Abstract
Pancreatic polypeptides such as neuropeptide Y (NPY) and peptide YY (PYY) exert profound, vagally mediated effects on gastrointestinal (GI) motility. Vagal efferent outflow to the GI tract is determined principally by tonic GABAergic synaptic inputs onto dorsal motor nucleus of the vagus (DMV) neurons, yet neither peptide modulates GABAergic transmission. We showed recently that opioid peptides appear similarly ineffective because of the low resting cAMP levels. Using whole cell recordings from identified DMV neurons, we aimed to correlate the influence of brainstem cAMP levels with the ability of pancreatic polypeptides to modulate GABAergic synaptic transmission. Neither NPY, PYY, nor the Y1 or Y2 receptor selective agonists [Leu,Pro]NPY or NPY(3–36) respectively, inhibited evoked inhibitory postsynaptic current (eIPSC) amplitude unless cAMP levels were elevated by forskolin or 8-bromo-cAMP, by exposure to adenylate cyclase-coupled modulators such as cholecystokinin octapeptide (sulfated) (CCK-8s) or thyrotropin releasing hormone (TRH), or by vagal deafferentation. The inhibition of eIPSC amplitude by [Leu,Pro]NPY or NPY(3–36) was stable for approximately 30 min following the initial increase in cAMP levels. Thereafter, the inhibition declined gradually until the agonists were again ineffective after 60 min. Analysis of spontaneous and miniature currents revealed that such inhibitory effects were due to actions at presynaptic Y1 and Y2 receptors. These results suggest that, similar to opioid peptides, the effects of pancreatic polypeptides on GABAergic transmission depend upon the levels of cAMP within gastric inhibitory vagal circuits.
Keywords: electrophysiology, gastrointestinal motility, vagus
INTRODUCTION
The nucleus of the tractus solitarius (NTS) receives sensory information from the GI tract via vagal afferent fibres. NTS neurons integrate and transfer this information to neurons of the dorsal motor nucleus of the vagus (DMV) using mainly GABA, glutamate and norepinephrine as neurotransmitters. Vagal preganglionic fibres originating from DMV neurons provide the modulated parasympathetic motor output to the sub-diaphragmatic viscera (Reviewed recently in1,2).
Among the many peptides that modulate vagal motor output to the GI tract, pancreatic polypeptides such as neuropeptide Y (NPY) and peptide YY (PYY), applied either by intravenous injection or by microinjection directly in the dorsal vagal complex (DVC; i.e. NTS, DMV and area postrema), have been shown to affect gastric motility, gastric and pancreatic secretion and intestinal transit time.3–13 Interestingly, the actions of PYY or NPY on gastric motility and secretion have been reported to be inhibitory3,6,14,15 as well as excitatory.6,8,16–18 In his investigation, Chen6 reported that the effects of NPY on gastric motility were excitatory under basal conditions, but became inhibitory if gastric motility was stimulated above baseline by 4th ventricular administration of a stable thyrotropin releasing factor (TRH)-analogue. These effects were interpreted as different actions at Y1 vs Y2 receptors, with activation of Y1 receptors by NPY causing GI stimulation while activation of Y2 receptors by PYY caused GI inhibition.6 The peptide’s affinity for the receptors as well as the second messenger pathway engaged by Y1 and Y2 receptor activation is, however, identical, i.e. a decrease in adenylate cyclase activity,19 making this interpretation quite puzzling and unlikely.
Recently, we provided experimental evidence showing that opioid receptors in the GABAergic synaptic connections between NTS and DMV neurons undergo rapid, but transient trafficking.1,20,21 Our model proposes that modulation of GABAergic, but not glutamatergic, synaptic transmission between the NTS and the DMV is dependent upon the ‘state of activation’ of brainstem circuits. More specifically, under normal conditions neuromodulators negatively coupled to adenylate cyclase, such as neuropeptide Y and peptide YY via actions at Y1 and Y2 receptors, cannot affect GABAergic synaptic transmission between the NTS and the DMV,22,23 possibly because of the low resting levels of cAMP within the inhibitory nerve terminals. Increasing the levels of cAMP with pharmacological tools, or relieving the dampening of cAMP induced by vagal afferent fibres allows receptor trafficking and uncovers the modulation of GABAergic currents by neurotransmitters or neuromodulators negatively coupled to adenylate cyclase. Indeed, this mechanism of action occurs when the effects of opioid peptides on the NTS–DMV synapses are analysed,20,21 a mechanism complementary to the direct effect of opioids on the soma of NTS neurons.24
The aim of this study was to investigate the effects of NPY and PYY on excitatory and inhibitory synaptic transmission within the DVC circuits controlling gastric motility under conditions of pharmacologically increased cAMP levels as well as following vagal deafferentation. We shall propose a mechanism of action that accounts for the contrasting effects on gastric motility of pancreatic polypeptides observed in vivo.
MATERIAL AND METHODS
Retrograde tracing
Retrograde tracers were applied to discrete regions of the rat stomach as described previously.23,25,26 Briefly, 14-day-old Sprague–Dawley rat pups of either sex were anesthetized deeply (2.5% isoflurane) in accordance with the Institutional Animal Care and Use Committee guidelines. Following laparotomy, crystals of the fluorescent tracer 1,1′-dioctadecyl-3,3,3′,3′-tetramethylinodcarbo-cyanine perchlorate (DiI) were applied to the serosal surface of the fundus, corpus or antrum/pyloric (A/P) areas along the major curvature of the stomach. The dye was embedded in place with fast hardening epoxy resin, the surgical area was flushed with saline and the wound closed with 5/0 suture. The animal recovered for 10–15 days prior to removal of the brainstem.
Electrophysiology
The protocol for brainstem slicing has been described previously.23,27 Briefly, rats were anesthetized with isoflurane before removal of the brainstem, which was then placed in oxygenated Krebs’ solution (see below) at 4 °C and cut in 4–6 coronal slices (300 μm-thick) spanning the rostro-caudal extent of the DVC. The slices were incubated in oxygenated Krebs’ solution at 35 ± 1 °C for at least 90 min prior to use.
A single slice was transferred to a perfusion chamber and held in place with a nylon mesh on the stage of a Nikon E600FN microscope equipped with tetramethylrhodamine isothiocyanate (TRITC) epifluorescent filters. The slice was maintained at 35 ± 1 °C by continuous perfusion with Krebs’ solution. Retrogradely-labelled, DiI filled DMV neurons were identified under TRITC epifluorescence. Recordings were conducted under brightfield illumination using differential interference contrast optics.
Whole cell recordings were made using pipettes of resistance 4–7 MΩ when filled with potassium solutions (see below) using a single electrode voltage clamp amplifier (Axoclamp 1D, Molecular Devices, Union City, CA). Data were filtered at 2 kHz, digitized via a Digidata 1320 interface and analysed utilizing pClamp9 software (Molecular Devices). Only recordings with a series resistance <15 MΩ were used.
Electrical stimulation
Bipolar tungsten stimulating electrodes (~125 μm tip separation) were placed in the subnuclei centralis or medialis of the NTS and used to evoke inhibitory or excitatory postsynaptic currents (eIPSCs and eEPSCs, respectively). IPSCs were recorded in DMV neurons perfused with Krebs’ containing the non-selective iono-tropic glutamate antagonist, kynurenic acid (1 mmol L−1) and voltage-clamped at −50mV. When recording EPSCs, neurons were held at −60 mV and the perfusing solution contained the GABA-A antagonist bicuculline (20 μmol L−1). The patch pipette contained lidocaine N-ethyl bromide (0.5 mmol L−1) to prevent antidromically-stimulated action potentials. Stimuli (10–500 μA, 0.1–0.5 ms) were applied every 20 s throughout the recording period to evoke submaximal currents.
