Abstract
Vago-vagal reflex circuits in the medulla are responsible for the smooth coordination of the digestive processes carried out from the oral cavity to the transverse colon. In this themes article, we concentrate mostly on electrophysiological studies concerning the extrinsic modulation of these vago-vagal reflex circuits, with a particular emphasis on two types of modulation, i.e., by “fast” classic neurotransmitters and by “slow” neuromodulators. These examples review two of the most potent modulatory processes at work within the dorsal vagal complex, which have dramatic effects on gastrointestinal function. The reader should be mindful of the fact that many more different inputs from other central nervous system (CNS) loci or circulating humoral factors add to this complex mix of modulatory inputs. It is likely that similar long-term modulations of synaptic transmission occur with other neurotransmitters and may represent an important mechanism for the integration and regulation of neuronal behavior. Of course, this fact strongly militates against the success of any single drug or approach in the treatment of motility disorders having a CNS component.
Keywords: thyrotropin-releasing hormone, tumor necrosis factor, gastric motility
The nucleus of the tractus solitarii (NTS) receives a host of gastrointestinal (GI) mechano- and chemosensory information via the vagus nerve (19). General visceral afferent signals in the vagus excite second-order NTS neurons. Several neurophysiological studies, including our own, suggest that the rapid, stimulus-locked excitation of NTS neurons resulting from activation of vagal afferents is caused by the synaptic release of glutamate (15, 22).
Neurophysiological, neuroimmunological, and neuropharmacological studies suggest that, in the main, NTS neurons inhibit dorsal motor nucleus of the vagus (DMV) neurons, i.e., those DMV neurons providing tonic excitatory (cholinergic) vagal input to the stomach (12, 21, 23). Recent studies also suggest that a subset of NTS neurons may also activate a different set of DMV neurons, forming an inhibitory nonadrenergic, noncholinergic (NANC) path to the gut (12, 20, 15). Thus stimuli that activate NTS neurons forming the vago-vagal control loop can cause gastroinhibition by removing cholinergic excitation and by activating a parallel NANC inhibitory path (Ref. 23; Fig. 1).
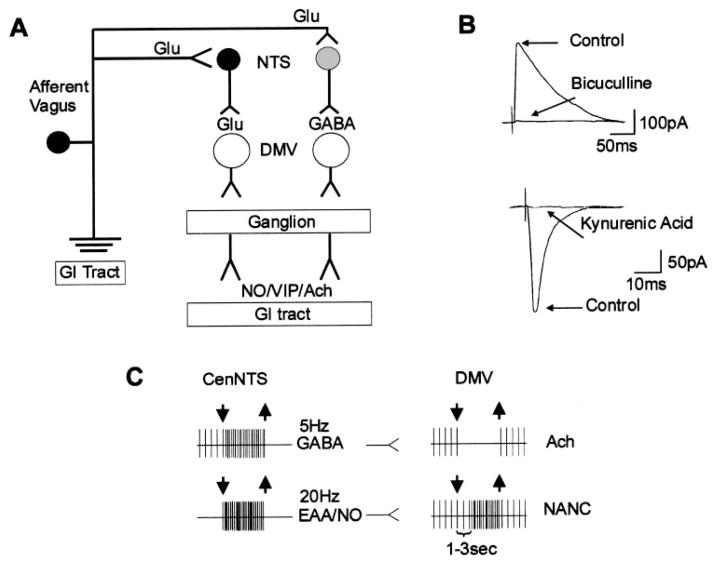
Fig. 1.
Schematic representation of vago-vagal reflexes. Vagal afferent fibers perceive sensory information from the gastrointestinal (GI) tract and, using glutamate (Glu) as the main neurotransmitter, activate nucleus tractus solitarii (NTS) neurons. The NTS then, using glutamate and GABA as the main neurotransmitters, conveys the information to the dorsal motor nucleus of the vagus (DMV) neurons, which, by means of nonadrenergic, noncholinergic (NANC) or cholinergic vagal efferent fibers, control the functions of the subdiaphragmatic upper GI tract (A). Electrical stimulation of the NTS evokes GABAergic or glutamatergic currents in the DMV (B). Electrophysiological (in vivo) traces show that the gastric relaxation obtained on esophageal stimulation induces a dramatic increase in the firing rate of NTS neurons in the subnucleus centralis (CenNTS) but also affects different sets of DMV neurons (C). One circuit, likely controlled by GABAergic NTS neurons, withdraws the cholinergic vagal output; the other circuit, likely controlled by glutamatergic NTS neurons, activates an inhibitory NANC path to the gut. The functional result is a gastroinhibition obtained by removal of cholinergic excitation and by activation of a parallel NANC inhibitory path. NO, nitric oxide; VIP, vasoactive intestinal polypeptide; EAA, excitatory amino acids.
