Abstract
Brainstem parasympathetic circuits that modulate digestive functions of the stomach are comprised of afferent vagal fibers, neurons of the nucleus tractus solitarius (NTS), and the efferent fibers originating in the dorsal motor nucleus of the vagus (DMV). A large body of evidence has shown that neuronal communications between the NTS and the DMV are plastic and are regulated by the presence of a variety of neurotransmitters and circulating hormones as well as the presence, or absence, of afferent input to the NTS. These data suggest that descending central nervous system inputs as well as hormonal and afferent feedback resulting from the digestive process can powerfully regulate vago-vagal reflex sensitivity. This paper first reviews the essential “static” organization and function of vago-vagal gastric control neurocircuitry. We then present data on the opioidergic modulation of NTS connections with the DMV as an example of the “gating” of these reflexes, i.e., how neurotransmitters, hormones, and vagal afferent traffic can make an otherwise static autonomic reflex highly plastic.
Keywords: dorsal motor nucleus of the vagus, nucleus tractus solitarius, gastrointestinal reflexes, plasticity
OVERVIEW
The gastrointestinal (GI) tract possesses an intrinsic nervous plexus that allows the intestine to have a considerable degree of independent neural control. The stomach and the esophagus, however, are almost completely dependent upon extrinsic nervous inputs arising from the central nervous system (CNS) (1–6). CNS control over the smooth and coordinated digestive functions of the stomach is mediated by parasympathetic and sympathetic pathways that either originate in, or are controlled by, neural circuits in the caudal brainstem.
Sympathetic control of the stomach stems from cholinergic preganglionic neurons in the intermediolateral column of the thoracic spinal cord (T6 through T9 divisions), which impinge on postganglionic neurons in the celiac ganglion, of which the catecholaminergic neurons provide the stomach with most of its sympathetic supply. Sympathetic regulation of motility primarily involves inhibitory presynaptic modulation of vagal cholinergic input to postganglionic neurons in the enteric plexus. The magnitude of sympathetic inhibition of motility is directly proportional to the level of background vagal efferent input (7). Recognizing that the GI tract is under the dual control of the sympathetic and parasympathetic nervous systems, we refer the reader to other comprehensive reviews on the role of the sympathetic control of gastric function (7–9). The present review focuses on the functionally dominant parasympathetic control of the stomach via the dorsal motor nucleus of the vagus (DMV) and the means by which this input is modulated, i.e., plasticity of vago-vagal reflexes.
ORGANIZATION OF THE BRAINSTEM VAGAL CIRCUITS CONTROLLING THE STOMACH
Visceral Afferent Inputs to Brainstem Gastric Control Circuits
Vagal afferent fibers carry a large volume of information about the physiological status of the gut directly to brainstem circuits regulating gastric function. The cell bodies of origin for this afferent sensory pathway are contained in the nodose ganglion. Neurons in the nodose ganglia are the cranial equivalent of dorsal root ganglion cells in that they are bipolar and connect the gut directly with nucleus tractus solitarius (NTS) neurons in the brainstem with no intervening synapse (reviewed recently in References 10–13).
There are several types of visceral receptors and afferent fibers (7, 9, 14–31). Independent of their functions or modalities, however, all vagal afferents use glutamate as the primary neurotransmitter to transfer information to the NTS (32–42).
Electrophysiological experiments have shown that glutamate released onto presumptive GI NTS neurons activates both NMDA and non-NMDA receptors (35, 41, 43), as do putative cardiovascular NTS neurons (36, 44, 45). The release of glutamate at this interface between peripheral perception (sensory afferent fibers) and central integration (NTS neurons) is open to modulation by a wide variety of transmitter and hormonal agonists, including glutamate itself (46–51), cholecystokinin (CCK) (52–54), leptin (55, 56), tumor necrosis factor (57), and ATP (58), to name but a few. These data strongly suggest that vago-vagal reflex functions are subject to pre- as well as postsynaptic modulation.
Nucleus Tractus Solitarius
The general hypothesis of a vago-vagal reflex connecting vagal afferent input, brainstem NTS neurons, and vagal efferent projections to the stomach was formulated some years ago and has been reviewed extensively (6, 10, 38, 59–66). The early models presented a framework to explain many of the salient features of brainstem reflex control of the stomach. Simply put, reflex action is initiated by stimulation of sensory vagal afferent pathways, which activate second-order NTS neurons via glutamate action on NMDA and non-NMDA receptors. These NTS neurons can use several different neurotransmitters to control the output from DMV cells, which, in turn, control gastric functions and complete the vago-vagal loop (Figure 1).
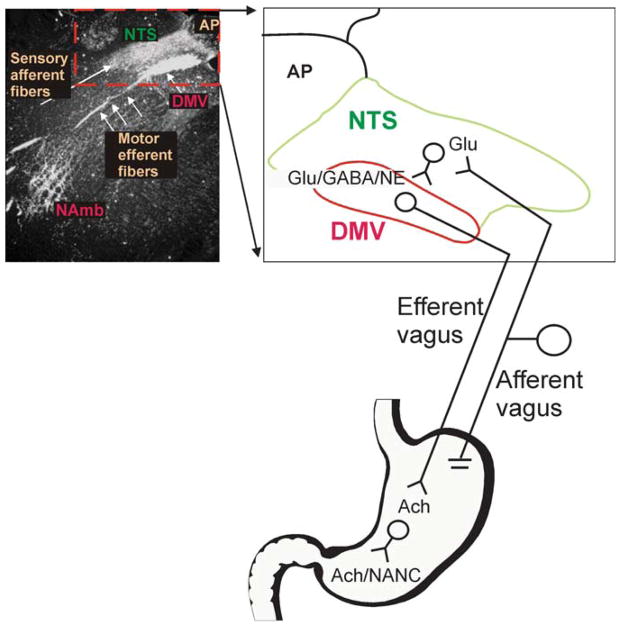
Figure 1.
Cytoarchitecture of the dorsal vagal complex. Darkfield photomicrograph of a coronal section of rat brainstem at the level of the area postrema/intermediate level following application of HRP crystals to the subdiaphragmatic vagus. Note the intense labeling of the nucleus tractus solitarius (NTS), dorsal motor nucleus of the vagus (DMV), and nucleus ambiguus (NAmb). An area of the photomicrograph (dotted line) has been expanded into cartoon form to allow a more detailed illustration of the brainstem circuitry of gastrointestinal (GI) vago-vagal reflexes. Note that vagal afferent neurons, whose cell bodies lie in the nodose ganglion, receive sensations from the GI tract. The central terminals of these afferent fibers enter the brainstem via the tractus solitarius and terminate within the NTS, utilizing principally glutamate as their neurotransmitter. These afferent signals are integrated by neurons of the NTS that project to, among other areas, the adjacent DMV using, mainly, glutamate, GABA, or NE as neurotransmitters. Neurons of the DMV are the preganglionic parasympathetic motoneurons that provide the motor output to the GI tract via the efferent vagus, where they release acetylcholine onto their postganglionic target. Postganglionic parasympathetic neurons are either excitatory [cholinergic (ACh)] or inhibitory [nonadrenergic, noncholinergic (NANC)].
Visceral sensory afferents are organized in an overlapping topographic manner within the NTS subnuclei. Terminal fields from the intestine are represented in the subnuclei commissuralis and medialis, the stomach sends its afferent inputs to the subnuclei medialis and gelatinosus, and the esophagus to the subnucleus centralis (66–70).
Although sensory inputs from distinct visceral areas, such as, for example, the aortic branches or gastric branches, do not seem to converge on single NTS neurons (71), the same subnucleus receives sensory information from more than one peripheral organ. Afferent terminals involved in arterial baroreflex circuits as well as terminals involved in vago-vagal gastric reflexes, for example, impinge upon neurons similarly located in the subnucleus medialis of the NTS (17, 38, 67, 70, 72–76). This overlap of the terminal fields from different organs within the NTS makes the anatomical and functional identification of individual neurons difficult.
The subnucleus centralis (cNTS), however, provides a convenient exception in that it seems to receive inputs from vagal afferent fibers originating almost exclusively in the esophagus (17, 38, 66, 67, 77), making it an excellent model for the study of a population of second-order neurons controlling esophageal-mediated reflexes. The cNTS of the rat is located between the tractus solitarius and the lateral third of the DMV at a level of approximately 0.5 to 1.5 mm rostral to the calamus scriptorius (70). Note that although the cNTS projects to the DMV as part of a classic example of a vago-vagal reflex, it also projects to areas that are not related to CNS control of gastric functions. Indeed, the cNTS projects to the nucleus ambiguus (NAmb), the reticular formation, and the ependymal layer of the fourth ventricle, as well as other NTS subnuclei (66). Thus, although cNTS neurons receive sensory information almost exclusively from the esophagus, these neurons control outputs related to gastric, esophageal, cardiovascular, deglutitive, and respiratory functions. Neurons of the cNTS may also be subject to plastic changes in function that translate into dramatic shifts in the reflex responses to afferent input dependent upon, and appropriate to, the feeding status of the animal.
Dorsal Motor Nucleus of the Vagus
The parasympathetic motor supply to the stomach is provided by the efferent vagus nerve originating from neurons located in the NAmb and DMV. The NAmb contains the soma of cells contributing to the vagal control of the esophagus, upper esophageal sphincter, and cardiorespiratory system. We refer the reader to recent reviews dealing with the NAmb (6, 8, 63, 77, 78).
The cell bodies for the great majority of parasympathetic efferent fibers that project to the upper GI tract originate along the whole rostro-caudal extent of the DMV (79–81). The DMV is a paired structure in the dorsal caudal medulla adjacent to the central canal, the majority of whose cells project to neurons in the myenteric plexus or onto interstitial cells of Cajal (ICC) of the upper GI tract (19, 82–85), with the highest density of efferent fibers terminating in the stomach (87).
Retrograde tracing experiments have determined that the DMV is organized in medio-lateral columns spanning its rostro-caudal extent (79–81). Efferent projections originating from soma located in the medial portions of the nucleus form both the dorsal and ventral gastric vagal branches. Neurons in the lateral portions of the DMV send axons to the vagal celiac and accessory celiac branches, whereas scattered neurons in the left DMV provide axons to the hepatic branch. The medio-lateral organization of the columns is not maintained when the target organs of the various vagal branches are considered. In fact, the two gastric branches innervate the stomach, a portion of the proximal duodenum, and some visceral structures such as the pancreas; the celiac branches innervate the GI tract from the duodenum to the transverse colon; and the hepatic branch innervates parts of the stomach, liver, and proximal duodenum (87, 88).
Although the DMV may not be organized in a rigid organotypic manner, evidence suggests that the vagal innervation of the stomach is segregated within the DMV by function. For example, descending vagal pathways responsible for causing gastric contractions versus gastric relaxation [i.e., the nonadrenergic, non-cholinergic (NANC) pathway] appear to be localized in different regions of the DMV. That is, putative NANC-pathway neurons appear to be located in the caudomedial and rostrolateral divisions of the DMV, whereas the gastroexcitatory neurons are located in the more rostral and medial divisions of the DMV (66, 89).
The DMV is comprised of neuronal populations that are nonhomogeneous with respect to morphological features such as soma size and shape, number of dendritic branches, and extent of dendritic arborization (90–96). The significance of these morphological differences is not well understood, although a possible explanation is discussed below.
Huang and colleagues (95) have hypothesized that neuronal DMV subgroups in the human may form functional units innervating specific organs; our studies in the rat suggest a similar organization. Combined retrograde tracing, whole cell patch clamp, and postrecording neuronal reconstruction techniques show a functional and morphological correlation between DMV neurons and the peripheral target organs that they innervate (92). Specifically, in the rat, DMV neurons projecting to the gastric fundus have a smaller soma size and fewer dendritic branches than neurons projecting to the corpus, duodenum, or cecum; neurons that project to the cecum have the largest soma out of all these. Additionally, there is evidence to support a relationship between structure and function of neurons in the rat DMV; e.g., neurons responsive to gastric or intestinal distension can be distinguished into separate morphological groups (96). Furthermore, these different DMV subgroups produce different profiles of extracellular recorded action potentials. This last observation suggests that DMV neurons engaged in different functions may possess different membrane properties.