Spontaneous and miniature IPSCs
Spontaneous and miniature IPSCs (sIPSCs and mIPSCS, respectively) were recorded at −50 mV using KCl as the current carrier (see below). To block sodium currents and action potential dependent synaptic transmission, tetrodotoxin (TTX, 1 μmol L−1) was included in the perfusion solution when recording mIPSCs. mIPSCs and sIPSCs were analysed with Mini Analysis Program (Synaptosoft, Leonia, NJ, USA).
Vagal deafferentation
The surgery for supranodose afferent rhizotomy was described previously.21,26,28,29 Briefly, rats were anesthetized with a mixture of ketamine/xylazine/acepromazine (80/1.6/5 mg/kg i.p.) and placed in a stereotaxic frame. Muscle tissue was blunt dissected to expose the occipital bone and the first cervical vertebra. After shaving the bone, the three supranodose vagal dorsal afferent rootlets were visualized on the right vagal trunk and sectioned with a 27-gauge surgical needle under microscopic guidance. In sham deafferented animals, the procedure was identical but the vagal rootlets were not sectioned. The effectiveness of the surgery was assessed by injecting 1 μL of 5% rhodamine dextran in both the nodose ganglia and observing the fluorescent labelling in brainstem slices 3 days later.
Drug application and statistical analysis
Drugs were dissolved in the perfusing Krebs’ at concentrations demonstrated previously to be effective.25 Agonists were applied until the response plateaued; neurons were allowed to recover fully between drug additions. Antagonists were perfused for at least 5 min prior to agonist reapplication. Each neuron served as its own control, i.e., the response was assessed before and after drug addition using the Students t-test. Intergroup comparisons were analysed with the chi-square test. A minimum ±10% variation of eIPSC or eEPSC amplitude was arbitrarily taken as indicative of an effect. Results are expressed as mean ± SEM and only responding neurons were included in the statistical analysis. Significance was defined as P < 0.05.
Drugs and solutions
Krebs’ solution (mmol L−1): 126 NaCl; 25 NaHCO3; 2.5 KCl; 1.2 MgCl; 2.4 CaCl2; 1.2 NaH2PO4 and 11 dextrose, maintained at pH 7.4 by bubbling with O2/CO2 (95%/5%).
Intracellular potassium gluconate solution (mmol L−1): 128 potassium gluconate; 10 KCl; 0.3CaCl2; 1 MgCl2; 10 Hepes; 1 EGTA; 2 Na2ATP; 0.25 NaGTP, adjusted to pH 7.35 with KOH.
Intracellular potassium chloride solution (mmol L−1): 140 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 10 EGTA, 2 ATP-Na, 0.25 GTP-Na, adjusted to pH 7.35 with HCl.
1,1′-dioctadecyl-3,3,3′,3′-tetramethylinodcarbocyanine perchlorate was purchased from Invitrogen Corp., (Eugene, OR); NPY, PYY, [Leu31,Pro34]NPY and NPY(3–36) were purchased from Bachem (King of Prussia, PA). All other chemicals were purchased from Sigma Chemical Company (St. Louis, MO, USA).
RESULTS
Recordings were made from 100 gastric-projecting DMV neurons (34-fundus, 33 corpus- and 33-AP projecting neurons) from 43 rats. As quantitative or qualitative differences were not apparent in the responses of DMV neurons, the results were pooled.
Pancreatic polypeptides do not modulate inhibitory synaptic transmission in naïve slices
As reported previously,23 perfusion of the slice with NPY (100 nmol L−1; N = 21) or PYY (100 nmol L−1; N = 18) did not affect the amplitude of eIPSCs. Furthermore, selective agonist for the Y1 receptor, [Leu31Pro34]NPY, or the Y2-receptor, NPY (3–36) (both 100 nmol L−1; N = 22 and N = 33, respectively), did not alter eIPSC amplitude in any of the neurons to which the peptide was applied (Fig. 1A). Results are detailed in Table 1.
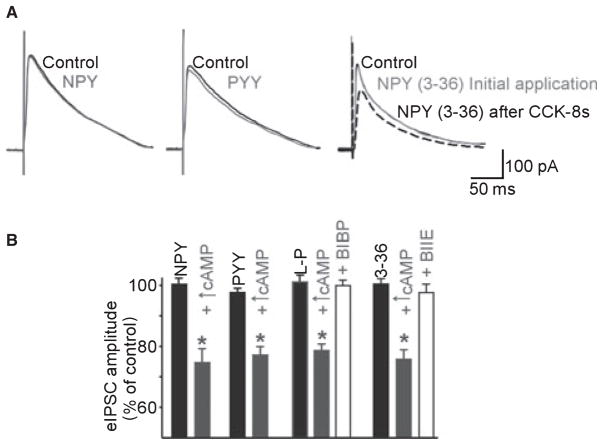
Figure 1.
Increasing the levels of cAMP ‘uncovers’ responses to pancreatic polypeptides in previously unresponsive neurones. (A) Representative traces of evoked inhibitory postsynaptic currents (eIPSC) recorded in DMV neurons. Superfusion with pancreatic polypeptides (in this example NPY, PYY or the Y2 selective agonist NPY (3–36), all at 100 nmol L−1) had no effect on the amplitude of the eIPSC. In the representative trace to the right, following a 5 min exposure to 100 nmol L−1 CCK-8s, subsequent re-application of the agonists reduced the amplitude of the eIPSC. Traces are the average of 3–6 originals each. Holding potential: −50 mV. Perfusing solution contains 1 mmol L−1 kynurenic acid. (B) Summary of the effects of alterations in the cAMP pathway in cells where pancreatic polypeptides per se did not reduce the amplitude of the eIPSC. After 5 min of incubation with forskolin, 8-Br-cAMP, CCK-8s or TRH and washout, subsequent applications of pancreatic polypeptides reduced the amplitude of eIPSC in a subpopulation of DMV neurons. The effects of the Y1 receptor selective agonist [Leu31-Pro34]NPY were antagonized by pretreatment with the Y1 receptor-selective antagonist BIBP3226. Similarly, the effects of the Y2 receptor selective agonist NPY (3–36) were antagonized by pretreatment with the Y2 receptor-selective antagonist BIIE0246. *P < 0.05 vs the agonist alone.
Table 1.
Increasing cAMP levels uncovers the ability of pancreatic polypeptides to inhibit evoked IPSC amplitude
| Untreated | Following pharmacological increase of cAMP levels | Following rhizotomy | |
|---|---|---|---|
| Control | 223 ± 27 pA | 173 ± 26.7 pA | Not tested |
| NPY | 227 ± 28 pA | 132 ± 23.4 pA | |
| Responsive (%amplitude vs control) | 0/21 neurons (101 ± 2%) | 7/12 neurons (75 ± 4%)* | |
| Control | 281 ± 30 pA | 211 ± 45.8 pA | Not tested |
| PYY | 275 ± 29 pA | 168 ± 40.1 pA | |
| Responsive (% amplitude vs control) | 0/18 neurons (98 ± 1%) | 7/8 neurons (77 ± 3%)* | |
| Control | 241 ± 24 pA | 200 ± 22.0 pA | 213 ± 24.4 pA |
| [Leu31Pro34]NPY | 247 ± 25 pA | 158 ± 19.0 pA | 155 ± 19.4 pA |
| Responsive (% amplitude vs control) | 0/22 neurons (103 ± 2%) | 11/17 neurons (79 ± 2%)* | 11/20 neurons (72 ± 3%)* |
| Control | 223 ± 15 pA | 219 ± 19.9 pA | 259 ± 26.6 pA |
| NPY (3–36) | 222 ± 14 pA | 166 ± 15.6 pA | 200 ± 24.0 pA |
| Responsive (% amplitude vs control) | 0/33 neurons (100 ± 2%) | 16/22 neurons (76 ± 3%)* | 14/19 neurons (76 ± 3%)* |
P < 0.05 vs control.
