Abstract
Sensory information from the gastrointestinal (GI) tract is transmitted centrally via primary afferents that terminate within the nucleus of the tractus solitarius (NTS) and utilize glutamate as their major neurotransmitter. Neurons of the NTS integrate this sensory information and transmit it to parasympathetic preganglionic neurons of the dorsal motor nucleus of the vagus (DMV), as well as to other areas, using principally glutamate, GABA and norepinephrine as neurotransmitters. Although susceptible to modulation by a vast array of neurotransmitters, the glutamatergic NTS to DMV synapse seems to play a minor role in the tonic modulation of gastric vagal reflexes. GABAergic neurotransmission between the NTS and DMV, however, is of critical importance as its in vivo blockade induces dramatic effects on gastric tone, motility and secretion. In in vitro experiments, however, this synapse appears initially resistant to modulation by most exogenously applied neuromodulators.
Using opioid peptides as a model, this review will discuss the remarkable plasticity of the NTS–DMV GABAergic synapse. Modulation of this synapse appears dependent upon the levels of cAMP within the brainstem circuit. In particular, this review will outline how vagal afferent inputs appear to dampen the cAMP–PKA system via tonic activation of metabotropic glutamate receptors. Removal of vagal sensory input, coincident activation of the cAMP –PKA system, or inhibition of group II metabotropic glutamate receptors, allows receptor trafficking to occur selectively at the level of the NTS–DMV GABAergic synapse. Thus, we propose that the state of activation of vagal sensory inputs determines the gastric motor response via selective engagement of GABAergic synapses.
This mini-review is based upon a presentation given at the International Society for Autonomic Neuroscience meeting in Marseille, France in July 2005.
Keywords: DMV, Opioid receptors, Receptor trafficking, Plasticity, Vagus, cAMP
1. Elements of a vago-vagal reflex
Sensory information from the GI tract is perceived, encoded and relayed to the central nervous system (CNS) by vagal afferent neurons, the cell bodies of which lie in the paired nodose ganglia. The central projections of these vagal afferent neurons enter the CNS via the tractus solitarius and terminate within NTS (Travagli et al., 2006). Despite exhibiting distinct sensory modalities, discrete neurochemical characteristics and diverse nerve fiber conduction properties, the central terminals of all vagal afferent neurons use glutamate as their principal neurotransmitter to transfer information to the NTS (Andresen and Kunze, 1994; Jean, 2001). NTS neurons respond to vagal afferent inputs via activation of both NMDA as well as non-NMDA glutamate receptors (Andresen and Yang, 1990; Baptista et al., 2005; Smith et al., 1998). NTS neurons assimilate these sensory inputs with converging inputs from other brainstem and higher CNS centers, as well as bringing their own unique biophysical and synaptic properties to bear in shaping the resultant output signal. Those NTS neurons involved in GI vagal reflexes project to the DMV, the neurons of which are the preganglionic parasympathetic motoneurons that provide the motor output to the GI tract via the efferent vagus (reviewed in Travagli et al., 2006).
NTS neurons comprise many different neurochemical phenotypes but, despite this, electrophysiological and functional studies have shown that DMV neurons involved in GI vago-vagal reflexes are controlled primarily by glutamatergic, GABAergic or catecholaminergic inputs from the NTS (Rogers et al., 2003; Travagli et al., 2006). Less is known about catecholaminergic transmission between the NTS and DMV, although electrical stimulation of the NTS (subnucleus commissuralis) has been shown to evoke a noradrenergic α2 mediated inhibitory current in DMV neurons, while electrical stimulation of the NTS between the medialis and centralis subnuclei evokes an excitatory α1 mediated current in gastric-projecting DMV neurons (Fukuda et al., 1987) (Browning and Travagli, unpublished observations). In contrast, many studies over several years have demonstrated that electrical stimulation of various NTS subnuclei elicit glutamatergic excitatory and GABAergic inhibitory currents in DMV neurons (Browning and Travagli, 1999; Browning et al., 2003; Davis et al., 2004; Travagli et al., 1991; Willis et al., 1996). Regardless of the incontrovertible evidence of glutamatergic and catechoaminergic transmission from the NTS to DMV, it appears that the majority of gastric vago-vagal reflexes are mediated via GABAergic transmission at the NTS–DMV synapse. Microinjections of glutamatergic or catecholaminergic antagonists into the dorsal vagal complex (DVC, i.e., NTS plus DMV) do not exert noticeable effects on gastric motility or tone unless GABAergic synaptic transmission is also blocked (Rogers et al., 2003; Sivarao et al., 1998). In contrast, microinjection of the GABAA receptor selective antagonist, bicuculline, into the same area induces substantial increases in gastric motility, tone and secretion (reviewed in Travagli et al., 2006).
