Abstract
Herein we examine the potential of a nitrile-containing proprionic acid moiety as an electrophile for covalent attack by the active site cysteine residue of caspase 1. The syntheses of several cyanopropanate containing small molecules based upon the optimized peptidic scaffold of the prodrug VX-765 were accomplished and found to be potent inhibitors of caspase 1 (IC50s ≤ 1 nM). Examination of these novel small molecules versus a caspase panel demonstrated an impressive degree of selectivity for caspase 1 inhibition. Assessment of hydrolytic stability and selected ADME properties highlighted these agents as potentially useful tools for studying caspase 1 down-regulation in various settings including in vivo analyses.
Keywords: Inhibitor; enzymes; prodrugs; peptides; caspase 1 inhibitor; Cysteine proteases; Caspase 1; VX-765; VRT-043198; covalent modifiers,; nitrile caspase inhibitors
Introduction
Caspases are cysteine proteases with a strict specificity for cleaving peptide sequences C-terminal to aspartic acids residues.1 Currently, 12 caspase isozymes have been identified in humans with numerous reported activities.1,2 Caspases are often subcategorized as either pro-apoptotic or pro-inflammatory enzymes. A prominent member of the pro-inflammatory class is caspase 1 (also known as interleukin-converting enzyme or ICE) which is responsible for the proteolytic activation of interleukin (IL)-1β and IL-18.3 IL-1β and IL-18 are cytokines that play a major role in the immune response and within numerous autoimmune and inflammatory diseases.4 Caspase 1 is constitutively and inducibly expressed in immune response elements such as T cells, macrophages and neutrophils.3,5 Procaspase 1 is known to associate with several multi-protein complexes capable of responding to numerous external stimuli suggesting that caspase 1 is a major regulator of the inflammation response.6
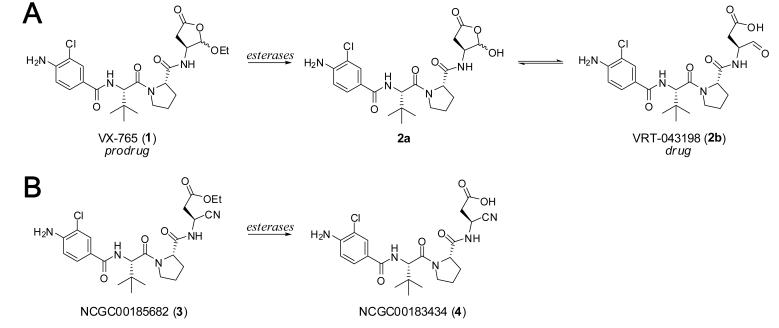
Targeting proteases and specifically caspases via small molecule therapeutics is an active area of research.7-10 Small molecule inhibitors of selected proteases have entered the clinic and many have received approval. Inhibitors of caspase 1 are sought for intervention strategies within ischemic disorders, Huntington's disease, amyotrophic lateral sclerosis (ALS), rheumatoid arthritis, osteoarthritis, inflammatory bowel disease and sepsis. To date, at least three caspase 1 inhibitors have entered clinical evaluation including Pralnacasan (VX-740), IDN-6556 and VX-765. All three agents are active site inhibitors that act through reversible (Pralnacasan and VX-765) or irreversible (IDN-6556) covalent modification of the catalytic cysteine residue. VX-765 (1) is a prodrug that requires esterase cleavage of the 5-ethoxydihydrofuran-2(3H)-one moiety to yield the aldehyde functionality of the drug VRT-043198 (2b) which acts as a potent electrophile for attack by the active site cysteine thiol (Figure 1).11 The remainder of the VX-765 (1) molecule establishes key binding contacts with caspase 1 that enhance the potency of the interaction and confer a modest degree of selectivity. Reports demonstrate that VX-765 (1) and VRT-043198 (2b) are capable of blocking lipopolysaccharide-stimulated IL-1β and IL-18 release from human peripheral blood mononuclear cells (PBMC's), whole blood and in mice (both IP and PO administration).11 Additional in vivo studies clearly showed mitigation of numerous inflammatory markers and models.11 In 2004, Vertex Pharmaceuticals reported that VX-765 had entered a phase II clinical study targeting psoriasis. Subsequent reports on the clinical development of VX-765 have yet to be released.
Figure 1.
A. The structure of VX-765 (1) and schematic representation of esterase cleavage of the 5-ethoxydihydrofuran-2(3H)-one moiety to yield the active drug VRT-043198 (2b). B. The structure of NCGC00185682 (3) and putative esterase cleavage of the ethyl-3-cyanopropanoate moiety to yield active agent NCGC00183434 (4).
The design of small molecule inhibitors of cysteine proteases relies heavily on covalent modification of the active site cysteine through reaction with the highly nucleophilic thiolate.12-14 Electrophilic ‘warhead’ moieties suitable for this modification include the aforementioned aldehyde, Michael acceptors (for instance vinyl sulfones), α-halo ketones, epoxides and nitriles. In particular, nitrile-based cysteine protease inhibitors have found utility versus cathepsin K15, TbCatB16 and cruzain.17 Covalently modifying small molecules rely upon binding which optimally aligns the reactive thiolate nucleophile and the ‘warhead’ electrophile. Oballa et al recently described a general method to gauge the electrophilic character of the CN functionality and reported calculated reaction energies for diversely substituted nitriles.18 In general, heterocyclic nitriles (for instance triazine and pyridine nitriles) scored as strong electrophiles, α-aminonitriles were modest electrophiles and phenolic and aliphatic nitriles were calculated as relatively weak electrophiles. Based upon successes surrounding nitrile based cysteine protease inhibitors and the potential alignment of reactivities between a nitrile containing Asp mimetic and the active site thiol we endeavored to examine the potential of the 3-cyanopropanoic acid moiety within a known caspase 1 inhibitor scaffold. Fairlie and coworkers included a 3-cyanopropanoic acid containing dipeptide in a study aimed at discovering caspase 1 inhibitors but reported that it had no activity.19 Several patents cover various small molecules with 3-cyanopropanoic acids but do not go into detail regarding the activities of these agents.20-22 From these limited studies, we felt that the promise of cyanopropanoates as caspase inhibitors remained in question. To explore the potential of this functional moiety, we took advantage of the peptidic scaffold of VX-765. Further, we incorporated the ethyl-3- cyanopropanoate to mimic the prodrug qualities associated with VX-765 (Figure 1).
Results and Discussion
Design and Synthesis
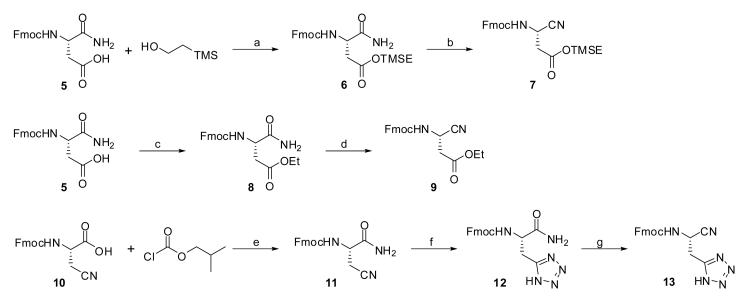
Appropriately substituted ethyl-3-cyanopropanoate derivatives are not commercially available and therefore required synthetic elaboration. As we desired to explore both an active and prodrug form of our conceived molecule, we examined alternative protecting group strategies for the acid side chain (Scheme 1). Commercially available Fmoc protected D-isoasparagine (5) offered a convenient entry point to both required building blocks. Treatment of 5 with 2-(trimethylsilyl)ethanol, EDC and DMAP in methylene chloride provided the TMSE protected 6 in good yield. Conversion of 6 to nitrile 7 was accomplished by treatment with trifluoroacetic anhydride and Hunig's base. A similar sequence was used to produce the ethyl ester 9. In addition to the ester prodrug and the active cyanopropionic acid it was of interest to explore carboxylic acid mimetics. As such, we undertook the synthesis of a tetrazole version of the key ethyl-3-cyanopropanoate moiety. Here, we utilized the previously reported Fmoc protected (S)-2-amino-3-cyanopropanoic acid (10).23 Conversion to amide 11 was required prior to formation of the tetrazole 12. The amide was formed via the mixed anhydride followed by treatment with ammonium hydroxide. Tetrazole formation was accomplished via microwave irradiation of the nitrile 11 and TMS-azide in the presence of dibutylstannanone.24 Dehydration to nitrile 13 was accomplished in a manner analogous to 7 and 9.