FAST SYNAPTIC CONNECTIONS BETWEEN NTS AND DMV
In vitro neurophysiological studies have shown that GABA and/or glutamate are the principal NTS sources of fast synaptic input responsible for regulating the excitability of DMV neurons (3, 23). Although the NTS subnuclei responsible for such discrete effects were not identified, such responses (i.e., GABA only, glutamate only, or both) were determined by the position of the stimulating electrode within the NTS. Willis et al. (28) showed the existence of a monosynaptic excitatory pathway from NTS to DMV, with excitation of DMV neurons being mediated by activation of at least three different glutamatergic receptors, N-methyl-D-aspartate (NMDA), amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA), and kainate. The actions of fast neurotransmitters in NTS and DMV vis-à-vis control of digestion were the subject of another recent themes article (11).
These intrinsic fast synaptic connections between vagal afferents, NTS, and DMV are capable of coordinating numerous digestive processes through vago-vagal reflex mechanisms. However, it is clear that these intrinsic circuits can be dominated by the effects of extrinsic CNS inputs as well as by circulating signal molecules. The most potent known examples of extrinsic control over intrinsic gastric vago-vagal circuitry are reviewed here with a particular emphasis on electrophysiological studies.
DESCENDING CNS MODULATION OF BRAIN STEM VAGO-VAGAL CIRCUITS
Numerous sources of descending CNS input to DVC reflex circuits have been described over the last 20 years; reviews of this literature are available else-where (1, 7, 21). However, the combined thyrotropin-releasing hormone (TRH)- and serotonin (5-HT)-containing projection from the medullary raphe deserves attention here because it serves as an example of how modulatory inputs from a single CNS source can appropriate the function of a gastric control reflex by acting at multiple sites within the basic reflex circuit. TRH and 5-HT together can produce profound increases in gastric motility and acid secretion. The over-all physiological role for TRH-5-HT in the control of the DVC is not proven. However, it is possible that this pathway acts to augment digestive processes to produce core heating as an emergency response to cold stress (21).
TRH effects on digestion are ultimately caused by a significant increase in the activity of cholinergic DMV efferents (12, 21, 23). This increase in vagal cholinergic output to the stomach is reinforced at several levels within the DVC by TRH. 1) TRH activates DMV neurons directly by modulating calcium-dependent potassium conductances (25). 2) TRH causes disinhibition of DMV neurons by acting to inhibit neurons in the NTS directly (3, 14), which, in turn, provide inhibitory GABAergic input to the DMV (23). 3) TRH potentiates the effects of neurotransmitters that also act to inhibit NTS neurons through the modulation of NTS transduction pathways. This latter example of TRH action involves the synergistic interaction with 5-HT, which is also released from raphe projections ending on the DVC. The combined TRH-5-HT interaction provides a clear example of how a “slow” modulatory effect of one signal (TRH) can completely alter the dynamics of gastric vago-vagal reflex control circuitry (3).
NEUROPHYSIOLOGY OF 5-HT AND TRH ACTIONS IN THE DVC TO CONTROL GASTRIC FUNCTIONS
It has long been known that 5-HT- and TRH-containing fibers originating from the medullary raphe nuclei (particularly the raphe obscurus) provide robust input to the DVC and, in consequence, influence GI reflex activity (12, 13, 15, 17). Exogenous application of those substances, both alone and in combination, has been studied in vivo and in vitro. TRH and 5-HT release from the raphe nuclei onto DVC neurons primarily regulate vagally mediated acid secretion and tone and motility of the GI tract (12).
5-HT
The importance of 5-HT in the neurophysiology of the DVC resides in its powerful modulation of intrinsic vago-vagal reflex circuit activity rather than any direct effects on vagal efferent neurons per se. In fact, even though microinjection of 5-HT in rat DVC has limited effects on gastric function, its coadministration with TRH induces a powerful, synergistic increase of gastric activity (13) that was not explained until recently (4).
5-HT effects in the DVC have been studied intensely in recent years with both in vivo and in vitro tools. Exogenous application of 5-HT in the DVC with in vivo extracellular recordings provided evidence of diverse, and puzzling, responses of DVC neurons. In the NTS, 5-HT excited ~40% of the neurons studied but also inhibited ~30% and had no effect on ~20%, the excitatory effect being mediated by 5-HT1A and 5-HT2 agonists (26). 5-HT1A receptors are negatively coupled to the cAMP pathway, one effect of which is the opening of potassium channels causing direct cellular hyperpolarization (i.e., inhibition). The 5-HT1A-mediated excitation observed in NTS suggests that it was obtained as a consequence of disfacilitation of an inhibitory input, whereas the 5-HT2-mediated excitation was a consequence of a direct neuronal depolarization.
