Abstract
Cholecystokinin (CCK) is released from enteroendocrine cells after ingestion of nutrients and induces multiple effects along the gastrointestinal tract, including gastric relaxation and short-term satiety. We used whole cell patch-clamp and immunohistochemical techniques in rat brain stem slices to characterize the effects of CCK. In 45% of the neurons of nucleus tractus solitarius subnucleus centralis (cNTS), perfusion with the sulfated form of CCK (CCK-8s) increased the frequency of spontaneous excitatory currents (sEPSCs) in a concentration-dependent manner (1–300 nM). The threshold for the CCK-8s excitatory effect was 1 nM, the EC50 was 20 nM, and Emax was 100 nM. The excitatory effects of CCK-8s were still present when the slices were preincubated with tetrodotoxin or bicuculline or when the recordings were conducted with Cs+ electrodes. Pretreatment with the CCK-A receptor antagonist, lorglumide (1 μM), antagonized the effects of CCK-8s, whereas perfusion with the CCK-B preferring agonist CCK-8 nonsulfated (CCK-ns, 1 μM) did not affect the frequency of sEPSCs. Similarly, pretreatment with the CCK-B receptor antagonist, triglumide (1 μM), did not prevent the actions of CCK-8s. Although the majority (i.e., 76%) of CCK-8s unresponsive cNTS neurons had a bipolar somata shape and were TH-IR negative, no differences were found in either the morphological or the neurochemical phenotype of cNTS neurons responsive to CCK-8s. Our results suggest that the excitatory effects of CCK-8s on terminals impinging on a subpopulation of cNTS neurons are mediated by CCK-A receptors; these responsive neurons, however, do not have morphological or neurochemical characteristics that automatically distinguish them from nonresponsive neurons.
INTRODUCTION
Vagal sensory afferent fibers enter the brain stem by the tractus solitarius and terminate in a viscerotopically organized manner in the subnuclei of the nucleus tractus solitarius (NTS) (Altschuler et al. 1989; Barraco et al. 1992; Broussard and Altschuler 2000; Rogers and McCann 1993; Rogers et al. 1999). The same NTS subnucleus may receive information from gastrointestinal, cardiovascular, and/or respiratory areas (Barraco et al. 1992). The subnucleus centralis (cNTS), which is located adjacent to the tractus solitarius, however, receives inputs from vagal afferent fibers originating almost exclusively from the esophagus (Altschuler et al. 1989; Broussard and Altschuler 2000; Jean 2001; Rogers et al. 1999), thus making it an excellent model for the study of gastrointestinal second order neurons.
We have shown that esophageal distension increases the firing rate of neurons in the cNTS and induces gastric relaxation (Rogers et al. 1999). More recently, we have reported that esophageal distention induces cFos activation in the majority of cNTS neurons that express tyrosine-hydroxylase immuno-reactivity (TH-IR) (Rogers et al. 2003). A vast array of studies have shown that sensory vagal information to NTS neurons is excitatory and uses, principally, excitatory amino acids such as glutamate (Andresen and Yang 1990; Glatzer et al. 2003; Jean 2001; Kawai and Senba 1996; Lachamp et al. 2003; Lu and Bieger 1998; Smith et al. 1998; Yen et al. 1999).
Cholecystokinin (CCK) is released from small intestinal enteroendocrine cells in response to nutrients (Liddle 1994; Moran et al. 2001; Ritter 2004). CCK then, mainly by CCK-A but possibly also by CCK-B receptors, induces multiple effects along the gastrointestinal tract, including gastric relaxation, as well as reduction of food intake (Moran and Kinzig 2004; Raybould and Tache 1998; Reidelberger 1994; Ritter 2004; Woods 2004). A vast amount of literature supports the possibility that CCK acts in a paracrine manner on vagal sensory neurons (Berthoud and Patterson 1996; Blackshaw and Grundy 1990; Davison and Clarke 1988; Raybould and Tache 1988). Physiological and pharmacological data, however, also support the effects of CCK on sites other than peripheral vagal afferent terminals, including the nodose ganglion itself and the dorsal vagal complex (DVC, i.e., the dorsal motor nucleus of the vagus, DMV, the NTS, and area postrema) (Blevins et al. 2000; Mercer and Beart 1997; Reidelberger et al. 2004; Rinaman et al. 1995; Simasko and Ritter 2003; Zheng et al. 2005).
In an electrophysiological study of DVC neurons, Branchereau et al. (1992, 1993) reported that application of CCK induced either an excitation, an inhibition, or a biphasic excitatory-inhibitory effect. These authors also concluded that the effects of CCK-8s were determined by activation of both CCK-A and CCK-B receptors, although their recordings did not distinguish between NTS and DMV neurons.
We have reported recently that CCK-8s excites subpopulations of DMV neurons by an action mediated by CCK-A receptors (Zheng et al. 2005). In the same study we showed that the effects of CCK-8s were attenuated by pretreatment with the synaptic transmission blocker tetrodotoxin (TTX), suggesting that synaptic inputs onto DMV neurons or onto neurons, such as NTS cells, projecting to DMV could also be excited by CCK-8s.
The aims of this work were to 1) characterize the pharmacological response to exogenous CCK-8s of terminals impinging on cNTS neurons and 2) characterize the cNTS neurons responsive and unresponsive to CCK with respect to their morphological and TH-IR neurochemical properties.
METHODS
Research reported in the present manuscript conforms fully to National Institute of Health guidelines and was approved by the Pennington Biomedical Research Center–LSU System Animal Care and Use Committee.
Electrophysiology
The method of slicing the brain stem has already been described (Travagli et al. 1991). Briefly, 25- to 35-day-old Sprague–Dawley rats of either sex were anesthetized with halothane (abolition of the foot pinch withdrawal reflex) before being killed by severing the blood vessels in the chest. The brain stem was removed and glued to the platform of a vibratome, and three coronal slices (300 μm thick) were cut starting from the posterior area postrema moving rostrally. The slices were stored at least 1 h in oxygenated (95% O2-5% CO2) Krebs’ solution (see Composition of solutions) at 30°C before use. A single slice was then transferred to a perfusion chamber (volume 500 μl; Michigan Precision Instruments, Parma, MI), kept in place with a nylon mesh and maintained at 35 ± 1°C by perfusion with warmed Krebs’ solution at a rate of 2.5–3.0 ml min−1.