Notwithstanding the large volume of evidence supporting GABA as the principal neurotransmitter at the NTS–DMV synapse in vago-vagal reflexes, studies in our laboratory demonstrated that modulation of GABAergic transmission at the synapse was extremely limited. In fact, while glutamatergic transmission could be modulated by a variety of neurotransmitter and neuromodulators (Bertolino et al., 1997; Browning et al., 2002, 2003; Browning and Travagli, 1999; Davis et al., 2003), GABAergic synaptic transmission proved resistant to the majority of these substances (Browning et al., 2002, 2003; Browning and Travagli, 1999). This led us to ask the question: what possible benefit would there be in having such an important synapse that is resistant to modulation? To answer this question, we used A-opioid peptides as a model system with which we could investigate the modulation of NTS–DMV synaptic transmission. Several reasons led us to this choice, including the well-defined pharmacology of opioid peptides. Furthermore, the activation of central μ-opioid receptors induces gastric relaxation, decreases gastric acid secretion, inhibits intestinal transit and increases feeding. Some of these effects may certainly be due to the actions of opioid peptides outside the brainstem, but evidence also suggests the involvement of opioid neurotransmission within the DVC, from local NTS neurons as well as from neurons in the amygdala and periaqueductal grey (Burks et al., 1987; Fox and Burks, 1988; Gué et al., 1989; Kotz et al., 1997). Thus, μ-opioid peptides are not just a convenient model system, but also a physiologically relevant one.
2. cAMP levels determine the state of activation of NTS GABAergic nerve terminals
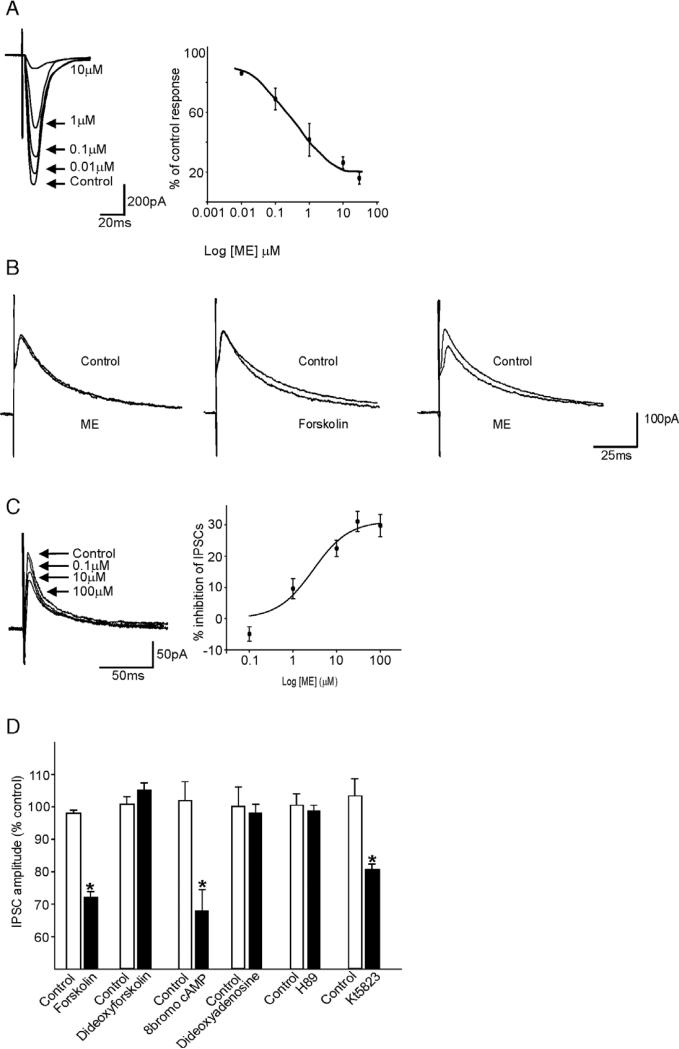
A series of experiments conducted in this laboratory determined that, under normal conditions, neuromodulators such as opioid peptides are unable to inhibit GABAergic transmission between the NTS and the DMV because of a low resting activity of the cAMP–PKA pathway in GABAergic NTS nerve terminals (Browning et al., 2002, 2004). Following activation of this pathway, either by activating adenylate cyclase (by forskolin, TRH or CCK), by inhibiting phosphodiesterase-mediated degradation of cAMP (IBMX), or by a using non-hydrolysable form of cAMP (8-bromo-cAMP), “uncovered” the ability of μ-opioid receptor agonists to act at presynaptic receptors to inhibit GABAergic synaptic transmission. Conversely, inhibition of the cAMP–PKA pathway using adenylate cyclase inhibitors (dideoxyadenosine), or PKA inhibitors (Rp-cAMPs or H-89), prevented the ability of adenylate cyclase activators to uncover these presynaptic inhibitory actions of opioid peptides (Fig. 1). The rapid (5 min) time-course of μ-opioid receptor uncovering, and its transient (1 h) nature led us to suggest that activation of the cAMP–PKA pathway caused a trafficking of internalized μ-opioid receptors to the cell membrane; immunohistochemical studies confirmed this hypothesis as did the finding