Indeed, several investigators have reported a large array of unevenly distributed membrane currents in the DMV (92, 94, 97–100). Using the current clamp configuration, Browning et al. (92) showed that gastric-projecting DMV neurons can be easily distinguished from intestinal-projecting DMV neurons by the characteristic shape and duration of the after-hyperpolarization (AHP). That is, intestinal projecting neurons have a much larger and slower AHP, in contrast to the smaller and faster AHP of gastric-projecting neurons. This characteristic implies that gastric DMV neurons are more prone to change membrane potential in response to synaptic inputs than larger DMV neurons that project to other targets. This is of particular relevance, as one of the distinguishing features of DMV neurons is that they maintain a spontaneous, slow (1–2 pulses sec−1) pacemaker-like activity (65, 101, 102). This pacemaker activity implies that afferent inputs altering the membrane potential of these neurons by even a few mV, in either direction, cause dramatic changes to the vagal motor output (97, 103). One immediate implication is that the stomach, at rest, is controlled by a tonic vagal efferent outflow provided by small gastric-projecting DMV cells, the vagal motor output of which is continuously sculpted by impinging inputs. Conversely, larger intestinal DMV neurons need more robust synaptic inputs to achieve the same membrane displacement. This size principle, widely accepted for spinal motoneuron activation and recruitment (104), states that neurons with the smallest cell bodies have the lowest threshold for synaptic activation and, therefore, can be activated by weaker synaptic inputs. The resting membrane potential of gastric smooth muscle is nonuniform, with a gradient from approximately −48 mV in the proximal stomach to −70 mV in the distal stomach (105). The threshold for smooth muscle contraction is approximately −50 mV (106). The myenteric plexus of the stomach essentially serves as a follower of vagal efferent input (107): There is a high degree of fidelity for activation of gastric myenteric neurons by vagal efferent inputs (108). As a result, the proximal stomach is highly sensitive to minute changes in input from either the cholinergic excitatory branch or the NANC inhibitory branch of the DMV, and slight changes in DMV activity in either division translate into dramatic effects on gastric function.
Synaptic Connections Between the Nucleus Tractus Solitarius and the Dorsal Motor Nucleus of the Vagus
The majority of NTS neurons do not possess pacemaker activity; their inputs onto DMV neurons must be driven and are modulated by synaptic activity, either from the afferent vagus, from other CNS areas, or via circulating hormones (10, 41, 53, 57, 65, 109–118). By being subject to a vast array of modulatory activity, the synaptic connections between NTS and DMV by implication play a major role in shaping the vagal efferent output.
There are numerous NTS neuronal phenotypes that can potentially contribute input to the DMV and induce potent effects on vagally mediated gastric function (17, 119–134). Despite the presence of this vast array of neurotransmitters in various NTS subnuclei, electrophysiological data show that the NTS primarily controls the DMV through glutamatergic (37, 61, 101, 135, 136), GABAergic (61, 101, 137), and catecholaminergic (138–140) inputs. Electrical stimulation of the NTS subnucleus commissuralis, for example, evokes noradrenergic α2-mediated inhibitory potentials in approximately 10% of the DMV neurons (140), whereas an α1-mediated current can be evoked by stimulation of the A2 area between the subnuclei medialis and cNTS of the NTS (K.N. Browning & R.A. Travagli, unpublished data). Additionally, stimulation of other portions of the NTS evokes both inhibitory GABA-mediated or/and excitatory glutamate-mediated currents in DMV neurons (61, 101, 103, 141–144). Pharmacological and immunocytochemical experiments have determined that GABA released onto DMV neurons activates GABA-A receptor subtypes (101, 135, 146–148).
Most of the NTS-induced inhibition of the DMV is mediated by GABA, interacting with the GABA-A receptor; conversely, most of the excitation delivered to the DMV by the NTS is mediated by glutamate interacting with both NMDA and non-NMDA receptors (101, 144, 146, 149–152). Recent data from our laboratory show a major functional role of catecholamines (likely originating from the A2 area) in both excitatory and inhibitory control of DMV. Application of glutamate or catecholamine agonists in the dorsal vagal complex (DVC) has profound effects on gastric motility and tone (114, 138, 152–154). Glutamate injected into the medial portion of the DMV causes a brisk gastric excitation, whereas norepinephrine injections cause gastroinhibition, perhaps owing to the simultaneous activation of the NANC inhibitory pathway and inhibition of the cholinergic gastroexcitatory pathway (66, 138, 139).
Microinjections of glutamate and catecholamine antagonists in the same area, however, do not induce noticeable effects on gastric motility and tone unless GABAergic transmission is blocked (37, 135, 138). Conversely, microinjections of GABA antagonists into the DVC induce profound excitatory effects on esophageal motility and on gastric motility and secretion (135, 147, 148). Taken together, these data suggest that the DMV output is restrained by a tonic GABAergic input arising, most likely, from the NTS. Furthermore, they suggest that factors modulating GABAergic inputs from the NTS to the DMV may have a significant impact on vagal reflex control of the stomach. By contrast, glutamatergic and catecholaminergic inputs do not seem to play a major role in setting tonic vagal output to the GI tract but rather seem to be invoked phasically by specific reflexes. Thus, NTS neurons that display some type of spontaneous activity use GABA as their main neurotransmitter. Glutamatergic and adrenergic NTS neurons are probably silent unless activated by afferent input.
Vagal Efferent (Motor) Control
To better appreciate the role of the efferent vagus in controlling GI functions, one has to consider the short-term effects of vagotomy (reviewed recently in References 9 and 155). Acute vagotomy induces an increase in fundic tone and a decrease in antral motility, impairing the reservoir function of the stomach. The conflicting effects of vagotomy suggested to Pavlov that the vagus nerve controlled gastric motility through both inhibitory and excitatory pathways, a concept that has received ample support over the past 100 years.
The vast majority (>95%) of DMV neurons are cholinergic, i.e., choline acetyl-transferase immunoreactive (156). Some DMV neurons also express immunoreactivity for nitric oxide synthase (NOS) or catecholamines [e.g., tyrosine hydroxylase (TH)] (89, 156–159). Although the projections of NOS- (158) or TH- (159) positive DMV neurons target selective areas of the stomach, the physiological significance of this detail is not clear. Interestingly, in both rodent (66, 89) as well as in feline (160) models, these markers for alternate neurotransmitter synthesis (i.e., TH and NOS) tend to coincide with the location of neurons involved in the inhibitory effect of vagal input to the stomach. For example, NOS-containing DMV neurons are found in the extreme caudal and rostrolateral portions of the DMV. Stimulation of these areas evoke gastric relaxation (66, 89, 160). This is far from establishing a connection between NOS- (or TH-) positive neurons and gastroinhibition; the correlation, however, is quite intriguing.
Using an intact vagus-gastric myenteric plexus in vitro preparation, Schemann & Grundy (107) demonstrated that stimulation of vagal preganglionic fibers, likely originating from DMV somata, induces exclusively cholinergic nicotinic potentials. These data indicate that acetylcholine, interacting with nicotinic receptors, is the principal neurotransmitter released from vagal efferent terminals and that preganglionic vagal fibers excite only enteric neurons. Because activation of vagal efferent fibers can induce both excitatory as well as inhibitory effects on gastric smooth muscles (18, 38, 62, 66, 160–180), it is clear that both excitatory and inhibitory postganglionic neuroeffectors are released from enteric neurons in response to excitatory vagal input. Note that the inhibitory vagal action on the stomach is directed toward the control of motility and tone but not of gastric secretion (181–185).
The principal excitatory postganglionic neurotransmitter is acetylcholine acting on muscarinic receptors in gastric smooth muscle, ICC, and parietal cells. Activation of muscarinic receptors depolarize smooth muscle and the ICC to augment the smooth-muscle activity that drives peristalsis and tone (1, 2, 4, 26, 186–190).
Inhibitory postganglionic neurons comprise the NANC path between the nicotinic preganglionic vagal efferent fibers and gastric smooth muscle and ICCs. The two most likely candidates mediating this connection are nitric oxide and vasoactive intestinal polypeptide, although other mediators such as adenosine and/or serotonin (5HT) have also been implicated (84, 162, 164, 165, 169–171, 176, 179, 191–194). Activation of this vagal NANC path produces a profound relaxation of the proximal stomach and depresses motility in the antrum. Together, these excitatory and inhibitory vagal efferent control mechanisms provide the brainstem with the tools to exert a very fine control of gastric motility (10).
Vago-Vagal Reflexes
Afferent input to the NTS is organized in a distinct viscerotopic manner: The intestine is represented in the subnuclei commissuralis and medialis, the stomach in the subnuclei medialis and gelatinosus, and the esophagus in the cNTS (67–69). Elucidation of this viscerotopic organization of afferent information, together with the knowledge that the DMV may be organized in a more functional manner, led to the hypothesis that vago-vagal reflex functions are organized in a sort of functional matrix (82). According to this hypothesis, contributions of vagal afferent input from different regions of the gut lead to different patterns of gut-directed vagal efferent outflow. Accordingly, afferents serving one gut region synapse on a subset of NTS neurons that, in turn, terminate on an appropriate collection of DMV neurons, completing the reflex. Medial NTS neurons (i.e., the cells that receive vagal afferent input from the stomach and intestine) possess long mediolaterally oriented dendrites (~600 μm) that extend across the terminal zones of all gastrointestinal afferent inputs (195). A priori, such an arrangement tends to favor a convergence of vagal afferent input of different modalities and different visceral loci onto select populations of NTS neurons. Selected NTS neurons provide dominant local synaptic control over the appropriate DMV projections to the stomach (61, 82) and determine a specific pattern of gastric control (59, 60, 108). In general, stimulation of the proximal GI (i.e., esophagus, fundic stomach) vagal afferents activates some gastric-projecting vagal fibers while inhibiting others. By contrast, activation of vagal afferents from more distal sites in the antrum and intestine results in inhibition of practically all vagal outflow to the stomach (see reviews in References 10, 61, 62, and 108). In combination with other physiological studies, we see that vagal afferent input integrated by the NTS ultimately evokes gastroinhibition by either the withdrawal of cholinergic tone (i.e., generated by the pacemaker activity of DMV neurons), the activation of the vagal NANC pathway, or a combination of both, as described above.
This model provides the framework for the hardware responsible for coordinating vago-vagal responses. In its static form, one may presume that activation of any given afferent elicits the same hard-wired efferent response. Recent studies have, however, revealed a high degree of plasticity in available responses, such as in the vago-vagal reflex control over the stomach. One agonist signal may “gate” another, and the tonic effects of vagal afferent input may “gate” agonist responses. As mentioned above, DMV neurons are spontaneously active and highly sensitive to inputs from the NTS. This means that influences modulating NTS input to the DMV can have potent effects on the regulation of gastric motility. Although many examples of presynaptic modulation of the NTS are available, we focus below on the regulation of opioidergic presynaptic signaling, as it is perhaps the best-developed example.
Agonist and Afferent “Gating” of Opiate Effects on Connections Between the NTS and DMV
CNS opioid release is proportional to the anticipated quality of a reward (196–198). With respect to feeding, opioid release is associated with the expected quality of food to be consumed. It is clear that activation of central opioidergic mechanisms is associated with the consumption of large amounts of palatable food. In evolutionary terms, rapid consumption of scarce, palatable food was desirable, in that palatability is an excellent predictor of caloric density or metabolic usefulness. There is some evidence suggesting that opiates can act in the dorsal vagal complex to augment feeding as well as to coordinate gastric function in anticipation of large (and in evolution, scarce) palatable meals (199–201). It would be advantageous, under these circumstances, to have a “cephalic-phase” mechanism by which opioids could augment digestive processes in order to assimilate high-quality food as quickly as possible. It would be especially convenient if the effects of opioids to accelerate digestion could be gated or regulated by the presence of other agonists whose release would signal either imminent feeding or that feeding is taking place. In this way, the effects of opioids on digestion would work best while taking food rewards, but not during the taking of other rewards.
Activation of central opioid receptors, abundant also in the DVC, may signal the consumption of palatable food and elicit gastric relaxation (199, 200, 202–208). Enhancement of proximal gastric relaxation during feeding would assist in the assimilation of large, palatable meals. Teleologically speaking, such a mechanism would explain why celebrants at Thanksgiving dinner may complain that they can’t eat another bite of green beans, yet mysteriously “find room” for pie and ice cream. Although this laboratory group did not set out specifically to investigate the hedonic mechanism of gastric relaxation in response to palatable meals, our basic neurophysiological results do suggest that opioid effects within the DVC to provoke gastric relaxation are gated by factors associated with the taking of meals.
