Abstract
Activation of esophageal mechanosensors excites neurons in and near the central nucleus of the solitary tract (NSTc). In turn, NSTc neurons coordinate the relaxation of the stomach [i.e., the receptive relaxation reflex (RRR)] by modulating the output of vagal efferent neurons of the dorsal motor nucleus of the vagus (DMN). The NSTc area contains neurons with diverse neurochemical phenotypes, including a large population of catecholaminergic and nitrergic neurons. The aim of the present study was to determine whether either one of these prominent neuronal phenotypes was involved in the RRR. Immunohistochemical techniques revealed that repetitive esophageal distension caused 53% of tyrosine hydroxylase-immunoreactive (TH-ir) neurons to colocalize c-Fos in the NSTc. No nitric oxide synthase (NOS)-ir neurons in the NSTc colocalized c-Fos in either distension or control conditions. Local brain stem application (2 ng) of α-adrenoreceptor antagonists (i.e., α1-prazosin or α2-yohimbine) significantly reduced the magnitude of the esophageal distension-induced gastric relaxation to ~55% of control conditions. The combination of yohimbine and prazosin reduced the magnitude of the reflex to ~27% of control. In contrast, pretreatment with either the NOS-inhibitor NG-nitro-L-arginine methyl ester or the β-adrenoceptor antagonist propranolol did not interfere with esophageal distension-induced gastric relaxation. Unilateral microinjections of the agonist norepinephrine (0.3 ng) directed at the DMN were sufficient to mimic the transient esophageal-gastric reflex. Our data suggest that noradrenergic, but not nitrergic, neurons of the NSTc play a prominent role in the modulation of the RRR through action on α1- and α2-adrenoreceptors. The finding that esophageal afferent stimulation alone is not sufficient to activate NOS-positive neurons in the NSTc suggests that these neurons may be strongly gated by other central nervous system inputs, perhaps related to the coordination of swallowing or emesis with respiration.
Keywords: vagus, brain stem, c-Fos
The receptive relaxation reflex (RRR) is an important mechanism that increases gastric volume and reduces intragastric pressure to ensure that swallowed food is efficiently transported from esophagus to stomach (9, 25, 30, 33). A number of investigators have shown that this potent proximal gastric relaxation is triggered by the activation of low-threshold vagal afferent mechanosensors in the esophagus (33). The reflex requires intact vago-vagal connections between the esophagus, brain stem, and stomach (30). Several anatomical tracing studies have shown that vagal afferent projections from the esophagus terminate in and near the central division of the nucleus of the solitary tract (NSTc) (1, 4, 13). Previous investigations have shown that neurons in the NSTc are intensely activated by low-level esophageal distension (7, 30, 33).
Much of the neurophysiological and anatomical work done on esophageal afferents and the NSTc has focused on the role these entities play in the production of the swallowing motor program and in coordinating the act of swallowing with respiration and cardiovascular function (6, 27). Other studies have demonstrated a dense projection from the NSTc area to the nucleus ambiguus (NA), the source of vagal motor neurons that project to the esophagus, lower esophageal sphincter, and heart (10, 16, 20). The NSTc-NA pathway is clearly involved in the production of swallowing as well as the regulation of the glottis and heart rate during deglutition (3, 6, 22).
The relationship between the NSTc and the vagal motor neurons that control the stomach has only recently been addressed. Physiologically guided nanoinjections of retrograde and anterograde tracer onto NSTc neurons that respond to esophageal distension demonstrate that these neurons project heavily throughout the full anterior-posterior extent of the dorsal motor nucleus of the vagus (DMN), the primary source of preganglionic autonomic control over the stomach (30).
Vagal reflex control over gastric tone and motility is affected by modulating the activity of two antagonistic vagal efferent projections. Vagal efferent-mediated increases in gastric tone and motility occur after activation of cholinergic neurons in the gastric enteric plexus by loosely aggregated preganglionic neurons in the DMN (30, 32). Conversely, rapid gastroinhibition can result from the inhibition of these DMN neurons. Indeed, it is well known that intestinal, gastric, and esophageal distension causes an abrupt cessation in the tonic firing of DMN neurons, which coincides with the rapid onset of a reduction in gastric tone and motility (30, 31). Activation of gastric and esophageal distension-sensitive afferent fibers can also produce a potent gastroinhibition through the activation of a vagal nonadrenergic, noncholinergic (NANC) pathway to the fundus (30, 34, 35). Our previous neurophysiological studies suggest that putative NANC path-inhibitory neurons in the DMN are excited by esophageal distension (30).
Little else is known about the mechanisms by which NSTc neurons produce changes in DMN neurons that result in the RRR. The NSTc area contains a number of different neuronal phenotypes. Two neurochemical phenotypes that are especially prominent are noradrenergic and nitrergic (20, 21). The core of the NSTc contains an especially dense concentration of nitric oxide synthase (NOS) neurons, whereas tyrosine hydroxylase-immunoreactive (TH-ir) neurons are found throughout the NST. Of particular interest is the subset of TH-ir neurons found encircling the NSTc-NOS neurons. Previous immunohistochemical studies have shown that virtually all of these TH-containing neurons near the NSTc also express dopamine β-hydroxylase and are, therefore, norepinephrine (NE)-producing neurons (37).
Both of these NST cell types (nitrergic and noradrenergic) have been implicated in the control of a variety of autonomic functions (5), although not the RRR. Indeed, these TH-ir and NOS-ir neurons could receive a large amount of input from esophageal vagal afferents (1). With the combined use of immunohistochemical and in vivo physiological methods, we aimed to determine 1) the distribution and neurochemical identification of NST neurons activated by esophageal distension and 2) the relative influence of these pathways in the modulation of the RRR.
METHODS
Drugs and Chemicals
Animals were anesthetized with thiobutabarbital (Inactin, Sigma; 150–200 mg/kg ip). This long-term anesthetic has been shown not to interfere with brain stem autonomic reflexes (8) or the activation of DVC neurons after exposure to cytokines (11, 12, 17).
Histological processing of the medullary brain stem for c-Fos production required primary c-Fos antibody (AB-5; rabbit cFos, 1:20,000; Oncogene Science Diagnostics, La Jolla, CA) and biotinylated goat, anti-rabbit IgG (1:600; Vector Labs, Burlingame, CA). Amplification of antibody-antigen reactions required incubation with Vector Elite avidin-biotin-peroxidase complex (1:600; Vector Labs) followed by Vector Nova red peroxidase detection reagents (Vector Labs). Phenotypic identification required either mouse anti-rat tyrosine hydroxylase (TH) primary antibody (T1299, Sigma, St. Louis, MO) or mouse anti-rat neuronal NOS primary antibody (N2280, Sigma) and biotinylated horse, anti-mouse IgG (1: 600; Vector Labs). Amplification of these antibody-antigen reactions required incubation with Vector Elite avidin-biotin-peroxidase complex (1:600; Vector Labs) followed by Vector SG peroxidase detection reagents (Vector Labs).
The antagonists for α1-adrenergic receptors (prazosin), α2-adrenergic receptors (yohimbine), or β-adrenergic receptors (propranolol) as well as the NOS synthesis inhibitor NG-nitro-L-arginine methyl ester hydrochloride (L-NAME) were purchased from Sigma. These drugs were made fresh daily immediately before use at a concentration of 1 mg/ml in sterile PBS. The adrenergic agonist NE (Receptor Research Chemicals, Baltimore, MD) was made fresh daily at a concentration of 50 mM in sterile PBS.