Scheme 1.
Conditions and reagents: (a) EDC, DMAP, CH2Cl2, 6 h (59%);(b) TFAA, DIPEA, CH2Cl2, 0 °C, 30 min. (90%); (c) thionyl chloride (excess), EtOH, 0 °C (95%); (d) TFAA, DIPEA, CH2Cl2, 0 °C, 30 min. (86%); (e) NMM, DME, then NH4OH (73%); (f) TMSN3, Bu2SnO (0.6 equiv.), toluene, μW, 100 °C, 1h (77%); (g) TFAA, DIPEA, CH2Cl2, 0 °C, 30 min. (80%).
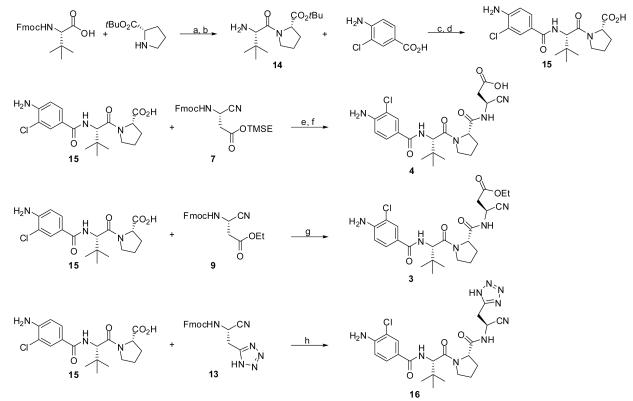
With appropriately substituted/protected cyanopropanoate building blocks we next turned our attention to the trimer core of VX-765 (Scheme 2). Both Fmoc protected L-tert-leucine and tert-butyl-L-prolinate are commercially available and were easily coupled via treatment with EDC and HOBt. Fmoc removal was effected by treatment with DBU resulting in the protected dimer 14. Coupling of 14 with 4-amino-3-chlorobenzoic acid was accomplished using HATU and Hunig's base in DMF. TFA mediated removal of the tert-butyl group yielded the carboxylic acid 15. A single pot deprotection-coupling sequence was used to generate the desired final products. Treatment of 7, 9 and 13 with DBU in DMF effected deprotection to the free amines to which 15, Hunig's base and finally HATU were sequentially added to yield the coupled products. The generation of 4 further required TBAF mediated removal of the TMSE group.
Scheme 2.
Conditions and reagents: (a) EDC, HOBt, DMF, rt, 8 h; (b) DBU, CH2Cl2, rt(71% over 2 steps);(c) HATU, DIPEA, DMF, rt, 2 h; (d)TFA,CH2Cl2 (1:1), rt, 4 h (85% over 2 steps); (e) DBU, DMF, 5 min. then 15, HATU, DIPEA, DMF, 0 °C, 2h; (f) TBAF, THF, 0 °C (72% over 2 steps); (g) DBU, DMF, 5 min. then 15, HATU, DIPEA, DMF, 0 °C, 2h (91%); (h) DBU, DMF, 5 min. then 15, HATU, DIPEA, DMF, 0 °C, 2h (82%).
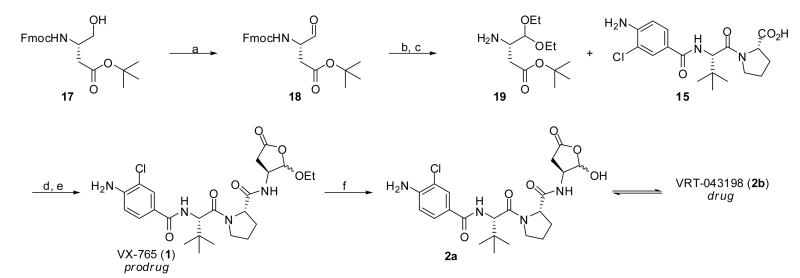
In order to compare the activities of our newly synthesized compounds to VX-765 (1) and VRT-043198 (2b) we undertook the synthesis of these agents as well. In 2008, Magdziak and coworkers reported a synthesis of 1 that relied upon a well engineered Pdcatalyzed coupling of a vinyl bromide of the ethoxyfuranone and the amide of a Cbz-protected proline amide.25 For our purposes it was convenient to begin with the orthogonally protected D-β-homoserine 17 which is transformed to the aldehyde 18 via the Parikh-Doering oxidation in good yield (Scheme 3).26 Conversion to the diethyl acetal and removal of the Fmoc protecting group provided the free amine 19 in modest yields over 2 steps. Standard coupling of 19 and 15 with HATU and Hunig's base was followed by treatment with TFA in dry methylene chloride to generate the 5-ethoxydihydrofuranone present in 1. In our hands, generation of hemiketal 2a/VRT-043198 (2b) was accomplished by treating 1 with HCl in a THF/water mixture.
Scheme 3.
Conditions and reagents: (a) SO3-pyridine, Hunig's Base CH2Cl2, DMSO (75%); (b) (EtO)3CH, PPTS, EtOH, 50 °C, 24 h; (c) DBU, CH2Cl2, (63% over 2 steps); (d) 19, DMF, 5 min. then 15, HATU, DIPEA, DMF, 0 °C, 1h; (e) TFA, CH2Cl2, 1 h (1:1 dr, 72% over 2 steps); (f) HCl, THF/H2O (quant.).
In vitro pharmacology
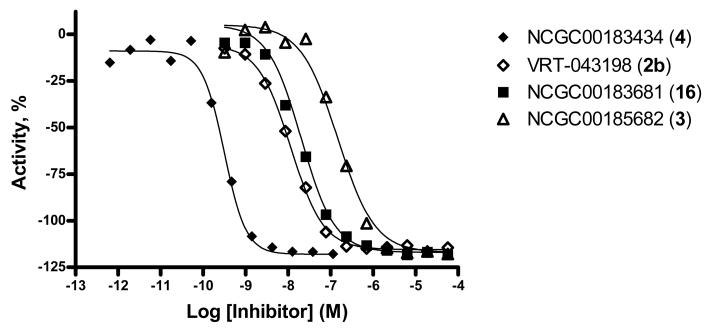
With the needed compounds in hand, we turned our attention to their biochemical capabilities. Our first evaluations were aimed at determining each agent's inhibition potency against caspase 1. For this purpose we utilized a well established protocol whereby caspase 1 activity is measured utilizing 2 nM enzyme in the presence and absence of compound and a caged fluorescent peptide substrate (Ac-LEHD-AMC). Compounds were examined across a titration series (0.65 pM → 57.5 μM) and data was recorded via fluorescence detection over 20 min, following a pre-incubation period of 15 minutes at room temperature using 5 μM of peptide substrate. We examined VRT-043198 (2b), NCGC00185682 (3), NCGC00183434 (4) and the tetrazole NCGC00183681 (16) and the results are displayed in figure 2. VRT-043198 (2b) was confirmed as a potent caspase 1 inhibitor with an IC50 value of 11.5 nM. NCGC00183434 (4) which contains the key cyanopropanoate moiety was found to inhibit caspase 1 with an impressive IC50 value of 0.316 nM. We were further gratified to find that the ethyl ester 3 and tetrazole 16 retained impressive potencies versus caspase 1 (IC50 = 144.7 nM and IC50 = 20.4 nM, respectively). The KI value of 4 was estimated to be 0.4 nM for caspase 1 using a competitive inhibition model (see Supporting Information).