In vivo and in vitro recordings in the DMV have shown that subpopulations of these cells are excited by 5-HT2-5-HT3 and 5-HT4 agonists (3, 24), whereas 5-HTA agonist excited ~20% but inhibited ~66% of DMV neurons (27). By studying the effects of 5-HT on identified GI-projecting DMV neurons, our group showed (3) that the slow postsynaptic excitatory effect of 5-HT on the DMV membrane was mediated only by 5-HT2 receptor activation. This result is in contrast with excitatory effects of 5-HT1 agonists on DMV neurons reported by Wang et al. (27). However, in a sub-population of DMV neurons, 5-HT, by acting on 5-HT1A receptors probably located on the NTS terminals, decreased the amplitude of electrically evoked inhibitory GABA-mediated currents (3). This may provide an explanation for the puzzling excitatory effects observed in vivo on 5-HT1A activation as well as supplying evidence for an in vivo tonic GABA-mediated inhibition of DMV neurons, and, most important, it could explain the synergistic effects observed by the combination of 5-HT with TRH.
TRH
There is a great deal of evidence suggesting that TRH is a powerful neurotransmitter/neuromodulator in the central vagal regulation of GI function (12, 21). In vivo and in vitro electrophysiological studies showed that TRH influences GI function via a direct excitation of DMV neurons (14, 25) and via inhibition of the neuronal activity of NTS gastric inflation-sensitive cells (14). If, for example, the TRH-mediated inhibition of NTS neurons is targeted to GABA-containing cells projecting to the DMV, release of TRH in the DVC would result in both a direct excitation and a disinhibition of (cholinergic) DMV neurons with a consequent extremely powerful increase in vagal motor output. Such an effect could explain the synergistic action on gastric function observed with the combination of 5-HT and TRH in vivo (13, 30). In support of this theory, there is evidence that these synergistic effects are not produced as a consequence of direct action of the agonists on the DMV (24).
Using in vitro pharmacological and electrophysiological techniques, Browning and Travagli (4) have explained, at the cellular level, this longstanding mystery of the synergistic interaction of TRH and 5-HT in the DVC. These authors showed that the role of TRH is to “prime” NTS neurons to the action of 5-HT. In fact, after exposure to low concentrations of TRH that them-selves do not cause any detectable electrophysiological activity but at the same time are sufficient to activate the cAMP-protein kinase A (PKA) pathway, subsequent exposure to 5-HT, or 5-HT1A agonists, inhibits GABAergic synaptic transmission in all DMV neurons, including those that were previously unresponsive to 5-HT (Fig. 2). As a consequence of such a disfacilitation of GABAergic inputs from NTS to DMV neurons, TRH (or any neurotransmitter/drug that increases the levels of cAMP; Fig. 2) and 5-HT increase the firing frequency of DMV neurons, an effect that would result in an increase of vagal parasympathetic outflow. Although TRH did not increase the magnitude of the 5-HT-induced inhibition of synaptic transmission to individual neurons, the “priming” of NTS neurons by TRH did increase the number of neurons to which 5-HT inhibited synaptic transmission. The larger inhibition of GABAergic synaptic transmission from NTS to DMV exerted by 5-HT results in the removal of the inhibitory “brake” that NTS has on DMV neurons and, in consequence, enhances the cholinergic (excitatory) input to the stomach with a severalfold increase in gastric parameters. Keeping in mind the possibility that in the in vitro slice preparation the levels of neuronal activation and GABAergic inputs into DMV might be lower than in vivo, such a dramatic increase in gastric function in response to a moderate reduction in the GABAergic input implies that the NTS plays a vital role in depressing the efferent output from DMV.
Fig. 2.