Experiment 1: Immunohistochemical Procedures
All experimental protocols were performed according to the guidelines set forth by the National Institutes of Health and were approved by the Institutional Animal Care and Use Committees at the Pennington Biomedical Research Center and the University of Michigan Health Sciences Center.
Male Long-Evans rats (Charles River Laboratories, Wilmington, MA; n = 37) weighing between 250 and 500 g were anesthetized with thiobutabarbital (150–200 mg/kg ip). A tracheal catheter was placed to maintain a clear airway. Animals were mounted in a stereotaxic frame. An esophageal distension balloon was constructed from a 1.5-cm length of 1 mm OD, 0.5 mm ID silicone tubing (AM Systems, Seattle, WA). The balloon tubing was connected via polyethylene tubing (PE-50) to a modified small animal respirator (Harvard Instruments, Cambridge, MA). The tubing and 1-ml respirator cylinder were filled with water. The respirator was programmed to cyclically inflate the balloon to a final volume of 160 μl (i.e., 2.5 mm distended diameter). Our previous study (30) showed that this distension produced a transmural pressure increase of <14 mmHg. This degree of distension activates vagal mechanosensors, but not spinal nociceptors (33). Surgically prepared, anesthetized rats were mounted in the stereotaxic frame.
A distal esophageal distension group (n = 16) had the balloon placed orally in the esophagus such that the tip was located 1 cm above the esophageal hiatus in the thoracic esophagus. The balloon was then distended and relaxed every 10 s (50% duty cycle) for 90 min. A control group of rats (n = 13) did not receive the esophageal balloon but the oral cavity was manipulated as if the balloon/catheter were to be placed in the esophagus.
At the end of the 90-min stimulation period, the animals rested for an additional 30 min to allow for maximal c-Fos activation of NST neurons. At that time, rats were transcardially perfused with PBS, followed by 4% paraformaldehyde in PBS. Brain stems were then removed to a solution of 4% paraformaldehyde plus 20% sucrose for overnight postfixation. Brain stems were cut on a freezing microtome into 40-μm sections.
All sections through the medullary brain stem were saved and processed for the demonstration of nuclear c-Fos protein, a marker for prolonged and significant neuronal excitation (28). This protocol is available in detail elsewhere (18, 28). Briefly, all tissue sections were rinsed in PBS and incubated in sodium borohydride and hydrogen peroxide to eliminate remaining fixative and to block endogenous peroxidase. Sections were then rinsed and blocked in 5% normal goat serum, rerinsed in PBS, and then incubated in rabbit, anti-rat c-Fos primary antibody overnight at room temperature on a shaker table. Sections were rinsed in PBS and incubated with bio-tinylated goat, anti-rabbit secondary antibody. Sections were rinsed in PBS and incubated in Vector ABC peroxidase reagent, followed by the Vector peroxidase chromogen Nova red. c-Fos staining was revealed in this protocol as brick red nuclei (Figs. 1 and 3). Omission of primary antibody or incubation with inappropriate secondary antibody produced no c-Fos label.
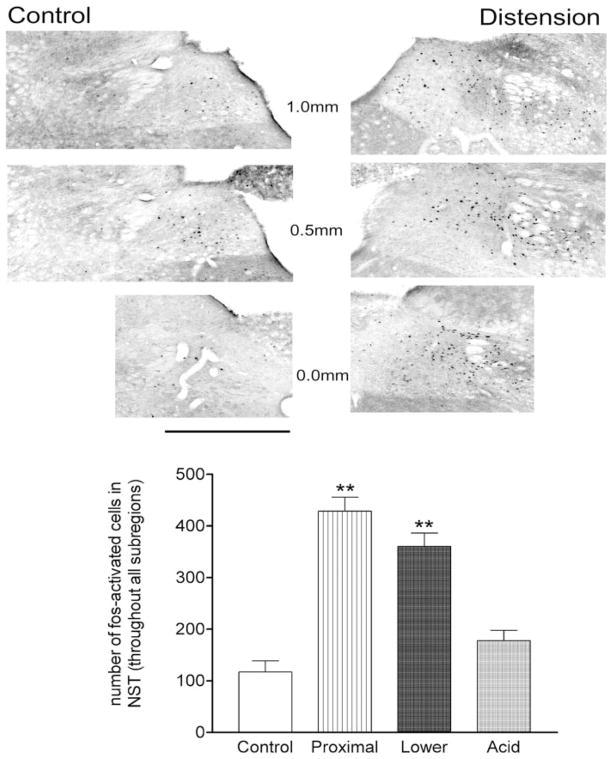
Fig. 1.
Illustration of c-Fos-immunoreactivity (ir) in control vs. esophageal distension cases. Rostral-caudal sections are described in terms of distance (in mm) with respect to calamus scriptorum. Scale bar = 500 μm. Bar graph: overall average number of c-Fos-ir-labeled neurons throughout the nucleus of the solitary tract (NST) in control vs. esophageal distension or acidification cases. ANOVA F3,33 = 26.0; P < 0.0001; Dunnett’s posttest for comparisons against control: **P < 0.001.
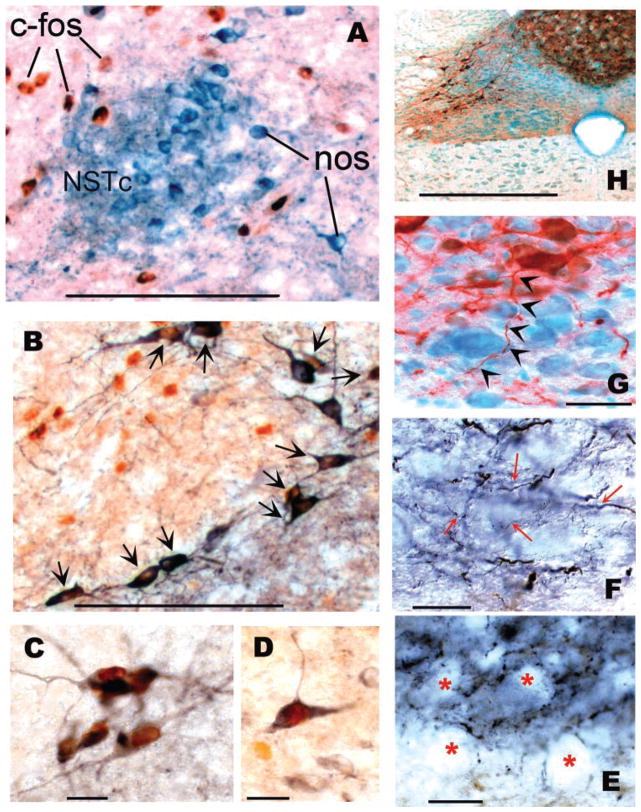
Fig. 3.