that the ‘uncovering’ or insertion of presynaptic μ-opioid receptors was inhibited by the Golgi-disturbing agent, brefeldin A (Browning et al., 2004). The inhibition of evoked IPSC amplitude by μ-opioid receptor agonists, or other neurotransmitters, is noticeably limited to a decrease of approximately 30–40% in peak current amplitude. One has to keep in mind, however, that DMV neurons are tonically active (Marks et al., 1993; McCann and Rogers, 1992; Travagli et al., 1991) and a membrane shift of even a few mV, such as that resulting from a small reduction in tonic synaptic current input, results in dramatic effects on the vagal motor output.
Fig. 1.
μ-opioid mediated inhibition of GABAergic but not glutamatergic synaptic transmission in the rat brainstem is dependent upon activation of the cAMP–PKA pathway. A: In a gastric-projecting rat DMV neuron, electrical stimulation of the adjacent NTS induced glutamatergic evoked excitatory postsynaptic currents (eEPSCs) that were inhibited by the μ-opioid agonist, Met-Enkephalin (ME) in a concentration-dependent manner. B: In contrast, in a gastric-projecting rat DMV neuron, electrical stimulation of the adjacent NTS induced GABAergic evoked inhibitory postsynaptic currents (IPSCs) that were unresponsive initially to ME (10μM; left). Following superfusion with the adenylate cyclase activator forskolin (10μM) which itself had no effect on eIPSC amplitude (middle), re-application of ME (10μM) induced an inhibition in eIPSC amplitude (right). C: Following superfusion with forskolin (10μM), ME induced a concentration-dependent inhibition in eIPSCs in an initially unresponsive neuron. D: The “uncovering” of the presynaptic inhibitory actions of ME was dependent upon increasing cAMP levels, as shown by the inhibition in eIPSC amplitude by the adenylate cyclase activator forskolin, but not its inactive analog, dideoxyforskolin, by the stable cAMP analog, 8-bromocAMP, but not by the adenylate cyclase inhibitor, dideoxyforskolin. Furthermore, the ME-induced inhibition in eIPSC amplitude was dependent on the PKA pathway as shown by the blocking of its effect following application of the PKA inhibitor, H89, but not the PKG inhibitor, K15823. *P<0.05 vs. control. Results are expressed as mean±S.E.M.
We theorized, therefore, that the ongoing levels of either cAMP or the level of activity of the cAMP–PKA system within GABAergic NTS nerve terminals determines their “state of activation” as far as their ability to be modulated by many neurotransmitters or neuromodulators is concerned. Under normal conditions, when cAMP levels in GABAergic nerve terminals appears to be low, neurotransmitters/modulators such as opioid peptides are unable to inhibit GABAergic transmission from the NTS to the DMV. Following activation of the cAMP–PKA pathway, and an increase in their “state of activation” however, these otherwise internalized μ-opioid receptors are rapidly and transiently translocated to the GABAergic nerve terminal membrane (Browning et al., 2004).