Figure 2.
Complete response curves for VRT-043198 (2b) ( IC50 = 11.5 nM), NCGC00185682 (3) (
IC50 = 11.5 nM), NCGC00185682 (3) ( IC50 = 144.7 nM), NCGC00183434 (4) (◆ IC50 = 0.316 nM)and the tetrazole NCGC00183681 (16) (■ IC50 = 20.4 nM).
IC50 = 144.7 nM), NCGC00183434 (4) (◆ IC50 = 0.316 nM)and the tetrazole NCGC00183681 (16) (■ IC50 = 20.4 nM).
Having established that the nitrile containing 4 is a potent inhibitor of caspase 1, we next turned our attention to the selectivity of these agents. Randle and coworkers have reported the Ki values of 2b versus caspases 1, 3, 4, 6, 7, 8 and 9 and versus granzyme B, cathepsin B and trypsin.11 This report presents evidence that 2b is nearly equipotent versus caspases 1 and 4 (Ki = <1 nM) and modestly potent versus caspases 8, 6 and 9 (100 nM, 560 nM and 1030 nM, respectively) while possessing little activity against the remaining targets. We entered 2b, 3, 4 and 16 within a commercial panel of caspases offered by Reaction Biology Corporation.27 The results are shown in Table 1. This data confirmed the potent inhibitory capacity of 2b versus caspase 1 (IC50 = 0.204 nM), however the IC50 values found versus caspase 4 (IC50 = 14.5 nM) and caspase 8 (IC50 = 3.3 nM) differed slightly from the reported Ki values.11 The results against caspase 6 (IC50 = > 10,000 nM) and caspase 9 (IC50 = 5.07 nM) were significantly different from those reported by Randle and coworkers.11 The Reaction Biology Corporation panel also included caspase 5 (IC50 = 10.6 nM), caspase 10 (IC50 = 66.5 nM) and caspase 14 (IC50 = 58.5 nM) and the data presented here represents the first disclosure of the IC50 values for 2b versus these targets. The results for 4 demonstrated an impressive potency against caspase 1 (IC50 = 0.023 nM) and a similar selectivity profile as 2b. The only prominent divergence between the selectivity profiles of 4 and 2b was a sharp drop in the ability to inhibit caspase 14 (IC50 = 801 nM and IC50 = 58.5 nM, respectively). The caspase 1 inhibition data generated in this panel for 3 and 16 was similar to the data generated in our caspase 1 assay with reported IC50 values of 43.4 nM and 2.58 nM, respectively. A particularly interesting aspect of these molecules was the high selectivity for caspase 1. NCGC00183681 (16) registered an IC50 value of 91.5 nM versus caspase 9. All other activities were above the 1 μM threshold. In addition to the primary molecules of this study, we were interested in establishing cyanopropanoates as general caspase directing ‘warhead’ for future utility in the search for other potent and selective small molecule inhibitors of caspases. As such, we included the general nitrileAsp directing group into the common peptide caspase inhibitors YVAD. The resulting agent YVAD-CN (20) was profiled and the results clearly demonstrate that cyanopropanoates represent a general moiety for reversible, covalent modification of caspases.
Table 1.
IC50 values for selected compounds versus caspase panel.
| Compound | Caspase 1 (nM) |
Caspase 3 (nM) |
Caspase 4 (nM) |
Caspase 5 (nM) |
Caspase 6 (nM) |
Caspase 7 (nM) |
Caspase 8 (nM) |
Caspase 9 (nM) |
Caspase 10 (nM) |
Caspase 14 (nM) |
|---|---|---|---|---|---|---|---|---|---|---|
| VRT-043198 (2b) (Drug) |
0.204 | > 10000 | 14.5 | 10.6 | > 10000 | > 10000 | 3.3 | 5.07 | 66.5 | 58.5 |
|
3 (Nitrile ester) |
43.4 | > 10000 | > 10000 | 1570 | > 10000 | > 10000 | > 10000 | 1610 | > 10000 | > 10000 |
|
4 (Nitrile acid) |
0.023 | > 10000 | 13.8 | 3.60 | > 10000 | > 10000 | 25.2 | 2.17 | 89.7 | 801 |
|
16 (Nitrile tetrazole) |
2.58 | > 10000 | 1380 | 1300 | > 10000 | > 10000 | > 10000 | 91.5 | > 10000 | > 10000 |
|
20 YVAD-CN |
2.16 | > 10000 | 114 | 29.0 | > 10000 | > 10000 | 726 | 297 | 187 | 116 |
| Ac-LEHD-CHO (standard) |
15.0 | ND | 81.7 | 21.3 | ND | ND | 3.82 | 49.2 | 40.4 | 134 |
| Ac-DEVD-CHO (standard) |
ND | 3.04 | ND | ND | 122 | 3.54 | ND | ND | ND | ND |
Data was generated by Reaction Biology (http://www.reactionbiology.com/). Data is presented as an IC50's using a (Z-LEHD)2-R110 tetrapeptide substrate for caspase 1, 4, 5, 8, 9, 10, 14 and a (Z-DEVD)2-R110 tetrapeptide substrate for caspase 3, 6 and 7. Data represents the results from three separate experiments.
Physical Properties
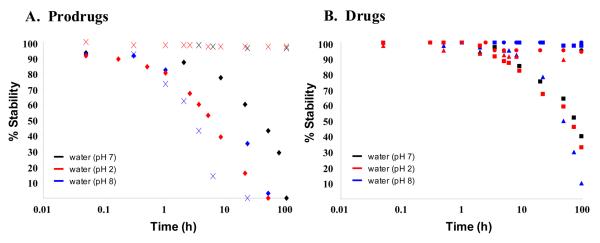
Based upon the data provided in this panel, it was clear that these agents represent important new tools for caspase 1 inhibition. However, the contributing functional groups for these agents (i.e. ethyl acetals, aldehydes, nitriles and esters) are all subject to hydrolysis in various conditions. It was paramount to fully understand their stability profile to appreciate their utility as molecular probes or even clinically used agents. Therefore, we examined 1, 2b, 3, 4 and 16 within an aqueous degradation study at neutral (pH 7), acidic (pH 2), and basic (pH 8) conditions. The study was conducted by monitoring the degradation of each agent by LCMS analysis at various time points over 96 hours (Figure 3). The prodrug 1 showed moderate degradation in water with over 50% of the compound decomposed after 48 hours. This degradation was amplified in both basic and acidic conditions. Conversely, the active agent 2b was very stable in both neutral and acidic conditions and its degradation at pH 8 was moderate. The potent 4 was exceedingly stable in basic conditions and its stability in neutral and acidic conditions was moderate to good (degradation of 50% in both conditions after 72 hours). The ethyl ester 3 was exceptionally stable in neutral and acidic conditions (no degradation noted), however, it was fully degraded in basic conditions after 22 hours (presumably due to saponification of the ester). Finally, the tetrazole 16 was found to be resistant to degradation in all conditions. Interestingly, this data suggests that 1 may have a short half-life as an oral agent due to its instability in acidic conditions such as those found in the gastric environment (40% degradation after 3.5 hours at pH 2). In contrast, this data highly suggests that 3 and 16 will be suitable reagents for all manner of examinations (cell based and in vivo studies) and even the highly active 4 will persist beyond 24 hours.
Figure 3.