When we analyzed the cellular mechanisms of μ-opioid actions in the DVC, our group first showed that endogenous opioid peptides inhibit all of the glutamatergic but none of the GABAergic currents between the NTS and the DMV (141). The selectivity of the effects of opioids on the brainstem circuitry was explained by the presence of μ-opioid receptors on the surface of glutamatergic, but not GABAergic, profiles impinging on DMV neurons (141) (Figure 2). It is possible, however, to induce an inhibitory effect of opioids on approximately 60% of the GABAergic currents by activating the cAMP-PKA pathway either via forskolin or via pretreatment with hormones such as thyrotropin-releasing hormone (TRH) or CCK. TRH and CCK are well-known markers of cephalic-phase digestive functions (10, 209) and the consumption of fatty meals, respectively (210).
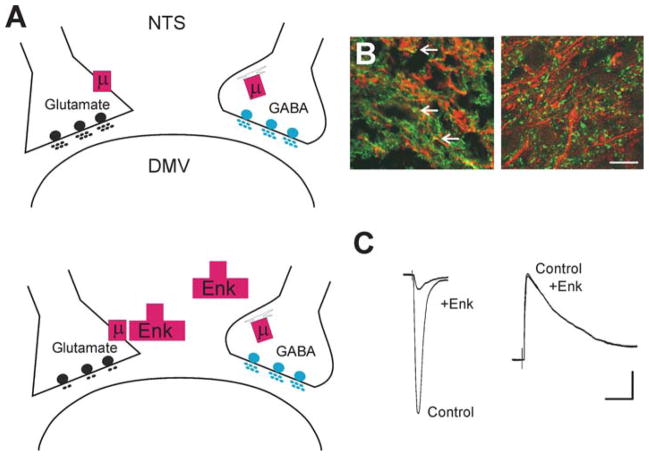
Figure 2.
Differential modulation of synaptic transmission. Depiction of the synaptic connections between the NTS and the DMV. (A) Under control conditions, the μ-opioid receptor (μ) is expressed on the nerve terminal of glutamatergic NTS profiles apposing DMV neurons; by contrast, on GABAergic nerve terminals, the μ-opioid receptor is internalized and associated with the Golgi apparatus (upper panel). Opioid agonists (e.g., Enk) can act to inhibit glutamate synaptic transmission, but not GABAergic transmission (lower panel). (B) High-power photomicrographs of the cloned μ-opioid receptor (MOR1)-immunoreactivity (-IR; TRITC filters, red) and γ-glutamyl glutamate-IR (Glu-IR; FITC filters, green; left panel) used as a marker for glutamate nerve terminals, and glutamic acid decarboxylase-IR (GAD-IR; FITC filters, green; right panel) used as a marker for GABAergic nerve terminals in the rat DVC. Note that MOR1-IR is colocalized with glutamate nerve terminals (yellow; arrows) but not with GABA nerve terminals. Scale bar: 15 μm. (C) Representative traces of evoked excitatory postsynaptic currents (eEPSCs) (left panel) and evoked inhibitory postsynaptic currents (eIPSCs) (right panel) in gastric-projecting DMV neurons voltage-clamped at −60 mV and −50 mV, respectively, evoked by electrical stimulation of the NTS. Perfusion with Enk inhibits the eEPSCs but not eIPSCs. Scale bar: 50 pA and 30 ms.
The increase in cAMP-PKA activity in NTS neurons promotes fast (within five minutes) but short-lasting (approximately 60 minutes) trafficking of μ-opioid receptors from intracellular compartments to the outer membrane of the GABAergic terminals. Once on the surface of the synaptic terminal, opioid receptors are available for activation by the μ-agonist, resulting in a decrease in the net charge of the GABAergic current (211). This mechanism may explain, in part, feeding-related changes in the effectiveness of central gastroinhibitory action of opiates. Opioid receptors are normally available for interaction with their endogenous ligand on a limited population of glutamatergic synapses impinging on DMV neurons of the cholinergic (gastroexcitatory) pathway. Inhibition of this glutamatergic input induces a partial cholinergic withdrawal in the stomach and, by consequence, a modest inhibition of gastric motility. Activation of the cAMP-PKA pathway in the NTS, for example by peptides such as TRH or CCK, induces trafficking of μ-opioid receptors on GABA terminals synapsing on DMV neurons that comprise the NANC pathway (211). Opioid agonists, by reducing the GABAergic input to these DMV neurons, increase the vagal output of NANC neurotransmitters to the target organ and, by consequence, further enhance its inhibition. By this means, the effectiveness of opiates to provoke gastroinhibition can be greatly amplified by the action of other agonists.
Agonist modulation of receptor trafficking may also be responsible for the dramatic and very different effects neuropeptide Y (NPY) has on gastric motility in the fed versus fasted state. Briefly, a vagally mediated increase in gastric motility and secretion is observed when NPY is administered centrally to fasted rats (212, 213), but gastric motility is reduced when NPY is administered to fed rats (213, 214). The cellular explanation of this intriguing difference in gastric responses to central administration of NPY is probably similar to those explanations regarding the effects of opioids at the level of the GABAergic synapse between the NTS and the DMV (62, 141, 143, 211, 215). Our group has shown recently that the GABAergic NTS synapse with DMV neurons is subject to receptor trafficking modulation by a number of neurotransmitter and hormone agonists, including TRH, CCK, 5HT, norepinephrine, pancreatic polypeptides, and opiates (103, 211, 216–221).
In another hypothesis, an additional type of short-term plasticity, determined by the state of activation of vagal afferent fibers, also influences receptor availability at the level of the DMV-NTS GABAergic synaptic connection. This, in turn, allows the vago-vagal reflex circuits to adapt their responses to the immediate physiological needs of the animal, i.e., to different phases of the digestive process. As mentioned above, this hypothesis is quite appealing because it suggests that multiple factors regulating the release of glutamate from vagal afferents (e.g., CCK, leptin, tumor necrosis factor, and glutamate itself) may modulate basic reflex functions.
Preliminary data from our group are starting to provide explanations for the cellular and structural mechanisms that underlie the capability of this circuit to adapt its responses to the visceral afferent state of activation. Although this state of activation is reflected in the levels of activity of the cAMP-PKA pathway in the GABAergic NTS nerve terminals impinging on DMV neurons, it is set, principally, by vagal afferent fiber inputs onto NTS neurons and/or terminals. We came to this conclusion by observing that following vagal sensory deafferentation, either by perivagal capsaicin or by selective sensory rhizotomy, opioids reduced the GABAergic currents between the NTS and the DMV, but without the need for increasing the activity of the cAMP-PKA pathway (215). These data imply that vagal sensory afferent inputs chronically dampen the level of activity of the cAMP-PKA pathway at the level of the GABAergic synapses between the NTS and the DMV, making the synapse unavailable for modulation. As mentioned above, glutamate is the main neurotransmitter of vagal afferent fibers impinging on brainstem circuits (32–35, 37–42, 44, 45). Following its release, glutamate activates both ionotropic and metabotropic receptors (mGluRs). All metabotropic glutamate receptor subunits identified to date have been found in the DVC (50). The presence of group II and III mGluRs, both of which play a role in the modulation of brainstem vagal circuits (47–49, 222, 223), is of particular interest, because their activation decreases the levels of cAMP (224). We have recently investigated whether these receptor groups are involved in setting the levels of cAMP-PKA activation in GI brainstem circuits and, by consequence, in determining the availability of the NTS-DMV GABAergic synapse to modulation.
Our data indicate that group II mGluRs, but not group III mGluRs, are involved in this type of modulation of the GI brainstem circuits. In fact, pretreatment with selective mGluR group II antagonists “primes” the μ-opioid receptors on GABAergic NTS-DMV synapses, making it possible for opioids to modulate the inhibitory currents without the need to pharmacologically increase the activity of the cAMP-PKA pathway (K.N. Browning, Z.L. Zheng, & R.A. Travagli, submitted manuscript).
In summary, sensory vagal afferent fibers use group II mGluRs to dampen the activity of the cAMP-PKA pathway in GABAergic NTS nerve terminals. While held in check, the low levels of cAMP-PKA activity keep the μ-opioid receptors in inaccessible intracellular compartments such that enkephalins do not have a modulatory effect on the NTS-DMV GABAergic currents. The use of glutamate both to activate the sensory vagal pathways and at the same time keep the GABAergic NTS neurons in a nonmodulatory state is an apparent conundrum. In fact, it implies that glutamate released by sensory vagal afferent fibers exerts opposite functions via simultaneously acting at ionotropic receptors (which carry information about the visceral organs and prepare the circuit for a sophisticated level of modulation to allow the appropriate response) and at mGluRs (which dampen the brainstem GABAergic circuitry and prevent its modulation by neurotransmitters such as pancreatic polypeptides, opioids, indolamines, and catecholamines).
We have to keep in mind, however, that group II mGluRs may be located perisynaptically (225) and may be activated by the release of glutamate that leaks from vagal afferent terminals on NTS neurons in an action potential–independent manner. If this is the case, then mGluRs and ionotropic glutamate receptors may coexist without interfering with each others’ functions; furthermore, peptides impinging on NTS neurons, such as TRH and CCK, that increase the activity of the cAMP-PKA pathway may overcome the tonic activation of group II mGluRs, increase the levels of cAMP, and induce altered sensitivity of neural circuits to a variety of inputs. The role of glutamate activation of group II mGluR, then, may be to change the synaptic state of the NTS-DMV GABAergic circuit. The widespread use by different neurotransmitters of the cAMP-PKA pathway argues in favor of its utilization in a general manner in the control of vago-vagal circuits. Also, depending on whether the affected GABAergic NTS-DMV synapse controls cholinergic excitatory pathways or NANC inhibitory pathways, a decrease in synaptic transmission may result in an increased or decreased vagal motor output, respectively. The physiological correlate of these experimental conditions is modulation of the NTS-DMV GABAergic synapse by neurotransmitters that are coupled to adenylate cyclase and that generate cAMP. Alterations in sensory inputs from the GI tract, such as those following activation of vago-vagal reflexes or changes in the feeding status, for example, are expected to modify the ability of tonic GABAergic inputs controlling the vagal brainstem circuits to be modulated. For example, the constant perception of ongoing GI tract activity exerts a tonic inhibition of cAMP-PK pathways in the DVC. Activation of vago-vagal reflexes or changes in the state of activation (i.e., from fasted to fed or vice versa) may change the levels of cAMP and influence the ability of circulating hormones or locally released neurotransmitters to modulate inhibitory synaptic transmission between the NTS and the DMV (Figure 3).
Figure 3.
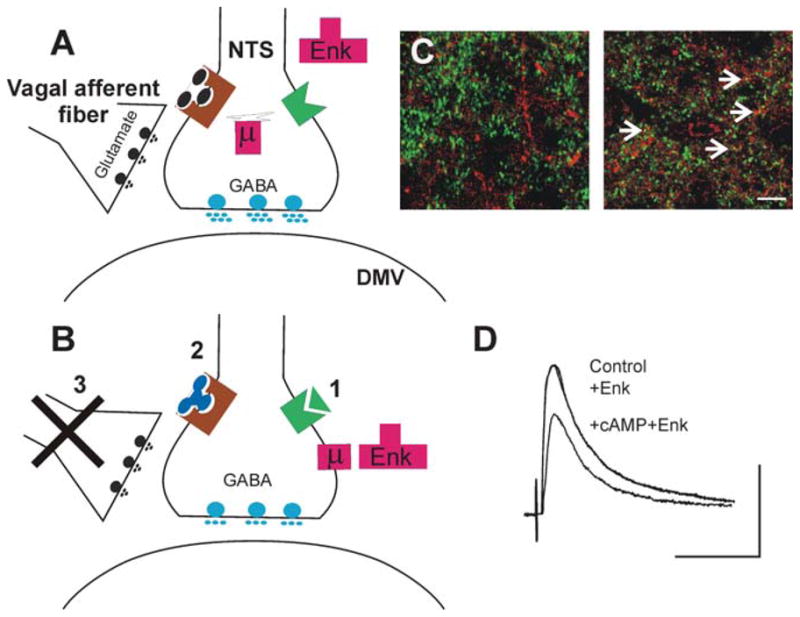
Increasing the levels of cAMP in the brainstem induces receptor trafficking in NTS nerve terminals. (A) In control conditions, i.e., when the levels of cAMP are low either because the tonic release of glutamate from vagal afferent fibers activates group II metabotropic glutamate receptors (left) or because Gαpled receptors are not activated (right), the terminals of GABAergic neurons in the NTS store μ-opioid receptors (μ) in internal compartments associated with the Golgi complex. In this situation, μ-opioid agonists (e.g., Enk) cannot modulate the release of GABA onto DMV neurons. (B) Following increases in cAMP levels within the GABAergic nerve terminal, for example, by (1) activation of a receptor coupled to Gα., TRH or CCK, (2) antagonism of group II metabotropic glutamate receptors, or (3) removal of tonic vagal afferent input, μ-opioid receptors are released from the Golgi apparatus and translocated to the nerve terminal membrane, where opioid agonists can inhibit GABA synaptic transmission between the NTS and the DMV. (C) High-power photomicrographs of the cloned μ-opioid receptor (MOR1)-immunoreactivity (-IR; TRITC filters, red) and glutamic acid decarboxylase-IR (GAD-IR; FITC filters, green) used as a marker for GABAergic nerve terminals in the rat DVC. In control conditions (left panel), note the absence of MOR- and GAD-IR colocalized profiles. Following vagal afferent rhizotomy (right panel), many nerve terminals show MOR- and GAD-IR colocalized profiles (yellow; arrows). Images represent three-dimensional reconstructions from Z-stack image series. Scale bar: 10 μm. (D) Representative traces of eIPSCs evoked in a gastric-projecting DMV neuron voltage-clamped at −50 mV. Perfusion with Enk does not affect the amplitude of IPSCs evoked by electrical stimulation of the NTS. Following five minutes’ perfusion with substances that increase the cAMP levels, however, reapplication of Enk reduces the amplitude of the eIPSCs. Scale bar: 50 ms and 200 pA.