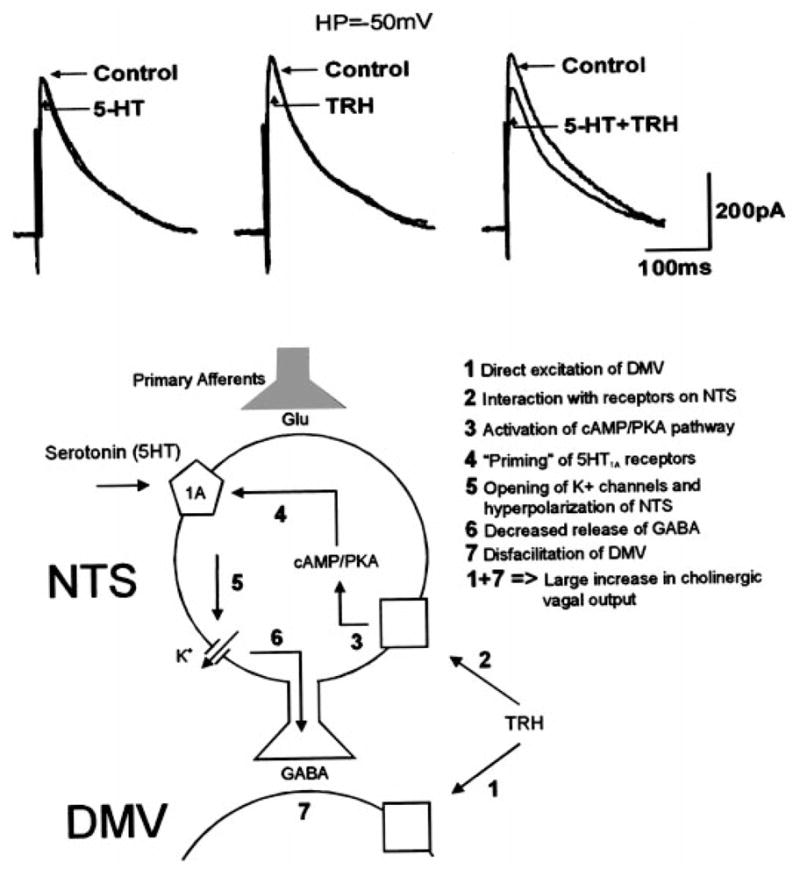
Top: in ~50% of DMV neurons, GABAergic (inhibitory out-ward) currents evoked by electrical stimulation of the NTS are not affected by serotonin (5-HT) or thyrotropin-releasing hormone (TRH). However, subsequent reapplication of 5-HT in combination with TRH reduced the amplitude of the evoked, inhibitory currents in the DMV. Bottom: simplified diagram of the relationship between 5-HT and TRH in the modulation of the function of gastric vago-vagal reflexes. PKA, protein kinase A.
DMV neurons are spontaneously active, and small increases in their membrane potential can produce a dramatic increase in action potential firing frequency. As a consequence, the cholinergic (excitatory) output to the stomach would be greatly enhanced, with a resultant dramatic increase in gastric motility and secretion. In the rat, under normal conditions a tonic GABAergic input, likely from the NTS, acts as a tonic brake on DMV output (12). Our data show that a reduction in this GABAergic inhibitory input results in an amplification of cholinergic excitatory tone to the GI tract. Thus any slight modulation of the NTS-DMV synapse has the potential to induce robust changes in GI function such as the ones seen with TRH and 5-HT.
The end result is that TRH and 5-HT “commandeer” the medullary gastric reflex control circuitry to produce maximal activation of gastric motility and secretion. Under normal circumstances, activation of DMV efferents increases gastric function; this increase in activity is monitored by vagal afferents. The resultant excitation of vagal afferents would normally act to inhibit further DMV activity through negative vago-vagal feedback mechanisms (14, 15, 21). However, in this case, TRH uncouples the reflex through multisite inhibition of NTS neurons and through direct activation of the DMN. Thus the activation and disinhibition of the DMV by TRH evokes a dramatic increase in motility and secretion.
The previous discussion briefly reviews one of the most potent modulatory processes at work within the DVC to alter the reflex control of gastric function. The reader should be mindful of the fact that 20 or more phenotypically distinct descending inputs from other CNS loci also add to this complex mix of modulatory inputs to the DVC (7). Of course, this fact strongly militates against the success of any single drug or approach in the treatment of motility disorders having a CNS component. As if these extrinsic CNS influences on the DVC were not confounding enough, it has become clear over the past several years that DVC circuits controlling gastrointestinal functions are also subject to dramatic modulation by signal molecules in the circulation.
CONTROL OF VAGO-VAGAL REFLEX FUNCTION BY HUMORAL “AFFERENTS”: DVC AS A CIRCUMVENTRICULAR ORGAN
Dendrites from neurons of the NTS and DMV penetrate areas devoid of a vascular diffusion barrier such as the area postrema and the ependymal lining of the fourth ventricle. Furthermore, the vascular supply of the NTS and portions of the DMV is composed of fenestrated capillaries. This arrangement defines the DVC as a circumventricular organ (CVO; Refs. 2 and 21). It is not surprising, then, that large blood-borne proteins and peptides (e.g., hormones, serum albumin, IgG, complement) have direct access to the DVC. Blood-borne horseradish peroxidase (HRP; mol wt 44,000) for example, can be seen in all CVOs and adjacent brain parenchyma (e.g., the DVC) within 10 min after intravenous injection of HRP (2). Thus cells and processes of both the DMV and NTS may be continuously exposed to circulating peptides and proteins (2, 21).