Aqueous stability of prodrugs VX-765 (1)(◆) and NCGC00185682 (3)(X) and drugs VRT-043198 (2b)(▲), NCGC00183434 (4)(■) and NCGC00183681 (16)(●) at neutral (pH 7 - black), acidic (pH 2 - red), and basic (pH 8 - blue) conditions.
Given the aqueous stability of these new agents, it was of interest to examine selected ADME properties for chosen compounds. As such, 1, 2b, 3, 4 and 16 were submitted to Cyprotex28 for a profile of bi-directional Caco-2 permeability, plasma protein binding (both human and rat) and microsomal stability (both human and rat) studies (Table 2). All agents possessed relatively low A to B permeability, however, the prodrug 1 and the ester 3 had moderately better levels. The high B to A levels reported for 1 and 3 highly suggested an active transport mechanism and a control experiment with verapamil confirmed that these agents are substrates for Pgp efflux. Unsurprisingly, the free acids 2b and 4 and the tetrazole 16 had significantly higher free fractions in both human and rat protein binding assays relative to the more hydrophobic prodrug 1 and ethyl ester 3. The clearance rates (Clint) and t1/2 for 2b, 3, 4 and 16 were all moderate. The ester 3 was noted to possess a slight degree of degradation in liver microsomes without NADPH as a cofactor suggesting a non-enzymatic related degradation mechanism. The prodrug 1 possessed minimal ability to be metabolized by liver microsomes and a t1/2 of >9400 minutes. It is unknown how this extended stability affects this agent's toxicity profile.
Table 2.
In vitro ADME propertiesa for selected compounds.
| Compound | Caco (A2B)b Papp (×10−6cms−1) |
Caco (B2A)b Papp (×10−6 cms−1) |
Protein Bindingc fraction unbound |
Microsomal Stabilityd CLint (μL/min/mg protein) |
Microsomal Stabilityd t1/2 (min) |
|---|---|---|---|---|---|
| VX-765 (2a) (Prodrug) |
0.797 | 32.7 | 0.006 | 0.147 | 9430 |
| VRT-043198 (2b) (Drug) |
ND | 0.173 | 0.420 | 6.72 | 206 |
|
3 (Nitrile ester) |
0.445 | 9.59 | 0.071 | 27.4 | 50.7 |
|
4 (Nitrile acid) |
0.144 | 0.060 | 0.431 | 10.3 | 134 |
|
18 (Nitrile tetrazole) |
0.130 | 0.193 | 0.243 | 9.38 | 148 |
Data was generated by Cyprotex (http://www.cyprotex.com/home/).
Caco-2 permeability assay over 3 separate experiments with 2 separate internal control groups. Agents were also profiled in the presence of the known Pgp substrate verapamil and data from these experiments highly suggested that 2a and 3 were substrates for efflux by Pgp.
Plasma protein binding assays were performed using an equilibrium dialysis method in 100% plasma (profiles versus human and rat plasma were obtained: see the supporting information section for rat plasma binding data).
Microsomal stability was profiled alongside internal control compounds, minus NADPH and minus compound (profiles versus human and rat plasma were obtained: see the supporting information section for rat plasma binding data).
Modeling
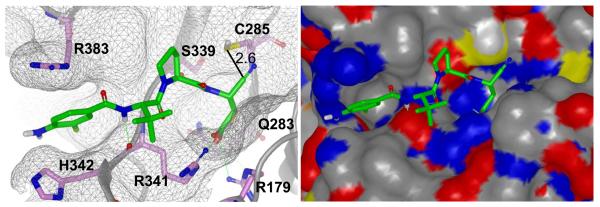
Finally, we examined the binding mechanism of these agents through molecular modeling. Several crystal structures of caspase 1 exist including structures with reversible and non-reversible inhibitors (PDB codes: 1BMQ, 1IBC, 1ICE, 1RWK, 1RWM, 1RWN, 1RWO, 1RWP, 1RWV, 1RWW, 1RWX, 1SC1, 1SC3, 1SC4, 2FQQ, 2H48, 2HBQ, 2HBR, 2HBY, 2HBZ, 2FQR, 2FQS, 2FQU, 2FQZ).29-32 We identified 2HBQ as the best template for 4 (2HBQ is a co-crystal of caspase 1 and Z-VAD-FMK). We applied the presumption of a covalent reversible mechanism of inhibition when building a model for binding of 4. The nitrile carbon was therefore held at a proximal distance (2.6 Å) from the catalytic cysteine residue (C285) by constraint docking and flexibility was granted to the remainder of the small molecule to achieve an optimal binding pose using FRED.33 The results are shown in figure 4 and demonstrate complementarity between the peptidic fragment of 4 and the peptide binding domain of caspase 1. Key interactions were noted for the acid moiety and arginine residues 341 and 179 in similar fashion to other Asp containing small molecule caspase 1 inhibitors. While direct interrogation of a covalent interaction between the nitrile and C285 was not pursued in our model, this representation does illustrate the open binding cavity that accommodates the tetrahedral intermediate that forms as a result of covalent binding with aldehyde based inhibitors (a mimetic of the hemithiolacetal intermediate associated with transition state 1 (TS1) during proteolysis). In contrast, covalent interactions between a thiol and a nitrile form a thioimidate intermediate that mimics transition state 2 (TS2) of an enzymatic proteolytic event between a cysteine proteases and a substrate. Ménard and coworkers examined aldehyde and nitrile inhibitors of papain and found that the thioimidate intermediate engages the oxyanion hole interaction in a manner that more closely mimics the natural process of hydrolysis during proteolysis.34 This may have consequences for both the binding affinity of nitrile based cysteine proteases inhibitors and their ultimate resolution through hydrolysis of the thioimidate intermediate.
Figure 4.
Molecular model (ribbon and space filling) of NCGC00183434 (4) bound to caspase 1.
Conclusion
Here, we set out to examine the potential of nitriles as a ‘warhead’ functionality for reversible, covalent modification of the active cysteine residue of caspases. The synthesis of a cyanopropanoate version of the clinically used caspase 1 inhibitor VRT-043198 (2b) was accomplished. Several related nitrile containing agents were examined and found to be highly potent and selective caspase 1 inhibitors. Interestingly, the Asp carboxylic acid moiety was found convey a large potency enhancement to NCGC00183434 (4), however ester and tetrazole versions of this compound were also found to be potent and selective caspase 1 inhibitors. Aqueous solubility studies and profiles of selected ADME properties also suggested that these novel agents possessed appropriate properties to be utilized as molecular probes of caspase 1 and, potentially within in vivo settings.
Experimental Section
Caspase Assay
Caspase-1 was dispensed (3 μL) to a 1536-well black solid-bottom microplate (Greiner Bio-One, Monroe, NC) using a BioRAPTR (Beckman Coulter, Fullerton, CA) nanoliter dispenser for automated addition of reagents, for final 2 nM enzyme concentration. Assay buffer was 50 mM HEPES, pH 7.4, containing 1 M sodium citrate, 100 mM NaCl, 0.1 mM EDTA, 10 mM DTT, 0.01 % CHAPS, 1 % DMSO, and 0.1 mg/mL BSA. Compound was transferred in 23 nL volume of DMSO stock to enzyme in each well using a pintool transfer station (Kalypsys Inc, San Diego, CA), and plates were incubated for 15 min at room temperature. Each compound was added as a 12-point titration in duplicate. Substrate was dispensed (1 μL) for final concentration of 5 μM Ac-LEHDAMC, and the initial reaction rate was monitored over 20 min at room temperature. Free AMC was measured by fluorescence detection (λex 340 nm, λem 450) with a ViewLux charge-coupled device-based imager (PerkinElmer, Waltham, MA). Percent inhibition was calculated from the median values of the uninhibited and the uncatalyzed controls, and IC50 values were determined by fitting the concentration-response data with a four-parameter Hill equation35 as previously described (http://www.ncgc.nih.gov/pub/openhts/curvefit/).