This, then, led to the next question: why should GABAergic, but not glutamatergic, transmission within the DVC be regulated in such a manner? We theorized that since GI vago-vagal reflexes can function independently of inputs from higher brain centers, the vagal afferent inputs themselves might act to keep cAMP levels within GABAergic NTS nerve terminals low. Accordingly, neuromodulators such as opioid peptides would have no effect on GABAergic transmission at the NTS–DMV synapse unless the afferent input was removed or overcome by coincident activation of the cAMP–PKA pathway.
3. Vagal afferent nerves control cAMP levels within GABAergic NTS nerve terminals
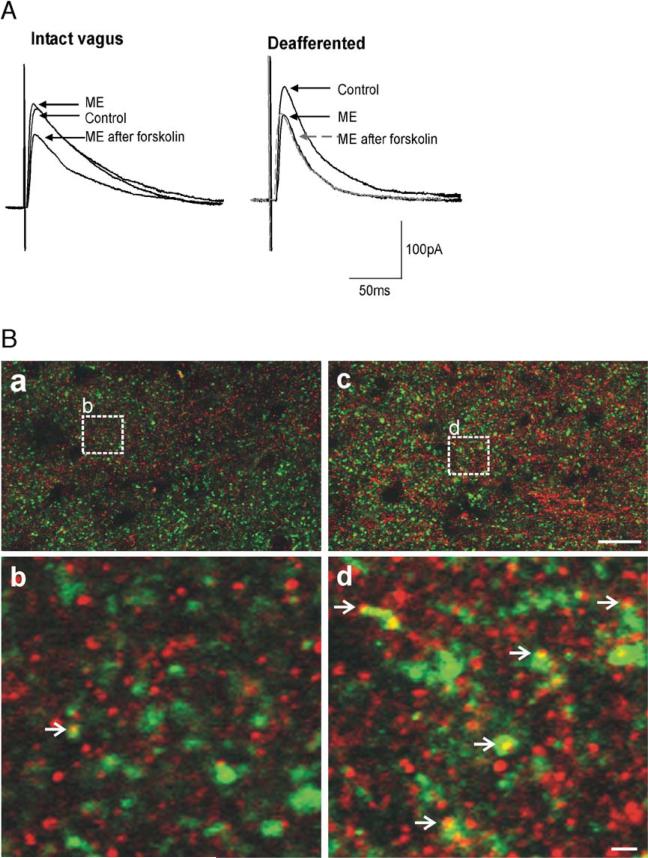
By selectively removing the vagal afferent inputs, either by chemical C-fiber ablation (Holzer, 1991) or by surgical rhizotomy, we were able to demonstrate that opioid peptides inhibit GABAergic synaptic transmission at the NTS–DMV synapse by activation of presynaptic μ-opioid receptors, without the prior need for activation of the cAMP–PKA pathway (Fig. 2). Immunohistochemical studies confirmed these electrophysiological results by demonstrating that, following vagal deafferentation, μ-opioid receptors can be localized on GABAergic profiles within the DVC (Fig. 2).
Fig. 2.
Vagal afferent input controls cAMP levels in GABAergic nerve terminals. A: In a gastric-projecting DMV neuron from a vagally intact rat, ME (10μM) induced an inhibition in eIPSC amplitude only following activation of the adenylate cyclase pathway (e.g. superfusion with forskolin (10μM) as shown; left). In contrast, in a rat in which the vagal afferent nerves were removed either chemically (perivagal capsaicin application) or surgically (afferent rootlet rhizotomy), ME (10μM) inhibited eIPSC amplitude without the need for prior activation of adenylate cyclase (right). Indeed, further activation of adenylate cyclase (e.g. forskolin, 10μM) resulted in no further inhibition by ME. B: Vagal deafferentation increases expression of μ-opioid receptor (MOR) on GABAergic profiles. MOR-IR: red; Glutamic acid decarboxylase-IR (GAD-IR; used to label GABAergic nerve terminals): green; profiles double-labeled for MOR and GAD: yellow. Panel a. Low magnification micrograph illustrating a portion of the DVC from the control (vagally intact) side of the brainstem. Panel b. High magnification micrograph illustrating the detail of a MOR-IR profile co-localized with GAD-IR (open arrowhead). The magnification is insert “b” shown in panel A. Panel c. Low magnification, micrograph illustrating a portion of the DVC from the contralateral (deafferented) side of the same sliceasin Panel A. Panel d. High magnification micrograph illustrating the detail of several MOR-IR profiles co-localized with GAD-IR (open arrowhead). The magnification is insert “d” shown in panel B. Scale bar=20μm in a and c; 2μm in b and d.