Montage of c-Fos, tyrosine hydroxylase (TH), and nitric oxide synthase (NOS) immunohistochemical results. A: illustration of NOS-ir neurons in the core of the central nucleus of the solitary tract (NSTc). Although this area receives esophageal afferent fibers, no NOS-ir neurons here were c-Fos-positive labeled after repeated esophageal distension. Blue-black = NOS-ir; red-brown = c-Fos-ir. B: this section was stained for TH and c-Fos immunoreactivity. Note the high density of TH-ir neurons (arrows) that have been c-Fos activated in response to esophageal distension. These cells surround the NOS-ir core of the NSTc demonstrated in A. Although additional c-Fos-positive cells can be seen as brown nuclei in this core region, they did not react to NOS immunostaining. Blue-black = TH-ir; red-brown = cFos-ir. C: higher magnification image of a cluster of TH-c-Fos-ir neurons in the NSTc. D: A small but nonsignificant number of cells in the medial NST demonstrate NOS-cFos-ir double label after esophageal distension. Blue-black = NOS-ir; red-brown = c-Fos-ir. E: immunohisto-chemical staining shows that dorsal motor nucleus (DMN) neurons (“vacant” regions in this DIC photomicrograph marked by red asterisks) are surrounded by NOS-ir terminal arborizations. However, our physiological studies did not suggest that NOS plays a role in the receptive relaxation reflex (RRR; refer to Fig. 5). Blue-black = NOS-ir fibers. F: TH-ir fibers coursing through the DMN denoted by red arrows. Blue-black = TH-ir fibers. G: NST TH-ir neuron (red) sending an axon (denoted by black arrowheads) to the DMN (methyl green). H: low-power micrograph of the dorsal medulla showing the TH-ir (red-brown) neurons and the “haze” of fine TH-ir fibers in the DMN. Scale bars: A, B = 100 μm; C, D = 20 μm; E, F, G = 10 μm; H = 500 μm.
Sections were then divided into three lots, with each lot containing a full series of sections through the brain stem. One of the three lots that were processed for c-Fos was mounted on subbed slides, dried, and placed under a cover slip (see Fig. 1). In addition to immunostaining for c-Fos activation, the two other lots were also processed for either TH or neuronal NOS. Sections were rinsed and blocked with 5% horse serum, rerinsed, and followed with either mouse anti-rat TH primary antibody or mouse anti-rat NOS primary antibody. Sections were incubated on a shaker table overnight at 8°C, then rinsed and incubated in Vector ABC reagent, rerinsed, and reacted with the Vector peroxidase chromogen SG. Cytoplasmic staining for TH or NOS is blue-black using this method. This cytoplasmic chromogen contrasts well with the nuclear Nova red stain that identifies c-Fos-activated neurons (Fig. 3). Occasionally, TH- or NOS-ir material was processed with Nova red and counterstained with methyl green (Vector Laboratories). Sections were viewed, analyzed, and photographed with a Nikon E800 microscope coupled with a Zeiss Axiocam CCD camera.
Analysis of the location of c-Fos-positive neurons
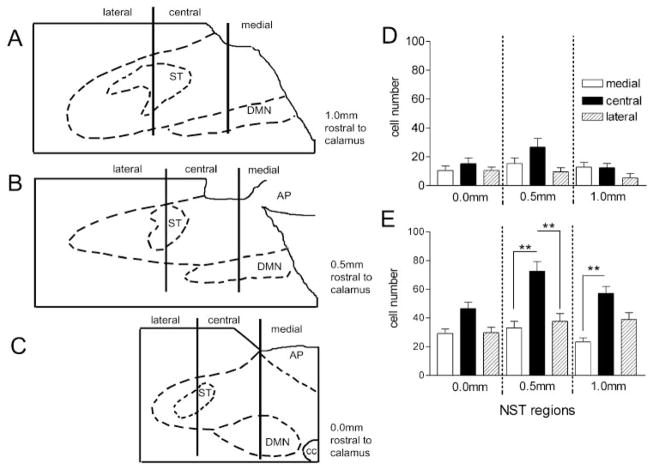
For quantitative purposes, the subregions of the solitary nucleus were divided as shown in Fig. 2 and subsequently schematized into row and column regions of interest. Anterior-posterior “rows” were sampled/binned at three levels: calamus (0.0), 0.5 mm rostral to calamus at the level of the anterior portion of the area postrema (+0.5), and 1.0 mm rostral to calamus (+1.0). This rostralmost section, just anterior to the rostral edge of the area postrema, is a principal region of termination of esophageal vagal afferents, according to several earlier papers (1, 30). The medial-lateral (“columns”) extent of the NST was divided into a “lateral” portion (i.e., all of the NST lateral to the midpoint of the solitary tract), a “central” portion (i.e., the NST between the midpoint of the solitary tract and the midpoint of the DMN), and a “medial” portion (i.e., between the midpoint of the DMN and the medialmost extreme of the NST; see scheme in Figs. 1 and 2).
Fig. 2.
Distribution of c-Fos-ir neurons in different subregions of the NST. A–C: line drawings of micrographs in Fig. 1 showing the schema used for the identification of different NST divisions for the bar graphs D and E. D: c-Fos distribution (c-Fos-ir cells counted per section) in unstimulated controls. No significant between-group differences. E: c-Fos distribution in rats receiving esophageal distension stimuli. All subregions of the NST are activated by distension, as seen by the increase in c-Fos-positive neurons. Within any given rostral-caudal level, the central subregion expressed more c-Fos (F8,135 = 11.21, P = 0.044; Bonferroni selected comparisons **P < 0.001).
In the c-Fos-only processed sets, all representative sections [i.e., those containing the NST from anterior-posterior levels (rows)] were evaluated for the presence of c-Fos-labeled cells across the three subregions (as defined above). Inclusion of c-Fos-labeled neurons required that the nuclei be a minimum of 6 μm in diameter and had to exhibit a nucleolus. These criteria guaranteed that staining artifacts and nuclear fragments would not be included in the count. An investigator unaware of the experimental condition being analyzed counted c-Fos-stained nuclei; a second observer verified the counts. The agreement between counts of the two observers was within 10%. Data in Fig. 1 represent the averaged total number (±SE) of c-Fos-activated neurons throughout the NST.
Given that the total number of c-Fos-activated cells in the NST in response to distal esophageal distension was markedly increased compared with the control condition, a plot of the distribution of c-Fos-positive neurons across and through the NST was constructed. A one-way ANOVA was performed on the numbers of c-Fos-positive cells per row and column segment. Bonferroni posttests on selected, appropriate pairs of segments followed. Statistical significance was defined at P < 0.05. (Fig. 2; data represent the average number of c-Fos-labeled neurons within any given subregion per histological section per animal.)
Analysis and quantification of the c-Fos-double-immunostained neurons (i.e., c-Fos + TH positive and c-Fos + NOS positive) were restricted to the central portion of the NST because 1) this region has been demonstrated to be the primary relay area for esophageal afferent information (1, 30) and 2) our analysis showed that the anterior, central divisions of the NST were significantly more activated by esophageal distension than the other divisions (Fig. 2). Data from this analysis are reported as the average number (per histological section) of either TH- or NOS-identified neurons in the central NST and the average number of identified (TH or NOS) neurons that were also c-Fos positive (Fig. 4).
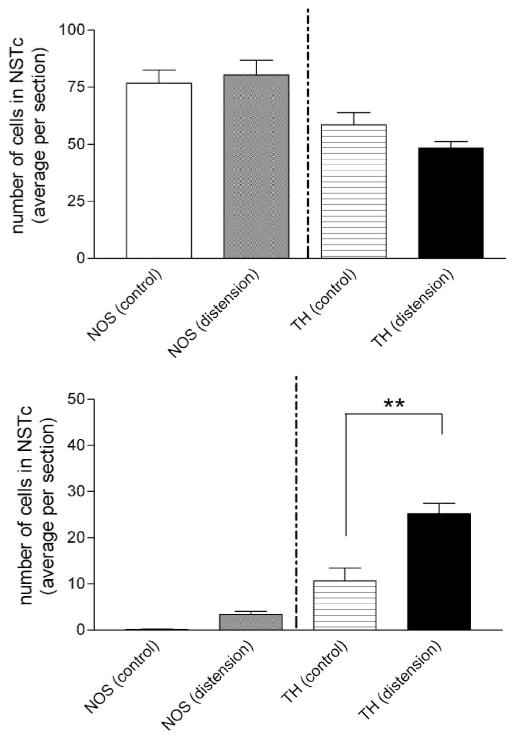
Fig. 4.