Whole cell recordings were conducted on putative cNTS neurons identified according to their location in close proximity (within 100 μm) to the tractus solitarius at a level encompassing the midrostral area postrema and the portion of NTS up to approximately 0.5 mm rostral of the anterior portion of the area postrema itself. Recordings were made with patch pipettes (resistance 6–8 MΩ) filled with a potassium or cesium gluconate solution (see Composition of solutions) by using an Axoclamp 2B amplifier (Axon Instruments, Union City, CA). Data were sampled at 10 kHz and filtered at 2 kHz, digitized by a Digidata 1200C interface (Axon Instruments), acquired, stored, and analyzed on an IBM PC using pClamp 8 software (Axon Instruments) and Mini Analysis software (Jaejin Software, Leonia, NJ). Recordings were accepted only if the series resistance was <15 MΩ. In addition, the action potential evoked after injection of depolarizing current must have had amplitude of ≥50 mV and the membrane potential had to return to the baseline value after the action potential afterhyperpolarization (AHP).
Drugs were applied to the bath by a series of manually operated valves at concentrations shown previously to be effective. Neurons were allowed to recover fully between additions of agonists (minimum washout period of 10 min). Antagonists were superfused for ≥5 min before reapplication of the agonist. Each neuron served as its own control when assessing the effects of antagonists, i.e., in each neuron the effect of any drug was assessed before and after antagonist. Cells were classified as CCK-8s responsive if perfusion with 100 nM CCK-8s increased the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) by a minimum of 50% above baseline. The concentration–response curve for CCK-8s was constructed from neurons in which at least three concentrations were tested.
At the end of recording, Neurobiotin (2.5% wt/vol) was injected into the neuron (0.3-nA depolarizing pulse, 600-ms duration, every 2 s) for 15–20 min to permit postfixation reconstruction. Slices were then immersed in Zamboni's fixative (see Composition of solutions) and stored at 4°C until analyzed.
Immunohistochemistry
The slice was cleared of fixative by washing it repeatedly in phosphate-buffered saline (PBS) before incubation for 18–24 h at 4°C in Phosphate Buffer Solution–Triton-X bovine serum albumin (PBSTX-BSA; see Composition of solutions) containing mouse anti-tyrosine hydroxylase (1:1,000). Slices were then washed in PBS-TX and incubated for 2 h at 37°C with PBS-TX-BSA containing secondary antibodies (goat anti-mouse conjugated with Alexa 488–FITC 1:500, TH staining, and streptavidin–Texas Red 1:100 to visualize the neurobiotin-filled neuron). The slices were then mounted in Fluoromount-G (Southern Biotechnology Associates, Birmingham, AL) to reduce fading and analyzed for immunofluorescence using a Zeiss 510 confocal scanning laser microscope equipped with a Kr/Ar-ion laser with filters for the selective visualization of Texas Red and FITC.
Morphological reconstructions
Slices were cleared of fixative in PBS-TX and kept at 4°C until the injected neurobiotin was visualized using a cobalt–nickel enhancement of the Avidin D–horseradish peroxidase (Avidin D–HRP) technique as described previously (Browning et al. 1999; Martinez-Pena y Valenzuela et al. 2004). Briefly, slices were incubated in Avidin D–HRP solution (see Composition of solutions) for 2 h. After a 15-min rinse in PBS and subsequent incubation for 15–20 min in Avidin D–HRP and DAB solutions (see Composition of solutions), the slice was incubated for an additional 15 min in the presence of 3% H2O2. The slice was then rinsed in PBS, placed on a gelatin-coated coverslip, air dried, cleared in alcohol and xylene, and mounted in Permount.
Three-dimensional reconstructions of individual neurobiotin-labeled neurons, digitized at a final magnification of ×600, were made using Neurolucida software (Microbrightfield, Williston, VT). Each reconstruction was verified using the software for “mathematical completeness.” The optical and physical compression of the slice that may occur is corrected by rescaling the section to 300 μm (the original thickness at the time of sectioning).
The morphological features that were assessed include: soma area and diameter, form factor (a measure of circularity for which a value of 1 indicates a perfect circle and 0 indicates a line; form factor = 4πa × 1/p2, where a is the soma area and p is the perimeter of the soma in the horizontal plane), whether the cell has bipolar or multipolar somata, number of segments (i.e., branching of dendrites), branch order, and extension in the x- and y-axes. Data analysis was performed as described previously (Browning et al. 1999; Martinez-Pena y Valenzuela et al. 2004).
Composition of solutions
Krebs’ (in mM): NaCl 126, NaHCO3 25, KCl 2.5, MgCl2 1.2, CaCl2 2.4, NaH2PO4, 1.2 and dextrose 11, maintained at pH 7.4 by bubbling with 95% O2 and 5% CO2.
Intracellular solution (in mM): K gluconate 128, KCl 10, CaCl2 0.3, MgCl2 1, Hepes 10, EGTA 1, ATP 2, GTP 0.25. Adjusted to pH 7.35 with KOH.
Intracellular solution (in mM): Cs gluconate 140, NaCl 10, CaCl2 0.3, Hepes 10, EGTA 1, ATP 2, GTP 0.25. Adjusted to pH 7.35 with NaOH.
Zamboni's fixative (in mM): 1.6% (wt/vol) paraformaldehyde, KH2PO4 19, and Na2HPO4 · 7H2O 100, in 240 ml saturated picric acid–1,600 ml H2O; adjusted to pH 7.4 with HCl.
PBS-TX (in mM): NaCl 115, Na2HPO4 · 7H2O 75, KH2PO4 7.5, and 0.3% Triton X-100.
Avidin D–HRP solution: 0.002% Avidin D–HRP in PBS containing 1% Triton X-100; 0.05% DAB in PBS containing 0.5% gelatin supplemented with 0.025% CoCl2 and 0.02% NiNH4SO4.
Drugs and chemicals
Tyrosine hydroxylase antibodies were purchased from Immunostar (Hudson, WI); Alexa conjugated secondary antibodies were purchased from Molecular Probes (Eugene, OR); neurobiotin was purchased from Vector Labs (Burlingame, CA). All other drugs were purchased from Sigma (St. Louis, MO). Stock solutions were freshly prepared and diluted to the final concentration in Krebs’ solution just before use.
Statistical analysis
Results are expressed as means ± SE with significance defined as P < 0.05. Results were compared before and after drug administration, with each neuron serving as its own control (Student's paired t-test). Intergroup comparisons were conducted using the Student's grouped t-test or the χ2 test.
RESULTS
We conducted electrophysiological studies on 187 neurons of the cNTS; in 19 of these neurons we obtained complete morphological reconstructions, whereas in a further 96 neurons we were able to obtain the reconstruction only of the somata shape. We also analyzed the neurochemical phenotype of 54 of the recorded cNTS neurons.