These data confirm our previous observation that pancreatic polypeptides do not modulate the GABAergic currents between NTS and DMV neurons.
Pancreatic polypeptides decrease the amplitude of eIPSC following elevation of cAMP levels
To determine whether the inability of pancreatic polypeptides to modulate GABAergic synaptic transmission was due to low resting levels of cAMP within inhibitory nerve terminals, the peptide effects were re-assessed after exposure to agents that either activate adenylate cyclase or increase cAMP levels (forskolin and 8-bromo-cAMP) as well as after exposure to neurotransmitters/neuromodulators known to activate adenylate cyclase (TRH and CCK-8s, via activation of CCKA receptors).30–33
Neurons were first perfused with NPY (N = 12), PYY (N = 8), [Leu31Pro34]NPY (N = 17) or NPY (3–36) (N = 22), none of which altered the amplitude of the eIPSC. Following wash-out and 5 min recovery, the brainstem slices were perfused for 5 min with forskolin (10 μmol L−1; N = 12), TRH (10 μmol L−1; N = 8), CCK-8s (100 nmol L−1; N = 17) or 8-Br-cAMP (1 mmol L−1; N = 4). All of these treatments increased the amplitude of the eIPSC. The amplitude of eIPSC returned to baseline values within 2–3 min of washout. A second application of the pancreatic polypeptide was conducted after the eIPSC amplitude returned to baseline. As the effectiveness of cAMP elevating agents to induce receptor trafficking was similar with respect to both the percentage of responsive neurons as well as the amplitude of the response, the data have been pooled.
During the second perfusion with these agonists, an inhibitory effect of NPY on the eIPSC amplitude was uncovered in 7 of the 12 neurons tested, while PYY decreased the eIPSC amplitude in 7 of 8 neurons, [Leu31Pro34]NPY in 11 of 17 neurons and NPY (3–36) in 16 of 22 neurons (chi-square test P > 0.05; Fig. 1B). The results are summarized in Table 1.
To determine whether the uncovering of the effects of pancreatic polypeptides were specific to GABAergic synapses or occurred also on glutamatergic synapses, the effects of NPY were tested in seven DMV neurons. In four of these neurons, perfusion with NPY decreased the amplitude of the eEPSC to 71 ± 4.5% and to 74 ± 1.0% of control before and after perfusion with forskolin (P > 0.05). In the remaining three neurons, the amplitude of the eEPSC was 102 ± 2.6% and 95 ± 2.2% of control before and after perfusion with forskolin (P > 0.05). Data not shown.
The inhibition of eIPSC by pancreatic polypeptides is mediated via actions at Y1 and Y2 receptors
Following pretreatment with forskolin, 8-Br-cAMP or CCK-8s (N = 6) as described above, perfusion with [Leu31Pro34]NPY reduced the amplitude of the eIPSC to 78 ± 2.5% of control (P < 0.05). Such inhibitory effects of [Leu31Pro34]NPY were prevented by pretreatment with the Y1 receptor selective antagonist, BIBP3226 (100 nmol L−1; 100 ± 1.9% of control, P > 0.05 vs control; P < 0.05 vs inhibition in the absence of antagonist).25,34,35
Similarly, following pretreatment with forskolin or 8-Br-cAMP (N = 4) as described above, NPY (3–36) reduced the amplitude of the eIPSC to 80 ± 2.3% of control (P < 0.05). Such inhibitory effects were prevented by pretreatment with the Y2 receptor selective antagonist, BIIE 0246 (100 nmol L−1; 98 ± 2.5% of control, P > 0.05 vs control; P < 0.05 vs inhibition in the absence of antagonist Fig. 1).
Time course of the effects of Y1 and Y2 agonists
The time course of recovery from Y1 or Y2 receptor-induced inhibition of GABAergic neurotransmission was assessed in 6 neurons. After 5 min perfusion with either CCK-8s or 8-Br-cAMP, the uncovering agent was washed out. Re-application of either [Leu31Pro34]NPY or NPY (3–36) induced a 21 ± 1.4% inhibition in eIPSC amplitude (P < 0.05). The agonist was then reapplied again at defined time points. The magnitude of the inhibition was 26 ± 2.4% and 21 ± 4.0% at 15 and 30 min, declining gradually thereafter such that 45 min after application, the agonists induced a 14 ± 3.1% inhibition and at 60 min there was no longer any inhibition in eIPSC amplitude (i.e. 96 ± 5.4% of control; Fig. 2).
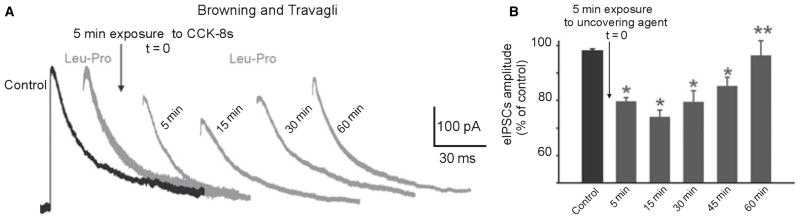
Figure 2.
Time course of the uncovering effects of agents that increase cAMP levels. (A) Representative traces of evoked inhibitory postsynaptic currents (eIPSC) recorded in DMV neurons. Superfusion with NPY (3–36) had no effect on the amplitude of the eIPSC. Following 5 min perfusion with CCK-8s and wash out (t = 0), repeated application of NPY (3–36) induced an inhibition in eIPSCs amplitude that was stable for approximately 15 min. By 30 min, NPY (3–36) was still able to induce an inhibition, albeit smaller, in eIPSC’s amplitude. By 60 min after CCK-8s application, NPY (3–36) was once again unable to inhibit eIPSC amplitude. Traces are the average of 3–6 originals each. Holding potential: −50 mV. Perfusing solution contains 1 mmol L−1 kynurenic acid. The stimulation artefact and rising portion of the eIPSC have been erased digitally for purpose of clarity. (B) Summary graphic showing the time course of the uncovering effects of agents that increase cAMP levels. *P < 0.05 vs control; **P < 0.05 vs 5 min.
Effects of pancreatic polypeptides on inhibitory synaptic transmission are mediated via actions at presynaptic receptors
In the 23 neurons tested in control conditions, the amplitude and frequency of sIPSC were not altered by perfusion with [Leu31Pro34]NPY. In eight out of 12 neurons tested, however, pretreatment with CCK-8s, TRH or forskolin uncovered an inhibitory effect of [Leu31Pro34]NPY on sIPSC frequency (P < 0.05) but not amplitude (P > 0.05). Similarly, when mIPSC were analysed following pretreatment with CCK-8s, their frequency, but not amplitude, was decreased (P < 0.05) following perfusion with [Leu31Pro34]NPY in three of the 5 neurons tested. Data are summarized in Table 2.