Esophageal distension causes a significant increase in the number of TH-c-Fos-double-immunostained neurons in the central division of the NST. Top: plots of the average number of TH or NOS neurons per histological section in control vs. esophageal distension conditions. There was no difference in the number of either phenotype in control vs. distension groups. Bottom: esophageal distension caused a significant increase in the number of TH-containing neurons that also demonstrated c-Fos activation (F3,40 = 42.0; P < 0.0001; selected Bonferroni posttests **P < 0.001). There was no significant increase in the number of NOS-ir/c-Fos-ir-double-labeled neurons.
The above experiments yielded unexpected results regarding the distribution of c-Fos-NOS-ir in the NSTc after esophageal distension. Thus these results prompted us to add two additional experimental groups. One was a proximal esophageal distension group (n = 4), where the esophageal stimulation balloon was placed orally just below the larynx and was cyclically distended as described above. Another group of rats (n = 4) received acid stimulation of the distal esophagus. A length of PE-50 tubing was orally placed in the esophagus to end at the level of the hiatus (26); 10 μl of a pH 1 solution of HCl was infused through this cannula into the esophagus every 10 min for 90 min.
All tissue processing and analyses of these groups were identical to those described above. Data in Fig. 1 represent the averaged total number (±SE) of c-Fos-activated neurons throughout the NST across all four groups. These data were subjected to a one-way ANOVA; statistical significance was defined at P < 0.05. Dunnett’s posttest for comparisons against control followed. The distributions of c-Fos-activated neurons within (i.e., across and through the subregions of) the NST for these additional two groups were plotted and analyzed as described above for the control and distal esophageal stimulation groups.
Experiment 2: Motility Measurement Methods in Vivo
The following set of experiments was designed to either interrupt the integrity of the esophageal-gastric receptive relaxation reflex (via the use of presumptive antagonists) or fictively mimic the reflex (via the microinjection of presumptive agonists directly into the DVC). The initial surgical and experimental preparations were identical for both groups with the exception of the delivery of the drugs in question.
All rats used in gastric motility measurement experiments were food deprived for 16 h before instrumentation. Rats were anesthetized with thiobutabarbital and fitted with a tracheal cannula, as described above. Uniformity in gastric preload conditions was accomplished by a latex balloon made from the small finger of a size 6 surgeon’s glove and attached to a 10-cm length of 2 mm OD silicone tubing. The balloon was installed into the empty fundus through a purse-string suture fistula in the greater curvature of the antrum. A miniature strain gauge (RB, Madison, WI) was sutured to the exterior of the fundus so as to align the strain-measuring elements with circular smooth muscle fibers. Care was taken to ensure that the strain gauges were attached to the fundus without stretching the stomach. Application of 2 ml of water to the gastric balloon produced a mild, but consistent, 1-g distension preload strain on the fundus (34). This arrangement produced a consistent distension against which the fundus, equipped with a strain gauge, could relax. Strain gauge signals and balloon pressure signals were routed through a personal computer-based signal-analysis system that digitized and stored the records.
The rat was positioned in a stereotaxic frame; removal of the occipital skull plate exposed the dorsal brain stem. The dura and arachnoid meninges were carefully removed to facilitate delivery of antagonists or agonists.
Functional verification of the results of experiment 1 required that we antagonize the potential effects of either NO or NE in the DVC. Previous work from this laboratory has shown that neurons in the NSTc area responsive to esophageal distension project throughout the entire DMN. The DMN is a relatively large, flattened tubular structure ~2-mm long (anterior-posterior; AP), 0.5 mm wide (medio-lateral; ML), and 0.1 mm deep (dorsoventral; DV). Of course, the RRR vago-vagal reflex circuits are duplicated bilaterally in the medulla. A single pair of even relatively large (40 nl) injections of antagonist would cover only a small fraction of the total volume of the DMN. This factor makes a “negative result” (i.e., no change in the RRR amplitude) uninterpretable. Furthermore, antagonizing putative NSTc reflex inputs to the DMN with multiple microinjections is not realistic. To do so would require the nearly simultaneous application of four 40 nl volumes of antagonist to either side. Therefore, we chose instead to deliver the antagonists (which did not have basal effects on gastric motility) via the floor of the fourth ventricle to maximize the exposure of the DMN to the antagonist and block (i.e., override) the endogenous pharmacological basis of a stimulated reflex.
Noradrenergic or Nitrergic Antagonists
On the basis of the results of experiment 1, subpopulations of immunologically identified (i.e., TH or NOS) NST neurons are activated by esophageal distension and may play a role in the RRR. Therefore, these experiments were designed to interrupt the normal esophageal distension-induced gastric reflex by applying the presumptive antagonists onto the floor of the fourth ventricle, thereby maximizing the exposure of the antagonist(s) to the full extent of the NST-DMN interface.
All stereotaxic and surgical preparations were as described above (n = 25). As described in experiment 1 above, an esophageal balloon cannula was placed in the distal esophagus and connected to the dome of a pressure transducer. The balloon, tubing, and transducer dome were filled with water. A 1-ml syringe was connected with the transducer dome to distend the esophageal balloon (160 μl final volume) during the esophageal stimulation.
Once all surgical preparations were completed and the esophageal cannula was in place, basal fundic activity was monitored for 1 h before starting the stimulation protocol. The fundic balloon was then inflated to 2 ml, producing a modest distension of the fundus (i.e., consistent 1-g distension preload strain). Simultaneously to the gastric preload, 2 μl of PBS were microdropped onto the floor of the fourth ventricle directly above the dorsal vagal complex (DVC). Ten minutes later, the esophageal balloon was distended (i.e., onset of esophageal stimulation) for 1 min and then released. The gastric balloon preload was relieved 5 min after the stimulation was delivered. This session constituted the control or basal RRR; thus each animal served as its own control for comparison purposes. One hour after this control RRR was elicited, the protocol was repeated, with the exception that one of the following antagonists or inhibitors were microdropped (2 ng in a 2 μl volume) onto the dorsal surface of the DVC: 1) prazosin (α1-adrenoceptor antagonist), 2) yohimbine (α2-adrenoceptor antagonist), 3) propranolol (nonselective β-adrenoceptor antagonist), 4) L-NAME (NO synthesis inhibitor), or 5) prazosin + yohimbine (2 ng each; total volume 2 μl).
Antagonists: In Vivo Data Analysis
The change in fundic tone elicited by the esophageal distension was evaluated by averaging the strain gauge output for the minute before and during the esophageal distension. The difference in fundic tone, before and during distension, represents the magnitude of the RRR.
Calculating a difference score between control and drug treatment conditions and multiplying this score by 100 determined the effects of antagonist application to the DVC on the elicited RRR. That is, a fundic relaxation index of 100% is indicative of no change to the control reflex due to the drug, whereas an index of 50% indicates a loss in the relaxation reflex by 50% (Fig. 5). The raw data were subjected to a one-way ANOVA; post hoc comparisons were made against the set control value of 100 (i.e., “no change” in reflex magnitude) using the Dunnett’s test for comparisons to control. Statistical significance was defined at P < 0.05.