CCK increases the frequency of EPSC by CCK-A receptors
When recorded at a holding potential of −50 mV, sEPSC had a frequency of 1.5 ± 0.16 events s−1 and 27.2 ± 1.26 pA amplitude (n = 173). These events were abolished by perfusion with 1 mM kynurenic acid (n = 8), suggesting they were mediated by glutamate.
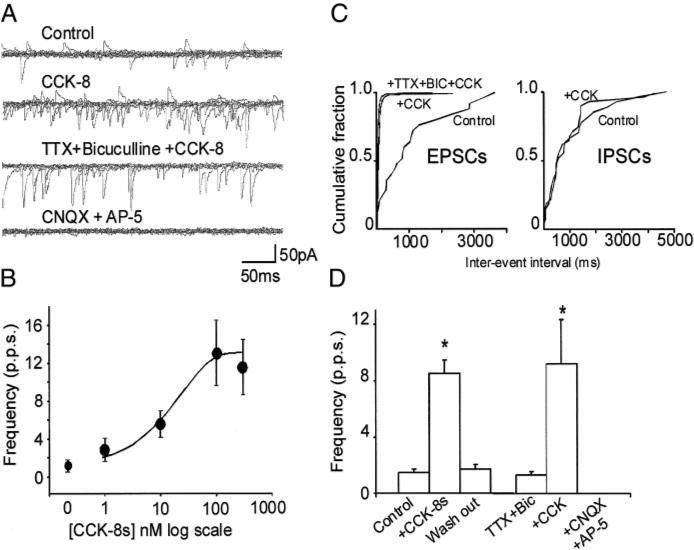
Perfusion with CCK-8s induced a concentration-dependent increase in the frequency of sEPSCs (Fig. 1). For example, 100 nM CCK-8s increased both the frequency and amplitude of sEPSC in 78 of the 173 cells tested (i.e., 45%), leaving unaffected the remaining 95 neurons. In detail, perfusion with 100 nM CCK-8s increased the frequency of sEPSC from 1.5 ± 0.21 events s−1 in Krebs’ to 8.5 ± 0.91 events s−1 after 2 min of perfusion with CCK-8s (P < 0.05); similarly, the amplitude of sEPSC was 27.2 ± 1.15 and 35.4 ± 1.61 pA in control and CCK-8s, respectively (P < 0.05). Both frequency and amplitude of sEPSC returned to baseline values within 5 min of washout (Fig. 1).
FIG. 1.
A: representative traces showing spontaneous excitatory postsynaptic currents (EPSCs) in control conditions (top); perfusion with 100 nM of sulfated cholecystokinin (CCK-8s) increased the frequency of spontaneous EPSCs but did not alter the frequency of spontaneous inhibitory postsynaptic current (IPSC) (middle); the effects of CCK-8s were still present after tetrodotoxin (TTX; 1 μM) and bicuculline (50 μM) pretreatment (middle, low) but not CNQX (10 μM) or AP-5 (30 μM) (bottom). B: concentration–response curve for the CCK-8s–induced increase in spontaneous EPSC (n = 5 for each data point). C: computer-generated graphics from the same neuron as in A showing that CCK-8s increased the frequency of EPSCs but not IPSCs. D: graphics summarizing the effects of 100 nM CCK-8s on frequency of spontaneous (left) and miniature (right) EPSCs. *P < 0.05 vs. control.
In cells unresponsive to CCK-8s, the frequency of sEPSC was 1.5 ± 0.23 and 1.4 ± 0.21 events s−1 in control and 100 nM CCK-8s, respectively (P > 0.05); similarly the amplitude of the sEPSC was 27.2 ± 2.09 and 25.6 ± 1.44 pA in control and CCK-8s, respectively (n = 89; P > 0.05).
Perfusion with CCK-8s increased both the frequency (thus denoting a possible presynaptic site of action) and the amplitude (in conditions of synaptic blockade, this observation is indicative of a postsynaptic site of action) of sEPSC. We conducted a series of experiments in which the postsynaptic component of the CCK-8s action (Appleyard et al. 2005; Branchereau et al. 1992, 1993) was prevented by using an intracellular solution in which K-gluconate was substituted with the nonselective gK blocker, Cs-gluconate. Six of the 14 cells tested responded to 100 nM CCK-8s with an increase of sEPSC frequency from 1.2 ± 0.62 to 10.7 ± 4.18 events s−1 (P < 0.05); in these cells, the amplitude of the sEPSC was 33.7 ± 2.26 and 45.4 ± 6.51 pA, in control and CCK-8s, respectively (P > 0.05). The eight remaining cells were unresponsive to CCK-8s. These data suggest a presynaptic site of action of CCK-8s.
We also analyzed the effects of CCK-8s in the presence of TTX, which blocks action potential–dependent transmitter release. In five cells, 100 nM CCK-8s increased the frequency and amplitude of the events from 1.8 ± 0.87 to 9.2 ± 3.06 events s−1 and from 28.6 ± 1.53 to 40.5 ± 5.30 pA, in control and CCK-8s, respectively (P < 0.05 for both). After a 5-min perfusion with TTX (0.3–1 μM), subsequent perfusion with 100 nM CCK-8s in the presence of TTX increased the frequency of miniature EPSC (mEPSC) from 1.7 ± 1.11 to 3.5 ± 1.08 events s−1 (P < 0.05 vs. TTX alone; P > 0.05 vs. CCK-8s alone). In these cells, however, the amplitude of the mEPSCs was not altered by perfusion with CCK-8s (29.8 ± 2.15 and 32.6 ± 3.60 pA in TTX alone and in TTX + CCK-8s, respectively (P > 0.05). These data suggest that the presynaptic site of action of CCK-8s is directed on terminals apposing cNTS neurons.
In some cNTS neurons, we observed the presence of spontaneous inhibitory postsynaptic currents (IPSCs) that were sensitive to bicuculline. In four cells, perfusion with 100 nM CCK-8s increased the frequency of sEPSC from 3.6 ± 2.37 to 18.4 ± 3.82 events s−1 (P < 0.05) but did not alter the frequency or amplitude of sIPSC (1 ± 0.18 and 0.8 ± 0.09 events s−1 and 33 ± 8.24 and 36 ± 12.21 pA in control and CCK-8s, respectively; P > 0.05; Vhold = −35 mV).
In a further six neurons, perfusion with 100 nM CCK-8s increased the frequency of sEPSC from 1.4 ± 0.20 to 13.3 ± 3.9 events s−1 (P < 0.05). Similarly, after 10 min perfusion with 1 μM TTX and/or 50 μM bicuculline, perfusion with CCK-8s increased the frequency of mEPSC from 1.3 ± 0.27 to 9.2 ± 3.15 events s−1 (P < 0.05). Subsequent perfusion of these cells with 10 μM CNQX and 30 μM AP-5 completely abolished mEPSC. These data suggest that the effects of CCK-8s involve an increase in glutamatergic but not GABAergic synaptic transmission.