Table 2.
Effects of pancreatic polypeptides on sIPSCs and mIPSCs frequency and amplitude
| sIPSC | Untreated sIPSC frequency, amplitude | Following pharmacological increase of cAMP levels frequency, amplitude |
|---|---|---|
| Control | 3.8 ± 1.1 events s−1, 84 ± 6.5 pA | 3 ± 0.8 events s−1, 84 ± 10.5 pA |
| [Leu31Pro34]NPY | 3.8 ± 1.1 events s−1, 84 ± 6.3 pA | 1.8 ± 0.5 events s−1, 82 ± 8.6 pA |
| Responsive (% vs control) | 0/23 neurons (102 ± 2.5%, 102 ± 2.3%) | 8/12 neurons (63 ± 7.2%*, 98 ± 2.9%) |
| Control | 5.2 ± 1.2 events s−1, 80 ± 5.2 pA | 4 ± 0.7 events s−1, 79 ± 9.5 pA |
| NPY (3–36) | 5.3 ± 1.2 events s−1, 82 ± 5.4 pA | 2.4 ± 0.7 events s−1, 78 ± 10.0 pA |
| Responsive (% vs control) | 0/23 neurons (102 ± 2.3%, 102 ± 2.5%) | 6/13 responsive (61 ± 10.2%*, 97 ± 2.0%) |
| mIPSC | Untreated sIPSC frequency, amplitude | Following pharmacological increase of cAMP levels frequency, amplitude |
| Control | 3.7 ± 2.8 events s−1, 49 ± 9.7 pA | 1.4 ± 0.4 events s−1, 53 ± 14.3 pA |
| [Leu31Pro34]NPY | 3.9 ± 0.3 events s−1, 50 ± 13.2 pA | 0.8 ± 0.3 events s−1, 52 ± 13.8 pA |
| Responsive (% vs control) | 0/5 responsive (112 ± 15.8%, 101 ± 7.5%) | 3/5 responsive (62 ± 3.8%*, 96 ± 0.4%) |
| Control | 1.6 ± 0.3 events s−1, 62 ± 12.5 pA | 1.3 ± 0.3 events s−1, 66 ± 14.9 pA |
| NPY (3–36) | 1.6 ± 0.3 events s−1, 60 ± 13.4 pA | 0.8 ± 0.2 events s−1, 65 ± 16.1 pA |
| Responsive (% vs control) | 0/5 responsive (98 ± 5.4%, 96 ± 3.4%) | 4/5 responsive (61 ± 9.7%*, 98 ± 2.9%) |
P < 0.05 vs control.
Similarly, in the 23 neurons tested in control conditions, the amplitude and frequency of sIPSC were not altered by perfusion with NPY (3–36). In six out of 13 neurons tested, however, pretreatment with CCK-8s, TRH or forskolin uncovered an inhibitory effect of NPY (3–36) on sIPSC frequency (P < 0.05) but not amplitude (P > 0.05). Similarly, when mIPSC were analysed following pretreatment with CCK-8s, their frequency, but not amplitude, was decreased (P < 0.05) following perfusion with NPY (3–36) in four of the 5 neurons tested (Fig. 3). Data are summarized in Table 2.
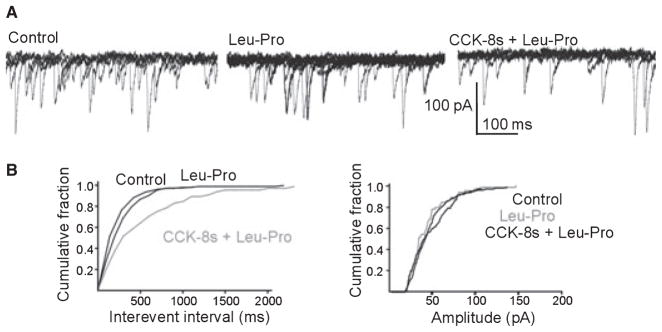
Figure 3.
Pretreatment with CCK-8s uncovers the [Leu31-Pro34]NPY-mediated decrease of the frequency but not the amplitude of spontaneous IPSCs. (A) Representative original traces showing that perfusion with 100 nmol L−1 [Leu31-Pro34]NPY does not modulate sIPSC frequency (middle trace). Following perfusion with, and recovery from, 100 nmol L−1 CCK-8s, re-perfusion of the same neuron with [Leu31-Pro34]NPY uncovered a significant decrease in sIPSC frequency. Recordings were conducted in symmetric chloride in the presence of 1 mmol L−1 kynurenic acid, 1 μmol L−1 tetro-dotoxin. HP = −50 mV. (B) Computer-generated averaged events from the traces in panel A showing that [Leu31-Pro34]NPY induced a decrease in the frequency (left side), but not in the amplitude (right side) of sIPSCs.
These data indicate that the inhibitory effects of NPY (3–36) and [Leu31Pro34]NPY were presynaptic, i.e. on GABAergic terminals impinging on DMV neurons.
Responses to multiple peptides indicates different membrane distribution of Y1 and Y2 receptors
Of 12 neurons in which the actions of both [Leu31-Pro34]NPY and NPY (3–36) were assessed after the inhibition of sIPSC was uncovered by pretreatment with forskolin, CCK-8s or TRH (N = 4 for each), both peptides were effective in reducing the frequency of sIPSC in 4 neurons. In a further 5 neurons, [Leu31-Pro34]NPY, but not NPY (3–36), inhibited sIPSC frequency while in the remaining 3 neurons, NPY (3–36), but not [Leu31Pro34]NPY, had any effect (data not shown).
These data indicate a different distribution of Y1 and Y2 receptors on gastric-projecting DMV neurons and suggest that these receptors may control different vagally-mediated effects on the GI tract.
After vagal deafferentation pancreatic polypeptides decrease eIPSC amplitude without the need for pharmacological increase of cAMP levels
As in naïve rats, the effects of pancreatic polypeptides did not show gastric regional differences, the data from fundus, corpus and A/P-projecting DMV neurons were thus pooled.
In deafferented rats, [Leu31Pro34]NPY inhibited the amplitude of eIPSC in naïve (i.e. not pretreated) brainstem slices in 12 of the 21 neurons tested. Data are summarized in Table 1. In the remaining nine neurons, perfusion with [Leu31Pro34]NPY did not affect the amplitude of the eIPSC (242 ± 34.8 and 237 ± 34.5 pA in control and after [Leu31Pro34]NPY, respectively; P > 0.05).
In deafferented rats, incubation with forskolin had no additional effect on the inhibitory actions of [Leu31Pro34]NPY. In three neurons [Leu31Pro34]NPY decreased the amplitude of eIPSC to 66 ± 13.0% and 65 ± 19.5% of control before or after incubation with forskolin respectively (P > 0.05). In three further neurons, perfusion with [Leu31Pro34]NPY had no effect on the eIPSC amplitude before or after forskolin treatment (98 ± 3.1% and 102 ± 2.5% of control, respectively; P > 0.05).
The effects of [Leu31Pro34]NPY were mediated by activation of Y1 receptors because perfusion with BIBP3226 abolished the inhibition of eIPSCs amplitude induced by [Leu31Pro34]NPY (78 ± 1.9% and 100 ± 2.1% of control prior and after BIPB3226, respectively; P < 0.05; N = 5) (Fig. 4).