Fig. 5.
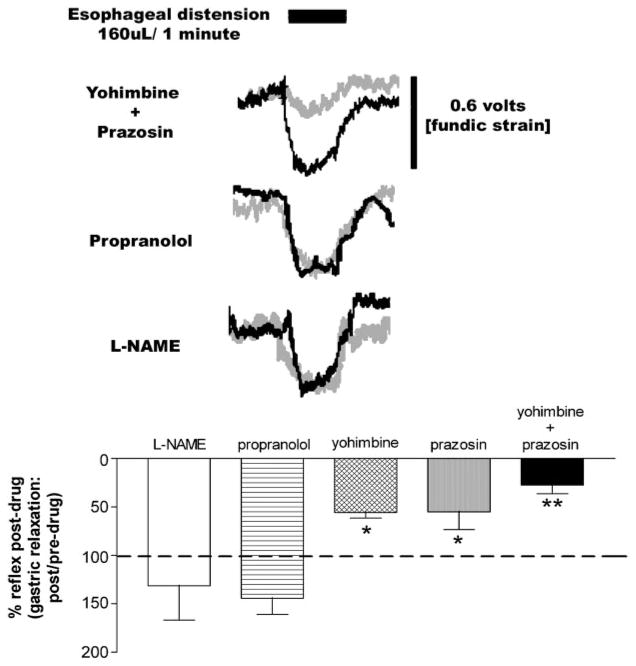
Esophageal distension in food-deprived animals results in RRR of the fundus. Top: sample raw motility records show the effects of receptor blockers on the RRR. Top trace indicates timing and duration of esophageal distension. Amplitude of the control (i.e., pre-drug) RRR is represented in the black traces. Amplitude of the RRR after any specific drug condition (listed at left of motility trace) is represented in gray. Bottom: bar graph summarizing the effects of adrenoceptor antagonists or NOS synthesis inhibition on the strength of the RRR. Data were normalized to a predrug control reflex = to 100%. ANOVA F5,44 = 13.0, P < 0.0001; Dunnett’s posttest, *P < 0.05, **P < 0.001.
Noradrenergic Agonists
Our observations from the preceding set of experiments revealed that only noradrenergic antagonists interrupted the normal RRR. To test the hypothesis that NE action in the DMN can elicit the same drop in gastric tone as seen with esophageal distension, relatively small (40 nl volume), unilateral injections of NE (50 mM; total dose = 0.3 ng = 2 pmol) into the DMN were made via micropipette. The logic of agonist application (via point microinjection) parallels that for antagonist application (via broad coverage). Specifically, point microinjection of a presumptive agonist into small areas of the DMN that are affected by NST input should be expected to elicit the full reflex. This is due to the extraordinary divergence of input to the enteric plexus from single vagal efferent fibers. Thus, to antagonize the RRR, broad coverage of the DMN is required for unambiguous effects. In contrast, nearly complete agonist effects can be expected from point applications to the DMN [e.g., thyrotropin releasing hormone (24), oxytocin (29), tumor necrosis factor-α (17)].
All stereotaxic and surgical preparations were as described above for the in vivo motility measurements, including the exposure of the brain stem surface. Once all surgical preparations were completed, basal fundic activity was monitored for 1 h. The fundic balloon was then inflated to 2 ml, producing a modest distension of the fundus (i.e., consistent 1-g distension preload strain). Within 5 min of the onset of the gastric preload, 40 nl of PBS were microinjected into the left DVC directly over the NSTc (coordinates relative to calamus scriptorum: AP = 0.3 mm rostral, ML = 0.3 mm off midline, DV = 500 μm below surface of medulla). The gastric balloon preload was relieved 5 min after the microinjection was delivered. This session constituted the control or basal motility response to microinjection; thus each animal served as its own control for comparison purposes (n = 4). One hour after this control response session, the protocol was repeated with the exception that 40 nl of 50 mM NE (total dose = 0.3 ng) was microinjected into the same injection site. At the end of this gastric motility/tone recording session, a micropipette (tip diameter ~20 μM, as above) filled with 1% Pontamine dye was lowered into the microinjection site, and 40 nl of dye was delivered to the site to mark the location for histological verification (Fig. 6).
Fig. 6.
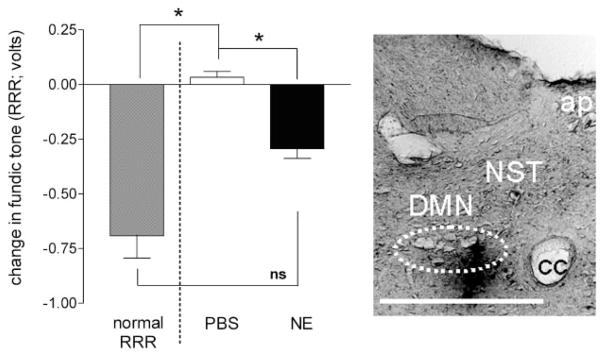
Unilateral injections of norepinephrine (NE; 40 nl volume; 2 pmol = 0.3 ng) into the left DVC of food-deprived animals equipped with a gastric preload balloon elicits a transient drop in gastric tone not unlike that seen during an RRR induced by mild esophageal distension (refer to Fig. 5). Left: demonstration of drop in fundic tone elicited by microinjection of either PBS or NE. Bar on far left of panel is an illustration of the average magnitude of drop in tone evoked during an RRR (ANOVA F2,29 = 5.28; P = 0.011; Tukey’s posttest *P < 0.05). Posttests indicate that there is no significant difference between the RRRs evoked in the first part of these studies and the drop in tone elicited by unilateral microinjections of NE. Right: microinjection of 1% Pontamine blue (40 nl volume) verifies location of micropipette in DVC. Scale bar = 200 μm. cc, central canal; ap, area postrema; DMN, dorsal motor nucleus; NST, nucleus of solitary tract.
NE Agonist: In Vivo Data Analysis
The change in fundic tone elicited by microinjection into the DVC was evaluated by averaging the strain gauge output for the minute before and after the microinjection. The difference in fundic tone after PBS vs. NE microinjection represents the magnitude of the fictive RRR. The difference scores (before vs. after DVC microinjection) of the PBS injections were compared with the difference scores obtained after microinjection of NE using paired t-test analysis. As an additional measure of comparison, the average magnitude of the normal RRR that was elicited by esophageal distension in the preceding set of experiments was compared with the magnitude of the average change in fundic tone elicited by unilateral microinjection of NE or PBS into the DVC (Fig. 6). These data were subjected to a one-way ANOVA; Tukey’s post hoc comparisons were made. Statistical significance was defined at P < 0.05.
RESULTS
Distribution of c-Fos-Labeled Neurons in the NST After Esophageal Distension
Periodic esophageal distension resulted in a significant increase in the numbers of c-Fos-positive neurons throughout the entire NST (Fig. 1; ANOVA F3,33 = 26.0; P < 0.0001; Dunnett’s posttest for comparisons against control: **P < 0.001). The distribution of c-Fos-positive neurons after esophageal distension was higher in the central regions of the NST than in the lateral or medial subregions at any given rostral-caudal plane (Fig. 2; ANOVA F8,135 = 11.21; P < 0.05, Bonferroni post hoc comparisons, P < 0.05).