The response to CCK-8s did not show tachyphylaxis. In four cells, perfusion with 100 nM CCK-8s increased the frequency of sEPSC from 1.7 ± 1.08 to 9.4 ± 3.28 events s−1, in control and CCK-8s, respectively (P < 0.05); after a 5-min washout, a second administration of 100 nM CCK-8s increased the frequency of sEPSC from 2.3 ± 1.5 to 10.1 ± 5.15 events s−1 (P < 0.05 vs. control; P > 0.05 vs. the first administration of CCK-8s).
In six cells that responded to 100 nM CCK-8s with an increase in sEPSC frequency from 1.6 ± 0.43 to 12.6 ± 3.02 events s−1 in control and CCK-8s, respectively (P < 0.05), a 5-min perfusion with the selective CCK-A receptor antagonist, lorglumide (1 μM), which per se did not have any effect on the frequency of sEPSC, antagonized the effects of CCK-8s. The frequency of sEPSC was 1.7 ± 0.63 and 1.7 ± 0.69 events s−1 in the presence of lorglumide alone and lorglumide + CCK-8s, respectively (P > 0.05; Fig. 2). Conversely, pretreatment with the selective CCK-B receptor antagonist, triglumide (1 μM), failed to antagonize the CCK-8s–induced increase in sEPSC frequency. In five cells that responded to CCK-8s with an increase in sEPSC frequency from 1.7 ± 0.50 to 11.9 ± 3.62 events s−1, in control and CCK-8s, respectively (P < 0.05), in the presence of triglumide, CCK-8s increased sEPSC frequency from 1.8 ± 0.62 to 8.10 ± 2.25 events s−1 in triglumide alone and triglumide + CCK-8s, respectively (P < 0.05 vs. triglumide alone; P > 0.05 vs. CCK-8s alone; Fig. 2). These data suggest that the excitatory effects of CCK-8s are mediated by CCK-A, but not CCK-B, receptors.
FIG. 2.
A: representative traces showing spontaneous EPSCs in control conditions (left); after 10-min perfusion with the CCK-B receptor antagonist, triglumide (1 μM), perfusion with 100 nM CCK-8s still increased the frequency of spontaneous EPSCs (middle); the effects of CCK-8s, however, were antagonized by 10-min perfusion with the CCK-A receptor antagonist, lorglumide (1 μM; right). Traces are from the same nucleus tractus solitarius subnucleus centralis (cNTS) neuron. Holding potential = −50 mV. B: graphics summarizing the effects of 100 nM CCK-8s on frequency of spontaneous EPSCs in the presence of the selective CCK-A (right) or CCK-B (left) receptor antagonists. *P < 0.05 vs. control.
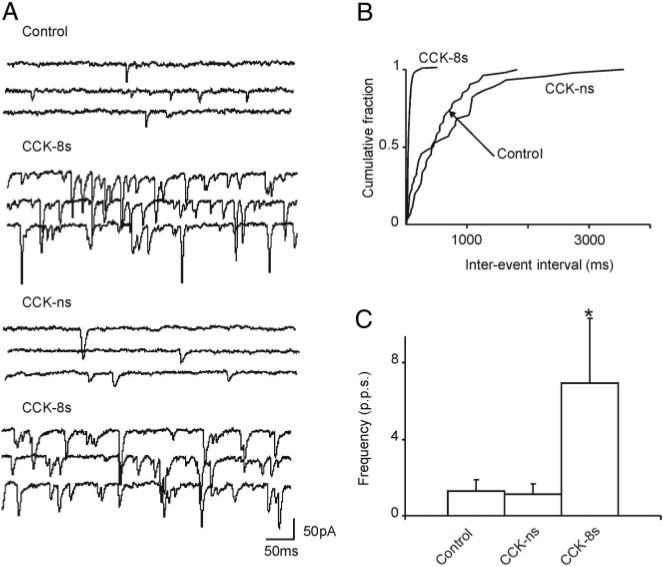
The involvement of CCK-A receptors only in the CCK-8s–mediated increase of sEPSC frequency was further supported by experiments conducted with the CCK-B preferring agonist non–CCK-ns. In three cells in which CCK-8s increased the frequency of sEPSC from 1.3 ± 0.6 to 6.9 ± 3.39 events s−1, perfusion with 1 μM CCK-ns had no effect on the sEPSC frequency (1.3 ± 0.6 to 1.15 ± 0.5 events s−1 in control and CCK-ns, respectively) (Fig. 3).
FIG. 3.
A: representative traces showing spontaneous EPSCs in control conditions, perfusion with 100 nM CCK-8s increased the frequency of spontaneous EPSCs; after a 10-min washout of CCK-8s, perfusion with the CCK-B preferring agonist, nonsulfated cholecystokinin (CCK-ns; 1 μM) did not increase the frequency of spontaneous EPSCs; however, perfusion with 100 nM CCK-8s immediately after CCK-ns still increased the frequency of spontaneous EPSCs. Holding potential = −50 mV. B: computer-generated graphics from the same neuron as in A showing that CCK-8s, but not CCK-ns, increased the frequency of spontaneous EPSCs. C: graphics summarizing the effects of 100 nM CCK-8s and 1 μM CCK-ns on frequency of spontaneous EPSCs. *P < 0.05 vs. control.