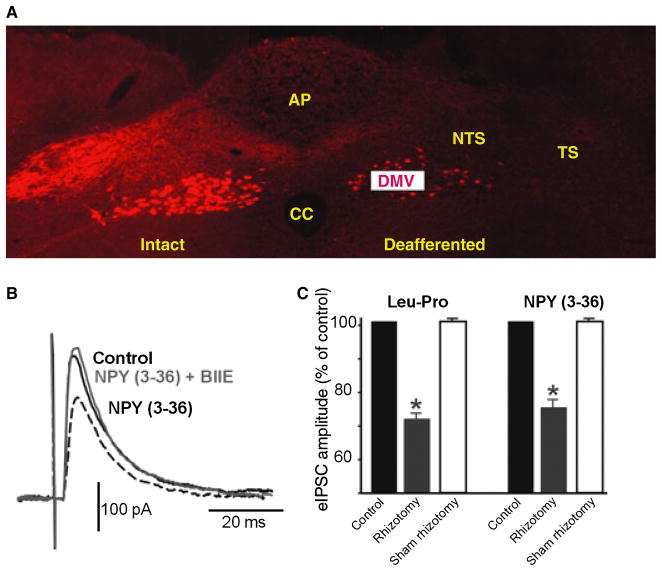
Figure 4.
Perfusion with NPY (3–36) inhibits neurons from deafferented rats without the need to increase the levels of cAMP pharmacologically. (A) Coronal section at an intermediate level of the vagal complex. Note the dense innervation of rodamine dextran labelled vagal afferent nerve terminals within the vagally intact brainstem side (left) compared with the almost complete absence of labelling on the deafferented side of the brainstem (right). Note also the presence of vagal preganglionic motoneurons labelled in both sides of the brainstem, indicating the selectivity of the surgical deafferentation procedure. AP, area postrema; DMV, dorsal motor nucleus of the vagus; NTS, nucleus tractus solitarius; TS, tractus solitarius; CC, central canal. (B) Representative trace showing that, in a slice from a deafferented rat, perfusion with NPY (3–36) reduced the amplitude of the eIPSC without the need to increase pharmacologically the levels of cAMP. The effects were antagonized by pretreatment with BIIE0246, indicating that they were mediated through activation of Y2 receptors. Traces are the average of 3–6 originals each. Holding potential: −50 mV. Perfusing solution contains 1 mmol L−1 kynurenic acid. (C) Summary graphic showing that in a subpopulation of DMV neurons, the inhibitory effects of [Leu31-Pro34]NPY and NPY (3–36) were present in deafferented but not sham operated rats. *P < 0.05 vs control.
In slices from deafferented animals, NPY (3–36) reduced the amplitude of eIPSC in 12 of the 17 neurons tested. Data are detailed in Table 1. In the remaining nine neurons, perfusion with NPY (3–36) did not affect the amplitude of the eIPSC (243 ± 48.3 and 248 ± 51.7 pA in control and after NPY (3–36), respectively; P > 0.05).
Furthermore, in deafferented rats, incubation with forskolin had no additional effect on the inhibitory actions of NPY (3–36). In two neurons NPY (3–36) decreased the amplitude of eIPSC to 79 ± 1.1% and 82 ± 0.7% of control before or after incubation with forskolin (P > 0.05). In three other neurons, perfusion with NPY (3–36) had no effect on the eIPSC amplitude before or after forskolin treatment (102 ± 1.8% and 107 ± 1.2% of control, respectively; P > 0.05).
These effects of NPY (3–36) were mediated by activation of Y2 receptors because perfusion with BIIE 0246 abolished the inhibition of the eIPSCs amplitude induced by NPY (3–36) (77 ± 1.9% and 100 ± 3.1% of control prior and after BIIE 0246, respectively; P < 0.05; N = 5) (Fig. 4).
These data suggest that vagal deafferentation increases cAMP levels allowing pancreatic polypeptides to modulate GABAergic synapses. It further indicates that in slices from deafferented rats, contrary to slices from vagally intact conditions, increasing the activity of adenylate cyclase with forskolin does not induce an additional increase in the response to pancreatic poly-peptides.
To verify that the ability of pancreatic polypeptides to inhibit GABAergic synaptic transmission following vagal deafferentation was, indeed, a direct result of the nerve interruption, the ability of [Leu31Pro34]NPY and NPY (3–36) to inhibit eIPSC was examined in 26 neurons from 5 sham operated rats. As in control slices, in all 26 neurons recorded perfusion with Y1 or Y2 agonists were unable to inhibit the amplitude of eIPSC. In 19 out of 26 of these neurons, the effects of [Leu31Pro34]NPY or NPY (3–36) were uncovered by forskolin (N = 6), CCK (N = 4) or 8-Br-cAMP (N = 4) (Fig. 4).
DISCUSSION
In the present manuscript we have shown that NPY and PYY activate Y1 and/or Y2 receptors on a subpopulation of inhibitory synaptic connections between the NTS and the DMV only if the levels of cAMP are increased, either with pharmacological agents or following vagal deafferentation. The actions of Y1 and Y2 to inhibit excitatory glutamatergic synaptic transmission between NTS and DMV neurons is unaffected by cAMP levels.
As DMV neurons controlling gastrointestinal motility are spontaneously active (27, reviewed in (2)), it is feasible that even a small change in their membrane potential, such as the one induced by a ca. 30% decrease in GABAergic input, would increase action potential firing and consequent amplification of the vagal efferent output modulating gastric functional parameters. Indeed, recent in vivo experiments have suggested that the tonic GABAergic inhibition is a predominant influence on rat DMV neurons.36,37
PYY and NPY have long been known to have vagally-mediated actions on gastrointestinal functions, including gastric motility.6,9,14,16,38 PYY appears to play a major role in the ‘ileal brake’ phenomenon, i.e. as digesta are transported in the distal small intestine, PYY is released into the circulation by intestinal endocrine cells, readily crosses the blood brain barrier and decreases gastric motility and tone via a vagally-mediated mechanism, principally via activation of Y2 receptors in the DVC (reviewed recently in 10). In contrast to the hormonal actions of PYY, NPY serves the more classical role of neurotransmitter in the DVC, where both NPY-immunoreactive terminals of hypo-thalamic origin, local NPY-immunoreactive neurons as well as Y1 and Y2 receptor binding sites are relatively abundant.39–43
While we have not measured cAMP levels within individual brainstem neurons or nerve terminals, the current experiments lead us to hypothesize that the levels of cAMP determine the modulation of GABAergic, but not glutamatergic, synapses by NPY and PYY between NTS and DMV. Our previous studies have led us to hypothesize that, under basal (non-stimulated) conditions, cAMP levels within GABAergic brainstem vagal circuits are low. By consequence, activation of receptors negatively coupled to adenylate cyclase, such as Y1 and Y2 receptors, exerts no effect upon GABAergic synaptic transmission. Elevation of cAMP levels either directly (e. g. via exposure to the non-hydrolysable analogue 8-bromo-cAMP) or indirectly (e.g. via activation of adenylate cyclase by forskolin or Gs coupled receptors) increases cAMP levels sufficient to allow observation of Y1 and Y2-receptor mediated actions on inhibitory synaptic transmission. TRH and CCK were used in the present study to demonstrate neurohormone or neurotransmitter-mediated activation of adenylate cyclase because both peptides are known to exert profound effects upon vagally-mediated gastrointestinal functions through brainstem sites of action.2,44–46 While the CCK is most commonly associated with the Gq-mediated activation of the phospholipase C pathway, CCK1 (or CCKA) receptors have also been shown to be coupled to Gs in several tissues, resulting in activation of adenylate cyclase and an increase in cAMP levels.31,47–49 We have shown previously that CCK-8s exerts its vagally-mediated actions on gastric functions via activation of brainstem CCKA receptors28,46,50,51 and, importantly, CCK-induced activation of adenylate cyclase has been demonstrated in vagal afferent neurons as well as within the dorsal vagal complex.52–54
This type of action is similar to that described recently for opioid peptides 20–22 and it may provide a mechanistic explanation for the opposing responses to in vivo DVC application of these polypeptides.6,8,14–18,55 In basal conditions, DVC microinjection of PYY and NPY increases motility. If, however, baseline gastric motility is stimulated via 4th ventricular administration of a TRH analogue,6 for example, or the rats are fasted for 25 h,9 microinjection of pancreatic polypeptides decreases gastric motility.