Although gut hormones can effect significant changes in GI function by acting on vagal afferents (19), physiological studies of the CNS effects of insulin, pancreatic polypeptide (PP), and peptide YY (PYY) suggest that gut hormones can also exert control over digestion by acting directly on neurons of the DVC (21). For example, PP (mol wt 5,000) is released exclusively from specialized pancreatic islet cells after meals. PP regulates pancreatic secretion and gastric motility by acting directly on neurons within the DVC to control vagal efferent outflow to the viscera (21). As it happens, “hormones” produced by the immune system can also dramatically affect the function of neurons that comprise the gastric vago-vagal reflex control circuit.
TUMOR NECROSIS FACTOR-α AND CENTRAL NEURAL CONTROL OF GASTROINTESTINAL FUNCTION
Immune system activation and the cytokine production associated with a variety of infectious and auto-immune disorders can be modeled by systemic injection of the endotoxin lipopolysaccharide (LPS). LPS is a constituent of the outer membrane of gram-negative bacteria and can induce the production and release of cytokines such as tumor necrosis factor (TNF)-α from macrophages, T cells, and glia (2). TNF-α has been recognized as the primary mediator of the symptoms of septic shock and cachexia associated with the immune activation of disease and LPS injection (see Ref. 6 for review). Gastric stasis is a common feature of experimental treatments causing immune activation and is highly correlated with elevated levels of TNF-α (10). Likewise, multiple disease states that have elevated TNF-α production in common also demonstrate gastric stasis, nausea, emesis, and anorexia (9, 10, 29).
There are multiple routes by which TNF-α may come into contact with neurons in the DVC. In addition to the free diffusion afforded to circulating TNF-α by fenestrated capillaries in the DVC (2), TNF-α readily gains access to the brain and spinal cord through specific, parallel transport mechanisms that TNF-α itself upregulates. (18). TNF-α is also generated in the brain in large quantities by astrocytes, microglia infiltrating macrophages and T cells after brain injury and neuroinfection (18).
The brain stem DVC region has the highest level of TNF-α binding sites in the CNS, and in situ hybridization studies suggest that these binding sites may be P55 TNF-α receptors (16). Vagal afferent fibers projecting to the NTS may also be activated by peripheral cytokines; however, these sensors may be sufficient but not necessary for the NTS to detect circulating or injected cytokines itself (5, 8, 9).
TNF-α ACTION IN DVC TO INHIBIT GASTRIC MOTILITY
Direct application of subfemtomole doses of TNF-α to the DVC produced a highly significant and dose-dependent inhibition of centrally stimulated gastric motility; this effect is vagally mediated (Ref. 9; Fig. 3). Further-more, endogenous production and release of cytokines in response to systemic LPS challenge suppressed centrally evoked and vagally mediated increases in gastric motility. Elevated plasma TNF-α levels were essential for this suppression (10). Preliminary studies in our laboratory suggest that local adsorption and elimination of TNF-α from the DVC with mobile TNF-α receptor constructs (i.e., huTNFR:fc, Immunex) eliminates the gastric stasis induced by peripheral LPS administration. FOS immunohistochemical techniques demonstrated that peripheral vagal afferents are not essential for the detection of plasma TNF-α by neurons in the DVC; that is, peripherally generated cytokines can activate DVC neurons directly, independent of any effect on peripheral afferents (8).
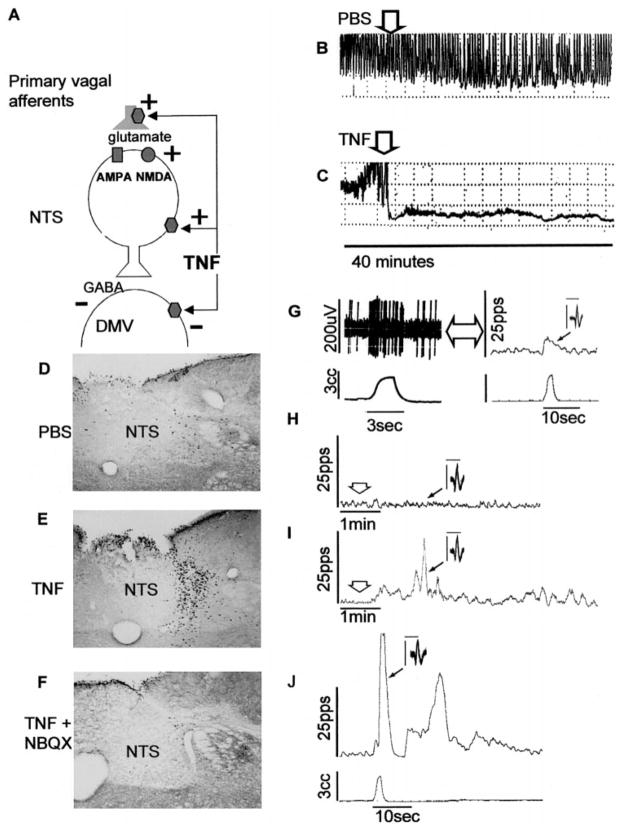
Fig. 3.