These results suggest that vagal afferent nerve fibers control, or at least modulate, cAMP levels within NTS GABAergic nerve terminals. The question then arose: how do vagal afferents modulate the “state of activation” of NTS GABAergic nerve terminals? As mentioned earlier, vagal afferent terminals use glutamate as their main neurotransmitter. While activation of ionotropic (NMDA and non-NMDA) receptors is vitally important in the transmission of vagal sensory information, metabotropic glutamate receptors (mGluR) have also been identified in the rat brainstem and their longer time-course of action implies they are more likely to play a role in the continuing modulation of levels of second messengers, such as cAMP (Glaum and Miller, 1992, 1993a,b; Hay et al., 1999). In particular, both group II and III mGluRs have been identified on vagal afferent nerve terminals, and both are located primarily at presynaptic sites where they act as autoreceptors, decreasing the release of neurotransmitters (Chen and Bonham, 2005; Chen et al., 2002; Jin et al., 2004). Both group II and II mGluRs are negatively coupled to adenylate cyclase and, thus, could potentially play a role in controlling the “state of activation” of GABAergic NTS nerve terminals (Cartmell and Schoepp, 2000; Conn and Pin, 1997).
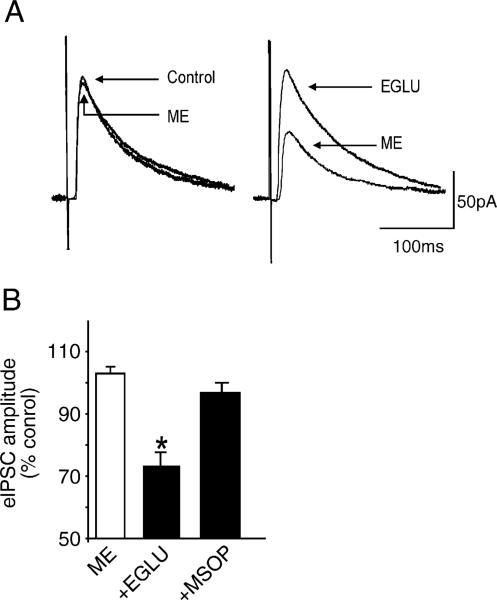
Our recent data has suggested that ongoing vagal afferent activation of group II but not group III mGluR acts as a ‘brake’ on the cAMP–PKA pathway in NTS GABAergic circuits (Browning and Travagli, 2005). Acute blockage of group II mGluR increases cAMP–PKA activity resulting in translocation of μ-opioid receptors to the membrane of GABAergic NTS nerve terminals where, following activation by μ-opioid peptides, they inhibit synaptic transmission. Vagal deafferentation removes the tonic activation of these receptors hence eliminates the need for coincident activation of the cAMP–PKA pathway prior to modulation of GABAergic synaptic transmission (Fig. 3).
Fig. 3.
Group II metabotropic glutamate receptors (mGluR) act as a “brake” on cAMP levels. A: ME (10μM) has no effect on eIPSC amplitude in a gastric-projecting DMV neuron (left). Following superfusion with the group II mGluR selective antagonist, EGLU (200μM), subsequent re-application of ME decreased eIPSC amplitude (right). B: Histogram illustrating that ME (10μM) inhibits eIPSC amplitude following superfusion with the group II mGluR selective antagonist, EGLU (200μM), but not the group III mGluR selective antagonist, MSOP (500μM). *P<0.05 vs. control. Results are expressed as mean±S.E.M.
4. Physiological significance
The NTS plays a major role in the integration and regulation of homeostasis and visceral reflexes. The NTS–DMV synapse represents a convenient model, therefore, to investigate the central components of vagovagal reflexes as well as central signal integration and synaptic modulation. As far as GI vago-vagal reflexes are concerned, GABA is the principal neurotransmitter at the NTS–DMV synapse yet initial studies led to the assumption that this synapse was resistant to modulation. More recent studies, however, led us to hypothesize that the “state of activation” of these inhibitory synapses determines their availability for modulation, and that ongoing vagal afferent activation of group II mGluR controls this state of activation. Such regulation appears, initially, to be unwieldy and cumbersome, but on closer inspection may provide a convenient, on-demand control of the pri ncipal neurotransmitter within GI central vagal brainstem circuits. Since their vagal afferent terminals already produce and release glutamate (at ionotropic receptors on NTS somata), this also provides an efficient modulation of vagal reflexes without the need for additional neurotransmitters.
