Abstract
Objective
To develop an animal model of intraocular tuberculosis (TB) with features of pulmonary TB and extrapulmonary dissemination to the eye.
Methods
Hartley strain guinea pigs were infected via an aerosol route with virulent Mycobacterium tuberculosis. One group of guinea pigs was infected with a relatively low bacterial inoculum and received no treatment. A second group of guinea pigs received high-dose infection and were treated with the first-line anti-TB drugs isoniazid, rifampin, and pyrazinamide. Development of ocular TB lesions was documented by histological analysis, acid-fast staining, and real-time polymerase chain reaction for M tuberculosis DNA.
Results
Untreated guinea pigs developed pulmonary and extrapulmonary TB. Ocular TB, primarily involving the uvea, developed in 5 of 12 eyes (42%). Uveal granulomatous lesions showed the presence of acid-fast organisms and M tuberculosis DNA. In treated animals, none of the 8 eyes examined revealed the presence of acid-fast organisms; however, there was mild nongranulomatous uveitis in 4 eyes.
Conclusions
Mycobacterium tuberculosis delivered via aerosol to guinea pigs results in extrapulmonary dissemination to the eye. Of significance, intraocular changes in this model include granulomatous inflammation and the presence of acid-fast organisms, as seen in human cases of ocular TB.
Clinical Relevance
The guinea pig model may provide greater insight into the pathogenesis of intraocular TB and assist in the development of novel modalities to treat this global infectious disease.
Mycobacterium tuberculosis infects one-third of the global population and claims 2 million lives annually.1 Although the rate of infection varies geographically, it roughly reflects the economic status of a nation. Tuberculosis (TB) is hyperendemic in Africa, with more than 250 cases per 100 000 population, and there are 100 to 249 cases per 100 000 population in Southeast Asia, China, and Russia.2 The rate of infection is relatively low (<10 cases per 100 000 population) in North America, certain European countries, and Australia. However, because of population migration from highly endemic regions, the citizens of countries with low endemic TB rates remain at risk for this important infection.
Extrapulmonary TB can occur during primary infection or after reactivation of a latent focus. In the United States, approximately 20% of patients with TB, but more than half of patients with human immunodeficiency virus or AIDS, have extrapulmonary TB; the risk of dissemination correlates with the degree of immunosuppression.3,4 After inhalation into the lungs, the tubercle bacilli may disseminate hematogenously or via the lymphatic system to most organ systems, including the eye. Although tuberculous ocular changes may occur in conjunction with active pulmonary infection, they are commonly seen in isolation, without clinical evidence of pulmonary disease.5 The diagnosis of ocular TB may be challenging in patients with isolated ocular findings, particularly in the absence of invasive diagnostic procedures, such as analysis of ocular fluids and/or tissue by mycobacterial culture, histological analysis, and nucleic acid amplification techniques.
Of the various ophthalmic changes seen in patients with TB, uveitis is the most common clinical feature5 Among patients with uveitis, the prevalence of intraocular TB is about 10% in developing countries and less than 1% in the Los Angeles area.5,6 In both the developing and the developed world, a common clinical manifestation of intraocular TB is posterior uveitis mimicking various uveitis entities, including multifocal choroiditis, serpiginous choroiditis, retinal vasculitis, mass lesion in the choroid, or subretinal abscess.5,7–9 Establishing a diagnosis of TB in these cases may be challenging because many patients may lack systemic or pulmonary symptoms and some may have a chest radiograph without signs of TB and a negative response on a tuberculin skin test. Although polymerase chain reaction (PCR)-based detection of M tuberculosis DNA in intraocular fluids can be useful, the sensitivity of this molecular technique appears to be suboptimal.5,10 In most instances, the diagnosis of TB uveitis is a clinical one, usually made retrospectively when patients respond to empirically administered anti-TB agents.5 Because anti-TB therapy can be associated with significant adverse effects, including drug-induced hepatitis, accurate etiologic diagnosis is important.