A: summary of possible sites of action for tumor necrosis factor-α (TNF-α) to modulate gastric vago-vagal reflex functions. TNF-α activation of NTS neurons is dependent on glutamate neurotransmission, although direct action of TNF-α to activate NTS neurons and to inhibit DMV neurons cannot yet be ruled out. B and C: effects of DVC injections of 20 nl of PBS (B) or 20 fM TNF-α in 20 nl of PBS (C) on maximal centrally stimulated gastric motility. D–F: TNF-α activation of NTS neurons appears to be dependent on glutamate neurotransmission. D: 20 nl of PBS injected into the NTS has no effect to increase cFOS expression in the NTS. E: 2 fM TNF-α in 20 nl of PBS injected into the NTS dramatically increases cFOS production in the NTS. F: the glutamate receptor antagonist NBQX (0.2 pM) combined with TNF-α completely blocks the NTS activation by TNF-α. G–J: effects of TNF-α on a gastric vago-vagal reflex circuit NTS neuron. G: 3-cm3 balloon distension of the stomach activates an NTS neuron recorded in vivo; raw record on left, processed rate meter record of the same data on right. Injection of 3 nl of PBS (H) onto recorded neuron has no effect on NTS activity, but TNF-α (I) produces a significant activation of the cell. J: 20 min after recovery of baseline excitability after the TNF-α injection in I, NTS neuron response to gastric distention is strongly potentiated. AMPA, amino-3-hydroxy-5-methyl-4-isoxazole propionate; NMDA, N-methyl-D-aspartate; pps, pulses per second.
Neurophysiological studies validate the hypothesis that TNF-α can act directly in the DVC to regulate gastric function. TNF-α potently activates specific NTS neurons identified as belonging to gastric vago-vagal control circuits. Subsequent to TNF-α exposure, these NTS neurons demonstrated a long-term potentiated response to vagal afferent input. The “fast” neurotransmitter between vagal afferents and the NTS is very likely to be glutaminergic (Refs. 5 and 15; Fig. 3). Preliminary in vitro voltage- and current-clamp recordings showed that TNF-α induced large inward (i.e., depolarizing) currents in NTS neurons. These results are comparable to the activation of NTS neurons seen in the in vivo electrophysiological experiments cited above. Finally, in vivo neurophysiological recordings from physiologically identified “gastric” DMV neurons show that these cells are inhibited by TNF.
Preliminary studies using cFOS histochemical studies support the suggestion that the action of TNF-α on the NTS may depend, to a significant extent, on the modulation of the effectiveness of glutamate neuro-transmission between vagal afferents and the NTS. Femtomolar doses of TNF-α nanoinjected into the NTS dramatically increase cFOS labeling in the NTS. This increase in NTS activation is blocked by the coadmin-istration of either the AMPA or NMDA receptor antagonists NBQX and MK-801, respectively (Fig. 3).
Together, these studies provide compelling evidence that TNF-α can act within the DVC to potently inhibit gastric function, probably by modulating glutamate release from presynaptic vagal afferents ending on “gastric” NTS neurons in the DVC. However, the complete details of the neurophysiological mechanism(s) by which TNF-α evokes changes in DVC neuronal function are not yet clear. Working these details out will be essential to correcting deficits in gastric function due to cytokine release. This is because inactivating the specific neural pathways that TNF-α affects, rather than systemically altering TNF-α production or action, may provide a better approach to dealing with the cytokine-mediated degradation of digestive functions. Systemic alteration of TNF-α levels, whether planned or accidental, can lead to disastrous unintended consequences because of the pleiotropic nature of TNF-α action (6, 10). An extreme example of this may be found in the teratogenic effects caused by the use of thalidomide (a TNF-α synthesis inhibitor) for the treatment of “morning sickness” and its highly effective suppression of TNF-α-induced nausea and stasis (10).
Acknowledgments
We thank the National Institute of Diabetes and Digestive and Kidney Diseases (DK-55530, DK-52142, DK-56373) and the National Science Foundation (IBN-9816662) for their support.