Gastric motility is modulated by two distinct vagal efferent pathways originating from the preganglionic cholinergic motoneurons of the DMV. At the postganglionic level, one pathway comprises cholinergic neurons; excitation of this pathway increases gastric motility while inhibition of this pathway (i.e. withdrawal of tonic cholinergic input) decreases gastric motility. Postganglionic neurons of the second pathway use NANC neurotransmitters and, when activated, reduce gastric motility (reviewed recently in 2).
Although we do not yet have definitive proof, when combined with the available literature, the data presented herein lead us to suggest the following scenario: in the intact animal under non-stimulated (i.e. fasted) conditions, the motility of the gastrointestinal tract is negligible and involved mostly with housekeeping purposes.56,57 In these conditions, one could hypothesize that the afferent vagal fibres impinging onto brainstem circuits release only small quantities of glutamate onto second order NTS neurons.58,59 It is important to note that the affinity of metabotropic receptors (mGluR) for glutamate is several-fold higher than that of ionotropic receptors, hence mGluR will be activated even under conditions of low glutamate release. We have demonstrated previously that the vagal afferent-mediated dampening of cAMP levels in brainstem vagal circuits occurs via activation of group II mGluR.26 We have shown recently25 that in a naive (non-stimulated) DVC slice preparation, NPY and PYY decrease the amplitude of glutamatergic, but not GABAergic, currents between NTS and DMV. If, as we propose, this condition is similar to a non-stimulated in vivo preparation, then the PYY and NPY-induced reduction of excitatory inputs to the DMV can result in an increase in gastric motility and tone only via withdrawal of NANC inhibition to the stomach.
As a consequence of food ingestion or a prolonged, stressful situation such as fasting, however, many neurotransmitters and neuromodulators, such as CCK or TRH, are released and contribute to the proper implementation of the digestive processes. These neuroactive substances overcome the effects of mGluR activation, increase the cAMP levels in the DVC21 and ‘prime’ the NTS–DMV GABAergic circuits, whose strength is now decreased by pancreatic polypeptides. Thus, as microinjections of NPY or PYY in stimulated conditions decrease gastric motility and tone,5,14 this can be obtained only by a reduction (withdrawal) of the vagal cholinergic tone. Indeed, Kobashi and colleagues showed that atropine pretreatment antagonized the NPY-induced decrease in tone.14
This cAMP-controlled arrangement of the circuits would be a fast, convenient and metabolically inexpensive way to permit the adaptation of vagal gastrointestinal circuits to the ever-changing environmental conditions and fulfil the requirements for proper digestive processes.
As neither all the DMV neurons are responsive to these variations in cAMP levels, nor all the neurons respond to a Y1 and Y2 agonist challenge, it appears that the GABAergic circuits involved in these adaptations comprise a highly selective population of both NTS and DMV neurons. Indeed, the concept of NTS and DMV neurons as relay stations has been challenged also in regard to the circuits that comprise NTS neurons controlling cardiovascular or respiratory reflexes.60–65
In conclusion, the present in vitro study suggests an hypothetical mechanistic explanation for the puzzling and apparently contradictory in vivo gastrointestinal responses to central application of pancreatic polypeptides. Under basal conditions, NPY and PYY stimulate gastrointestinal motility presumably via disinhibition of NANC vagal output to the stomach. After stimulation, however, cAMP levels within brainstem GABAergic circuits are assumed to increase, allowing pancreatic polypeptides to modulate inhibitory transmission to DMV neurons. The result of this inhibition is withdrawal of cholinergic tone to the stomach, decreasing gastric motility.
Thus, the effects of hormones and peptides on gastrointestinal processes may be altered radically depending upon the activation state of vagal brainstem circuits and must be considered in the context of ongoing homeostatic and physiological digestive processes in order to reach an accurate and reliable conclusion regarding their actions.
The postprandial symptoms of functional dyspepsia could then be related to an unbalanced secretion of neuromodulators positively coupled with adenylate cyclase which keeps the vagal circuits in a constant state of activation and induces untimely gastric motor responses.
Acknowledgments
Supported by DK#55530.
We would like to thank Cesare M., Zoraide Travagli and W. Nairn Browning for support and encouragement.
Footnotes
COMPETING INTERESTS
The authors have no competing interests.
References
- 1.Browning KN, Travagli RA. Short-term receptor trafficking in the dorsal vagal complex: an overview. Auton Neurosci. 2006;127:2–8. doi: 10.1016/j.autneu.2006.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adrian TE, Savage AP, Sagor GR, et al. Effect of peptide YY on gastric, pancreatic, and biliary function in humans. Gastroenterology. 1985;89:494–9. doi: 10.1016/0016-5085(85)90442-1. [DOI] [PubMed] [Google Scholar]
- 4.Ashby D, Bloom SR. Recent progress in PYY research – an update report for 8th NPY meeting. Peptides. 2007;28:198–202. doi: 10.1016/j.peptides.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Chen CH, Rogers RC, Stephens RL., Jr Intracisternal injection of peptide YY inhibits gastric emptying in rats. Reg Pept. 1996;61:95–8. doi: 10.1016/0167-0115(95)00143-3. [DOI] [PubMed] [Google Scholar]
- 6.Chen CH, Stephens RL, Jr, Rogers RC. PYY and NPY control of gastric motility via action on Y1 and Y2 receptors in the DVC. Neurogastroenterol Motil. 1997;9:109–16. doi: 10.1046/j.1365-2982.1997.d01-26.x. [DOI] [PubMed] [Google Scholar]
- 7.Fujimiya M, Inui A. Peptidergic regulation of gastrointestinal motility in rodents. Peptides. 2001;21:1565–82. doi: 10.1016/s0196-9781(00)00313-2. [DOI] [PubMed] [Google Scholar]
- 8.Geoghegan JG, Lawson DC, Cheng CA, Opara E, Taylor IL, Pappas TN. Intracerebroventricular neuropeptide Y increases gastric and pancreatic secretion in the dog. Gastroenterology. 1993;105:1069–77. doi: 10.1016/0016-5085(93)90951-8. [DOI] [PubMed] [Google Scholar]
- 9.Kobashi M, Shimatani Y, Shirota K, Xuan SY, Mitoh Y, Matsuo R. Central neuropeptide Y induces proximal stomach relaxation via Y1 receptors in the dorsal vagal complex of the rat. Am J Physiol Regul Integr Comp Physiol. 2006;290:R290–7. doi: 10.1152/ajpregu.00423.2005. [DOI] [PubMed] [Google Scholar]