Our understanding of pulmonary TB pathogenesis has been assisted greatly by the availability of well-established animal models.11 In contrast, the pathogenesis of intraocular TB remains poorly understood, partly because of the lack of adequate animal models that accurately simulate human disease. Although experimental ocular TB has been reported in rabbits, primates, guinea pigs, and other animals, the ocular infection was induced either by direct intraocular administration or by intracarotid injection of the infectious agent.12–18 Such models are deficient because they do not reproduce the natural route of infection through the lungs. In this study, we attempted to develop a model of ocular TB resulting from hematogenous dissemination of M tuberculosis following aerosol delivery of the organisms to guinea pig lungs.19 All such exposed animals developed pulmonary TB, and several also showed ocular involvement, with histopathological features virtually identical to those observed in human ocular TB. We also assessed the efficacy of systemically administered standard anti-TB drugs in preventing intraocular changes in this model.
METHODS
Six Hartley strain guinea pigs (weight range, 300–350 g each) were infected by the aerosol route with virulent M tuberculosis strains CDC1551 or H37Rv using the Inhalation Exposure System (Glas-Col LLC, Terre Haute, Indiana), calibrated to deliver 103 to 104 colony-forming units (CFUs) per lung on the day following infection. Animals were humanely killed on day 56 after infection, and lung and spleen tissues were cultured for M tuberculosis. The lungs were homogenized in 10 to 20 mL of phosphate-buffered saline using a Kinematica Polytron Homogenizer (Bohemia, New York) with a 12-mm generator (Brinkmann Instruments, Inc, Westbury, New York) within a BSL-III Glovebox Cabinet (Germfree Laboratories, Ormond Beach, Florida), as previously described.19 The spleens were homogenized using glass homogenizers. Organ homogenates were plated on Middlebrook 7H11 selective plates containing carbenicillin, polymyxin B, amphotericin B, and trimethoprim (BD Biosciences, Sparks, Maryland) for CFU determination; log-transformed CFU values were used to calculate means and standard errors (Table 1). Sections of lung and the enucleated eyes were formalin fixed and processed for paraffin embedding, hematoxylin-eosin staining, and Ziehl-Neelsen acid-fast staining. Paraffin-embedded, 100-μm sections of each guinea pig globe were processed for quantitative PCR using the following M tuberculosis-specific primers: forward primer 5′-AGGCGAACCCTGCCCAG-3′ and reverse primer IS7 5′-GATCGCTGATCCGGCCA-3′, which are known to amplify a 122-base pair fragment of IS6110 multicopy element (GenBank accession number X52471).
Table 1.
Ocular Involvement in Guinea Pigs With Systemic Tuberculosis
| Animal No. | Granulomatous Inflammation | Location | AFB | qPCR |
|---|---|---|---|---|
| CDC1 | ||||
| OD | No | NA | No | ND |
| OS | Yes | Choroid | No | 287a |
| CDC 2 | ||||
| OD | Yes | Choroid | No | 315a |
| OS | No | NA | No | ND |
| CDC 3 | ||||
| OD | No | NA | No | ND |
| OS | Yes | Choroid | Yes | 1244a |
| CDC 4 | ||||
| OD | Yes | Choroid | No | ND |
| OS | No | NA | No | ND |
| H37Rv 5 | ||||
| OD | No | NA | No | ND |
| OS | Yes | Limbus | Yes | 883a |
| H37Rv 6 | ||||
| OD | No | NA | No | ND |
| OS | No | NA | No | ND |
Abbreviations: AFB, acid-fast bacilli (detected by acid-fast stain); CDC, Mycobacterium tuberculosis CDC1551; H37Rv, M tuberculosis H37Rv; NA, not applicable; ND, not detected; OD, right eye; OS, left eye; qPCR, quantitative polymerase chain reaction.
Mycobacterial genome load per 1 μg of total tissue DNA.
A second group of 4 guinea pigs were aerosol-infected with M tuberculosis strain H37Rv at an implantation inoculum of approximately 105 CFUs per lung. Oral therapy was initiated 14 days after infection and consisted of isoniazid (60 mg/kg), rifampin (100 mg/kg), and pyrazinamide (300 mg/kg) in 40% sucrose. Guinea pig doses were based on pharmacokinetic data modeling of the human area under the plasma concentration-time curve for each drug.20 Antibiotic therapy was administered 5 times weekly for the first 14 days, then twice weekly for the remainder of the experiment, and eye samples were evaluated on day 56 after antibiotic treatment. The enucleated eyes were submitted to paraffin embedding and hematoxylin-eosin and Ziehl-Neelsen acid-fast staining. Ocular tissues for these experiments were obtained from animals included in a larger TB chemotherapy study, the results of which will be published separately.
