of alpha B-crystallin in epithelial tumours: a useful tumour marker? J Pathol. 1994;174:209–15. doi: 10.1002/path.1711740310. [DOI] [PubMed] [Google Scholar]
- 8.Kato S, Hirano A, Kato M, Herz F, Ohama E. Comparative study on the expression of stress-response protein (srp) 72, srp 27, alpha B-crystallin and ubiquitin in brain tumours. An immunohistochemical investigation. Neuropathol Appl Neurobiol. 1993;19:436–42. doi: 10.1111/j.1365-2990.1993.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 9.Mineva I, Gartner W, Hauser P, et al. Differential expression of alphaB-crystallin and Hsp27–1 in anaplastic thyroid carcinomas because of tumor-specific alphaB-crystallin gene (CRYAB) silencing. Cell Stress Chaperones. 2005;10:171–84. doi: 10.1379/CSC-107R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klemenz R, Scheier B, Muller A, Steiger R, Aoyama A. Alpha B crystallin expression in response to hormone, oncogenes and stress. Verh Dtsch Ges Pathol. 1994;78:34–5. [PubMed] [Google Scholar]
- 11.Aoyama A, Steiger RH, Frohli E, et al. Expression of alpha B-crystallin in human brain tumors. Int J Cancer. 1993;55:760–4. doi: 10.1002/ijc.2910550511. [DOI] [PubMed] [Google Scholar]
- 12.Moyano JV, Evans JR, Chen F, et al. AlphaB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J Clin Invest. 2005;116:261–70. doi: 10.1172/JCI25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khurana RN, Parikh JG, Saraswathy S, Wu GS, Rao NA. Mitochondrial oxidative DNA damage in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2008;49:3299–304. doi: 10.1167/iovs.07-1607. [DOI] [PubMed] [Google Scholar]
- 14.Rao NA, Hidayat AA, McLean IW, Zimmerman LE. Sebaceous carcinomas of the ocular adnexa: a clinicopathologic study of 104 cases, with five-year follow-up data. Hum Pathol. 1982;13:113–22. doi: 10.1016/s0046-8177(82)80115-9. [DOI] [PubMed] [Google Scholar]
- 15.Shields JA, Demirci H, Marr BP, Eagle RC, Jr, Shields CL. Sebaceous carcinoma of the ocular region: a review. Surv Ophthalmol. 2005;50:103–22. doi: 10.1016/j.survophthal.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Uehara F, Ohba N. MUC1 and sialoglycan expression associated with cytotoxic T lymphocyte infiltration in eyelid malignant tumors. Jpn J Ophthalmol. 2002;46:237–43. doi: 10.1016/s0021-5155(02)00472-0. [DOI] [PubMed] [Google Scholar]
- 17.Arrigo AP, Simon S, Gibert B, et al. Hsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targets. FEBS Lett. 2007;581:3665–74. doi: 10.1016/j.febslet.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Andley UP. Crystallins in the eye: function and pathology. Prog Retin Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Concannon CG, Gorman AM, Samali A. On the role of Hsp27 in regulating apoptosis. Apoptosis. 2003;8:61–70. doi: 10.1023/a:1021601103096. [DOI] [PubMed] [Google Scholar]