- 10.Maljaars PW, Peters HP, Mela DJ, Masclee AA. Ileal brake: a sensible food target for appetite control. A review. Physiol Behav. 2008;95:271–81. doi: 10.1016/j.physbeh.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Masuda M, Tomita H, Okubo K, Miyasaka K. Vagal efferent nerve-dependent inhibitory action of pancreatic polypeptide and peptide YY in conscious rats: comparison with somatostatin. J Auton Nerv Syst. 1994;50:131–8. doi: 10.1016/0165-1838(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 12.Naruse S, Kitagawa M, Ishiguro H, Hayakawa T. Feedback regulation of pancreatic secretion by peptide YY. Peptides. 2002;23:359–65. doi: 10.1016/s0196-9781(01)00612-x. [DOI] [PubMed] [Google Scholar]
- 13.Yang H. Central and peripheral regulation of gastric acid secretion by peptide YY. Peptides. 2002;23:349–58. doi: 10.1016/s0196-9781(01)00611-8. [DOI] [PubMed] [Google Scholar]
- 14.Ishiguchi T, Amano T, Matsubayashi H, Tada H, Fujita M, Takahashi T. Centrally administered neuropeptide Y delays gastric emptying via Y2 receptors in rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1522–30. doi: 10.1152/ajpregu.2001.281.5.R1522. [DOI] [PubMed] [Google Scholar]
- 15.Yang H, Kawakubo K, Wong H, Ohning G, Walsh J, Tache Y. Peripheral PYY inhibits intracisternal TRH-induced gastric acid secretion by acting in the brain. Am J Physiol Gastrointest Liver Physiol. 2000;279:G575–81. doi: 10.1152/ajpgi.2000.279.3.G575. [DOI] [PubMed] [Google Scholar]
- 16.Chen CH, Rogers RC. Central inhibitory action of peptide YY on gastric motility in rats. Am J Physiol. 1995;269:R787–92. doi: 10.1152/ajpregu.1995.269.4.R787. [DOI] [PubMed] [Google Scholar]
- 17.Yang H, Li WP, Reeve JR, Rivier J, Tache Y. PYY-preferring receptor in the dorsal vagal complex and its involvement in PYY stimulation in gastric acid secretion in rats. Br J Pharmacol. 1998;123:1549–54. doi: 10.1038/sj.bjp.0701767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoneda M, Yokohama S, Tamori K, Sato Y, Nakamura K, Makino I. Neuropeptide Y in the dorsal vagal complex stimulates bicarbonate-dependent bile secretion in rats. Gastroenterology. 1997;112:1673–80. doi: 10.1016/s0016-5085(97)70050-7. [DOI] [PubMed] [Google Scholar]
- 19.Michel MC, Beck-Sickinger A, Cox H, et al. XVI. International union of pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1999;50:143–50. [PubMed] [Google Scholar]
- 20.Browning KN, Kalyuzhny AE, Travagli RA. Mu-opioid receptor trafficking on inhibitory synapses in the rat brainstem. J Neurosci. 2004;24:9344–52. doi: 10.1523/JNEUROSCI.1676-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Browning KN, Zheng Z, Gettys TW, Travagli RA. Vagal afferent control of opioidergic effects in rat brainstem circuits. J Physiol. 2006;575:761–76. doi: 10.1113/jphysiol.2006.111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Browning KN, Kalyuzhny AE, Travagli RA. Opioid peptides inhibit excitatory but not inhibitory synaptic transmission in the rat dorsal motor nucleus of the vagus. J Neurosci. 2002;22:2998–3004. doi: 10.1523/JNEUROSCI.22-08-02998.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Browning KN, Renehan WE, Travagli RA. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. J Physiol. 1999;517:521–32. doi: 10.1111/j.1469-7793.1999.0521t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glatzer NR, Derbenev AV, Banfield BW, Smith BN. Endomorphin-1 modulates intrinsic inhibition in the dorsal vagal complex. J Neurophysiol. 2007;98:1591–9. doi: 10.1152/jn.00336.2007. [DOI] [PubMed] [Google Scholar]
- 25.Browning KN, Travagli RA. Neuro-peptide Y and peptide YY inhibit excitatory synaptic transmission in the rat dorsal motor nucleus of the vagus. J Physiol. 2003;549:775–85. doi: 10.1113/jphysiol.2003.042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Browning KN, Travagli RA. Functional organization of presynaptic metabotropic glutamate receptors in vagal brainstem circuits. J Neurosci. 2007;27:8979–88. doi: 10.1523/JNEUROSCI.1105-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol. 1991;260:G531–6. doi: 10.1152/ajpgi.1991.260.3.G531. [DOI] [PubMed] [Google Scholar]
- 28.Baptista V, Browning KN, Travagli RA. Effects of cholecystokinin-8s in the nucleus tractus solitarius of vagally deafferented rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1092–100. doi: 10.1152/ajpregu.00517.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan S, Browning KN, Coleman FH, et al. Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J Neurosci. 2008;28:4957–66. doi: 10.1523/JNEUROSCI.5398-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker JL, Dufy B, Harrington JW, et al. Signals transduced by g-aminobutyric acid in cultured central nervous system neurons and thyrotropin releasing hormone in clonal pituitary cells. Ann NY Acad Sci. 1987;494:1–38. doi: 10.1111/j.1749-6632.1987.tb29477.x. [DOI] [PubMed] [Google Scholar]
- 31.Dufresne M, Seva C, Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev. 2006;86:805–47. doi: 10.1152/physrev.00014.2005. [DOI] [PubMed] [Google Scholar]
- 32.Gershengorn MC. Mechanism of signal transduction by TRH. Ann N Y Acad Sci. 1989;553:191–6. doi: 10.1111/j.1749-6632.1989.tb46641.x. [DOI] [PubMed] [Google Scholar]
- 33.Wank SA. G protein-coupled receptors in gastrointestinal physiology. I. CCK receptors: an exemplary family. Am J Physiol. 1998;274:G607–13. doi: 10.1152/ajpgi.1998.274.4.g607. [DOI] [PubMed] [Google Scholar]
- 34.Jacques D, Cadieux A, Dumont Y, Quirion R. Apparent affinity and potency of BIBP3226, a non-peptide neuropeptide Y receptor antagonist, on purported neuropeptide Y Y1, Y2 and Y3 receptors. Eur J Pharmacol. 1995;278:R3–5. doi: 10.1016/0014-2999(95)00179-o. [DOI] [PubMed] [Google Scholar]
- 35.Rudolf K, Eberlein W, Engel W, et al. The first highly potent and selective non-peptide neuropeptide Y Y1 receptor antagonist: BIBP3226. Eur J Pharmacol. 1994;271:R11–3. doi: 10.1016/0014-2999(94)90822-2. [DOI] [PubMed] [Google Scholar]
- 36.Herman MA, Cruz MT, Sahibzada N, Verbalis J, Gillis RA. GABA signaling in the nucleus tractus solitarius sets the level of activity in dorsal motor nucleus of the vagus cholinergic neurons in the vagovagal circuit. Am J Physiol Gastrointest Liver Physiol. 2009;296:G101–11. doi: 10.1152/ajpgi.90504.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sivarao DV, Krowicki ZK, Hornby PJ. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol Motil. 1998;10:305–13. doi: 10.1046/j.1365-2982.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- 38.Fujimiya M, Itoh E, Kihara N, Yamamoto I, Fujimura M, Inui A. Neuropeptide Y induces fasted pattern of duodenal motility via Y2 receptors in conscious fed rats. Am J Physiol Gastrointest Liver Physiol. 2000;278:G32–8. doi: 10.1152/ajpgi.2000.278.1.G32. [DOI] [PubMed] [Google Scholar]