SUMMARY
GI brainstem circuits that are part of vago-vagal reflexes are comprised of sensory afferent fibers whose terminals impinge on NTS neurons. The NTS neurons then project to DMV cells, which in turn provide the preganglionic efferent fibers controlling cholinergic excitatory and NANC inhibitory postganglionic cells. The strategic location outside the blood-brain barrier of portions of brainstem vagal circuits makes them accessible to a multitude of circulating hormones, cytokines, and chemokines that can dramatically alter vago-vagal reflex responsiveness. Add to this the dense and phenotypically diverse descending projections from anterior forebrain structures to the dorsal vagal complex (8), and it becomes clear that the number of mechanisms by which vago-vagal reflex selectivity and sensitivity can be modulated is virtually limitless.
The presynaptic modulation, and hence plasticity, of these circuits allows for a finely tuned control over gastric functions. It is now apparent that these circuits are not static entities devoted to the relay of unprocessed information between the brain and the gut. Indeed, by controlling the levels of activity of the cAMP-PKA pathway in synaptic terminals, circulating molecules, descending neurotransmitter inputs, and primary afferent traffic can act on brainstem circuits to affect the sensitivity of the synapses to modulation. If variations of the cAMP levels are determined, for example by the substances circulating in different situations such as during fasting or following a meal or by differing levels of visceral afferent traffic, then the net effect of activation of a particular circuit can vary according to the cAMP levels within the synapse itself. This implies, then, that gross differences in the performance of gastric control reflexes that occur at different times of the digestive process may result from variations in the cAMP levels at GABAergic NTS-DMV synapses.
In conclusion, we are just starting to understand the cellular mechanisms that underlie the exquisite coordination of digestive processes with ongoing and anticipated changes in behavior as a consequence of the diversity of input. The work to be conducted in the years to come must investigate the circulating factors that can cause rapid changes in brain-gut control through their direct action on brainstem vagal reflex control circuits and the cellular entities involved in this control. A multitude of possible modulatory mechanisms exist within these circuits to guarantee speed, precision, and flexibility in the control of digestive processes.
Acknowledgments
The authors would like to thank the NIH (grants nos. DK-55530, DK-56373, and DK-52142) and NSF (grant no. IBN-0456291) for their support. We also thank Cesare M. Travagli, Hans Hermann, and Lois and Richard F. Rogers for their support and encouragement.
Contributor Information
R. Alberto Travagli, Email: travagra@pbrc.edu.
Gerlinda E. Hermann, Email: hermange@pbrc.edu.
Kirsteen N. Browning, Email: brownik@pbrc.edu.
Richard C. Rogers, Email: rogersrc@pbrc.edu.
LITERATURE CITED
- 1.Wood JD. Physiology of the enteric nervous system. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven; 1987. pp. 67–110. [Google Scholar]
- 2.Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996;334:1106–15. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- 3.Costa M, Brookes SJH, Hennig GW. Anatomy and physiology of the enteric nervous system. Gut. 2000;47:15–19. doi: 10.1136/gut.47.suppl_4.iv15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bornstein JC, Costa M, Grider JR. Enteric motor and interneuronal circuits controlling motility. Neurogastroenterol Motil. 2004;16(Suppl 1):34–38. doi: 10.1111/j.1743-3150.2004.00472.x. [DOI] [PubMed] [Google Scholar]
- 5.Goyal RK, Paterson WG. Esophageal motility. In: Wood JD, editor. Handbook of Physiology—The Gastrointestinal System I. Bethesda, MD: Am. Phys. Soc; 1989. pp. 865–908. [Google Scholar]
- 6.Chang HY, Mashimo H, Goyal RK. Musings on the wanderer: What’s new in our understanding of vago-vagal reflex?: IV Current concepts of vagal efferent projections to the gut. Am J Physiol Gastrointest Liver Physiol. 2003;284:G357–66. doi: 10.1152/ajpgi.00478.2002. [DOI] [PubMed] [Google Scholar]
- 7.Roman C, Gonella J. Extrinsic control of digestive tract motility. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven; 1987. pp. 507–54. [Google Scholar]
- 8.Blessing WW. The Lower Brainstem and Bodily Homeostasis. New York: Oxford Univ. Press; 1997. [Google Scholar]
- 9.Rogers RC, Hermann GE, Travagli RA. Brainstem control of the gastric function. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. San Diego, CA: Elsevier; 2005. In press. [Google Scholar]
- 10.Rogers RC, McTigue DM, Hermann GE. Vagovagal reflex control of digestion: afferent modulation by neural and “endoneurocrine” factors. Am J Physiol. 1995;268:G1–10. doi: 10.1152/ajpgi.1995.268.1.G1. [DOI] [PubMed] [Google Scholar]
- 11.Browning KN. Excitability of nodose ganglion cells and their role in vago-vagal reflex control of gastrointestinal function. Curr Opin Pharmacol. 2003;3:613–17. doi: 10.1016/j.coph.2003.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Browning KN, Mendelowitz D. Musings on the wanderer: what’s new in our understanding of vago-vagal reflexes?: II Integration of afferent signaling from the viscera by the nodose ganglia. Am J Physiol Gastrointest Liver Physiol. 2003;284:G8–14. doi: 10.1152/ajpgi.00322.2002. [DOI] [PubMed] [Google Scholar]
- 13.Zhuo H, Ichikawa H, Helke CJ. Neurochemistry of the nodose ganglion. Prog Neurobiol. 1997;52:79–107. doi: 10.1016/s0301-0082(97)00003-8. [DOI] [PubMed] [Google Scholar]
- 14.Berthoud HR, Kressel M, Raybould HE, Neuhuber WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol. 1991;191:203–12. doi: 10.1007/BF00187819. [DOI] [PubMed] [Google Scholar]
- 15.Berthoud HR, Powley TL. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol. 1992;319:261–76. doi: 10.1002/cne.903190206. [DOI] [PubMed] [Google Scholar]
- 16.Neuhuber WL, Kressel M, Stark A, Berthoud HR. J Auton Nerv Syst. 1998;70:92–102. doi: 10.1016/s0165-1838(98)00034-4. (Abstr) [DOI] [PubMed] [Google Scholar]
- 17.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 18.Mayer EA. The physiology of gastric storage and emptying. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven; 1994. pp. 929–76. [Google Scholar]
- 19.Berthoud HR, Patterson LM, Zheng H. Vagal-enteric interface: vagal activation-induced expression of c-Fos and p-CREB in neurons of the upper gastrointestinal tract and pancreas. Anat Rec. 2001;262:29–40. doi: 10.1002/1097-0185(20010101)262:1<29::AID-AR1008>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Sengupta JN. An overview of esophageal sensory receptors. Am J Med. 2000;108 (Suppl 4a):87S–9S. doi: 10.1016/s0002-9343(99)00344-7. [DOI] [PubMed] [Google Scholar]
- 21.Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil. 2004;16 (Suppl 1):28–33. doi: 10.1111/j.1743-3150.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- 22.Zagorodnyuk V, Chen B, Brookes S. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534:255–68. doi: 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blackshaw LA, Grundy D, Scratcherd T. Involvement of gastrointestinal mechano- and intestinal chemoreceptors in vagal reflexes: an electrophysiological study. J Auton Nerv Syst. 1987;18:225–34. doi: 10.1016/0165-1838(87)90121-4. [DOI] [PubMed] [Google Scholar]
- 24.Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Rev. 2000;34:1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- 25.Zagorodnyuk VP, Brookes SJH. Transduction sites of vagal mechanore-ceptors in the guinea pig esophagus. J Neurosci. 2000;20:6249–55. doi: 10.1523/JNEUROSCI.20-16-06249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grundy D, Schemann M. The interface between the enteric and central nervous system. In: Tache Y, Wingate DL, Burks TF, editors. Innvervation of the Gut: Pathophysiological Implications. Boca Raton: CRC; 1993. pp. 157–66. [Google Scholar]
- 27.Powley TL, Phillips RJ. Musings on the wanderer: what’s new in our understanding of vago-vagal reflexes? I. Morphology and topography of vagal afferents innervating the GI tract. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1217–25. doi: 10.1152/ajpgi.00249.2002. [DOI] [PubMed] [Google Scholar]
- 28.Zagorodnyuk VP, Chen BN, Costa M, Brookes SJ. 4-aminopyridine- and dendrotoxin-sensitive potassium channels influence excitability of vagal mechano-sensitive endings in guinea-pig oesophagus. Br J Pharmacol. 2002;137:1195–206. doi: 10.1038/sj.bjp.0704964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut. 2002;51 (Suppl 1):i2–i5. doi: 10.1136/gut.51.suppl_1.i2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zagorodnyuk VP, Chen BN, Costa M, Brookes SJ. Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the guinea-pig oesophagus. J Physiol. 2003;553:575–87. doi: 10.1113/jphysiol.2003.051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirkup AJ, Brunsden AM, Grundy D. Receptors and transmission in the brain-gut axis: potential for novel therapies. 1 Receptors on visceral afferents. Am J Physiol Gastrointest Liver Physiol. 2001;280:G787–94. doi: 10.1152/ajpgi.2001.280.5.G787. [DOI] [PubMed] [Google Scholar]
- 32.Kawai Y, Senba E. Organization of excitatory and inhibitory local networks in the caudal nucleus of tractus solitarius of rats revealed in in vitro slice preparation. J Comp Neurol. 1996;373:309–21. doi: 10.1002/(SICI)1096-9861(19960923)373:3<309::AID-CNE1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Smith BN, Dou P, Barber WD, Dudek FE. Vagally evoked synaptic currents in the immature rat nucleus tractus solitarii in an intact in vitro preparation. J Physiol. 1998;512:149–62. doi: 10.1111/j.1469-7793.1998.149bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu WY, Bieger D. Vagal afferent transmission in the NTS mediating reflex responses of the rat esophagus. Am J Physiol. 1998;274:R1436–45. doi: 10.1152/ajpregu.1998.274.5.R1436. [DOI] [PubMed] [Google Scholar]
- 35.Glatzer NR, Hasney CP, Bhaskaran MD, Smith BN. Synaptic and morphologic properties in vitro of premotor rat nucleus tractus solitarius neurons labeled transneuronally from the stomach. J Comp Neurol. 2003;464:525–39. doi: 10.1002/cne.10831. [DOI] [PubMed] [Google Scholar]
- 36.Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol. 1990;259:H1307–11. doi: 10.1152/ajpheart.1990.259.4.H1307. [DOI] [PubMed] [Google Scholar]
- 37.Hornby PJ. Receptors and transmission in the brain-gut axis. II. Excitatory amino acid receptors in the brain-gut axis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1055–60. doi: 10.1152/ajpgi.2001.280.6.G1055. [DOI] [PubMed] [Google Scholar]