Morphological and neurochemical characteristics of cNTS neurons responsive or unresponsive to CCK-8s
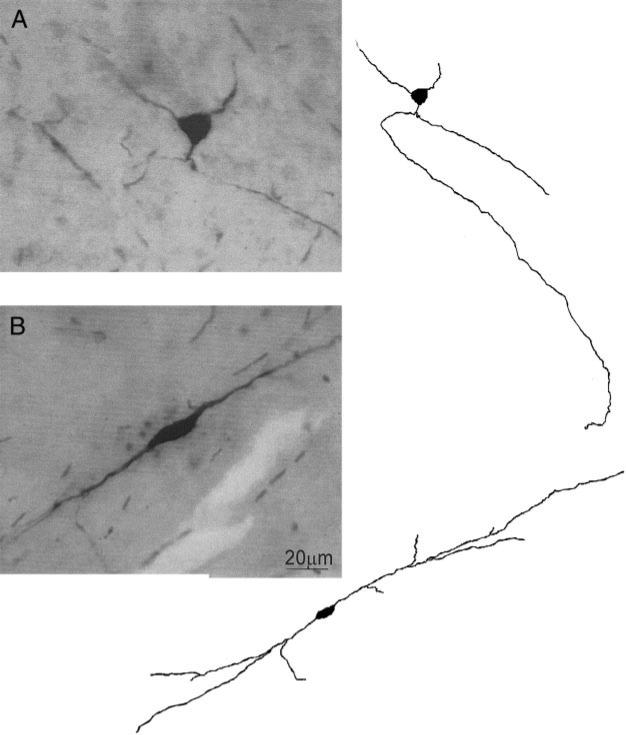
Thirty-seven of the 96 cNTS neurons (39%) in which we were able to reconstruct the somata shape responded to CCK-8s with an increase in sEPSC frequency, 16 of those 37 neurons (43%) had a multipolar soma shape, and the other 21 neurons (57%) had a bipolar soma shape. Forty-five of the 59 cNTS neurons (76%) unresponsive to CCK-8s had a bipolar soma shape; the remaining 14 (24%) were instead multipolar (P < 0.05 vs. responsive neurons, χ2 test) (Fig. 4).
FIG. 4.
A, left: micrograph of a cNTS neuron with multipolar somata shape; right: computer-aided reconstruction of the neuron on left. B, left: micrograph of a cNTS neuron with bipolar somata shape; right: computer-aided reconstruction of the neuron on left.
In the 19 cells in which we were able to conduct a complete morphological characterization, the morphological characteristics of cNTS neurons did not differ, apart from the number of segments, between cNTS neurons responsive (n = 8) or unresponsive (n = 11) to CCK-8s. The soma area was 126.5 ± 22.23 and 109.0 ± 13.73 μm2 (P > 0.05), the soma diameter was 18.6 ± 1.02 and 15.8 ± 0.99 μm (P > 0.05), the soma form factor was 0.66 ± 0.03 and 0.72 ± 0.03 (P > 0.05), the number of segments was 6.1 ± 0.44 and 4.7 ± 0.44 (P < 0.05), and the segment length was 117.1 ± 21.95 and 101.6 ± 13.99 μm (P > 0.05) in neurons responsive and nonresponsive to CCK-8s, respectively.
Fifteen of the 54 cNTS neurons (26%) in which we were able to conduct both a pharmacological and neurochemical characterization were TH-IR positive; the remaining 39 neurons (74%) were, instead, TH-IR negative. Of the 15 neurons responsive to CCK-8s in which we were also able to conduct an analysis of TH-IR, seven of them (47%) were TH-IR positive and eight (53%) were TH-IR negative. Conversely, 32 (82%) of the neurons nonresponsive to perfusion with 100 nM CCK-8s were TH-IR negative and only seven (18%) were TH-IR positive (P < 0.05 χ2 test; Fig. 5).
FIG. 5.
A: schematic diagram depicting the localization of cNTS neurons according to their soma shape (bipolar = square; multipolar = circle) and their response to perfusion with 100 nM CCK-8s (responsive to CCK-8s = white symbol; nonresponsive to CCK-8s = black symbol). For the purpose of intelligibility, not all the neurons have been reported in the graphic. TS, tractus solitarius; AP, area postrema; DMV, dorsal motor nucleus of the vagus; cc, central canal; IV Ventr., fourth ventricle. B: micrographs showing a postrecording immunohistochemical detection in a tyrosine-hydroxylase immunoreactive (TH-IR) positive (left) and in a TH-IR negative (right) cNTS neuron. Scale bar = 10 μm.
DISCUSSION
In the present report we have shown that: 1) perfusion with CCK-8s increases the frequency of glutamatergic spontaneous and miniature EPSCs in 45% of cNTS neurons; 2) perfusion with CCK-8s does not affect the frequency of sIPSC; 3) the CCK-8s effects on EPSCs are mediated by CCK-A receptors only; and, 4) cNTS neurons responsive to CCK-8s do not have unique morphological or immunocytochemical (i.e., TH-IR) characteristics that distinguish them from neurons that are unresponsive to CCK-8s, although the unresponsive neurons are more likely to have a bipolar soma shape and to be TH-IR negative.
Our data also suggest that functional CCK-A, but not CCK-B, receptors, are present on a subpopulation of fibers impinging on cNTS neurons. It is possible that by modulating the glutamatergic inputs onto NTS these CCK-A receptors contribute to the physiological role of CCK in the CNS. The response threshold of sEPSC to CCK-8s was 1 nM, a concentration close to that of gut-derived CCK, raising the possibility that CCK acting on NTS neurons is released postprandially, although not excluding the possibility that some other sources, such as local NTS neurons or projections from hypothalamus or amygdala, are involved.
The processing of autonomic sensory signals entering the CNS first occurs at the level of the synapse between the vagal afferent fibers and neurons of the NTS. As is the case for other NTS subnuclei (Andresen and Yang 1990; Glatzer et al. 2003; Kawai and Senba 1996; Lachamp et al. 2003; Lu and Bieger 1998; Smith et al. 1998; Yen et al. 1999). cNTS neurons also receive kynurenic-sensitive glutamatergic inputs from vagal afferent fibers averaging 1.5 ± 0.16 excitatory events s−1 (Baptista et al. 2005). These excitatory events are modulated by perfusion with CCK-8s that, by activation of CCK-A, but not CCK-B receptors, increases in a concentration-dependent manner the frequency of the spontaneous EPSCs on a subgroup of cNTS neurons.
Our evidence is the following. Perfusion with 100 nM CCK-8s significantly increased the frequency (thus denoting a possible presynaptic site of action) as well as the amplitude (in conditions of synaptic blockade, this observation is indicative of a postsynaptic site of action) of sEPSC in nearly 50% of the cNTS neurons. Support for the presynaptic site of action of the octapeptide is provided by our data showing that perfusion with CCK-8s also increased the frequency of sEPSC when the recordings were conducted with intracellular solutions containing cesium, which blocks the potassium currents affected by CCK-8s on postsynaptic membranes of brain stem vagal neurons (Branchereau et al. 1993; Zheng et al. 2005). These data, however, do not clarify whether CCK-8s affected glutamatergic terminals apposing directly the cNTS neuron or acted at other remote sites and were recorded on the cNTS neuron because of the intact glutamatergic circuitry in the slice. To partially circumvent the latter possibility, we conducted a series of experiments in the presence of TTX. After pretreatment with TTX, perfusion with CCK-8s still increased the frequency of mEPSCs. Some cNTS neurons also displayed the presence of bicuculline-sensitive GABAergic spontaneous currents; these sIPSC, however, were not affected by perfusion with CCK-8s. These data are strongly suggestive of a presynaptic effect of CCK-8s on glutamatergic terminals, some of which are very likely to be of vagal origin, apposing cNTS neurons directly, without interposed local interneurons. This is not meant to categorically exclude the possibility that CCK-8s is exciting sites remote to the recorded neuron but rather to support our interpretation (see following text) that CCK-8s mechanisms of action also involve vagal afferent terminals within the brain stem.