- 39.Harfstrand A, Fuxe K, Terenius L, Kalia M. Neuropeptide Y-immunoreactive perikarya and nerve terminals in the rat medulla oblongata: relationship to cytoarchitecture and catecholaminergic cell groups. J Comp Neurol. 1987;260:20–35. doi: 10.1002/cne.902600103. [DOI] [PubMed] [Google Scholar]
- 40.Hernandez EJ, Whitcomb DC, Vigna SR, Taylor IL. Saturable binding of circulating peptide YY in the dorsal vagal complex of rats. Am J Physiol. 1994;256:G511–6. doi: 10.1152/ajpgi.1994.266.3.G511. [DOI] [PubMed] [Google Scholar]
- 41.Leslie RA, McDonald TJ, Robertson HA. Autoradiographic localization of peptide YY and neuropeptide Y binding sites in the medulla oblongata. Peptides. 1988;9:1071–6. doi: 10.1016/0196-9781(88)90091-5. [DOI] [PubMed] [Google Scholar]
- 42.Sheikh SP. Neuropeptide Y and peptide YY: major modulators of gastro-intestinal blood flow and function. Am J Physiol. 1991;261:G701–15. doi: 10.1152/ajpgi.1991.261.5.G701. [DOI] [PubMed] [Google Scholar]
- 43.Whitcomb DC, Puccio AM, Vigna SR, Taylor IL, Hoffman GE. Distribution of pancreatic polypeptide receptors in the rat brain. Brain Res. 1997;760:137–49. doi: 10.1016/s0006-8993(97)00295-3. [DOI] [PubMed] [Google Scholar]
- 44.Owyang C, Logsdon CD. New insights into neurohormonal regulation of pancreatic secretion. Gastroenterology. 2004;127:957–69. doi: 10.1053/j.gastro.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Tache Y, Yang H. Role of medullary TRH in the vagal regulation of gastric function. In: Tache Y, Wingate DL, Burks TF, editors. Innvervation of the Gut: Pathophysiological Implications. Boca Raton: CRC Press; 1993. pp. 67–80. [Google Scholar]
- 46.Viard E, Zheng Z, Wan S, Travagli RA. Vagally-mediated, non paracrine effects of cholecystokinin-8s on rat pancreatic exocrine secretion. Am J Physiol Gastrointest Liver Physiol. 2007;293:G494–500. doi: 10.1152/ajpgi.00118.2007. [DOI] [PubMed] [Google Scholar]
- 47.Marino CR, Leach SD, Schaefer JF, Miller LJ, Gorelick FS. Characterization of cAMP-dependent protein kinase activation by CCK in rat pancreas. FEBS Lett. 1993;316:48–52. doi: 10.1016/0014-5793(93)81734-h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmer JM, Wood JD, Zafirov DH. Transduction of aminergic and peptidergic signals in enteric neurones of the guinea-pig. J Physiol. 1987;387:371–83. doi: 10.1113/jphysiol.1987.sp016578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willems PH, Tilly RH, De Pont JJ. Pertussis toxin stimulates cholecystokinin- induced cyclic AMP formation but is without effect on secretagogue-induced calcium mobilization in exocrine pancreas. Biochim Biophys Acta. 1987;928:179–85. doi: 10.1016/0167-4889(87)90119-4. [DOI] [PubMed] [Google Scholar]
- 50.Baptista V, Zheng Z, Coleman FH, Rogers RC, Travagli RA. Cholecystokinin octapeptide increases spontaneous glutamatergic synaptic transmission to neurons of the nucleus tractus solitarius centralis. J Neurophysiol. 2005;94:2763–71. doi: 10.1152/jn.00351.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wan S, Coleman FH, Travagli RA. Cholecystokinin-8s excites identified rat pancreatic-projecting vagal moto-neurons. Am J Physiol Gastrointest Liver Physiol. 2007;293:G484–92. doi: 10.1152/ajpgi.00116.2007. [DOI] [PubMed] [Google Scholar]
- 52.de LG, Dimaline R, Varro A, Dockray GJ. Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J Neurosci. 2007;27:2876–82. doi: 10.1523/JNEUROSCI.5508-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sutton GM, Duos B, Patterson LM, Berthoud HR. Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus. Endocrinology. 2005;146:3739–47. doi: 10.1210/en.2005-0562. [DOI] [PubMed] [Google Scholar]
- 54.Sutton GM, Patterson LM, Berthoud HR. Extracellular signal-regulated kinase 1/2 signaling pathway in solitary nucleus mediates cholecystokinin- induced suppression of food intake in rats. J Neurosci. 2004;24:10240–7. doi: 10.1523/JNEUROSCI.2764-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsuda M, Aono M, Moriga M, Okuma M. Centrally administered NPY inhibits gastric emptying and intestinal transit in the rat. Dig Dis Sci. 1993;38:845–50. doi: 10.1007/BF01295910. [DOI] [PubMed] [Google Scholar]
- 56.Hall KE, El Sharkawy TY, Diamant NE. Vagal control of migrating motor complex in the dog. Am J Physiol. 1982;243:G276–84. doi: 10.1152/ajpgi.1982.243.4.G276. [DOI] [PubMed] [Google Scholar]
- 57.Szurszewski JH. A 100-year perspective on gastrointestinal motility. Am J Physiol. 1998;274:G447–53. doi: 10.1152/ajpgi.1998.274.3.G447. [DOI] [PubMed] [Google Scholar]
- 58.Andresen MC, Kunze DL. Nucleus tractus solitarius – gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- 59.Smith BN, Dou P, Barber WD, Dudek FE. Vagally evoked synaptic currents in the immature rat nucleus tractus solitarii in an intact in vitro preparation. J Physiol. 1998;512:149–62. doi: 10.1111/j.1469-7793.1998.149bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bailey TW, Hermes SM, Andresen MC, Aicher SA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventro-lateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns. J Neurosci. 2006;26:11893–902. doi: 10.1523/JNEUROSCI.2044-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bailey TW, Hermes SM, Whittier KL, Aicher SA, Andresen MC. A-type potassium channels differentially tune afferent pathways from rat solitary tract nucleus to caudal ventro-lateral medulla or paraventricular hypothalamus. J Physiol. 2007;582:613–28. doi: 10.1113/jphysiol.2007.132365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bailey TW, Jin YH, Doyle MW, Andresen MC. Vanilloid-sensitive afferents activate neurons with prominent A-type potassium currents in nucleus tractus solitarius. J Neurosci. 2002;22:8230–7. doi: 10.1523/JNEUROSCI.22-18-08230.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonham AC, Chen CY, Sekizawa SI, Joad JP. Plasticity in the nucleus tractus solitarius (NTS) and its influence on lung and airway reflexes. J Appl Physiol. 2006;101:322–7. doi: 10.1152/japplphysiol.00143.2006. [DOI] [PubMed] [Google Scholar]
- 64.Bonham AC, McCrimmon DR. Neurones in a discrete region of the nucleus tractus solitarius are required for the Breuer–Hering reflex in rat. J Physiol. 1990;427:261–80. doi: 10.1113/jphysiol.1990.sp018171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jin YH, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci. 2004;24:4709–17. doi: 10.1523/JNEUROSCI.0753-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]