- 38.Jean A. Brainstem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–69. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 39.Lachamp P, Balland B, Tell F, Crest M, Kessler JP. Synaptic localization of the glutamate receptor subunit GluR2 in the rat nucleus tractus solitarii. Eur J Neurosci. 2003;17:892–96. doi: 10.1046/j.1460-9568.2003.02494.x. [DOI] [PubMed] [Google Scholar]
- 40.Yen JC, Chan JY, Chan SHH. Differential roles of NMDA and non-NMDA receptors in synaptic responses of neurons in nucleus tractus solitarii of the rat. J Neurophysiol. 1999;81:3034–43. doi: 10.1152/jn.1999.81.6.3034. [DOI] [PubMed] [Google Scholar]
- 41.Baptista V, Zheng ZL, Coleman FH, Rogers RC, Travagli RA. Characterization of neurons of the nucleus tractus solitarius pars centralis. Brain Res. 2005;1052:139–46. doi: 10.1016/j.brainres.2005.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tell F, Jean A. Activation of N-methyl-D-aspartate receptors induces endogenous rhythmic bursting activities in nucleus tractus solitarii neurons: an intra-cellular study on adult rat brainstem slices. Eur J Neurosci. 1991;3:1353–65. doi: 10.1111/j.1460-9568.1991.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang YT, Bieger D, Neuman RS. Activation of NMDA receptors is necessary for fast information transfer at brainstem vagal motoneurons. Brain Res. 1991;567:260–66. doi: 10.1016/0006-8993(91)90804-5. [DOI] [PubMed] [Google Scholar]
- 44.Aylwin ML, Horowitz JM, Bonham AC. NMDA receptors contribute to primary visceral afferent transmission in the nucleus of the solitary tract. J Neurophysiol. 1997;77:2539–48. doi: 10.1152/jn.1997.77.5.2539. [DOI] [PubMed] [Google Scholar]
- 45.Andresen MC, Kunze DL. Nucleus tractus solitarius—gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- 46.Jones NM, Monn JA, Beart PM. Type I and II metabotropic glutamate receptors regulate the outflow of [3H]D-aspartate and [14C]γ-aminobutyric acid in rat solitary nucleus. Eur J Pharmacol. 1998;353:43–51. doi: 10.1016/s0014-2999(98)00394-x. [DOI] [PubMed] [Google Scholar]
- 47.Chen CY, Ling EH, Horowitz JM, Bonham AC. Synaptic transmission in nucleus tractus solitarius is depressed by Group II and III but not Group I presynaptic metabotropic glutamate receptors in rats. J Physiol. 2002;538:773–86. doi: 10.1113/jphysiol.2001.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen CY, Bonham AC. Glutamate suppresses GABA release via presynaptic metabotropic glutamate receptors at baroreceptor neurones in rats. J Physiol. 2005;562:535–51. doi: 10.1113/jphysiol.2004.076885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Page AJ, Young RL, Martin CM, Umaerus M, O’Donnell TA, et al. Metabotropic glutamate receptors inhibit mechanosensitivity in vagal sensory neurons. Gastroenterology. 2005;128:402–10. doi: 10.1053/j.gastro.2004.11.062. [DOI] [PubMed] [Google Scholar]
- 50.Hay M, McKenzie H, Lindsley K, Dietz N, Bradley SR, et al. Heterogeneity of metabotropic glutamate receptors in autonomic cell groups of the medulla oblongata of the rat. J Comp Neurol. 1999;403:486–501. [PubMed] [Google Scholar]
- 51.Hay M, Hoang CJ, Pamidimukkala J. Cellular mechanisms regulating synaptic vesicle exocytosis and endocytosis in aortic baroreceptor neurons. Ann NY Acad Sci. 2001;940:119–31. doi: 10.1111/j.1749-6632.2001.tb03671.x. [DOI] [PubMed] [Google Scholar]
- 52.Simasko SM, Ritter RC. Cholecystokinin activates both A- and C-type vagal afferent neurons. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1204–13. doi: 10.1152/ajpgi.00132.2003. [DOI] [PubMed] [Google Scholar]
- 53.Appleyard SM, Bailey TW, Doyle MW, Jin YH, Smart JL, et al. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J Neurosci. 2005;25:3578–85. doi: 10.1523/JNEUROSCI.4177-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baptista V, Zheng Z, Coleman FH, Rogers RC, Travagli RA. Cholecystokinin octapeptide increases spontaneous gluta-matergic synaptic transmission to neurons of the nucleus tractus solitarius centralis. J Neurophysiol. 2005;94:2763–71. doi: 10.1152/jn.00351.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peters JH, McKay BM, Simasko SM, Ritter RC. Leptin-induced satiation mediated by abdominal vagal afferents. Am J Physiol Regul Integr Comp Physiol. 2005;288:R879–84. doi: 10.1152/ajpregu.00716.2004. [DOI] [PubMed] [Google Scholar]
- 56.Kaplan JM, Moran TH. Gastrointestinal signaling in the control of food intake. In: Stricker E, Woods S, editors. Neurobiology of Food and Fluid Intake, Vol. 14 of Handbook of Behavioral Neurobiology. New York: Kluwer Acad./Plenum Publ; 2004. pp. 275–305. [Google Scholar]
- 57.Emch GS, Hermann GE, Rogers RC. TNF-α activates solitary nucleus neurons responsive to gastric distention. Am J Physiol Gastrointest Liver Physiol. 2000;279:G582–86. doi: 10.1152/ajpgi.2000.279.3.G582. [DOI] [PubMed] [Google Scholar]
- 58.Jin YH, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci. 2004;24:4709–17. doi: 10.1523/JNEUROSCI.0753-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miolan JP, Roman C. The role of oesophageal and intestinal receptors in the control of gastric motility. J Auton Nerv Syst. 1984;10:235–41. doi: 10.1016/0165-1838(84)90018-3. [DOI] [PubMed] [Google Scholar]
- 60.Miolan JP, Roman C. Discharge of efferent vagal fibers supplying gastric antrum: indirect study by nerve suture technique. Am J Physiol. 1978;235:E366–73. doi: 10.1152/ajpendo.1978.235.4.E366. [DOI] [PubMed] [Google Scholar]
- 61.Travagli RA, Rogers RC. Receptors and transmission in the brain-gut axis: potential for novel therapies. V. Fast and slow extrinsic modulation of dorsal vagal complex circuits. Am J Physiol Gastrointest Liver Physiol. 2001;281:G595–601. doi: 10.1152/ajpgi.2001.281.3.G595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Travagli RA, Hermann GE, Browning KN, Rogers RC. Musings on the wanderer: What’s new in our understanding of vago-vagal reflexes?: III Activity-dependent plasticity in vago-vagal reflexes controlling the stomach. Am J Physiol Gastrointest Liver Physiol. 2003;284:G180–87. doi: 10.1152/ajpgi.00413.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goyal RK, Padmanabhan R, Sang Q. Neural circuits in swallowing and abdominal vagal afferent-mediated lower esophageal sphincter relaxation. Am J Med. 2001;111 (Suppl 8A):95S–105S. doi: 10.1016/s0002-9343(01)00863-4. [DOI] [PubMed] [Google Scholar]
- 64.Bieger D. The brainstem esophago-motor network pattern generator: a rodent model. Dysphagia. 1993;8:203–8. doi: 10.1007/BF01354539. [DOI] [PubMed] [Google Scholar]
- 65.McCann MJ, Rogers RC. Impact of antral mechanoreceptor activation on the vago-vagal reflex in the rat: functional zonation of responses. J Physiol. 1992;453:401–11. doi: 10.1113/jphysiol.1992.sp019235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rogers RC, Hermann GE, Travagli RA. Brainstem pathways responsible for oesophageal control of gastric motility and tone in the rat. J Physiol. 1999;514:369–83. doi: 10.1111/j.1469-7793.1999.369ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989;283:248–68. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- 68.Altschuler SM, Ferenci DA, Lynn RB, Miselis RR. Representation of the cecum in the lateral dorsal motor nucleus of the vagus nerve and commissural sub-nucleus of the nucleus tractus solitarii in rat. J Comp Neurol. 1991;304:261–74. doi: 10.1002/cne.903040209. [DOI] [PubMed] [Google Scholar]
- 69.Altschuler SM, Escardo J, Lynn RB, Miselis RR. The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology. 1993;104:502–9. doi: 10.1016/0016-5085(93)90419-d. [DOI] [PubMed] [Google Scholar]
- 70.Barraco R, El-Ridi M, Parizon M, Bradley D. An atlas of the rat subpostremal nucleus tractus solitarius. Brain Res Bull. 1992;29:703–65. doi: 10.1016/0361-9230(92)90143-l. [DOI] [PubMed] [Google Scholar]
- 71.Paton JFR, Li Y-W, Deuchars J, Kasparov S. Properties of solitary tract neurons receiving inputs from the sub-diaphragmatic vagus nerve. Neurosci. 2000;95:141–53. doi: 10.1016/s0306-4522(99)00416-9. [DOI] [PubMed] [Google Scholar]
- 72.Chan RKW, Peto CA, Sawchenko PE. Fine structure and plasticity of barosensitive neurons in the nucleus solitary tract. J Comp Neurol. 2000;422:338–51. [PubMed] [Google Scholar]
- 73.Bradley RM, Grabauskas G. Excitation, inhibition and synaptic plasticity in the rostral gustatory zone of the nucleus of the solitary tract. Ann NY Acad Sci. 1998;855:467–74. doi: 10.1111/j.1749-6632.1998.tb10607.x. [DOI] [PubMed] [Google Scholar]
- 74.Mifflin SW, Felder RB. Synaptic mechanisms regulating cardiovascular afferent inputs to solitary tract nucleus. Am J Physiol Heart Circ Physiol. 1990;28:H653–61. doi: 10.1152/ajpheart.1990.259.3.H653. [DOI] [PubMed] [Google Scholar]
- 75.Loewy AD, Spyer KM. Vagal pre-ganglionic neurons. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. New York/Oxford: Oxford Univ. Press; 1990. pp. 68–87. [Google Scholar]
- 76.Spyer KM. Neural organisation and control of the baroreceptor reflex. Rev Physiol Biochem Pharmacol. 1981;88:24–124. [PubMed] [Google Scholar]
- 77.Broussard DL, Altschuler SM. Brainstem viscerotopic organization of afferents and efferents involved in the control of swallowing. Am J Med. 2000;108:79S–86S. doi: 10.1016/s0002-9343(99)00343-5. [DOI] [PubMed] [Google Scholar]
- 78.Mendelowitz D. Advances in parasympathetic control of heart rate and cardiac function. News Physiol Sci. 1999;14:155–61. doi: 10.1152/physiologyonline.1999.14.4.155. [DOI] [PubMed] [Google Scholar]
- 79.Fox EA, Powley TL. Longitudinal columnar organization within the dorsal motor nucleus represents separate branches of the abdominal vagus. Brain Res. 1985;341:269–82. doi: 10.1016/0006-8993(85)91066-2. [DOI] [PubMed] [Google Scholar]
- 80.Shapiro RE, Miselis RR. The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol. 1985;238:473–88. doi: 10.1002/cne.902380411. [DOI] [PubMed] [Google Scholar]
- 81.Norgren R, Smith GP. Central distribution of subdiaphragmatic vagal branches in the rat. J Comp Neurol. 1988;273:207–23. doi: 10.1002/cne.902730206. [DOI] [PubMed] [Google Scholar]
- 82.Powley TL, Berthoud HR, Fox EA, Laughton W. The dorsal vagal complex forms a sensory-motor lattice: the circuitry of gastrointestinal reflexes. In: Ritter S, Ritter RC, Barnes CD, editors. Neuroanatomy and Physiology of Abdominal Vagal Afferents. Boca Raton, FL: CRC; 1992. pp. 55–79. [Google Scholar]
- 83.Beckett EA, McGeough CA, Sanders KM, Ward SM. Pacing of interstitial cells of Cajal in the murine gastric antrum: neurally mediated and direct stimulation. J Physiol. 2003;553:545–59. doi: 10.1113/jphysiol.2003.050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berthoud HR. Anatomical demonstration of vagal input to nicotinamide acetamide dinucleotide phosphate diaphorase-positive (nitrergic) neurons in rat fundic stomach. J Comp Neurol. 1995;358:428–39. doi: 10.1002/cne.903580309. [DOI] [PubMed] [Google Scholar]
- 85.Zheng H, Berthoud HR. Functional vagal input to gastric myenteric plexus as assessed by vagal stimulation-induced Fos expression. Am J Physiol Gastroin-test Liver Physiol. 2000;279:G73–81. doi: 10.1152/ajpgi.2000.279.1.G73. [DOI] [PubMed] [Google Scholar]