We apologize to our colleagues for the paucity of citations necessitated by the length restrictions of this brief review.
References
- 1.Blessing WW. The Lower Brainstem and Bodily Homeostasis. New York: Oxford Univ. Press; 1997. Eating and metabolism; pp. 323–372. [Google Scholar]
- 2.Broadwell RD, Sofroniew MV. Serum proteins bypass the blood-brain fluid barriers for extracellular entry to the CNS. Exp Neurol. 1993;120:245–263. doi: 10.1006/exnr.1993.1059. [DOI] [PubMed] [Google Scholar]
- 3.Browning KN, Travagli RA. Characterization of the in vitro effects of 5-hydroxytryptamine (5HT) on identified neurones of the rat dorsal motor nucleus of the vagus (DMV) Br J Pharmacol. 1999;128:1307–1315. doi: 10.1038/sj.bjp.0702908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Browning KN, Travagli RA. The peptide TRH uncovers the presence of presynaptic 5-HT1A receptors via activation of a second messenger pathway in the rat dorsal vagal complex. J Physiol (Lond) 2001;531:425–435. doi: 10.1111/j.1469-7793.2001.0425i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emch GS, Hermann GE, Rogers RC. TNF-α activates solitary nucleus neurons responsive to gastric distension. Am J Physiol Gastrointest Liver Physiol. 2000;279:G582–G586. doi: 10.1152/ajpgi.2000.279.3.G582. [DOI] [PubMed] [Google Scholar]
- 6.Galanos C, Fredenberg MA. Mechanisms of endotoxin shock and endotoxin hypersensitivity. Immunobiology. 1993;187:346–56. doi: 10.1016/S0171-2985(11)80349-9. [DOI] [PubMed] [Google Scholar]
- 7.Gillis RA, Quest JA, Pagani FD, Norman WP. Handbook of Physiology. The Gastrointestinal System. Motility and Circulation. pt. 1. I. Bethesda, MD: Am. Physiol. Soc; 1989. Control centers in the central nervous system for regulating gastrointestinal motility; pp. 621–683. sect. 6. chapt. 17. [Google Scholar]
- 8.Hermann GE, Emch GS, Tovar CA, Rogers RC. cFOS generation in the dorsal vagal complex is not dependent on the vagus nerve. Am J Physiol Regulatory Integrative Comp Physiol. 2001;280:R289–R299. doi: 10.1152/ajpregu.2001.280.1.R289. [DOI] [PubMed] [Google Scholar]
- 9.Hermann GE, Rogers RC. TNF alpha in the dorsal vagal complex suppresses gastric motility. Neuroimmunomodulation. 1995;2:74–81. doi: 10.1159/000096874. [DOI] [PubMed] [Google Scholar]
- 10.Hermann GE, Tovar CA, Rogers RC. Induction of endogenous tumor necrosis factor-α: suppression of centrally stimulated gastric motility. Am J Physiol Regulatory Integrative Comp Physiol. 1999;276:R59–R68. doi: 10.1152/ajpregu.1999.276.1.R59. [DOI] [PubMed] [Google Scholar]
- 11.Hornby PJ. Receptors and transmission in the brain-gut axis: potential for novel therapies. II. Excitatory amino acid receptors in the brain-gut axis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1055–G1060. doi: 10.1152/ajpgi.2001.280.6.G1055. [DOI] [PubMed] [Google Scholar]
- 12.Krowicki ZK, Hornby PJ. Hindbrain neuroactive substances controlling gastrointestinal function. In: Gaginella TS, editor. Regulatory Mechanism in Gastrointestinal Function. Boca Raton, FL: CRC; 1995. pp. 277–319. [Google Scholar]
- 13.McCann MJ, Hermann GE, Rogers RC. Dorsal medullary serotonin and gastric motility: enhancement of effects by thyrotropin-releasing hormone. J Auton Nerv Syst. 1988;25:35–40. doi: 10.1016/0165-1838(88)90005-7. [DOI] [PubMed] [Google Scholar]
- 14.McCann MJ, Hermann GE, Rogers RC. Thyrotropin-releasing hormone: effects on identified neurons of the dorsal vagal complex. J Auton Nerv Syst. 1989;26:107–112. doi: 10.1016/0165-1838(89)90158-6. [DOI] [PubMed] [Google Scholar]
- 15.McCann MJ, Rogers RC. Functional and chemical neuroanatomy of a gastric vago-vagal reflex. In: Tache Y, Wingate DL, Burks TF, editors. Innervation of the Gut: Pathophysiological Implications. Boca Raton, FL: CRC; 1994. [Google Scholar]