The presynaptic effects of CCK-8s were mediated by activation of CCK-A receptors only. In fact, pretreatment with the selective CCK-A receptor antagonist lorglumide prevented the increase in spontaneous EPSCs induced by 100 nM CCK-8s. Conversely, pretreatment with the selective CCK-B receptor antagonist triglumide was ineffective in attenuating the excitatory effects of CCK-8s. The lack of involvement of CCK-B receptors was further supported by the data showing that the CCK-B–preferring agonist CCK-ns, even when used at concentrations as high as 1 μM, was ineffective in modulating spontaneous EPSCs whose frequency was increased by CCK-8s.
Our data demonstrating that the effects of CCK-8s were mediated by CCK-A receptors are in agreement with results showing that, overall, the satiety and gastrointestinal effects of CCK-8s are mediated by CCK-A receptors (Glatzle et al. 2001; Lloyd et al. 1992; Moran and Kinzig 2004; Reidelberger 1994; Reidelberger et al. 2004). Of particular relevance are the data showing that CCK-A receptors are present in the NTS (Hill et al. 1987; Mercer and Beart 1997) and that the CCK-mediated effects at the level of the brain stem are mediated by activation of CCK-A receptors (Appleyard et al. 2005; Blevins et al. 2000; Glatzle et al. 2001; Reidelberger et al. 2004; Zheng et al. 2004).
In a recent report, Appleyard et al. (2005) used a POMC-GFP transgenic mouse to analyze the effects of CCK-8s on identified NTS cells. In their paper, using immunohistochemical and electrophysiological approaches, these authors showed that perfusion with CCK-8s excites all the tested POMC-positive NTS neurons by an action on CCK-A receptors. These data suggest that in the mouse caudal NTS, CCK-8s excites neurons most likely to be involved in bodily homeostasis. However, only a small population of neurons in the subnucleus commissuralis of the NTS express POMC, and POMC-positive cells are not present in the cNTS (Appleyard et al. 2005; Bronstein et al. 1992; Joseph et al. 1985; Palkovits et al. 1987). Conversely, cNTS neurons constitute two main neurochemical phenotypes: an outer shell of neurons that are TH-IR positive and an inner core of NOS-IR positive neurons (Rogers et al. 2003). Our recent data show that esophageal distention activates preferentially TH-IR positive cNTS neurons (Rogers et al. 2003), suggesting the possible presence of distinct subpopulations of cNTS neurons. It was thus somehow surprising that the subgroup of cNTS cells excited by CCK-8 does not appear to have distinctive morphological or neurochemical characteristics. We have observed, however, that neurons unresponsive to CCK-8s were more likely to have a bipolar somata shape and to be TH-IR negative. These data would suggest that esophageal distention activates a subpopulations of cNTS neurons that is neurochemically more homogeneous than that activated by CCK-8s.
The postprandial plasma concentration of CCK is in the picomolar range (Soudah et al. 1992) and a decrease in gastric motility was observed after intravenous injection of 10 pmol CCK-8s (in a 250-g rat, this would be approximately equivalent to a 7-nM concentration) (Takahashi and Owyang 1999). A threshold of 1 nM, as reported in the present manuscript, would give physiological, rather than pharmacological, relevance to our data. In fact, it is well known that in in vitro preparations there is a shift to the right of the concentration dependency to agonist by one or more orders of magnitude when compared with in in vivo preparations (Fatt and Katz 1952). Indeed, the most commonly used concentrations of CCK-8s in in vitro experiments are ≥100 nM (Appleyard et al. 2005; Branchereau et al. 1992, 1993; Plata-Salaman et al. 1988; Simasko and Ritter 2003). Furthermore, experiments conducted in slices would undoubtedly damage some afferent fibers, rendering them less responsive to modulatory agents.
Despite the potential physiological importance of our data, it is not clear how CCK would reach the brain stem to exert its actions because CCK does not readily cross the blood brain barrier. The dorsal vagal complex (DVC; i.e., NTS + DMV), however, has a rather porous blood brain barrier (Cottrell and Ferguson 2004) that allows the passage of molecules as large as, among others, TNFα or pancreatic polypeptides (Cox and Randich 2004; Emch et al. 2002; Rogers et al. 1995). Functional evidence that CCK crosses the “leaky ” blood brain barrier to affect vagal neurons has also been provided by several authors (Hommer et al. 1985; Reidelberger et al. 2004; Zheng et al. 2005). In fact, lesions of vagal pathways did not prevent the effects of systemic CCK administration on the NTS-nigral circuit, which was, instead, interrupted by lesion of medullary nuclei (Hommer et al. 1985). Also, the short-term satiety effects of CCK are antagonized by devazepide, a selective CCK-A receptor antagonist that crosses the blood brain barrier, but not by A70104, a selective CCK-A antagonist that does not cross the blood brain barrier (Reidelberger et al. 2004), and, finally, intraperitoneal CCK injection at low concentrations phosphorylates (i.e., activates) CCK-A receptors in the dorsal vagal complex (Zheng et al. 2005).
It is also possible that the effects of CCK on brain stem nuclei are determined by CCK acting as a local neurotransmitter or neuromodulator. Indeed, CCK-containing neural projections originating from both the NTS itself or, likely, the hypothalamus or amygdala are present throughout the rostrocaudal extent of the vagal complex (Kubota et al. 1986).
In summary, although the source of CCK in NTS has not been elucidated, our results support a possible physiologically relevant role to CCK as a neurotransmitter or neuromodulator, acting on CCK-A receptors, to indirectly excite subpopulations of cNTS neurons. When combined, these data seem to support the hypothesis that the actions of CCK-8s cannot be ascribed exclusively to activation of vagal fibers in the periphery, and a CNS–brain stem component should be considered.
ACKNOWLEDGMENTS
We thank Drs. Browning and Berthoud for comments on previous versions of the manuscript. We also thank C. M. Travagli for support and encouragement.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-55530 and DK-56373.