Chemistry
General Methods
All air or moisture sensitive reactions were performed under positive pressure of nitrogen with oven-dried glassware. Anhydrous solvents such as tetrahydrofuran (THF), toluene, dichloromethane, N,N-dimethylforamide (DMF), acetonitrile, methanol and triethylamine were obtained by purchasing from Sigma-Aldrich. Preparative purification was performed on a Waters semi-preparative HPLC. The column used was a Phenomenex Luna C18 (5 micron, 30 × 75 mm) at a flow rate of 45 mL/min. The mobile phase consisted of acetonitrile and water (each containing 0.1% trifluoroacetic acid). A gradient of 10% to 50% acetonitrile over 8 minutes was used during the purification. Fraction collection was triggered by UV detection (220 nM). Analytical analysis was performed on an Agilent LC/MS (Agilent Technologies, Santa Clara, CA). Method 1: A 7 minute gradient of 4% to 100% Acetonitrile (containing 0.025% trifluoroacetic acid) in water (containing 0.05% trifluoroacetic acid) was used with an 8 minute run time at a flow rate of 1 mL/min. A Phenomenex Luna C18 column (3 micron, 3 × 75 mm) was used at a temperature of 50°C. Method 2: A 3 minute gradient of 4% to 100% Acetonitrile (containing 0.025% trifluoroacetic acid) in water (containing 0.05% trifluoroacetic acid) was used with a 4.5 minute run time at a flow rate of 1 mL/min. A Phenomenex Gemini Phenyl column (3 micron, 3 × 100 mm) was used at a temperature of 50 °C. Purity determination was performed using an Agilent Diode Array Detector. Mass determination was performed using an Agilent 6130 mass spectrometer with electrospray ionization in the positive mode. 1H NMR spectra were recorded on Varian 400 MHz spectrometers. Chemical Shifts are reported in ppm with tetramethylsilane (TMS) as internal standard (0 ppm) for CDCl3 solutions or undeuterated solvent (DMSO-h6 at 2.49 ppm) for DMSO-d6 solutions. All of the analogs for assay have purity greater than 95% based on both analytical methods. High resolution mass spectrometry was recorded on Agilent 6210 Time-of-Flight LC/MS system. Confirmation of molecular formula was accomplished using electrospray ionization in the positive mode with the Agilent Masshunter software (version B.02).
(S)-2-(trimethylsilyl)ethyl 3-(((9H-fluoren-9-yl)methoxy)carbonylamino)-4-amino-4-oxobutanoate (6)
To (S)-3-(((9H-fluoren-9-yl)methoxy)carbonylamino)-4-amino-4-oxobutanoic acid (5) (17.29 g, 48.8 mmol, 1 eqiuvalent) and EDC (12.16 g, 63.4 mmol, 1.3 equivalents) suspended in dichloromethane (250 mL) was added 2-(trimethylsilyl)ethanol (8.39 mL, 58.6 mmol, 1.2 equivalents) followed by DMAP (8.35 g, 68.3 mmol, 1.4 equivalents). The suspension was stirred for 12 h, then quenched with aqueous sodium bicarbonate, washed twice with sodium bicarbonate, once with brine, dried over sodium sulfate and concentrated. The residue was purified by column chromatography using 9/1 - 1/9 hexane/EtOAc (v/v) gradient to give 6 as a white powder (13.09 g, 59% yield). 1H NMR (400 MHz, CHLOROFORM-d) δ ppm 7.72 (d, J=7.4 Hz, 2 H), 7.54 (d, J=7.4 Hz, 2 H), 7.36 (t, J=7.4 Hz, 2 H), 7.27 (t, J=7.4 Hz, 2 H), 6.38 (br. s., 1 H), 6.03 (br. s., J=8.2 Hz, 1 H), 5.80 (br. s., 1 H), 4.50 - 4.61 (m, 1 H), 4.35 - 4.50 (m, 2 H), 4.05 - 4.25 (m, 3 H), 2.92 (m, 1 H), 2.49 - 2.73 (m, 1 H), 0.85 - 1.03 (m, 2 H), 0.04 (s, 9 H). 13C (100 MHz, DMSO-d6): (ppm): 173.9, 171.9, 157.3, 145.3, 142.2, 129.2, 128.6, 126.8, 121.6, 67.3, 63.6, 52.7, 48.1, 38.0, 18.3, 0.00. [α]22D = −5 (c 1.0, MeOH).
(S)-2-(trimethylsilyl)ethyl 3-(((9H-fluoren-9-yl)methoxy)carbonylamino)-3-cyanopropanoate (7)
(S)-2-(trimethylsilyl)ethyl 3-(((9H-fluoren-9-yl)methoxy)carbonylamino)-4-amino-4-oxobutanoate (6) (3.62 g, 7.96 mmol, 1.0 equivalent) was dissolved in dichloromethane (50 mL) and cooled in an ice bath. Diisopropylethylamine (4.17 mL, 23.89 mmol, 3 equivalents) was added, followed by the dropwise addition of trifluoroacetic anhydride (2.25 mL, 15.93 mmol, 2 equivalents). The yellow solution was stirred for 30 minutes at which point TLC showed disappearance of the starting material and the reaction was quenched with aqueous sodium bicarbonate. After washing twice with sodium bicarbonate, once with brine, drying over sodium sulfate and concentrating, the residue was purified by column chromatography using 9/1 - 1/1 hexane/EtOAc (v/v) gradient to give 7 as an off-white powder (3.13 g, 90% yield). 1H NMR (400 MHz, DMSO-d6) δ ppm 8.23 (d, J=7.6 Hz, 1 H), 7.88 (d, J=7.4 Hz, 2 H), 7.68 (d, J=7.4 Hz, 2 H), 7.37 - 7.45 (m, 2 H), 7.32 (t, J=7.4 Hz, 2 H), 4.76 (q, J=7.6 Hz, 1 H), 4.35 - 4.46 (m, 2 H), 4.24 (t, J=6.5 Hz, 1 H), 4.02 - 4.21 (m, 2 H), 2.77 - 3.00 (m, 2 H), 0.85 - 1.05 (m, 2 H), 0.00 (s, 9 H). 13C (100 MHz, DMSO-d6): 170.0, 156.8, 145.1, 142.3, 129.2, 128.6, 126.6, 121.7, 120.4, 67.6, 64.4, 50.2, 48.1, 37.8, 18.3, 0.0. [α]22D = −26 (c 1.0, MeOH).
(S)-ethyl 3-(((9H-fluoren-9-yl)methoxy)carbonylamino)-4-amino-4-oxobutanoate (8)
A suspension of (S)-3-(((9H-fluoren-9-yl)methoxy)carbonylamino)-4-amino-4-oxobutanoic acid (5) (10 g, 28.2 mmol, 1 equivalent) in EtOH (100 ml) was cooled in an ice bath and thionyl chloride (20.6 mL, 282 mmol, 10 equivalents) was carefully added dropwise. After stirring at RT for 2 h the suspension becomes a clear solution. The solvents were removed and the residue was purified by column chromatography using a 9/1 - 1/9 hexane/EtOAc (v/v) gradient to give 8 as a white powder (10.25 g, 95%). 1H NMR (400 MHz, DMSO-d6) δ ppm 7.86 (d, J=7.6 Hz, 2 H), 7.67 (d, J=7.4 Hz, 2 H), 7.54 (d, J=8.4 Hz, 1 H), 7.38 (t, J=7.4 Hz, 2 H), 7.29 (t, J=7.5 Hz, 2 H), 7.08 (br. s., 2 H), 4.13 - 4.42 (m, 4 H), 3.93 - 4.11 (m, 2 H), 2.70 (dd, J=16.0, 5.3 Hz, 1 H), 2.49 - 2.59 (m, 1 H), 1.03 - 1.29 (m, 3 H). 13C (100 MHz, DMSO-d6): 172.8, 170.7, 156.2, 144.2, 141.1, 128.1, 127.5, 125.7, 120.5, 66.1, 60.5, 51.6, 47.1, 36.8, 14.5. [α]22D = −7 (c 1.0, MeOH).