- 16.Nadeau S, Rivest S. Effects of circulating tumor necrosis factor on the neuronal activity and expression of the genes encoding the tumor necrosis factor receptors (p55 and p75) in the rat brain: a view from the blood-brain barrier. Neuroscience. 1999;93:1449–1464. doi: 10.1016/s0306-4522(99)00225-0. [DOI] [PubMed] [Google Scholar]
- 17.Palkovits M, Mezey E, Eskay RL, Brownstein MJ. Innervation of the nucleus of the solitary tract and the dorsal vagal nucleus by thyrotropin-releasing hormone-containing raphe neurons. Brain Res. 1986;373:246–251. doi: 10.1016/0006-8993(86)90338-0. [DOI] [PubMed] [Google Scholar]
- 18.Pan W, Zadina JE, Harlan RE, Weber JT, Banks WA, Kastin AJ. TNF-alpha; a neuromodulator in the CNS. Neurosci Biobehav Rev. 1997;21:603–613. doi: 10.1016/s0149-7634(96)00047-4. [DOI] [PubMed] [Google Scholar]
- 19.Raybould HE. Vagal afferent innervation and the regulation of gastric motor function. In: Ritter S, Ritter R, Barnes CD, editors. Neuroanatomy and Physiology of Abdominal Vagal Afferents. Boca Raton, FL: CRC; 1992. pp. 193–220. [Google Scholar]
- 20.Rogers RC, Hermann GE, Travagli RA. Brainstem path-ways responsible for oesophageal control of gastric motility and tone in the rat. J Physiol (Lond) 1999;514:369–383. doi: 10.1111/j.1469-7793.1999.369ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers RC, McTigue DM, Hermann GE. Vagal control of digestion; modulation by central neural and peripheral endocrine factors. Neurosci Biobehav Rev. 1996;20:57–66. doi: 10.1016/0149-7634(95)00040-l. [DOI] [PubMed] [Google Scholar]
- 22.Smith BN, Dou P, Barber WD, Dudek FE. Vagally evoked synaptic currents in the immature rat nucleus tractus solitarii in an intact in vitro preparation. J Physiol (Lond) 1998;512:149–162. doi: 10.1111/j.1469-7793.1998.149bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Travagli RA, Browning KN, Gillis RA. Studies of the dorsal motor nucleus of the vagus, a major CNS center for controlling vagal outflow to the upper gastrointestinal tract. In: Quigley ENM, Pfeiffer RF, editors. Neurogastroenterology/Gastrointestinal Dysfunction in Neurologic Disease. Cambridge, UK: Cambridge Univ Press; In press. [Google Scholar]
- 24.Travagli RA, Gillis RA. Effects of 5-HT alone and its interaction with TRH on neurons in rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol. 1995;268:G292–G299. doi: 10.1152/ajpgi.1995.268.2.G292. [DOI] [PubMed] [Google Scholar]
- 25.Travagli RA, Gillis RA, Vicini S. Effects of thyrotropin-releasing hormone on neurons in rat dorsal motor nucleus of the vagus, in vitro. Am J Physiol Gastrointest Liver Physiol. 1992;263:G508–G517. doi: 10.1152/ajpgi.1992.263.4.G508. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Ramage AG, Jordan D. In vivo effects of 5-hy-droxytryptamine receptor activation on rat nucleus tractus solitarius neurones excited by vagal C-fibre afferents. Neurophar-macology. 1997;36:489–498. doi: 10.1016/s0028-3908(97)00063-4. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Jones JFX, Ramage AG, Jordan D. Effects of 5-HT and 5-HT1A receptor agonists and antagonists on dorsal vagal preganglionic neurones in anaesthetized rats: an ionophoretic study. Br J Pharmacol. 1995;116:2291–2297. doi: 10.1111/j.1476-5381.1995.tb15067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willis A, Mihalevich M, Neff RA, Mendelowitz D. Three types of postsynaptic glutamatergic receptors are activated in DMNX neurons upon stimulation of NTS. Am J Physiol Regulatory Integrative Comp Physiol. 1996;271:R1614–R1619. doi: 10.1152/ajpregu.1996.271.6.R1614. [DOI] [PubMed] [Google Scholar]
- 29.Winkler U, Jensen M, Manzke O, Schulz H, Diehl V, Engert A. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8) Blood. 1999;94:2217–2224. [PubMed] [Google Scholar]
- 30.Yoneda M, Taché Y. Serotonin enhances gastric acid response to TRH analog in dorsal vagal complex through 5-HT2 receptors in rats. Am J Physiol Regulatory Integrative Comp Physiol. 1995;269:R1–R6. doi: 10.1152/ajpregu.1995.269.1.R1. [DOI] [PubMed] [Google Scholar]