REFERENCES
- Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989;283:248–268. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol Heart Circ Physiol. 1990;259:H1307–H1311. doi: 10.1152/ajpheart.1990.259.4.H1307. [DOI] [PubMed] [Google Scholar]
- Appleyard SM, Bailey TW, Doyle MW, Jin YH, Smart JL, Low MJ, Andresen MC. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J Neurosci. 2005;25:3578–3585. doi: 10.1523/JNEUROSCI.4177-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista V, Zheng ZL, Coleman FH, Rogers RC, Travagli RA. Characterization of neurons of the nucleus tractus solitarius pars centralis. Brain Res. 2005;1052:139–146. doi: 10.1016/j.brainres.2005.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraco R, El-Ridi M, Parizon M, Bradley D. An atlas of the rat subpostremal nucleus tractus solitarius. Brain Res Bull. 1992;29:703–765. doi: 10.1016/0361-9230(92)90143-l. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM. Anatomical relationship between vagal afferent fibers and CCK-immunoreactive entero-endocrine cells in the rat small intestinal mucosa. Acta Anat (Basel) 1996;156:123–131. doi: 10.1159/000147837. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. J Auton Nerv Syst. 1990;31:191–202. doi: 10.1016/0165-1838(90)90185-l. [DOI] [PubMed] [Google Scholar]
- Blevins JE, Stanley BG, Reidelberger RD. Brain regions where cholecystokinin suppresses feeding in rats. Brain Res. 2000;860:1–10. doi: 10.1016/s0006-8993(99)02477-4. [DOI] [PubMed] [Google Scholar]
- Branchereau P, Bohme GA, Champagnat J, Morin-Surun M-P, Durieux C, Blanchard J-C, Roques BP, Denavit-Saubie M. CholecystokininA and cholecystokininB receptors in neurons of the brainstem solitary complex of the rat: pharmacological identification. J Pharmacol Exp Ther. 1992;260:1433–1440. [PubMed] [Google Scholar]
- Branchereau P, Champagnat J, Denavit-Saubie M. Cholecystokiningated currents in neurons of the rat solitary complex in vitro. J Neurophysiol. 1993;70:2584–2595. doi: 10.1152/jn.1993.70.6.2584. [DOI] [PubMed] [Google Scholar]
- Bronstein DM, Schafer MK, Watson SJ, Akil H. Evidence that beta-endorphin is synthesized in cells in the nucleus tractus solitarius: detection of POMC mRNA. Brain Res. 1992;587:269–275. doi: 10.1016/0006-8993(92)91007-2. [DOI] [PubMed] [Google Scholar]
- Broussard DL, Altschuler SM. Brainstem viscerotopic organization of afferent and efferents involved in the control of swallowing. Am J Med. 2000;108:79S–86S. doi: 10.1016/s0002-9343(99)00343-5. [DOI] [PubMed] [Google Scholar]
- Browning KN, Renehan WE, Travagli RA. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. J Physiol. 1999;517:521–532. doi: 10.1111/j.1469-7793.1999.0521t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell GT, Ferguson AV. Sensory circumventricular organs: central roles in integrated autonomic regulation. Regul Pept. 2004;117:11–23. doi: 10.1016/j.regpep.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Cox JE, Randich A. Enhancement of feeding suppression by PYY(3–36) in rats with area postrema ablations. Peptides. 2004;25:985–989. doi: 10.1016/j.peptides.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Davison JS, Clarke GD. Mechanical properties and sensitivity to CCK of vagal gastric slowly adapting mechanoreceptors. Am J Physiol Gastrointest Liver Physiol. 1988;255:G55–G61. doi: 10.1152/ajpgi.1988.255.1.G55. [DOI] [PubMed] [Google Scholar]
- Emch GS, Hermann GE, Rogers RC. Tumor necrosis factor-alpha inhibits physiologically identified dorsal motor nucleus neurons in vivo. Brain Res. 2002;951:311–315. doi: 10.1016/s0006-8993(02)03178-5. [DOI] [PubMed] [Google Scholar]
- Fatt P, Katz B. An analysis of the end plate potential recorded with an intracellular electrode. J Physiol. 1952;115:320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatzer NR, Hasney CP, Bhaskaran MD, Smith BN. Synaptic and morphologic properties in vitro of premotor rat nucleus tractus solitarius neurons labeled transneuronally from the stomach. J Comp Neurol. 2003;464:525–539. doi: 10.1002/cne.10831. [DOI] [PubMed] [Google Scholar]
- Glatzle J, Kreis ME, Kawano K, Raybould HE, Zittel TT. Postprandial neuronal activation in the nucleus of the solitary tract is partly mediated by CCK-A receptors. Am J Physiol Regul Integr Comp Physiol. 2001;281:R222–R229. doi: 10.1152/ajpregu.2001.281.1.R222. [DOI] [PubMed] [Google Scholar]
- Hill DR, Campbell NJ, Shaw TM, Woodruff GN. Autoradiographic localization and biochemical characterization of peripheral type CCK receptors in rat CNS using highly selective nonpeptide CCK antagonists. J Neurosci. 1987;7:2967–2976. doi: 10.1523/JNEUROSCI.07-09-02967.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer DW, Palkovits M, Crawley JN, Paul SM, Skirboll LR. Cholecystokinin-induced excitation in the substantia nigra: evidence for peripheral and central components. J Neurosci. 1985;5:1387–1392. doi: 10.1523/JNEUROSCI.05-06-01387.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean A. Brainstem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- Joseph SA, Pilcher WH, Knigge KM. Anatomy of the corticotropin-releasing factor and opiomelanocortin systems of the brain. Fed Proc. 1985;44:100–107. [PubMed] [Google Scholar]
- Kawai Y, Senba E. Organization of excitatory and inhibitory local networks in the caudal nucleus of tractus solitarius of rats revealed in in vitro slice preparation. J Comp Neurol. 1996;373:309–321. doi: 10.1002/(SICI)1096-9861(19960923)373:3<309::AID-CNE1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Takagi H, Morishima Y, Kaway Y, Smith AD. Relationship between catecholaminergic neurons and cholecystokinin-containing neurons in the caudal part of the dorsomedial medulla oblongata of the rat: light and electron microscopic observations by the mirror technique. Brain Res. 1986;370:343–348. doi: 10.1016/0006-8993(86)90491-9. [DOI] [PubMed] [Google Scholar]
- Lachamp P, Balland B, Tell F, Crest M, Kessler JP. Synaptic localization of the glutamate receptor subunit GluR2 in the rat nucleus tractus solitarii. Eur J Neurosci. 2003;17:892–896. doi: 10.1046/j.1460-9568.2003.02494.x. [DOI] [PubMed] [Google Scholar]