(S)-ethyl 3-(((9H-fluoren-9-yl)methoxy)carbonylamino)-3-cyanopropanoate (9)
(S)-ethyl 3-(((9H-fluoren-9-yl)methoxy)carbonylamino)-4-amino-4-oxobutanoate (8) (2.58 g, 6.75 mmol, 1.0 equivalent) was dissolved in dichloromethane (40 mL) and cooled in an ice bath. Diisopropylethylamine (3.53 mL, 20.24 mmol, 3 equivalents) was added, followed by the dropwise addition of trifluoroacetic anhydride (1.43 mL, 10.12 mmol, 2 equivalents). The yellow solution was stirred for 30 minutes at which point TLC showed disappearance of the starting material and the reaction was quenched with aqueous sodium bicarbonate, washed twice with sodium bicarbonate, once with brine, dried over sodium sulfate and concentrated. The residue was purified by column chromatography using 9/1 - 1/1 hexane/EtOAc (v/v) gradient to give 9 as an off-white powder (2.11 g, 86% yield). 1H NMR (400 MHz, DMSO-d6) δ ppm 8.19 (d, J=7.6 Hz, 1 H), 7.86 (d, J=7.6 Hz, 2 H), 7.65 (d, J=7.4 Hz, 2 H), 7.39 (t, J=7.3 Hz, 2 H), 7.30 (t, J=7.4 Hz, 2 H), 4.73 (q, J=7.6 Hz, 1 H), 4.39 (d, J=6.5 Hz, 2 H), 4.22 (d, J=6.3 Hz, 1 H), 4.07 (q, J=7.0 Hz, 2 H), 2.88 (m, 2 H), 1.15 (t, 3 H). 13C NMR (DMSO-d6) δ ppm: 168.9, 155.7, 144.1, 141.2, 128.1, 127.5, 125.5, 120.6, 119.3, 66.5, 61.2, 46.9, 39.4, 36.5, 14.4. [α]22D = −24 (c 0.5, 1/1 MeOH/CH2Cl2).
(S)-(9H-fluoren-9-yl)methyl 1-amino-3-cyano-1-oxopropan-2-ylcarbamate (11)
A suspension of (S)-2-(((9H-fluoren-9-yl)methoxy)carbonylamino)-3-cyanopropanoic acid (10) (5 g, 14.87 mmol, 1 equivalent) in DME (50 ml) was cooled in an ice bath. N-methylmorpholine (1.63 ml, 14.87 mmol, 1 equivalent) was added and the cooled suspension was stirred for 10 minutes. Isobutyl chloroformate (1.95 ml, 14.87 mmol, 1 equivalent) was added dropwise to the cooled solution and stirring was continued for an additional 10 minutes. Ammonium hydroxide (5.79 ml, 149 mmol, 10 equivalents) was then added to the cold suspension at which point the reaction was removed from the ice bath and allowed to warm to RT and stirred an additional 4 h. At this point the reaction was diluted with ethyl acetate (50 mL) and water (75 mL) was added. The mixture was stirred for 15 minutes the the organic layer was separated and washed twice with sodium bicarbonate, once with brine, dried over sodium sulfate and concentrated. The residue was purified by column chromatography using 9/1 - 1/99 hexane/EtOAc (v/v) gradient to give 11 as a white solid (3.64 g, 73% yield). 1H NMR (400 MHz, DMSO-d6) δ ppm 7.78 - 7.93 (m, 2 H), 7.56 - 7.76 (m, 2 H), 7.47 (br. s., 1 H), 7.39 (t, J=7.4 Hz, 2 H), 7.30 (t, J=7.4 Hz, 2 H), 4.20 - 4.39 (m, 3 H), 2.81 - 2.96 (m, 1 H), 2.57 - 2.81 (m, 1 H), 1.11 - 1.32 (m, 1 H). 13C NMR (DMSO-d6) δ ppm: 171.0, 156.2, 144.2, 141.1, 128.1, 127.5, 125.7, 120.6, 118.7, 66.4, 51.1, 47.0, 20.9 [α]22D = −10 (c 1.0, MeOH).
(S)-(9H-fluoren-9-yl)methyl 1-amino-1-oxo-3-(1H-tetrazol-5-yl)propan-2-ylcarbamate (12)
(S)-(9H-fluoren-9-yl)methyl 1-amino-3-cyano-1-oxopropan-2-ylcarbamate (11) (1 g, 2.98 mmol) and dibutylstannanone (0.445 g, 1.789 mmol) were added to a 20 mL Biotage microwave vial and suspended in toluene (20 ml). TMS-azide (0.910 ml, 6.86 mmol) was added and the flask was sealed with a Teflon cap. The sealed flask was submerged in an oil bath at 100 °C and stirred for 12 h. The suspension was transferred to a round bottom flask and the toluene was removed in vaccuo. Reverse phase chromotography gave 12 as a white fluffy powder (870 mg, 77% yield). 1H NMR (400 MHz, DMSO-d6) δ ppm 11.83 - 12.07 (br. s., 1 H), 7.87 (d, J=7.4 Hz, 2 H), 7.57 - 7.71 (m, 4 H), 7.40 (t, J=7.2 Hz, 2 H), 7.30 (t, J=7.2 Hz, 2 H), 7.19 (br. s., 1 H), 4.34 - 4.53 (m, 1 H), 4.11 - 4.30 (m, 2 H), 3.23 - 3.40 (m, 2 H), 3.07 - 3.19 (m, 1 H). 13C NMR (DMSO-d6) δ ppm: 172.3, 156.1, 144.2, 141.1, 128.1, 127.5, 125.8, 125.6, 120.5, 66.2, 53.3, 47.0, 26.2. [α]22D = −10 (c 0.3, MeOH).
(S)-(9H-fluoren-9-yl)methyl 1-cyano-2-(1H-tetrazol-5-yl)ethylcarbamate (13)
(S)-(9H-fluoren-9-yl)methyl 1-amino-1-oxo-3-(1H-tetrazol-5-yl)propan-2-ylcarbamate (12) (.75 g, 1.982 mmol) was dissolved in dichloromethane (10 ml) and cooled in an ice bath. Diisopropylethylamine (1.731 ml, 9.91 mmol) was added, followed by the dropwise addition of trifluoroacetic anhydride (0.700 ml, 4.96 mmol). The yellow solution was stirred for 30 minutes at which point TLC showed disappearance of the starting material and the reaction was quenched and washed with 5% HCl, dried over sodium sulfate and concentrated. The residue was purified by reverse phase chromatography to give 13 as a white powder (714 mg, 80% yield). 1H NMR (400 MHz, DMSO-d6) δ ppm 8.35 (d, J=7.8 Hz, 1 H), 7.78 - 7.95 (m, 2 H), 7.54 - 7.74 (m, 2 H), 7.34 - 7.46 (m, 2 H), 7.30 (t, J=7.3 Hz, 2 H), 6.33 - 6.83 (m, 1 H), 4.99 (dd, J=7.5 Hz, 1 H), 4.38 (d, J=6.7 Hz, 1 H), 4.13 - 4.28 (m, 2 H), 3.46 (m, 1 H), 1.18 (dd, 1 H). 13C NMR (100 MHz, DMSO-d6): 157.0, 155.7, 144.4, 141.2, 128.0, 127.5, 125.6, 120.6, 120.5, 65.4, 47.2, 46.9, 41.2. [α]22D = −11 (c 0.5, MeOH).