5. Conclusions
Recent studies from this and other laboratories have refuted the earlier assumption that GI vago-vagal reflexes are merely static relay circuits. Instead, brainstem vagal circuits show a remarkable degree of regulation, modulation and plasticity. By allowing the activity levels of a second messenger system such as the cAMP–PKA pathway to control the modulation of synaptic transmission, brainstem vagal circuits can be regulated by circulating messengers and other neurotransmitter systems as well as by ongoing vagal afferent activity. We are only just beginning to understand the mechanisms involved in the modulation of GI vago-vagal reflexes and, while the permutations for their modulation appear almost limitless, they certainly appear to be organized in a manner that ensures efficient, metabolically economic and meticulous control of digestive processes.
Acknowledgements
The authors would like to thank the NIH (DK 55530 and DK 56373) and NSF (IBN-04,56291) for their support. We also thank Cesare M. Travagli for support and encouragement.
References
- Andresen MC, Kunze DL. Nucleus tractus solitarius–gateway to neural circulatory control. Annu. Rev. Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am. J. Physiol. 1990;259:H1307–H1311. doi: 10.1152/ajpheart.1990.259.4.H1307. [DOI] [PubMed] [Google Scholar]
- Baptista V, Zheng ZL, Coleman FH, Rogers RC, Travagli RA. Characterization of neurons of the nucleus tractus solitarius pars centralis. Brain Res. 2005;1052:139–146. doi: 10.1016/j.brainres.2005.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino M, Vicini S, Gillis RA, Travagli RA. Presynaptic α2-adrenoceptors inhibit excitatory synaptic transmission in rat brain stem. Am. J. Physiol. 1997;272:G654–G661. doi: 10.1152/ajpgi.1997.272.3.G654. [DOI] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. Characterisation of the in vitro effects of 5-hydroxytryptamine (5-HT) on identified neurones of the rat dorsal motor nucleus of the vagus (DMV). Br. J. Pharmacol. 1999;128:1307–1315. doi: 10.1038/sj.bjp.0702908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. Digestive Disease Week. Chicago, IL: May 14–19, 2005. Differential distribution of metabotropic glutamate receptors within gastric vagal brainstem circuits. [Google Scholar]
- Browning KN, Kalyuzhny AE, Travagli RA. Opioid peptides inhibit excitatory but not inhibitory synaptic transmission in the rat dorsal motor nucleus of the vagus. J. Neurosci. 2002;22:2998–3004. doi: 10.1523/JNEUROSCI.22-08-02998.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Travagli RA, Neuropeptide Y, Peptide YY. Inhibit excitatory synaptic transmission in the rat dorsal motor nucleus of the vagus. J. Physiol. 2003;549:775–785. doi: 10.1113/jphysiol.2003.042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Kalyuzhny AE, Travagli RA. Mu-opioid receptor trafficking on inhibitory synapses in the rat brainstem. J. Neurosci. 2004;24:7344–7352. doi: 10.1523/JNEUROSCI.1676-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks TF, Galligan JJ, Hirning LD, Porreca F. Brain, spinal cord and peripheral site of action of enkephalins and other endogenous opioids on gastrointestinal motility. Gastroenterol. Clin. Biol. 1987;11:44B–51B. [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J. Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Chen CY, Bonham AC. Glutamate suppresses GABA release via presynaptic metabotropic glutamate receptors at baroreceptor neurones in rats. J. Physiol. 2005;562:535–551. doi: 10.1113/jphysiol.2004.076885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Ling Eh EH, Horowitz JM, Bonham AC. Synaptic transmission in nucleus tractus solitarius is depressed by Group II and III but not Group I presynaptic metabotropic glutamate receptors in rats. J. Physiol. 2002;538:773–786. doi: 10.1113/jphysiol.2001.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Davis SF, Williams KW, Xu W, Glatzer NR, Smith BN. Selective enhancement of synaptic inhibition by hypocretin (orexin) in rat vagal motor neurons: implications for autonomic regulation. J. Neurosci. 2003;23:3844–3854. doi: 10.1523/JNEUROSCI.23-09-03844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SF, Derbenev AV, Williams KW, Glatzer NR, Smith BN. Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res. 2004;1017:208–217. doi: 10.1016/j.brainres.2004.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox DA, Burks TF. Roles of central and peripheral mu, delta and kappa opioid receptors in the mediation of gastric acid secretory effects in the rat. JPET. 1988;244:456–462. [PubMed] [Google Scholar]