- Liddle RA. Regulation of cholecystokinin synthesis and secretion in rat intestine. J Nutr. 1994;124:1308S–1314S. doi: 10.1093/jn/124.suppl_8.1308S. [DOI] [PubMed] [Google Scholar]
- Lloyd KCK, Raybould HE, Walsh JH. Cholecystokinin inhibits gastric acid secretion through type “A” cholecystokinin receptors and somatostatin in rats. Am J Physiol Gastrointest Liver Physiol. 1992;263:G287–G292. doi: 10.1152/ajpgi.1992.263.3.G287. [DOI] [PubMed] [Google Scholar]
- Lu WY, Bieger D. Vagal afferent transmission in the NTS mediating reflex responses of the rat esophagus. Am J Physiol Regul Integr Comp Physiol. 1998;274:R1436–R1445. doi: 10.1152/ajpregu.1998.274.5.R1436. [DOI] [PubMed] [Google Scholar]
- Martinez-Pena y Valenzuela IM, Browning KN, Travagli RA. Morphological differences between planes of section do not influence the electrophysiological properties of identified rat dorsal motor nucleus of the vagus neurons. Brain Res. 2004;1003:54–60. doi: 10.1016/j.brainres.2003.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer LD, Beart PM. Histochemistry in rat brain and spinal cord with an antibody directed at the cholecystokininA receptor. Neurosci Lett. 1997;225:97–100. doi: 10.1016/s0304-3940(97)00197-3. [DOI] [PubMed] [Google Scholar]
- Moran TH, Kinzig KP. Gastrointestinal satiety signals. II. Cholecystokinin. Am J Physiol Gastrointest Liver Physiol. 2004;286:G183–G188. doi: 10.1152/ajpgi.00434.2003. [DOI] [PubMed] [Google Scholar]
- Moran TH, Ladenheim EE, Schwartz GJ. Within-meal gut feedback signaling. Int J Obes Relat Metab Disord. 2001;5:S39–S41. doi: 10.1038/sj.ijo.0801910. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Mezey E, Eskay RL. Pro-opiomelanocortin-derived peptides (ACTH/beta-endorphin/alpha-MSH) in brainstem baroreceptor areas of the rat. Brain Res. 1987;436:323–338. doi: 10.1016/0006-8993(87)91676-3. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR, Fukuda A, Oomura Y, Minami T. Effects of sulphated cholecystokinin octapeptide (CCK-8) on the dorsal motor nucleus of the vagus. Brain Res Bull. 1988;21:839–842. doi: 10.1016/0361-9230(88)90054-8. [DOI] [PubMed] [Google Scholar]
- Raybould HE, Tache Y. Cholecystokinin inhibits gastric motility and emptying via a capsaicin-sensitive vagal pathway in rats. Am J Physiol Gastrointest Liver Physiol. 1988;255:G242–G246. doi: 10.1152/ajpgi.1988.255.2.G242. [DOI] [PubMed] [Google Scholar]
- Reidelberger RD. Cholecystokinin and control of food intake. J Nutr. 1994;124:1327S–1333S. doi: 10.1093/jn/124.suppl_8.1327S. [DOI] [PubMed] [Google Scholar]
- Reidelberger RD, Hernandez J, Fritzsch B, Hulce M. Abdominal vagal mediation of the satiety effects of CCK in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1005–R1012. doi: 10.1152/ajpregu.00646.2003. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Hoffman GE, Dohanics J, Lee WW, Stricker EM, Verbalis JG. Cholecystokinin activates catecholaminergic neurons in the caudal medulla that innervate the paraventricular nucleus of the hypothalamus in rats. J Comp Neurol. 1995;360:246–256. doi: 10.1002/cne.903600204. [DOI] [PubMed] [Google Scholar]
- Ritter RC. Gastrointestinal mechanisms of satiation for food. Physiol Behav. 2004;81:249–273. doi: 10.1016/j.physbeh.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Hermann GE, Travagli RA. Brainstem pathways responsible for oesophageal control of gastric motility and tone in the rat. J Physiol. 1999;514:369–383. doi: 10.1111/j.1469-7793.1999.369ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RC, McCann MJ. Intramedullary connections of the gastric region in the solitary nucleus: a biocytin histochemical tracing study in the rat. J Auton Nerv Syst. 1993;42:119–130. doi: 10.1016/0165-1838(93)90043-t. [DOI] [PubMed] [Google Scholar]
- Rogers RC, McTigue DM, Hermann GE. Vagovagal reflex control of digestion: afferent modulation by neural and “endoneurocrine” factors. Am J Physiol Gastrointest Liver Physiol. 1995;268:G1–G10. doi: 10.1152/ajpgi.1995.268.1.G1. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Travagli RA, Hermann GE. Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. Am J Physiol Regul Integr Comp Physiol. 2003;285:R479–R489. doi: 10.1152/ajpregu.00155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simasko SM, Ritter RC. Cholecystokinin activates both A- and C-type vagal afferent neurons. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1204–G1213. doi: 10.1152/ajpgi.00132.2003. [DOI] [PubMed] [Google Scholar]
- Smith BN, Dou P, Barber WD, Dudek FE. Vagally evoked synaptic currents in the immature rat nucleus tractus solitarii in an intact in vitro preparation. J Physiol. 1998;512:149–162. doi: 10.1111/j.1469-7793.1998.149bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soudah HC, Lu Y, Hasler WL, Owyang C. Cholecystokinin at physiological levels evokes pancreatic enzyme secretion via a cholinergic pathway. Am J Physiol Gastrointest Liver Physiol. 1992;263:G102–G107. doi: 10.1152/ajpgi.1992.263.1.G102. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Owyang C. Mechanism of cholecystokinin-induced relaxation of the rat stomach. J Auton Nerv Syst. 1999;75:123–130. doi: 10.1016/s0165-1838(98)00181-7. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol. 1991;260:G531–G536. doi: 10.1152/ajpgi.1991.260.3.G531. [DOI] [PubMed] [Google Scholar]
- Woods SC. Gastrointestinal satiety signals. I. An overview of gastrointestinal signals that influence food intake. Am J Physiol Gastrointest Liver Physiol. 2004;286:G7–13. doi: 10.1152/ajpgi.00448.2003. [DOI] [PubMed] [Google Scholar]
- Yen JC, Chan JY, Chan SHH. Differential roles of NMDA and non-NMDA receptors in synaptic responses of neurons in nucleus tractus solitarii of the rat. J Neurophysiol. 1999;81:3034–3043. doi: 10.1152/jn.1999.81.6.3034. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Lewis MW, Travagli RA. In vitro analysis of the effects of cholecystokinin (CCK) on rat brainstem motorneurons. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1066–G1073. doi: 10.1152/ajpgi.00497.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]