- 86.Deleted in proof
- 87.Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol. 1991;260:R200–7. doi: 10.1152/ajpregu.1991.260.1.R200. [DOI] [PubMed] [Google Scholar]
- 88.Berthoud HR. The caudal brainstem and the control of food intake and energy balance. In: Stricker E, Woods S, editors. Neurobiology of Food and Fluid Intake, Vol. 14 of Handbook of Behavioral Neurobiology. New York: Kluwer Acad./Plenum Publ; 2004. pp. 195–240. [Google Scholar]
- 89.Krowicki ZK, Sharkey KA, Serron SC, Nathan NA, Hornby PJ. Distribution of nitric oxide synthase in rat dorsal vagal complex and effects of microinjection of NO compounds upon gastric motor function. J Comp Neurol. 1997;377:49–69. doi: 10.1002/(sici)1096-9861(19970106)377:1<49::aid-cne6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 90.Fox EA, Powley TL. Morphology of identified preganglionic neurons in the dorsal motor nucleus of the vagus. J Comp Neurol. 1992;322:79–98. doi: 10.1002/cne.903220107. [DOI] [PubMed] [Google Scholar]
- 91.Jarvinen MK, Powley TL. Dorsal motor nucleus of the vagus neurons: a multivariate taxonomy. J Comp Neurol. 1999;403:359–77. [PubMed] [Google Scholar]
- 92.Browning KN, Renehan WE, Travagli RA. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. J Physiol. 1999;517:521–32. doi: 10.1111/j.1469-7793.1999.0521t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martinez-Pena y Valenzuela IM, Browning KN, Travagli RA. Morphological differences between planes of section do not influence the electrophysiological properties of identified rat dorsal motor nucleus of the vagus neurons. Brain Res. 2004;1003:54–60. doi: 10.1016/j.brainres.2003.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Browning KN, Coleman FH, Travagli RA. Characterization of pancreas-projecting rat dorsal motor nucleus of the vagus neurons. Am J Physiol Gastroin-test Liver Physiol. 2005;288:G950–55. doi: 10.1152/ajpgi.00549.2004. [DOI] [PubMed] [Google Scholar]
- 95.Huang X, Tork I, Paxinos G. Dorsal motor nucleus of the vagus nerve: a cyto- and chemoarchitectonic study in the human. J Comp Neurol. 1993;330:158–82. doi: 10.1002/cne.903300203. [DOI] [PubMed] [Google Scholar]
- 96.Fogel R, Zhang X, Renehan WE. Relationships between the morphology and function of gastric and intestinal distention-sensitive neurons in the dorsal motor nucleus of the vagus. J Comp Neurol. 1996;364:78–91. doi: 10.1002/(SICI)1096-9861(19960101)364:1<78::AID-CNE7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 97.Travagli RA, Gillis RA. Hyperpolarization-activated currents IH and IKIR, in rat dorsal motor nucleus of the vagus neurons in vitro. J Neurophysiol. 1994;71:1308–17. doi: 10.1152/jn.1994.71.4.1308. [DOI] [PubMed] [Google Scholar]
- 98.Sah P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19:150–54. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- 99.Dean JB, Huang R-Q, Erlichman JS, Southard TL, Hellard DT. Cell-cell coupling occurs in dorsal medullary neurons after minimizing anatomical-coupling artifacts. Neuroscience. 1997;80:21–40. doi: 10.1016/s0306-4522(97)00016-x. [DOI] [PubMed] [Google Scholar]
- 100.Pedarzani P, Kulik A, Muller M, Ballanyi K, Stocker M. Molecular determinants of Ca2+-dependent K+ channel function in rat dorsal vagal neurones. J Physiol. 2000;527:283–90. doi: 10.1111/j.1469-7793.2000.t01-1-00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol. 1991;260:G531–36. doi: 10.1152/ajpgi.1991.260.3.G531. [DOI] [PubMed] [Google Scholar]
- 102.Marks JD, Donnelly DF, Haddad GG. Adenosine-induced inhibition of vagal motoneuron excitability: receptor subtype and mechanisms. Am J Physiol. 1993;264:L124–32. doi: 10.1152/ajplung.1993.264.2.L124. [DOI] [PubMed] [Google Scholar]
- 103.Browning KN, Travagli RA. The peptide TRH uncovers the presence of presynaptic 5-HT1A receptors via activation of a second messenger pathway in the rat dorsal vagal complex. J Physiol. 2001;531:425–35. doi: 10.1111/j.1469-7793.2001.0425i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–80. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- 105.Hunt JN. Mechanisms and disorders of gastric emptying. Annu Rev Med. 1983;34:219–29. doi: 10.1146/annurev.me.34.020183.001251. [DOI] [PubMed] [Google Scholar]
- 106.Szurszewski JH. Modulation of smooth muscle by nervous activity: a review and a hypothesis. Fed Proc. 1977;36:2456–61. [PubMed] [Google Scholar]
- 107.Schemann M, Grundy D. Electro-physiological identification of vagally innervated enteric neurons in guinea pig stomach. Am J Physiol. 1992;263:G709–18. doi: 10.1152/ajpgi.1992.263.5.G709. [DOI] [PubMed] [Google Scholar]
- 108.Grundy D, Schemann M. Motor control of the stomach. In: Brookes S, Costa M, editors. Innervation of the Gastrointestinal Tract. London: Taylor and Francis; 2002. pp. 57–102. [Google Scholar]
- 109.Champagnat J, Denavit-Saubie M, Grant K, Shen KF. Organization of synaptic transmission in the mammalian solitary complex, studied in vitro. J Physiol. 1986;381:551–73. doi: 10.1113/jphysiol.1986.sp016343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fortin G, Champagnat J. Spontaneous synaptic activities in rat nucleus tractus solitarius neurons in vitro: evidence for re-excitatory processing. Brain Res. 1993;630:125–35. doi: 10.1016/0006-8993(93)90650-c. [DOI] [PubMed] [Google Scholar]
- 111.Schild JH, Khushalani S, Clark JW, Andresen MC, Kunze DL, Yang M. An ionic current model for neurons in the rat medial nucleus tractus solitarii receiving sensory afferent input. J Physiol. 1993;469:341–63. doi: 10.1113/jphysiol.1993.sp019817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fan W, Andresen MC. Differential frequency-dependent reflex integration of myelinated and nonmyelinated rat aortic baroreceptors. Am J Physiol. 1998;275:H632–40. doi: 10.1152/ajpheart.1998.275.2.H632. [DOI] [PubMed] [Google Scholar]
- 113.Andresen MC, Doyle MW, Bailey TW, Jin YH. Differentiation of autonomic reflex control begins with cellular mechanisms at the first synapse within the nucleus tractus solitarius. Braz J Med Biol Res. 2004;37:549–58. doi: 10.1590/s0100-879x2004000400012. [DOI] [PubMed] [Google Scholar]
- 114.Gillis RA, Quest JA, Pagani FD, Norman WP. Control centers in the central nervous system for regulating gastrointestinal motility. In: Wood JD, editor. Handbook of Physiology. Section 6: The Gastrointestinal System Motility and Circulation. 2. Vol. 1. Bethesda, MD: Am. Physiol. Soc; 1989. pp. 621–83. [Google Scholar]
- 115.Dekin MS, Getting PA. Firing pattern of neurons in the nucleus tractus solitarius: modulation by membrane hyper-polarization. Brain Res. 1984;324:180–84. doi: 10.1016/0006-8993(84)90640-1. [DOI] [PubMed] [Google Scholar]
- 116.Dekin MS, Getting PA. In vitro characterization of neurons in the ventral part of the nucleus tractus solitarius. II. Ionic basis for repetitive firing patterns. J Neurophysiol. 1987;58:215–29. doi: 10.1152/jn.1987.58.1.215. [DOI] [PubMed] [Google Scholar]
- 117.Haddad GG, Getting PA. Repetitive firing properties of neurons in the ventral region of nucleus tractus solitarius. In vitro studies in adult and neonatal rat. J Neurophysiol. 1989;62:1213–24. doi: 10.1152/jn.1989.62.6.1213. [DOI] [PubMed] [Google Scholar]
- 118.Paton JFR, Rogers WT, Schwaber JS. Tonically rhythmic neurons within a cardiorespiratory region of the nucleus tractus solitarii of the rat. J Neurophysiol. 1991;66:824–38. doi: 10.1152/jn.1991.66.3.824. [DOI] [PubMed] [Google Scholar]
- 119.Maley BE. Immunohistochemical localization of neuropeptides and neurotransmitters in the nucleus solitarius. Chem Senses. 1996;21:367–76. doi: 10.1093/chemse/21.3.367. [DOI] [PubMed] [Google Scholar]
- 120.Ritchie TC, Westlund KN, Bowker RM, Coulter JD, Leonard RB. The relationship of the medullary catecholamine containing neurones to the vagal motor nuclei. Neuroscience. 1982;7:1471–82. doi: 10.1016/0306-4522(82)90258-5. [DOI] [PubMed] [Google Scholar]
- 121.Kalia M, Fuxe K, Hokfelt T, Johansson O, Lang R, et al. Distribution of neuropeptide immunoreactive nerve terminals within the subnuclei of the nucleus of the tractus solitarius of the rat. J Comp Neurol. 1984;222:409–44. doi: 10.1002/cne.902220308. [DOI] [PubMed] [Google Scholar]
- 122.Takagi H, Kubota Y, Mori S, Tateishi K, Hamaoka T, Tohyama M. Fine structural studies of cholecystokinin-8-like immunoreactive neurons and axon terminals in the nucleus of tractus solitarius of the rat. J Comp Neurol. 1984;227:369–79. doi: 10.1002/cne.902270307. [DOI] [PubMed] [Google Scholar]
- 123.Kalia M, Fuxe K, Goldstein M. Rat medulla oblongata. II. Dopaminergic, noradrenergic (A1 and A2) and adrenergic neurons, nerve fibers, and presumptive terminal processes. J Comp Neurol. 1985;233:308–32. doi: 10.1002/cne.902330303. [DOI] [PubMed] [Google Scholar]
- 124.Harfstrand A, Fuxe K, Terenius L, Kalia M. Neuropeptide Y-immunoreactive perikarya and nerve terminals in the rat medulla oblongata: relationship to cytoarchitecture and catecholaminergic cell groups. J Comp Neurol. 1987;260:20–35. doi: 10.1002/cne.902600103. [DOI] [PubMed] [Google Scholar]
- 125.Thor KB, Hill KM, Harrod C, Helke CJ. Immunohistochemical and biochemical analysis of serotonin and substance P colocalization in the nucleus tractus solitarii and associated ganglia of the rat. Synapse. 1988;2:225–31. doi: 10.1002/syn.890020309. [DOI] [PubMed] [Google Scholar]
- 126.Pickel VM, Chan J, Massari VJ. Neuropeptide Y-like immunoreactivity in neurons of the solitary tract nuclei: vesicular localization and synaptic input from GABAergic terminals. Brain Res. 1989;476:265–78. doi: 10.1016/0006-8993(89)91247-x. [DOI] [PubMed] [Google Scholar]
- 127.Caccatelli S, Seroogy KB, Millhorn DE, Terenius L. Presence of a dynorphin-like peptide in a restricted sub-population of catecholaminergic neurons in rat nucleus tractus solitarii. Brain Res. 1992;589:225–30. doi: 10.1016/0006-8993(92)91281-i. [DOI] [PubMed] [Google Scholar]
- 128.Ohta A, Takagi H, Matsui T, Hamai Y, Iida S, Esumi H. Localization of nitric oxide synthase-immunoreactive neurons in the solitary nucleus and ventrolateral medulla oblongata of the rat: their relation to catecholaminergic neurons. Neurosci Lett. 1993;158:33–35. doi: 10.1016/0304-3940(93)90605-k. [DOI] [PubMed] [Google Scholar]
- 129.Lynn RB, Hyde TM, Cooperman RR, Miselis RR. Distribution of bombesin-like immunoreactivity in the nucleus of the solitary tract and dorsal motor nucleus of the rat and human: colocalization with tyrosine-hydroxylase. J Comp Neurol. 1996;369:552–70. doi: 10.1002/(SICI)1096-9861(19960610)369:4<552::AID-CNE6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 130.Simonian SX, Herbison AE. Localization of neuronal nitric oxide synthase-immunoreactivity within sub-populations of noradrenergic A1 and A2 neurons in the rat. Brain Res. 1996;732:247–52. doi: 10.1016/0006-8993(96)00687-7. [DOI] [PubMed] [Google Scholar]