- Fukuda A, Minami T, Nabekura J, Oomura Y. The effects of noradrenaline on neurons in the rat dorsal motor nucleus of the vagus, in vitro. J. Physiol. 1987;393:213–231. doi: 10.1113/jphysiol.1987.sp016820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaum SR, Miller RJ. Metabotropic glutamate receptors mediate excitatory transmission in the nucleus of the solitary tract. J. Neurosci. 1992;12:2251–2258. doi: 10.1523/JNEUROSCI.12-06-02251.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaum SR, Miller RJ. Activation of metabotropic glutamate receptors produces reciprocal regulation of ionotropic glutamate and GABA responses in the nucleus of the tractus solitarius of the rat. J. Neurosci. 1993;13:1636–1641. doi: 10.1523/JNEUROSCI.13-04-01636.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaum SR, Miller RJ. Metabotropic glutamate receptors depress afferent excitatory transmission in the rat nucleus tractus solitarii. J. Neurophysiol. 1993;70:2669–2672. doi: 10.1152/jn.1993.70.6.2669. [DOI] [PubMed] [Google Scholar]
- Gué M, Junien JL, Bueno L. Central and peripheral opioid modulation of gastric relaxation induced by feeding in dogs. JPET. 1989;250:1006–1010. [PubMed] [Google Scholar]
- Hay M, McKenzie H, Lindsley K, Dietz N, Bradley SR, Conn PJ, Hasser EM. Heterogeneity of metabotropic glutamate receptors in autonomic cell groups of the medulla oblongata of the rat. J. Comp. Neurol. 1999;403:486–501. [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- Jean A. Brainstem control of swallowing: neuronal network and cellular mechanisms. Physiol. Rev. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- Jin YH, Bailey TW, Andresen MC. Cranial afferent glutamate heterosynaptically modulates GABA release onto second-order neurons via distinctly segregated metabotropic glutamate receptors. J. Neurosci. 2004;24:9332–9340. doi: 10.1523/JNEUROSCI.1991-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz CM, Billington CJ, Levine AS. Opioids in the nucleus of the solitary tract are involved in feeding i n the rat. Am. J. Physiol. 1997;272:R1028–R1032. doi: 10.1152/ajpregu.1997.272.4.R1028. [DOI] [PubMed] [Google Scholar]
- Marks JD, Donnelly DF, Haddad GG. Adenosine-induced inhibition of vagal motoneuron excitability: receptor subtype and mechanisms. Am. J. Physiol. 1993;264:L124–L132. doi: 10.1152/ajplung.1993.264.2.L124. [DOI] [PubMed] [Google Scholar]
- McCann MJ, Rogers RC. Impact of antral mechanoreceptor activation on the vago-vagal reflex in the rat: functional zonation of responses. J. Physiol. 1992;453:401–411. doi: 10.1113/jphysiol.1992.sp019235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RC, Travagli RA, Hermann GE. Noradrenergic neurons in the rat solitary nucleus participate in the esophageal–gastric relaxation reflex. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R479–R489. doi: 10.1152/ajpregu.00155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivarao DV, Krowicki ZK, Hornby PJ. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol. Motil. 1998;10:305–313. doi: 10.1046/j.1365-2982.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- Smith BN, Dou P, Barber WD, Dudek FE. Vagally evoked synaptic currents in the immature rat nucleus tractus solitarii in an intact in vitro preparation. J. Physiol. 1998;512:149–162. doi: 10.1111/j.1469-7793.1998.149bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am. J. Physiol. 1991;260:G531–G536. doi: 10.1152/ajpgi.1991.260.3.G531. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu. Rev. Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis A, Mihalevich M, Neff RA, Mendelowitz D. Three types of postsynaptic glutamatergic receptors are activated in DMNX neurons upon stimulation of NTS. Am. J. Physiol. 1996;271:R1614–R1619. doi: 10.1152/ajpregu.1996.271.6.R1614. [DOI] [PubMed] [Google Scholar]