In view of the observation that esophageal distension activated more c-Fos-positive neurons in the central division of the NST than in the other subregions, we concentrated our analysis of the neurochemical phenotype c-Fos-double-immunostained neurons in this area. Figure 3A shows that a relatively large number of c-Fos-activated neurons are located just outside the confines of the NOS core of the central division of the NST. This stands in sharp contrast with the observation in the adjacent histological section where numerous c-Fos-TH-positive NST neurons are seen to encircle the same core of presumably NOS-positive NSTc neurons (Fig. 3, B and C). Note that some NOS-ir neurons outside the dense core NOS-ir area of the NSTc did contain c-Fos-ir (Fig. 3D), but these were few in number. Although esophageal distension does not appear to activate many NOS-ir neurons in the NST, nevertheless a very dense plexus of presumptive NOS-ir terminal fibers are interwoven between DMN neurons. These fibers have a coarse appearance (Fig. 3E) compared with the equally dense, but fine, filamentous TH fibers that surround practically all neurons in the DMN (Fig. 3F). As Fig. 3G shows, single TH-containing fibers in the NST send short filamentous projections into the immediately subjacent DMN (Fig. 3, G and H).
An attempt was made to test whether a different location (i.e., proximal esophageal distension) or mode of stimulation (i.e., acid infusion) could activate neurons in the dense core NOS region of the NSTc. In neither set of stimulation conditions were NOS neurons in this region of the NSTc activated by these stimuli as demonstrated by c-Fos expression. Specifically, proximal esophageal distension produced a similar distribution pattern of c-Fos activation and the proportion of activated TH and NOS-positive NST neurons as was observed with distal esophageal distension. Therefore, these data were combined for analysis purposes. In contrast, repetitive infusion of small amounts of acid into the distal esophagus had no significant effect to elevate the number of c-Fos-positive neurons above control values, nor did it activate a significant number of NOS-positive neurons in the NSTc.
The total numbers of NOS-ir or TH-ir neurons found in the NSTc in either the control or esophageal distension groups are not different (Fig. 4). However, the number of activated TH-ir neurons (i.e., TH + c-Fos positive) responding to esophageal distension was significantly greater than control group (ANOVA F3,40 = 51.0; P < 0.0001; distension: 25 ± 2 vs. control: 11 ± 3; Bonferroni post hoc comparison, P < 0.001). There was no significant difference in the number of NOS-c-Fos-double-immunoreactive cells in the NSTc (Fig. 4; distension: 3 ± 1 vs. control: 0 ± 0; Bonferroni post hoc comparison P > 0.05).
Effects of Noradrenergic and NOS Antagonism on the RRR
Similar to our previous studies on the antrum (30), we observed that low-volume esophageal distensions produce brisk relaxations of the mildly distended fundus (Fig. 5). Application of either yohimbine or prazosin, but not propranolol or L-NAME, to the floor for the fourth ventricle significantly reduced the magnitude of the fundic relaxation elicited by esophageal distension compared with control (yohimbine 56 ± 6%, prazosin 55 ± 18% of control, respectively). The combination of yohimbine and prazosin produced a further reduction in the magnitude of the RRR (28 ± 9% of control; overall ANOVA F4,43 = 12.0; P < 0.0001; Dunnett’s posttest P < 0.05).
Effects of Microinjection of Noradrenergic Agonist into DVC
Microinjections (40 nl) of PBS into the DVC did not change fundic tone. In contrast, unilateral microinjections of NE (40 nl; 0.3 ng total dose) into the same site of the DVC evoked a transient relaxation (Fig. 6). Paired t-tests on these difference scores indicate that this drop in gastric tone after NE is highly significant (degrees of freedom = 3, t = 15.3; P = 0.0006). As a point of comparison, we reanalyzed the magnitude of the evoked relaxation after NE (or PBS) microinjection in the context of the magnitude of the “normal” RRR (i.e., mild esophageal distension as seen in the previous group of experiments) using a one-way ANOVA followed by Tukey’s posttests. This reanalysis reveals that, although the relaxation in fundic tone evoked after unilateral NE microinjection is significantly different to that evoked by PBS microinjection (Fig. 6; ANOVA F2,29 = 5.28; P = 0.011; Tukey’s posttest *P < 0.05), the magnitude of the drop in tone after NE microinjection it is not statistically different to the magnitude of the drop elicited by esophageal distension (i.e., NE vs. RRR).
DISCUSSION
The principal findings of this report are that 1) repetitive esophageal distension activates NST neurons in the central subdivision of the anterior solitary nucleus; 2) the majority of these central NST neurons that were activated by esophageal distension are TH positive; 3) the gastric relaxation induced by esophageal distension could be modulated (i.e., suppressed) by antagonism of both α1- and α2-adrenoreceptors; and 4) unilateral microinjection of NE into the DVC was sufficient to mimic the reflex. Remarkably, esophageal distension had no effect on activation of the core of NOS-ir neurons in the NSTc, an area that anatomical studies show receives substantial esophageal afferent input. Given that we did observe scattered NOS-ir neurons in the NST that were also c-Fos positive, it is not likely that the lack of c-Fos label was due to any inherent inability of NST NOS-ir neurons to produce c-Fos.
Our physiological results paralleled the immunohistochemical observations. Local brain stem application of α1- or α2-adrenoceptor (but not β-adrenoceptor) blockade reduced the magnitude of the reflex. Combined α1- and α2-adrenoceptor blockade nearly eliminated the RRR, whereas blockade of the synthesis of nitric oxide with L-NAME had no effect on the RRR. Last, unilateral microinjections of NE into the DVC were sufficient to transiently mimic the reflex drop in fundic tone seen with mild esophageal distension.
The possibility that local noradrenergic inputs to the DMN can influence vagal parasympathetic functions has been appreciated for some time. On the basis of their observations of the density of TH and dopamine β-hydroxylase (DβH) terminal fields in the DMN, Kahlia et al. (20) speculated that local adrenergic inputs from the A2 cell group (adjacent to the NSTc) should powerfully modulate vagal autonomic functions. Because there is a nearly 100% overlap of TH with DβH neurons in the rat DVC (37), it is very likely that these localized reflex inputs to the DMN are principally noradrenergic.
There have been relatively few investigations into the physiological impact of NE on DMN neurons and the functions they regulate. Fukuda and colleagues (14), performing “blind” DMN recordings in the in vitro slice preparation (i.e., the cells recorded from had not been previously identified or prelabeled), showed that some DMN neurons (32%) are inhibited by NE and this primary NE effect on DMN excitability was blocked by the α2-adrenoceptor antagonist yohimbine. Other (55%) DMN neurons are activated by NE, and this activation is suppressed by the α1-adrenoceptor antagonist prazosin. Fukuda et al. also found that β-adrenergic antagonists had no effect on blocking NE influences on DMN excitability. Bertolino and colleagues (2) demonstrated further that excitatory glutamatergic inputs to the DMN are inhibited by NE and that this presynaptic effect to inhibit DMN neuronal firing is also blocked by yohimbine. These earlier neurophysiological results are consistent with the present report. That is, both α2- and α1-adrenoceptor antagonists (yohimbine and prazosin, respectively) reduced the magnitude of the RRR compared with control observations in the same animal. α2-Adrenoceptor antagonism may reduce RRR magnitude by blocking inhibitory effects of NE directly on DMN neurons (14), which normally provide excitatory cholinergic input to the stomach. Furthermore, α2-adrenoceptor blockade may remove noradrenergic inhibition of glutamate inputs (2) to the same population of DMN neurons. This dual action of α2-adrenoceptor antagonists would disinhibit the activity of the excitatory cholinergic path to the stomach, resulting in a further blockade of the RRR. α1 Antagonism probably blocks the direct postsynaptic effects of NE to activate the NANC component of DMN projections to the stomach. Our present observation that the combination of α1- and α2-blockade further suppresses the RRR argues for the operation of two independent, yet parallel, α-adrenergic circuits in the DVC that are responsible for this reflex. Preliminary in vitro patch-clamp studies on identified gastric-projecting neurons in the brain stem slice preparation suggest further that NE can selectively activate or inhibit different populations of DMN neurons subserving different autonomic functions (Travagli RA, personal communication).