(S)-3-((S)-1-((S)-2-(4-amino-3-chlorobenzamido)-3,3-dimethylbutanoyl)pyrrolidine-2-carboxamido)-3-cyanopropanoic acid (4)
(S)-2-(trimethylsilyl)ethyl 3-(((9Hfluoren-9-yl)methoxy)carbonylamino)-3-cyanopropanoate (7) (1.200 g, 2.75 mmol) was dissolved in DMF (10 ml) cooled to 0 °C, and DBU (0.414 ml, 2.75 mmol) was added. The solution was stirred for 5 minutes at which point (S)-1-((S)-2-(4-amino-3-chlorobenzamido)-3,3-dimethylbutanoyl)pyrrolidine-2-carboxylic acid (1 g, 2.62 mmol), diisopropylethyl amine (0.595 ml, 3.40 mmol) and HATU (1.195 g, 3.14 mmol) were added sequentially to the cold solution. The reaction was stirred for 2 h at 0 °C, diluted with ethyl acetate (30 mL) and quenched with saturated aqueous sodium bicarbonate, washed twice with sodium bicarbonate, once with brine, dried over sodium sulfate and concentrated. The residue was purified by column chromatography to give the TMSE protected acid as a white powder. Deprotection was affected by treatment of this ester in THF (0.3 M solution) with TBAF (2 equivalents of a 1.0 M THF solution). The reaction was stirred for 1 h then diluted with ethyl acetate, washed with water, dried over sodium sulfate and concentrated. Reverse phase chromatography gave 4 as a white powder (902 mg, 72% yield over steps). 1H NMR (400 MHz, DMSO-d6) δ ppm 8.68 (d, J=7.4 Hz, 1 H), 7.79 (d, J=1.9 Hz, 1 H), 7.64 (d, J=9.0 Hz, 1 H), 7.56 (dd, J=8.6, 1.9 Hz, 1 H), 6.73 (d, J=8.6 Hz, 1 H), 5.95 (b, 2H), 4.83 (q, J=7.4 Hz, 1H), 4.64 (d, J=9.0 Hz, 1 H), 4.20 - 4.30 (dd, J= 8.2, 5.6 Hz 1 H), 3.69 - 3.80 (m, 1 H), 3.60 (m, 1 H), 2.79 (dd, J=6.7, 3.1 Hz, 2 H), 1.99 - 2.11 (m, 1 H), 1.63 - 1.96 (m, 4 H), 0.99 (s, 9 H) 13C NMR (100 MHz, DMSO-d6): 172.2, 170.6, 169.9, 165.8, 148.0, 129.4, 128.2, 122.1, 119.1, 116.4, 114.4, 59.7, 57.8, 48.2, 37.4, 36.6, 35.3, 29.5, 27.0, 25.1. LC/MS: Method 1, retention time: 4.595 min; Method 2, retention time: 3.591 min; HRMS: m/z (M+H+) = 477.1784 (Calculated for C22H28N5O5Cl = 477.1779). [α]22D = −65 (c 1.0, MeOH).
(S)-ethyl 3-((S)-1-((S)-2-(4-amino-3-chlorobenzamido)-3,3-dimethylbutanoyl)pyrrolidine-2-carboxamido)-3-cyanopropanoate (3)
(S)-ethyl 3-(((9H-fluoren-9-yl)methoxy)carbonylamino)-3-cyanopropanoate (9) (0.437 g, 1.200 mmol) was dissolved in DMF (5 ml) at RT and DBU (0.158 ml, 1.050 mmol) was added and stirred for 5 min upon which LCMS showed cleavaged of Fmoc. (S)-1-((S)-2-(4-amino-3-chlorobenzamido)-3,3-dimethylbutanoyl)pyrrolidine-2-carboxylic acid (0.382 g, 1 mmol) in diisopropylethyl amine (0.262 ml, 1.500 mmol) and HATU (0.456 g, 1.200 mmol) were added sequentially. The solution was stirred at 0 °C for 2 h,diluted with ethyl acetate (30 mL) and quenched with saturated aqueous sodium bicarbonate, washed twice with sodium bicarbonate, once with brine, dried over sodium sulfate and concentrated. Purification by reverse phase chromotography gave 3 as a white powder (460 mg, 91% yield). 1H NMR (400 MHz, DMSO-d6) δ ppm 8.61 - 8.74 (m, 1 H), 7.78 (d, J=2.0 Hz, 1 H), 7.64 (d, J=8.8 Hz, 1 H), 7.56 (dd, J=8.6, 2.0 Hz, 1 H), 6.73 (d, J=8.4 Hz, 1 H), 4.92 (q, J=7.0 Hz, 1 H), 4.65 (d, J=8.8 Hz, 1 H), 4.24 (dd, J=8.2, 5.7 Hz, 1 H), 4.08 (q, J=7.2 Hz, 2 H), 3.69 - 3.80 (m, 1 H), 3.55 - 3.66 (m, 1 H), 2.85 - 2.93 (m, 2 H), 1.97 - 2.10 (m, 1 H), 1.64 - 1.95 (m, 3 H), 1.17 (t, J=7.6 Hz, 3 H), 0.99 (s, 9 H). 13C NMR (100 MHz, DMSO-d6): 171.2, 170.1, 170.0, 164.9, 147.7, 129.4, 128.1, 122.1, 118.7, 116.9, 113.4, 61.1, 60.4, 56.8, 45.1, 37.1, 36.0, 35.1, 29.3, 28.4, 22.1, 15.6. Method 1, retention time: 5.011 min; Method 2, retention time: 3.981 min; HRMS: m/z (M+H+) = 505.2100 (Calculated for C24H32N5O5Cl = 505.2092). [α]22D = −35 (c 0.6, MeOH).
(S)-1-((S)-2-(4-amino-3-chlorobenzamido)-3,3-dimethylbutanoyl)-N-((S)-1-cyano-2-(1H-tetrazol-5-yl)ethyl)pyrrolidine-2-carboxamide (16)
(S)-(9H-fluoren-9-yl)methyl 1-cyano-2-(1H-tetrazol-5-yl)ethylcarbamate (13) (0.727 g, 2.016 mmol) was dissolved in DMF (9 ml) cooled to 0 °C, DBU (0.276 ml, 1.833 mmol) was added and the reaction was stirred for 5 minutes. (S)-1-((S)-2-(4-amino-3-chlorobenzamido)-3,3-dimethylbutanoyl)pyrrolidine-2-carboxylic acid (.7 g, 1.833 mmol), diisopropylethyl amine (0.480 ml, 2.75 mmol) and HATU (0.836 g, 2.200 mmol) were then added sequentially. The solution was stirred at 0 °C for 2 h,diluted with ethyl acetate (30 mL) and quenched with saturated aqueous sodium bicarbonate, washed twice with sodium bicarbonate, once with brine, dried over sodium sulfate and concentrated. Purification by reverse phase chromatography gave 16 as a white powder (755 mg, 82% yield). 1H NMR (400 MHz, DMSO-d6) δ ppm 8.87 (d, J=7.6 Hz, 1 H), 7.73 - 7.86 (m, 1 H), 7.61 - 7.73 (m, 1 H), 7.56 (dd, J=8.5, 1.9 Hz, 1 H), 6.73 (d, J=8.4 Hz, 1 H), 5.15 (q, J=7.4 Hz, 1 H), 4.64 (d, J=8.6 Hz, 1 H), 4.12 - 4.26 (m, 1 H), 3.93 - 4.05 (m, 2 H), 3.68 - 3.80 (m, 1 H), 3.53 - 3.67 (m, 2 H), 3.34 - 3.53 (m, 2 H), 1.97 - 2.10 (m, 1 H), 1.86 - 1.97 (m, 1 H), 1.75 - 1.86 (m, 1 H), 1.57 - 1.75 (m, 1 H), 0.99 (s, 9 H). 13C NMR (100 MHz, DMSO-d6): 172.3, 170.0, 165.9, 148.0, 129.4, 128.2, 122.1, 116.4, 114.4, 59.9, 57.8, 35.3, 29.5, 27.0, 25.1. LC/MS: Method 1, retention time: 4.645 min; Method 2, retention time: 3.596 min; HRMS: m/z (M+H+) = 501.2004 (Calculated for C22H28N9O3Cl = 501.2004). [α]22D = −23 (c 0.5, MeOH).