RESULTS
The mean (SE) implantation dose of untreated guinea pigs infected with M tuberculosis CDC1551 was 3.05 (0.05) log10 CFUs, whereas that of treated M tuberculosis H37Rv-infected animals was 4.24(0.15) log10 CFUs. By day 56 after infection, the mean (SE) lung and spleen values were 7.3(0.7) and 5.8(0.6) log10 CFUs, respectively, for M tuberculosis CDC 1551-infected guinea pigs and 6.7(0.5) and 5.8 (0.5) log10 CFUs, respectively, for M tuberculosis H37Rv-infected guinea pigs (Table 2). On day 56 after infection, gross examination of untreated guinea pig lungs revealed several nodules (Figure 1A). Histopathological evaluation of these nodules using acid-fast stains revealed necrotizing granulomatous inflammation and the presence of acid-fast bacilli (Figure 1B). Of 6 guinea pigs examined, 5 had unilateral granulomatous ocular inflammation on histological analysis (Table 1). Four eyes had uveal involvement (Figure 2), and 1 eye had a granuloma involving the limbus. Quantitative PCR was positive for M tuberculosis DNA in 3 eyes with uveal involvement and in the eye with the limbal granuloma. In contrast, acid-fast-positive bacteria were detected in 2 eyes with choroidal (Figure 2) and limbal granulomas. The remaining eyes were negative for M tuberculosis by acid-fast staining and PCR. The corresponding CFUs in the lung and spleen of each animal are summarized in Table 2.
Table 2.
Guinea Pig Lung and Spleen CFUs on Day 56 After Aerosol Infection
| Animal No. | CFUs, Log10 |
|
|---|---|---|
| Lung | Spleen | |
| CDC 1 | 7.2 | 6.0 |
| CDC 2 | 6.6 | 5.9 |
| CDC 3 | 7.2 | 4.9 |
| CDC 4 | 8.3 | 6.3 |
| H37Rv 5 | 6.9 | 5.2 |
| H37Rv 6 | 6.2 | 6.0 |
Abbreviations: CDC, Mycobacterium tuberculosis CDC1551; CFUs, colony-forming units; H37Rv, M tuberculosis H37Rv.
Figure 1.
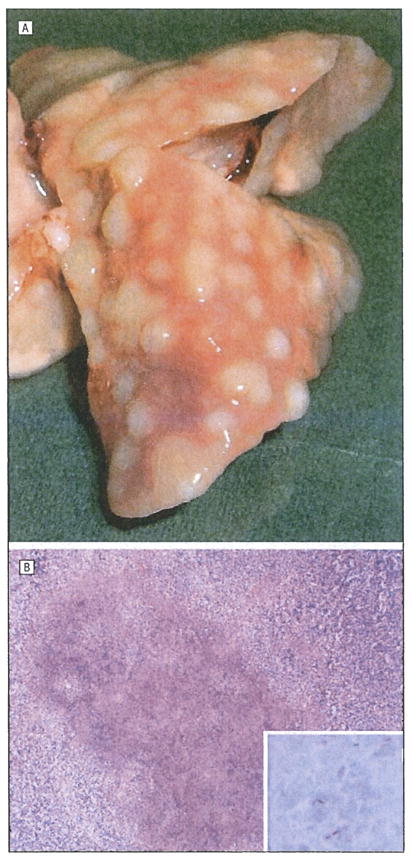
Guinea pigs were aerosol-infected with virulent Mycobacterium tuberculosis. All animals developed multiple pulmonary nodules (A). Histopathological evaluation of these lesions revealed necrotizing granulomatous inflammation (B) (hematoxylin-eosin, original magnification ×40). The inset shows acid-fast bacilli (Ziehl-Neelsen, original magnification ×1000).
Figure 2.