The combined results from a number of reports strongly suggested a role for NSTc-NOS neurons in the parasympathetic control of digestion. Krowicki and colleagues (21) have shown that microinjection of L-arginine (precursor to nitric oxide) significantly decreases intragastric pressure, whereas microinjection of the NOS inhibitor L-NAME increases intragastric pressure. These effects are vagally mediated. Anatomical evidence has shown that NOS neurons in the NSTc receive esophageal afferent input (1). These NOS-ir NSTc neurons certainly project to the NA and are also likely to be a source of nitrergic input to the DMN (1, 36). Indeed, our present results clearly show that the DMN is the potential recipient of a large volume of NOS terminal input (Fig. 3E). Previous studies show that neurons in the NSTc area are activated by esophageal distension (19, 23, 30). Neurons in this area project throughout the DMN and, therefore, are clearly in position to regulate gastric function as a consequence of these projections. Recordings made from the DMN show that medially located neurons in this nucleus are largely inhibited after esophageal distension, whereas laterally and posteriorly located DMN neurons are excited by esophageal distension. Given the anatomical literature, we had speculated that some of this reflex signal from NSTc to DMN might be carried by NOS-ir NSTc neurons (30).
However, none of these electrophysiological studies (19, 23, 30) could identify the phenotypes of NSTc neurons, only their location in the NSTc area. So, given the present anatomical and physiological results, it is possible that neurons recorded in the NSTc area that were responsive to esophageal distension may have been the TH and not the NOS phenotype. To resolve this apparent paradox, it may be necessary to consider the possibility that the NSTc requires a specific pattern of visceral afferent input to activate the NOS components of this nucleus. In a recent review, Goyal and colleagues (15) observed that modest electrical stimulation of the superior laryngeal nerve (SLN; a proximal branch of the afferent vagus) activates neurons in all subdivisions of the solitary nucleus except the core of nucleus centralis (15). This moderate level of afferent stimulation also caused localized gastroesophageal relaxation, but not esophageal peristalsis. Localized activation of the DMN was observed coincident with these limited effects; however, the nucleus ambiguus (NA) was not activated. In contrast, higher frequency stimulation of the SLN elicited a complete swallowing reflex (including esophageal peristalsis and sphincter relaxation); this response was accompanied by c-Fos activation of the core of NSTc and the NA.
In addition to patterned afferent modulation of the activity of the NSTc, as described above, it is likely that the NOS core of the NSTc is under the control of CNS inputs. That is, esophageal afferent input may modulate the activity of NSTc-NOS neurons, but a central pattern generator is required to excite them. Such a mechanism was suggested by the pioneering work of Jean (reviewed in 19). Jean concluded that the act of swallowing (initiated by the NSTc) required programmed input from other sites in the CNS. However, the “swallowing program” is modified by vagal afferent feedback from the esophagus. These observations, in combination with those we present in this manuscript, suggest that the activation of esophageal afferent pathways sufficient to produce gastric relaxation do not require the activation of the nitrergic core of the NSTc. Noradrenergic neurons in and near the NSTc carry out this particular reflex task. Activation of this nitrergic core of the NSTc is required to generate voluntary swallowing. Although vagal afferents can modulate this motor program, these inputs are not essential for program initiation (15).
Acknowledgments
We thank S. Hebert for superb technical assistance with the double immunocytochemical protocols. We also thank Dr. H. R. Berthoud for helpful discussions during these experiments. Finally, we acknowledge the support and encouragement of L. M. Rogers and R. F. Rogers.
Footnotes
Preliminary accounts of this work were previously presented: Travagli RA and Rogers RC. A proposed brainstem circuitry for the receptive relaxation reflex. Digestive Disease week. May 16–22, 1998. New Orleans, LA; Rogers RC, Bantikyan A, and Travagli RA. Involvement of catecholamines in the esophageal-gastric relaxation pathway. 30th Neuroscience Mtg. New Orleans, LA, 2000.
DISCLOSURES
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-52142, DK-55530, and DK-56373 and by the Metabolife Settlement Fund.
References
- 1.Altschuler SM, Bao XM, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989;283:248–268. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- 2.Bertolino M, Vicini S, Gillis R, Travagli A. Presynaptic α2-adrenoceptors inhibit excitatory synaptic transmission in rat brain stem. Am J Physiol Gastrointest Liver Physiol. 1997;272:G654–G661. doi: 10.1152/ajpgi.1997.272.3.G654. [DOI] [PubMed] [Google Scholar]
- 3.Bieger D. The brainstem esophagomotor network pattern generator: a rodent model. Dysphagia. 1993;8:203–208. doi: 10.1007/BF01354539. [DOI] [PubMed] [Google Scholar]
- 4.Bieger D, Hopkins DA. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J Comp Neurol. 1987;262:546–562. doi: 10.1002/cne.902620408. [DOI] [PubMed] [Google Scholar]
- 5.Blessing WW. The Lower Brainstem and Bodily Homeostasis. Oxford, UK: Oxford Univ. Press; 1997. Eating and metabolism; pp. 323–372. [Google Scholar]
- 6.Broussard DL, Altschuler SM. Central integration of swallow and airway-protective reflexes. Am J Med. 2000;108(Suppl 4a):62S–67S. doi: 10.1016/s0002-9343(99)00340-x. [DOI] [PubMed] [Google Scholar]
- 7.Browning KN, Travagli RA. Characterization of the in vitro effects of 5-hydroxytryptamine (5-HT) on identified neurones of the rat dorsal motor nucleus of the vagus (DMV) Br J Pharmacol. 1999;128:1307–1315. doi: 10.1038/sj.bjp.0702908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buelke-Sam J, Holson JF, Bazare JJ, Young JF. Comparative stability of physiological parameters during sustained anesthesia in rats. Lab Anim Sci. 1978;28:157–162. [PubMed] [Google Scholar]
- 9.Canon WB, Leib CW. The receptive relaxation of the stomach. Am J Physiol. 1911;29:267–273. [Google Scholar]
- 10.Cunningham ET, Jr, Sawchenko PE. A circumscribed projection from the nucleus of the solitary tract to the nucleus ambiguus in the rat: anatomical evidence for somatostatin-28-immunoreactive interneurons subserving reflex control of esophageal motility. J Neurosci. 1989;9:1668–1682. doi: 10.1523/JNEUROSCI.09-05-01668.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emch GS, Hermann GE, Rogers RC. TNF-α activates solitary nucleus neurons responsive to gastric distension. Am J Physiol Gastrointest Liver Physiol. 2000;279:G582–G586. doi: 10.1152/ajpgi.2000.279.3.G582. [DOI] [PubMed] [Google Scholar]