Supplementary Material
Acknowledgments
We thank Ms. Allison Mandich for critical reading of this manuscript and Mr. Paul Shinn for assistance with compound management. This research was supported by the Molecular Libraries Initiative of the National Institutes of Health Roadmap for Medical Research and the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
References
- 1.Earnshaw WC, Martins LM, Kaufmann SH. Annu. Rev. Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 2.Denault J-B, Salvesen GS. Chem. Rev. 2002;102:4489–4499. doi: 10.1021/cr010183n. [DOI] [PubMed] [Google Scholar]
- 3.Martinon F, Tschopp J. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Braddock M, Quinn A. Nat. Rev. Drug Discovery. 2004;3:1–10. doi: 10.1038/nrd1342. [DOI] [PubMed] [Google Scholar]
- 5.Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. Nat. Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu HB, Finlay BB. Cell Host & Microbe. 2008;4:198–208. doi: 10.1016/j.chom.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Turk B. Nat. Rev. Drug Discovery. 2006;5:785–799. doi: 10.1038/nrd2092. [DOI] [PubMed] [Google Scholar]
- 8.Howley B, Fearnhead HO. J. Cell. Mol. Med. 2008;12:1502–1516. doi: 10.1111/j.1582-4934.2008.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linton SD. Curr. Top. Med. Chem. 2005;5:1697–1717. doi: 10.2174/156802605775009720. [DOI] [PubMed] [Google Scholar]
- 10.Cornelis S, Kersse K, Festjens N, Lamkanfi M, Vandenabeele P. Curr. Pharm. Design. 2007;13:367–385. doi: 10.2174/138161207780163006. [DOI] [PubMed] [Google Scholar]
- 11.Wannamaker W, Davies R, Namchuk M, Pollard J, Ford P, Ku G, Decker C, Charifson P, Weber P, Germann UA, Kuida K, Randle JCR. J. Pharmacol. Exp. Ther. 2007;321:509–516. doi: 10.1124/jpet.106.111344. [DOI] [PubMed] [Google Scholar]
- 12.Rasnick D. Perspect. Drug Discovery Des. 1996;6:47–63. [Google Scholar]
- 13.Hernandez AA, Roush WR. Curr. Opin. Chem. Biol. 2002;6:459–465. doi: 10.1016/s1367-5931(02)00345-9. [DOI] [PubMed] [Google Scholar]
- 14.Vicik R, Busemann M, Baumann K, Schirmeister T. Curr. Top. Med. Chem. 2006;6:331–353. doi: 10.2174/156802606776287081. [DOI] [PubMed] [Google Scholar]
- 15.Falgueyret J-P, Oballa RM, Okamoto O, Wesolowski G, Aubin Y, Rydzewski RM, Prasit P, Riendeau D, Rodan SB, Percival MD. J. Med. Chem. 2001;44:94–104. doi: 10.1021/jm0003440. [DOI] [PubMed] [Google Scholar]
- 16.Mallari JP, Shelat AA, Obrien T, Caffrey CR, Kosinski A, Connelly M, Harbut M, Greenbaum D, McKerrow JH, Guy RK. J. Med. Chem. 2008;51:545–552. doi: 10.1021/jm070760l. [DOI] [PubMed] [Google Scholar]
- 17.Mott BT, Ferreira R, Simeonov A, Jadhav A, Ang K-H, Leister W, Shen M, Silveira JT, McKerrow JH, Inglese J, Austin CP, Thomas CJ, Shoichet BK, Maloney DJ. J. Med. Chem. in press DOI: 10.1021/jm901069a. [Google Scholar]
- 18.Oballa RM, Truchon J-F, Bayly CI, Chauret N, Day S, Crane S, Berthelette C. Bioorg. Med. Chem. Lett. 2007;17:998–1002. doi: 10.1016/j.bmcl.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 19.Le GT, Abbenante G, Madala PK, Hoang HN, Fairlie DP. J. Am. Chem. Soc. 2006;128:12396–12397. doi: 10.1021/ja0637649. [DOI] [PubMed] [Google Scholar]
- 20.Caprathe BW, Gilmore JL, Harter WG, Hays SJ, Knapp KM, Dostlan CR, Lunney EA, Para KS, Galatsis P, Thomas AJ. WO 9956765 PCT Int. Appl. 1999
- 21.Knobelsdorf J, Hays S, Stankovic CJ, Para KS, Connolly MK, Galatsis P, Harter W, Shahripor AB, Plummer MS, Lunney B, Janssen B, Fell JB. WO 2002089749 PCT Int. Appl. 2002
- 22.Batchelor MJ, Bebbington D, Bemis GW, Fridman WH, Gillespie RJ, Golec JMC, Lauffer DJ, Livingston DJ, Matharu SS, Mullican MD, Murcko MA, Murdoch R, Zelle RE. US5874424 PCT US. Appl. 1999
- 23.Sureshbabu VV, Venkataramanarao R, Naik SA, Chennakrishnareddy G. Tetrahedron Lett. 2007;48:7038–7041. [Google Scholar]
- 24.Wittenberger SJ, Donner BG. J. Org. Chem. 1993;58:4139–4141. [Google Scholar]
- 25.Tanoury GJ, Chen M, Dong Y, Forslund RE, Magdziak D. Org. Lett. 2008;10:185–188. doi: 10.1021/ol702532h. [DOI] [PubMed] [Google Scholar]
- 26.Parikh JP, Doering WE. J. Am. Chem. Soc. 1967;89:5505–5507. [Google Scholar]
- 27. http://www.reactionbiology.com/
- 28. http://www.cyprotex.com/home/
- 29.Wilson KP, Black J-AF, Thomason JA, Kim EE, Griffith JP, Navia MA, Murcko MA, Chambers SP, Aldape RA, Raybuck SA, Libingston DJ. Nature. 1994;370:270–275. doi: 10.1038/370270a0. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto Y, Anan H, Nakai E, Morihira K, Yonetoku Y, Kurihara H, Sakashita H, Terai Y, Takeuchi M, Shibanuma T, Isomura Y. Chem. Pharm. Bull. 1999;47:11–21. doi: 10.1248/cpb.47.11. [DOI] [PubMed] [Google Scholar]
- 31.Romanowski MJ, Scheer JM, O'Brien T, McDowell RS. Structure. 2004;12:1361–1371. doi: 10.1016/j.str.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Scheer JM, Romanowski MJ, Wells JA. Proc. Nat. Acad. Sci. U. S. A. 2006;103:7595–7600. doi: 10.1073/pnas.0602571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.OpenEye Scientific Software, Inc. Santa Fe, NM: http://www.eyesopen.com/ [Google Scholar]
- 34.Dufour E, Storer AC, Ménard R. Biochemistry. 1995;34:9136–9143. doi: 10.1021/bi00028a024. [DOI] [PubMed] [Google Scholar]
- 35.Hill AV. J. Physiol. (London) 1910;40:4–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.