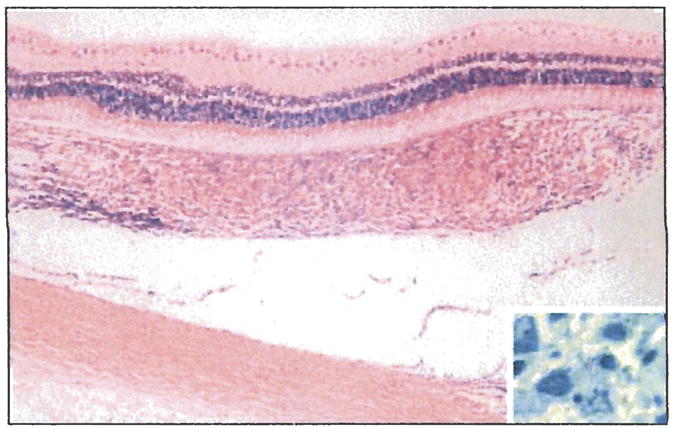
Granulomatous choroiditis in guinea pigs following aerosol infection with Mycobacterium tuberculosis (hematoxylin-eosin, original magnification ×240). The inset shows an acid-fast bacillus in the choroidal inflammation (Ziehl-Neelsen, original magnification ×600).
In the second group of guinea pigs treated with the standard anti-TB regimen rifampin, isoniazid, and pyrazinamide beginning on day 14 after aerosol infection, 1 guinea pig eye had a few lymphocytes in the ciliary body on day 56 after infection. Three other eyes had mild focal lymphocytic infiltration in the choroid, and no eyes showed granulomatous inflammation. All eyes had intact retinal pigment epithelium, retina, optic disc, and sclera without inflammatory cell infiltration. The chronic uveal inflammation was mild and was unlike the granulomatous uveal inflammation noted in the untreated animals. The results of acid-fast staining were negative for M tuberculosis in the eye sections of all guinea pigs treated with anti-TB therapy.
COMMENT
In the present study, all untreated animals exposed to virulent M tuberculosis by the aerosol route developed pulmonary lesions with evidence of dissemination to the spleen. Infected guinea pigs receiving no anti-TB therapy also developed granulomatous lesions in the eyes, in either the uveal tract or the limbus. Lesions were present in 5 of 12 eyes (42%), and acid-fast staining and/or quantitative PCR revealed the presence of M tuberculosis (Table 1). These observations indicate that guinea pigs infected with M tuberculosis via an aerosol route may offer a model to determine the pathogenesis of ocular TB, and TB uveitis in particular. The development of pulmonary TB in all animals and ocular lesions in several suggests that guinea pigs with ocular TB exhibit features similar to those seen in humans with TB. Moreover, histological analysis of the pulmonary and ocular lesions revealed granulomatous inflammation similar to that observed in humans with TB.
Diagnosis and treatment of intraocular TB continues to present challenges owing, in part, to a lack of pathological material and a lack of systematic study of the pathogenesis of intraocular TB. The scarcity of intraocular tissues obtained for diagnosis has impeded our understanding of the pathogenesis of intraocular TB. Moreover, in rare cases in which human uvea, retina, or enucleated eyes become available, the disease process is often that of late-stage TB. Thus, even extensive histological studies of such eyes may not provide a clear understanding of the early events in ocular TB pathogenesis. Such deficiencies can be adequately addressed in future studies by using the current animal model, which mimics human disease both clinically and histopathologically, to correlate ophthalmic findings with quantitative PCR examination of aqueous humor and culture of the affected intraocular tissue for M tuberculosis.
Although the present study provides a useful animal model, confirmed by histological changes, acid-fast staining, and quantitative PCR methods, it lacks revealing ophthalmic clinical findings, primarily because the infectious nature of the disease requires a special setup for ophthalmic examination. However, additional studies are in process for the documentation of clinical findings in guinea pigs exposed to M tuberculosis, using a setup that properly protects the ophthalmic clinical investigator from exposure to the infectious organisms. In such a setup, sequential ophthalmic examination of the infected guinea pigs can provide proper clinicopathological correlations and, ultimately, an improved understanding of the pathogenesis of intraocular TB.
In humans, ocular TB manifests clinically with features of inflammation involving either extraocular structures, such as the lacrimal system, conjunctiva, sclera, and cornea, or intraocular inflammation, such as posterior uveitis.3,21–24 Similarly, the guinea pig model reveals primarily posterior uveitis, followed by intermediate and limbal inflammation. Of significance, all these sites showed granulomatous inflammatory cell infiltration, and some showed the presence of acid-fast organisms. Although the number of samples was small, as expected, quantitative PCR appeared to be more sensitive at detecting the presence of M tuberculosis relative to acid-fast staining techniques. Such similarities in histopathological changes to those seen in human ocular TB indicate that guinea pigs exposed to M tuberculosis by the aerosol route offer an excellent model to address the pathogenesis of intraocular TB. However, the lack of vasculature in the guinea pig retina would preclude any study of retinal vasculitis pathogenesis in the guinea pig model.