- 12.Emch GS, Hermann GE, Rogers RC. TNF-α-induced c-Fos generation in the nucleus of the solitary tract is blocked by NBQX and MK-801. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1394–R1400. doi: 10.1152/ajpregu.2001.281.5.R1394. [DOI] [PubMed] [Google Scholar]
- 13.Fryscak T, Zenker W, Kantner D. Afferent and efferent innervation of the rat esophagus. A tracing study with horseradish peroxidase and nuclear yellow. Anat Embryol (Berl) 1984;170:63–70. doi: 10.1007/BF00319459. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda A, Minami T, Nabekura J, Oomura Y. The effects of noradrenaline on neurones in the rat dorsal motor nucleus of the vagus, in vitro. J Physiol. 1987;393:213–231. doi: 10.1113/jphysiol.1987.sp016820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goyal RK, Padmanabhan R, Sang Q. Neural circuits in swallowing and abdominal vagal afferent-mediated lower esophageal sphincter relaxation. Am J Med. 2001;111(Suppl 8A):95S–105S. doi: 10.1016/s0002-9343(01)00863-4. [DOI] [PubMed] [Google Scholar]
- 16.Hayakawa T, Zheng JQ, Yajima Y. Direct synaptic projections to esophageal motoneurons in the nucleus ambiguus from the nucleus of the solitary tract of the rat. J Comp Neurol. 1997;381:18–30. doi: 10.1002/(sici)1096-9861(19970428)381:1<18::aid-cne2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 17.Hermann G, Rogers RC. Tumor necrosis factor-alpha in the dorsal vagal complex suppresses gastric motility. Neuroimmunomodulation. 1995;2:74–81. doi: 10.1159/000096874. [DOI] [PubMed] [Google Scholar]
- 18.Hermann GE, Emch GS, Tovar CA, Rogers RC. c-Fos generation in the dorsal vagal complex after systemic endotoxin is not dependent on the vagus nerve. Am J Physiol Regul Integr Comp Physiol. 2001;280:R289–R299. doi: 10.1152/ajpregu.2001.280.1.R289. [DOI] [PubMed] [Google Scholar]
- 19.Jean A. Control of the central swallowing program by inputs from the peripheral receptors. A review. J Auton Nerv Syst. 1984;10:225–233. doi: 10.1016/0165-1838(84)90017-1. [DOI] [PubMed] [Google Scholar]
- 20.Kalia M, Fuxe K, Goldstein M. Rat medulla oblongata. III. Adrenergic (C1 and C2) neurons, nerve fibers and presumptive terminal processes. J Comp Neurol. 1985;233:333–349. doi: 10.1002/cne.902330304. [DOI] [PubMed] [Google Scholar]
- 21.Krowicki ZK, Sharkey KA, Serron SC, Nathan NA, Hornby PJ. Distribution of nitric oxide synthase in rat dorsal vagal complex and effects of microinjection of nitric oxide compounds upon gastric motor function. J Comp Neurol. 1997;377:49–69. doi: 10.1002/(sici)1096-9861(19970106)377:1<49::aid-cne6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 22.Loomis CW, Yao D, Bieger D. Characterization of an esophagocardiovascular reflex in the rat. Am J Physiol Regul Integr Comp Physiol. 1997;272:R1783–R1791. doi: 10.1152/ajpregu.1997.272.6.R1783. [DOI] [PubMed] [Google Scholar]
- 23.Lu WY, Bieger D. Vagovagal reflex motility patterns of the rat esophagus. Am J Physiol Regul Integr Comp Physiol. 1998;274:R1425–R1435. doi: 10.1152/ajpregu.1998.274.5.R1425. [DOI] [PubMed] [Google Scholar]
- 24.McCann MJ, Hermann GE, Rogers RC. Thyrotropin-releasing hormone: effects on identified neurons of the dorsal vagal complex. J Auton Nerv Syst. 1989;26:107–112. doi: 10.1016/0165-1838(89)90158-6. [DOI] [PubMed] [Google Scholar]
- 25.Miolan JP, Roman C. The role of oesophageal and intestinal receptors in the control of gastric motility. J Auton Nerv Syst. 1984;10:235–241. doi: 10.1016/0165-1838(84)90018-3. [DOI] [PubMed] [Google Scholar]
- 26.Partosoedarso ER, Blackshaw LA. Vagal efferent fibre responses to gastric and oesophageal mechanical and chemical stimuli in the ferret. J Auton Nerv Syst. 1997;66:169–178. doi: 10.1016/s0165-1838(97)00081-7. [DOI] [PubMed] [Google Scholar]
- 27.Paton JF, Li YW, Kasparov S. Reflex response and convergence of pharyngoesophageal and peripheral chemoreceptors in the nucleus of the solitary tract. Neuroscience. 1999;93:143–154. doi: 10.1016/s0306-4522(99)00098-6. [DOI] [PubMed] [Google Scholar]
- 28.Rinaman L, Verbalis JG, Stricker EM, Hoffman GE. Distribution and neurochemical phenotypes of caudal medullary neurons activated to express cFos following peripheral administration of cholecystokinin. J Comp Neurol. 1993;338:475–490. doi: 10.1002/cne.903380402. [DOI] [PubMed] [Google Scholar]
- 29.Rogers RC, Hermann GE. Oxytocin, oxytocin antagonist, TRH, and hypothalamic paraventricular nucleus stimulation effects on gastric motility. Peptides. 1987;8:505–513. doi: 10.1016/0196-9781(87)90017-9. [DOI] [PubMed] [Google Scholar]
- 30.Rogers RC, Hermann GE, Travagli RA. Brainstem pathways responsible for oesophageal control of gastric motility and tone in the rat. J Physiol. 1999;514:369–383. doi: 10.1111/j.1469-7793.1999.369ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers RC, McTigue DM, Hermann GE. Vagal control of digestion: modulation by central neural and peripheral endocrine factors. Neurosci Biobehav Rev. 1996;20:57–66. doi: 10.1016/0149-7634(95)00040-l. [DOI] [PubMed] [Google Scholar]
- 32.Schemann MGD. Electrophysiological identification of vagally innervated enteric neurons in guinea pig stomach. Am J Physiol Gastrointest Liver Physiol. 1992;263:G709–G718. doi: 10.1152/ajpgi.1992.263.5.G709. [DOI] [PubMed] [Google Scholar]
- 33.Sengupta JN, Kauvar D, Goyal RK. Characteristics of vagal esophageal tension-sensitive afferent fibers in the opossum. J Neurophysiol. 1989;61:1001–1010. doi: 10.1152/jn.1989.61.5.1001. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J Physiol. 1997;504:479–488. doi: 10.1111/j.1469-7793.1997.479be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Travagli RA, Rogers RC. Receptors and transmission in the brain-gut axis: potential for novel therapies. V. Fast and slow extrinsic modulation of dorsal vagal complex circuits. Am J Physiol Gastrointest Liver Physiol. 2001;281:G595–G601. doi: 10.1152/ajpgi.2001.281.3.G595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiedner EB, Bao X, Altschuler SM. Localization of nitric oxide synthase in the brain stem neural circuit controlling esophageal peristalsis in rats. Gastroenterology. 1995;108:367–375. doi: 10.1016/0016-5085(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 37.Willing AE, Berthoud HR. Gastric distension-induced c-fos expression in catecholaminergic neurons of rat dorsal vagal complex. Am J Physiol Regul Integr Comp Physiol. 1997;272:R59–R67. doi: 10.1152/ajpregu.1997.272.1.R59. [DOI] [PubMed] [Google Scholar]