- 131.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–80. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 132.Atkinson L, Batten TFC, Deuchars J. P2X2 receptor immunoreactivity in the dorsal vagal complex and area postrema of the rat. Neuroscience. 2000;99:683–96. doi: 10.1016/s0306-4522(00)00233-5. [DOI] [PubMed] [Google Scholar]
- 133.Lin L-H, Talman WT. N-methyl-D-aspartate receptors on neurons that synthetize nitric oxide in rat nucleus tractus solitarius. Neuroscience. 2000;100:581–88. doi: 10.1016/s0306-4522(00)00314-6. [DOI] [PubMed] [Google Scholar]
- 134.Yao ST, Barden JA, Lawrence AJ. On the immunohistochemical distribution of ionotropic P2X receptors in the nucleus tractus solitarius of the rat. Neuroscience. 2001;108:673–85. doi: 10.1016/s0306-4522(01)00438-9. [DOI] [PubMed] [Google Scholar]
- 135.Sivarao DV, Krowicki ZK, Hornby PJ. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol Motil. 1998;10:305–13. doi: 10.1046/j.1365-2982.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- 136.Derbenev AV, Stuart TC, Smith BN. Cannabinoids suppress synaptic input to neurones of the rat dorsal motor nucleus of the vagus nerve. J Physiol. 2004;559:923–38. doi: 10.1113/jphysiol.2004.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Williforl DJ, Ormsbee HS, Norman WP, Harmon JW, Garwey TQ, et al. Hindbrain GABA receptors influence parasympathetic outflow to the stomach. Science. 1981;214:193–94. doi: 10.1126/science.6269182. [DOI] [PubMed] [Google Scholar]
- 138.Rogers RC, Travagli RA, Hermann GE. Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. Am J Physiol Regul Integr Comp Physiol. 2003;285:R479–89. doi: 10.1152/ajpregu.00155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Martinez-Pena YVI, Rogers RC, Hermann GE, Travagli RA. Nore-pinephrine effects on identified neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol. 2004;286:G333–39. doi: 10.1152/ajpgi.00289.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fukuda A, Minami T, Nabekura J, Oomura Y. The effects of nora-drenaline on neurones in the rat dorsal motor nucleus of the vagus, in vitro. J Physiol. 1987;393:213–31. doi: 10.1113/jphysiol.1987.sp016820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Browning KN, Kalyuzhny AE, Travagli RA. Opioid peptides inhibit excitatory but not inhibitory synaptic transmission in the rat dorsal motor nucleus of the vagus. J Neurosci. 2002;22:2998–3004. doi: 10.1523/JNEUROSCI.22-08-02998.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lewis MW, Hermann GE, Rogers RC, Travagli RA. In vitro and in vivo analysis of the effects of corticotropin releasing factor on rat dorsal vagal complex. J Physiol. 2002;543:135–46. doi: 10.1113/jphysiol.2002.019281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Browning KN, Travagli RA. Neuropeptide Y and peptide YY inhibit excitatory synaptic transmission in the rat dorsal motor nucleus of the vagus. J Physiol. 2003;549:775–85. doi: 10.1113/jphysiol.2003.042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Willis A, Mihalevich M, Neff RA, Mendelowitz D. Three types of postsynaptic glutamatergic receptors are activated in DMNX neurons upon stimulation of NTS. Am J Physiol. 1996;271:R1614–19. doi: 10.1152/ajpregu.1996.271.6.R1614. [DOI] [PubMed] [Google Scholar]
- 145.Deleted in proof
- 146.Broussard DL, Li H, Altschuler SM. Colocalization of GABA A and NMDA receptors within the dorsal motor nucleus of the vagus nerve (DMV) of the rat. Brain Res. 1997;763:123–26. doi: 10.1016/s0006-8993(97)00344-2. [DOI] [PubMed] [Google Scholar]
- 147.Feng HS, Lynn RB, Han J, Brooks FP. Gastric effects of TRH analogue and bicuculline injected into dorsal motor vagal nucleus in cats. Am J Physiol. 1990;259:G321–26. doi: 10.1152/ajpgi.1990.259.2.G321. [DOI] [PubMed] [Google Scholar]
- 148.Washabau RJ, Fudge M, Price WJ, Barone FC. GABA receptors in the dorsal motor nucleus of the vagus influence feline lower esophageal sphincter and gastric function. Brain Res Bull. 1995;38:587–94. doi: 10.1016/0361-9230(95)02038-7. [DOI] [PubMed] [Google Scholar]
- 149.Corbett EK, Saha S, Deuchars J, McWilliam PN, Batten TF. Ionotropic glutamate receptor subunit immunoreactivity of vagal preganglionic neurones projecting to the rat heart. Auton Neurosci. 2003;105:105–17. doi: 10.1016/S1566-0702(03)00047-X. [DOI] [PubMed] [Google Scholar]
- 150.Laccasagne O, Kessler JP. Cellular and subcellular distribution of the amino-3-hydroxy-5-metyl-4-isoxazole proprionate receptor subunit GluR2 in the rat dorsal vagal complex. Neurosci. 2000;99:557–63. doi: 10.1016/s0306-4522(00)00204-9. [DOI] [PubMed] [Google Scholar]
- 151.Nabekura J, Ueno T, Katsurabayashi S, Furuta A, Akaike N, Okada M. Reduced NR2A expression and prolonged decay of NMDA receptor-mediated synaptic current in rat vagal motoneurons following axotomy. J Physiol. 2002;539:735–41. doi: 10.1113/jphysiol.2001.013379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sivarao DV, Krowicki ZK, Abrahams TP, Hornby PJ. Vagally-regulated gastric motor activity: evidence for kainate and NMDA receptor mediation. Eur J Pharmacol. 1999;368:173–82. doi: 10.1016/s0014-2999(99)00015-1. [DOI] [PubMed] [Google Scholar]
- 153.Pagani FD, Dimicco JA, Hamilton BL, Souza JD, Schmidt B, Gillis RA. Stress-induced changes in the function of the parasympathetic nervous system are mimicked by blocking GABA in the CNS of the cat. Neuropharmacology. 1987;26:155–60. doi: 10.1016/0028-3908(87)90203-6. [DOI] [PubMed] [Google Scholar]
- 154.Ferreira M, Jr, Sahibzada N, Shi M, Panico W, Niedringhaus M, et al. CNS site of action and brainstem circuitry responsible for the intravenous effects of nicotine on gastric tone. J Neurosci. 2002;22:2764–79. doi: 10.1523/JNEUROSCI.22-07-02764.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li Y, Owyang C. Musings on the wanderer: What’s new in our understanding of vago-vagal reflexes? V. Remodeling of vagus and enteric neural circuitry after vagal injury. Am J Physiol Gastrointest Liver Physiol. 2003;285:G461–69. doi: 10.1152/ajpgi.00119.2003. [DOI] [PubMed] [Google Scholar]
- 156.Armstrong DM, Manley L, Haycock JW, Hersh LB. Co-localization of choline acetyltransferase and tyrosine hydroxylase within neurons of the dorsal motor nucleus of the vagus. J Chem Neuroanat. 1990;3:133–40. [PubMed] [Google Scholar]
- 157.Kalia M, Fuxe K, Goldstein M, Harfstrand A, Agnati LF, Coyle JT. Evidence for the existence of putative dopamine-, adrenaline- and noradrenaline-containing vagal motor neurons in the brainstem of the rat. Neurosci Lett. 1984;50:57–62. doi: 10.1016/0304-3940(84)90462-2. [DOI] [PubMed] [Google Scholar]
- 158.Zheng ZL, Rogers RC, Travagli RA. Selective gastric projections of nitric oxide synthase-containing vagal brainstem neurons. Neuroscience. 1999;90:685–94. doi: 10.1016/s0306-4522(98)00586-7. [DOI] [PubMed] [Google Scholar]
- 159.Guo JJ, Browning KN, Rogers RC, Travagli RA. Catecholaminergic neurons in rat dorsal motor nucleus of vagus project selectively to gastric corpus. Am J Physiol Gastrointest Liver Physiol. 2001;280:G361–67. doi: 10.1152/ajpgi.2001.280.3.G361. [DOI] [PubMed] [Google Scholar]
- 160.Rossiter CD, Norman WP, Jain M, Hornby PJ, Benjamin SB, Gillis RA. Control of lower esophageal sphincter pressure by two sites in dorsal motor nucleus of the vagus. Am J Physiol. 1990;259:G899–906. doi: 10.1152/ajpgi.1990.259.6.G899. [DOI] [PubMed] [Google Scholar]
- 161.Abrahamsson H. Studies on the inhibitory nervous control of gastric motility. Acta Physiol Scand. 1973;390 (Suppl):1–38. [PubMed] [Google Scholar]
- 162.Abrahamsson H. Vagal relaxation of the stomach induced from the gastric antrum. Acta Physiol Scand. 1973;89:406–14. doi: 10.1111/j.1748-1716.1973.tb05535.x. [DOI] [PubMed] [Google Scholar]
- 163.Andrews PLR, Grundy D, Scratcherd T. Reflex excitation of antral motility induced by gastric distension in the ferret. J Physiol. 1980;298:79–84. doi: 10.1113/jphysiol.1980.sp013068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Goyal RK, Rattan S. VIP as a possible neurotransmitter of non-cholinergic non-adrenergic inhibitory neurons. Nature. 1980;288:378–80. doi: 10.1038/288378a0. [DOI] [PubMed] [Google Scholar]
- 165.Abrahamsson H. Non-adrenergic non-cholinergic nervous control of gastrointestinal motility patterns. Arch Int Pharmacodyn Ther. 1986;280:50–61. [PubMed] [Google Scholar]
- 166.Azpiroz F, Malagelada JR. Vagally mediated gastric relaxation induced by intestinal nutrients in the dog. Am J Physiol. 1986;251:G727–35. doi: 10.1152/ajpgi.1986.251.6.G727. [DOI] [PubMed] [Google Scholar]
- 167.Ito S, Ohga A, Ohta T. Gastric relaxation and vasoactive intestinal peptide output in response to reflex vagal stimulation in the dog. J Physiol. 1988;404:683–93. doi: 10.1113/jphysiol.1988.sp017313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Azpiroz F, Malagelada JR. Perception and reflex relaxation of the stomach in response to gut distention. Gastroenterol. 1990;98:1193–98. doi: 10.1016/0016-5085(90)90333-v. [DOI] [PubMed] [Google Scholar]
- 169.Desai KM, Zembowicz A, Sessa WC, Vane JR. Nitroxergic nerves mediate vagally induced relaxation in the isolated stomach of the guinea pig. Proc Natl Acad Sci USA. 1991;88:11490–94. doi: 10.1073/pnas.88.24.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Desai KM, Sessa WC, Vane JR. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature. 1991;351:477–79. doi: 10.1038/351477a0. [DOI] [PubMed] [Google Scholar]
- 171.Lefebvre RA, Hasrat J, Gobert A. Influence of NG-nitro-L-arginine methyl ester on vagally induced gastric relaxation in the anaesthetized rat. Br J Pharmacol. 1992;105:315–20. doi: 10.1111/j.1476-5381.1992.tb14252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lefebvre RA, Baert E, Barbier AJ. Influence of NG-nitro-L-arginine on non-adrenergic non-cholinergic relaxation in the guinea-pig gastric fundus. Br J Pharmacol. 1992;106:173–79. doi: 10.1111/j.1476-5381.1992.tb14311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Meulemans AL, Helsen LF, Schuurkes AJ. Role of NO in vagally-mediated relaxations of guinea-pig stomach. Naunyn-Schmiedebergs Arch Pharmacol. 1993;347:225–30. doi: 10.1007/BF00169272. [DOI] [PubMed] [Google Scholar]
- 174.Barbier AJ, Lefebvre RA. Involvement of the L-arginine: Nitric oxide pathway in nonadrenergic noncholinerigic relaxation of the cat gastric fundus. J Pharmacol Exp Ther. 1993;266:172–78. [PubMed] [Google Scholar]
- 175.Meulemans AL, Eelen JG, Schuurkes JA. NO mediates gastric relaxation after brief vagal stimulation in anesthetized dogs. Am J Physiol. 1995;269:G255–61. doi: 10.1152/ajpgi.1995.269.2.G255. [DOI] [PubMed] [Google Scholar]