In contrast to the untreated animals, the group of guinea pigs that received anti-TB agents showed an absence of granulomatous inflammation. These findings suggest that systemic administration of anti-TB drugs may be effective in preventing ocular TB. However, in some eyes of treated animals, a mild, nonspecific uveal inflammation in the absence of acid-fast organisms was observed histologically. Such residual inflammation may reflect a resolving process or persistent mild inflammatory immune responses directed against the infectious agent or bacterial antigens. Thus, a combination of anti-TB treatment with systemic corticosteroids may be required for complete resolution of TB uveitis. Future studies are required to investigate the efficacy of anti-TB drugs with or without administration of systemic corticosteroids in reversing histopathological changes, eliminating residual nongranulomatous uveitis and reducing intraocular CFUs following establishment of ocular TB disease.
Clearly, the current ocular TB animal model, by virtue of revealing various ocular manifestations that simulate human ocular TB, could provide a unique opportunity to address the pathogenesis of intraocular TB and to evaluate current and novel anti-TB agents in the treatment of this infectious disease. A clear understanding of the pathogenesis requires sequential study of ocular changes using classical histopathological analysis, immunohistological studies, and documentation of the bacterial load at the site of the ocular lesions, both by microbiological culture and by quantitative PCR. Although PCR using aqueous humor is the recommended investigation in human cases of clinically suspected intraocular TB, there is no validated data on the sensitivity and specificity of such an approach. Analysis of aqueous humor in the guinea pig TB model may offer an ideal system for determining the sensitivity and specificity of classical and real-time PCR in the diagnosis of intraocular TB. We believe that this animal model will contribute significantly to our understanding of human intraocular TB pathogenesis, as well as to the development of a rational and reliable approach to diagnose and treat ocular TB.
Acknowledgments
Funding/Support: This study was supported in part by grants EY 03040 (Dr Rao) and AI064229 (Dr Karakousis) from the National Institutes of Health and a TB Drug Accelerator grant from The Bill and Melinda Gates Foundation (Dr Karakousis and Jacques Grosset, MD).
Footnotes
Financial Disclosure: None reported.
References
- 1. [Accessed June 12, 2009.];World Health Organization tuberculosis fact sheet. http://www.who.int/mediacentre/factsheets/fs104/en/
- 2.Kaufmann SH, McMichael AJ. Annulling a dangerous liaison: vaccination strategies against AIDS and tuberculosis. Nat Med. 2005;11(4)(suppl):S33–S44. doi: 10.1038/nm1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Z, Kong Y, Wilson F, et al. Identification of risk factors for extrapulmonary tuberculosis [published online December 19, 2003] Clin Infect Dis. 2004;38 (2):199–205. doi: 10.1086/380644. [DOI] [PubMed] [Google Scholar]
- 4.Karakousis PC, Chaisson RE. Mycobacterial infections and HIV infection. In: Fishman AP, Elias JA, Fishman JA, Grippi MA, Senior RM, Pack AI, editors. Fishman’s Pulmonary Diseases and Disorders. 4. New York, NY: McGraw Hill; 2008. pp. 2487–2497. [Google Scholar]
- 5.Gupta V, Gupta A, Rao NA. Intraocular tuberculosis: an update. Surv Ophthalmol. 2007;52(6):561–587. doi: 10.1016/j.survophthal.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Henderly DE, Genstler AJ, Smith RE, Rao NA. Changing patterns of uveitis. Am J Ophthalmol. 1987;103(2):131–136. doi: 10.1016/s0002-9394(14)74217-5. [DOI] [PubMed] [Google Scholar]
- 7.Varma D, Anand S, Reddy AR, et al. Tuberculosis: an under-diagnosed aetiological agent in uveitis with an effective treatment. Eye. 2006;20(9):1068–1073. doi: 10.1038/sj.eye.6702093. [DOI] [PubMed] [Google Scholar]