- 176.Takahashi T, Owyang C. Vagal control of nitric oxide and vasoactive intestinal polypeptide release in the regulation of gastric relaxation in rat. J Physiol. 1995;484:481–92. doi: 10.1113/jphysiol.1995.sp020680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Curro D, Volpe AR, Preziosi P. Nitric oxide synthase activity and non-adrenergic non-cholinergic relaxation in the rat gastric fundus. Br J Pharmacol. 1996;117:717–23. doi: 10.1111/j.1476-5381.1996.tb15249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Krowicki ZK, Hornby PJ. Contribution of acetylcholine, vasoactive intestinal polypeptide and nitric oxide to CNS-evoked vagal gastric relaxation in the rat. Neurogastroenterol Motil. 1996;8:307–17. doi: 10.1111/j.1365-2982.1996.tb00269.x. [DOI] [PubMed] [Google Scholar]
- 179.Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accomodation reflex in rats. J Physiol. 1997;504:479–88. doi: 10.1111/j.1469-7793.1997.479be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Krowicki ZK, Sivarao DV, Abrahams TP, Hornby PJ. Excitation of dorsal motor vagal neurons evokes non-nicotinic receptor-mediated gastric relaxation. J Auton Nerv Syst. 1999;77:83–89. [PubMed] [Google Scholar]
- 181.Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2003;19:519–25. doi: 10.1097/00001574-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 182.Konturek PC, Konturek SJ, Ochmanski W. Neuroendocrinology of gastric H+ and duodenal HCO3− secretion: the role of brain-gut axis. Eur J Pharmacol. 2004;499:15–27. doi: 10.1016/j.ejphar.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 183.Tache Y, Yang H. Role of medullary TRH in the vagal regulation of gastric function. In: Tache Y, Wingate DL, Burks TF, editors. Innervation of the Gut: Pathophysiological Implications. Boca Raton: CRC; 1993. pp. 67–80. [Google Scholar]
- 184.Rogers RC, Hermann GE. Vagal afferent stimulation-evoked gastric secretion suppressed by paraventricular nucleus lesion. J Auton Nerv Syst. 1985;13:191–99. doi: 10.1016/0165-1838(85)90011-6. [DOI] [PubMed] [Google Scholar]
- 185.Raybould HE, Tache Y. Capsaicin-sensitive vagal afferent fibers and stimulation of gastric acid secretion in anesthetized rats. Eur J Pharmacol. 1989;167:237–43. doi: 10.1016/0014-2999(89)90584-0. [DOI] [PubMed] [Google Scholar]
- 186.Galligan JJ. Pharmacology of synaptic transmission in the enteric nervous system. Curr Opin Pharmacol. 2002;2:623–29. doi: 10.1016/s1471-4892(02)00212-6. [DOI] [PubMed] [Google Scholar]
- 187.Horowitz B, Ward SM, Sanders KM. Cellular and molecular basis for electrical rhythmicity in gastrointestinal muscles. Annu Rev Physiol. 1999;61:19–43. doi: 10.1146/annurev.physiol.61.1.19. [DOI] [PubMed] [Google Scholar]
- 188.Kunze WAA, Furness JB. The enteric nervous sytem and regulation of intestinal motility. Annu Rev Physiol. 1999;61:117–42. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- 189.Hirst GD, Ward SM. Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle. J Physiol. 2003;550:337–46. doi: 10.1113/jphysiol.2003.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sanders KM, Koh SD, Ordog T, Ward SM. Ionic conductances involved in generation and propagation of electrical slow waves in phasic gastrointestinal muscles. Neurogastroenterol Motil. 2004;16 (Suppl 1):100–5. doi: 10.1111/j.1743-3150.2004.00483.x. [DOI] [PubMed] [Google Scholar]
- 191.Abrahamsson H. Reflex adrenergic inhibition of gastric motility elicited from the gastric antrum. Acta Physiol Scand. 1974;90:14–24. doi: 10.1111/j.1748-1716.1974.tb05558.x. [DOI] [PubMed] [Google Scholar]
- 192.Lefebvre RA, De Vriese A, Smits GJM. Influence of vasoactive intestinal polypeptide and NG-nitro-L-arginine methyl ester on cholinergic neurotransmission in the rat gastric fundus. Eur J Pharmacol. 1992;221:235–42. doi: 10.1016/0014-2999(92)90707-b. [DOI] [PubMed] [Google Scholar]
- 193.Bojo L, Lefebvre RA, Nellgard P, Cassuto J. Involvement of vasoactive intestinal polypeptide in gastric reflex relaxation. Eur J Pharmacol. 1993;236:443–48. doi: 10.1016/0014-2999(93)90483-x. [DOI] [PubMed] [Google Scholar]
- 194.Boeckxstaens GE, Pelckmans PA, Bogers J, Bult H, De Man JG, et al. Release of nitric oxide upon stimulation of noad-renergic noncholinergic nerves in the rat gastric fundus. J Pharmacol Exp Ther. 1991;256:441–47. [PubMed] [Google Scholar]
- 195.Rogers RC, McCann MJ. Intramedullary connections of the gastric region in the solitary nucleus: a biocytin histo-chemical tracing study in the rat. J Auton Nerv Syst. 1993;42:119–30. doi: 10.1016/0165-1838(93)90043-t. [DOI] [PubMed] [Google Scholar]
- 196.Dum J, Herz A. Endorphinergic modulation of neural reward systems indicated by behavioral changes. Pharmacol Biochem Behav. 1984;21:259–66. doi: 10.1016/0091-3057(84)90224-7. [DOI] [PubMed] [Google Scholar]
- 197.Levine AS, Kotz CM, Gosnell BA. Sugars and fats: the neurobiology of preference. J Nutr. 2003;133:831S–4S. doi: 10.1093/jn/133.3.831S. [DOI] [PubMed] [Google Scholar]
- 198.Fullerton DT, Getto CJ, Swift WJ, Carlson IH. Sugar, opioids and binge eating. Brain Res Bull. 1985;14:673–80. doi: 10.1016/0361-9230(85)90117-0. [DOI] [PubMed] [Google Scholar]
- 199.Giraudo SQ, Kotz M, Billington CJ, Levine AS. Association between the amygdala and the nucleus of the solitary tract in μ-opioid induced feeding in the rat. Brain Res. 1998;802:184–88. doi: 10.1016/s0006-8993(98)00602-7. [DOI] [PubMed] [Google Scholar]
- 200.Kotz CM, Billington CJ, Levine AS. Opioids in the nucleus of the solitary tract are involved in feeding in the rat. Am J Physiol. 1997;272:R1028–32. doi: 10.1152/ajpregu.1997.272.4.R1028. [DOI] [PubMed] [Google Scholar]
- 201.Kotz CM, Glass MJ, Levine AS, Billington CJ. Regional effect of naltrex-one in the nucleus of the solitary tract in blockade of NPY-induced feeding. Am J Physiol Regulatory Integrative Comp Physiol. 2000;278:R499–503. doi: 10.1152/ajpregu.2000.278.2.R499. [DOI] [PubMed] [Google Scholar]
- 202.Burks TF, Galligan JJ, Hirning LD, Porreca F. Brain, spinal cord and peripheral sites of action of enkephalins and other endogenous opioids on gastrointestinal motility. Gastroenterol Clin Biol. 1987;11:44B–51B. [PubMed] [Google Scholar]
- 203.Del Tacca M, Bernardini C, Corsano E, Soldani G, Roze C. Effects of morphine on gastric ulceration, barrier mucus and acid secretion in pylorus-ligated rats. Pharmacology. 1987;35:174–80. doi: 10.1159/000138309. [DOI] [PubMed] [Google Scholar]
- 204.Fox DA, Burks TF. Roles of central and peripheral μ, δ, and κ opioid receptors in the mediation gastric acid secretory effects in the rat. J Pharmacol Exp Ther. 1988;244:456–62. [PubMed] [Google Scholar]
- 205.Farkas E, Jansen ASP, Loewy AD. Periaqueductal gray matter projection to vagal preganglionic neurons and the nucleus tractus solitarius. Brain Res. 1997;764:257–61. doi: 10.1016/s0006-8993(97)00592-1. [DOI] [PubMed] [Google Scholar]
- 206.Liubashina O, Jolkkonen E, Pitkanen A. Projections from the central nucleus of the amygdala to the gastric related area of the dorsal vagal complex: a Phaseolus vulgaris-leucoagglutinin study in rats. Neurosci Lett. 2000;291:85–88. doi: 10.1016/s0304-3940(00)01392-6. [DOI] [PubMed] [Google Scholar]
- 207.Moriwaki A, Wang JB, Svingos A, Van Bockstaele EJ, Cheng P, et al. μ-opiate receptor immunoreactivity in rat central nervous system. Neurochem Res. 1996;21:1315–31. doi: 10.1007/BF02532373. [DOI] [PubMed] [Google Scholar]
- 208.Pickel VM, Colago EEO. Presence of μ-opioid receptors in targets of efferent projections from the central nucleus of the amygdala to the nucleus of the solitary tract. Synapse. 1999;33:141–52. doi: 10.1002/(SICI)1098-2396(199908)33:2<141::AID-SYN4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 209.Martinez V, Barrachina MD, Ohning G, Tache Y. Cephalic phase of acid secretion involves activation of medullary TRH receptor subtype 1 in rats. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1310–19. doi: 10.1152/ajpgi.00222.2002. [DOI] [PubMed] [Google Scholar]
- 210.Moran TH. Gut peptides in the control of food intake: 30 years of ideas. Physiol Behav. 2004;82:175–80. doi: 10.1016/j.physbeh.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 211.Browning KN, Kalyuzhny AE, Travagli RA. μ-opioid receptor trafficking on inhibitory synapses in the rat brainstem. J Neurosci. 2004;24:9344–52. doi: 10.1523/JNEUROSCI.1676-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yoneda M, Yokohama S, Tamori K, Sato Y, Nakamura K, Makino I. Neuropeptide Y in the dorsal vagal complex stimulates bicarbonate-dependent bile secretion in rats. Gastroenterology. 1997;112:1673–80. doi: 10.1016/s0016-5085(97)70050-7. [DOI] [PubMed] [Google Scholar]
- 213.Chen CH, Stephens RL, Jr, Rogers RC. PYY and NPY control of gastric motility via action on Y1 and Y2 receptors in the DVC. Neurogastroenterol Motil. 1997;9:109–16. doi: 10.1046/j.1365-2982.1997.d01-26.x. [DOI] [PubMed] [Google Scholar]
- 214.Matsuda M, Aono M, Moriga M, Okuma M. Centrally administered NPY inhibits gastric emptying and intestinal transit in the rat. Dig Dis Sci. 1993;38:845–50. doi: 10.1007/BF01295910. [DOI] [PubMed] [Google Scholar]
- 215.Browning KN, Kalyuzhny AE, Travagli RA. State dependent activation of brainstem circuits by opioids. Presented at Neurosci. Meet., 33rd; New Orleans. 2003. [Google Scholar]
- 216.Travagli RA, Browning KN. Electrophysiological properties of identified pancreatic neurons of the rat dorsal motor nucleus of the vagus. Presented at Neurosci. Meet., 31st; San Diego. 2001. [Google Scholar]
- 217.Browning KN, Travagli RA. NPY in the rat dorsal motor nucleus of the vagus (DMV) inhibit excitatory but not inhibitory synaptic transmission. Presented at Dig. Dis. Week; Atlanta. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Browning KN, Travagli RA. State dependent activation of brainstem circuits by opioids. Presented at Dig. Dis. Week; Orlando, FL. 2003. [Google Scholar]
- 219.Browning KN, Gillis RA, Travagli RA. State dependent activation of brainstem circuits by opioids. Presented at Neurosci. Meet., 33rd; New Orleans. 2003. [Google Scholar]
- 220.Browning KN, Travagli RA. Modulation of brainstem circuits by pancreatic polypeptides. Presented at Int. Soc. Auton. Neurosci; Marseille. 2005. [Google Scholar]
- 221.Travagli RA, Browning KN. Short term receptor trafficking in the dorsal vagal complex. Presented at Int. Soc. Auton. Neurosci; Marseille. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jin YH, Bailey TW, Andresen MC. Cranial afferent glutamate heterosynaptically modulates GABA release onto second-order neurons via distinctly segregated metabotropic glutamate receptors. J Neurosci. 2004;24:9332–40. doi: 10.1523/JNEUROSCI.1991-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Browning KN, Travagli RA. Differential distribution of metabotropic glutamate receptors within gastric vagal brainstem circuits. Presented at Dig. Dis. Week; Chicago. 2005. [Google Scholar]
- 224.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–37. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 225.Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907 . doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]; Annual Review of Physiology. 2006;68 [Google Scholar]