- 8.Gupta V, Gupta A, Arora S, Bambery P, Dogra MR, Agarwal A. Presumed tubercular serpiginouslike choroiditis: clinical presentations and management. Ophthalmology. 2003;110(9):1744–1749. doi: 10.1016/S0161-6420(03)00619-5. [DOI] [PubMed] [Google Scholar]
- 9.Babu RB, Sudharshan S, Kumarasamy N, Therese L, Biswas J. Ocular tuberculosis in acquired immunodeficiency syndrome. Am J Ophthalmol. 2006;142 (3):413–418. doi: 10.1016/j.ajo.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 10.Ortega Larrocea G, Bobadilla del Valle M, Ponce de Leon A, Sifuentes Osornio J. Nested polymerase chain reaction for Mycobacterium tuberculosis DNA detection in aqueous and vitreous of patients with uveitis. Arch Med Res. 2003;34(2):116–119. doi: 10.1016/S0188-4409(02)00467-8. [DOI] [PubMed] [Google Scholar]
- 11.Flynn JL, Chan J. Animal models of tuberculosis. In: Rom WN, Garay SM, editors. Tuberculosis. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. pp. 237–250. [Google Scholar]
- 12.Finnoff WC. The relation of tuberculosis to chronic uveitis. Am J Ophthalmol. 1931;14(12):1208–1227. [Google Scholar]
- 13.Woods AC. Experimental studies on the pathogenesis and treatment of ocular tuberculosis. Br J Ophthalmol. 1949;33(4):197–228. doi: 10.1136/bjo.33.4.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woods AC, Wood R, Senterfit LB. Studies in experimental ocular tuberculosis, XVIII: the effect of cortisone combined with specific antibacterial therapy on experimental oculartuberculosis in the immune-allergic rabbit. AMA Arch Opthalmol. 1958;59(4):559–578. [PubMed] [Google Scholar]
- 15.Lepri G, Capalbi S. Influence of terramycin on the course of experimental ocular tuberculosis: comparative activity of terramycin, streptomycin, and both antibiotics combined. Br J Ophthalmol. 1952;36(2):75–80. doi: 10.1136/bjo.36.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capuano SV, III, Croix DA, Pawar S, et al. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect Immun. 2003;71(10):5831–5844. doi: 10.1128/IAI.71.10.5831-5844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woods AC, Burky EL, Friedenwald JS. Experimental studies of ocular tuberculosis. Arch Ophthalmol. 1938;19(2):245–250. [Google Scholar]
- 18.Walsh GP, Tan EV, Dela Cruz EC, et al. The Philippine cynomolgus monkey (Mucaca fascicularis) provides a new non-human primate model of tuberculosis that resembles human disease. Nat Med. 1996;2(4):430–436. doi: 10.1038/nm0496-430. [DOI] [PubMed] [Google Scholar]
- 19.Klinkenberg LG, Sutherland LA, Bishai WR, Karakousis PC. Metronidazole lacks activity against Mycobacterium tuberculosis in an in vivo hypoxic granuloma model of latency. J Infect Dis. 2008;198(2):275–283. doi: 10.1086/589515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karakousis PC, Parry Z, Klinkenberg LG, Pinn ML, Nuermberger E, Grosset J. A guinea pig model of TB chemotherapy. Paper presented at: US Japan Cooperative Medical Sciences Program 43rd Tuberculosis and Leprosy Research Conference; July 10, 2008; Baltimore, MD. [Google Scholar]
- 21.Sheu SJ, Shyu JS, Chen LM, Chen YY, Chirn SC, Wang JS. Ocular manifestations of tuberculosis. Ophthalmology. 2001;108(9):1580–1585. doi: 10.1016/s0161-6420(01)00693-5. [DOI] [PubMed] [Google Scholar]
- 22.Morimura Y, Okada AA, Kawahara S, et al. Tuberculin skin testing in uveitis patients and treatment of presumed intraocular tuberculosis in Japan. Ophthalmology. 2002;109(5):851–857. doi: 10.1016/s0161-6420(02)00973-9. [DOI] [PubMed] [Google Scholar]
- 23.Donahue HC. Ophthalmic experience in a tuberculosis sanitorium. Am J Ophthalmol. 1967;64(4):742–748. doi: 10.1016/0002-9394(67)92860-7. [DOI] [PubMed] [Google Scholar]
- 24.Rao NA, Saraswathy S, Smith RE. Tuberculous uveitis: distribution of Mycobacterium tuberculosis in the retinal pigment epithelium. Arch Ophthalmol. 2006;124(12):1777–1779. doi: 10.1001/archopht.124.12.1777. [DOI] [PubMed] [Google Scholar]


