Abstract
Mammalian homologues of Drosophila Trp form plasma membrane channels that mediate Ca2+ influx in response to activation of phospholipase C and internal Ca2+ store depletion. Previous studies showed that human Trp3 is activated by inositol 1,4,5-trisphosphate (IP3) receptors (IP3Rs) and identified interacting domains, one on Trp and two on IP3R. We now find that Trp3 binds Ca2+-calmodulin (Ca2+/CaM) at a site that overlaps with the IP3R binding domain. Using patch-clamp recordings from inside-out patches, we further show that Trp3 has a high intrinsic activity that is suppressed by Ca2+/CaM under resting conditions, and that Trp3 is activated by the following: a Trp-binding peptide from IP3R that displaces CaM from Trp3, a myosin light chain kinase Ca2+/CaM binding peptide that prevents CaM from binding to Trp3, and calmidazolium, an inactivator of Ca2+/CaM. We conclude that inhibition of the inhibitory action of CaM is a key step of Trp3 channel activation by IP3Rs.
Ca2+ release from internal stores and the consequent influx from external space are important steps of Ca2+ signaling that follows the stimulation of phospholipase C. Ca2+ release is triggered by the binding of inositol 1,4,5-trisphosphate (IP3) to IP3 receptors (IP3Rs), and the depletion of Ca2+ from the stores in turn activates Ca2+ influx via a so-called capacitative Ca2+ entry mechanism (1–3). Therefore, the term store-operated channels (SOCs) is often used to describe the channels responsible for the Ca2+ influx. Although the molecular makeup of SOCs is not known, recent studies indicate that Drosophila Trp (transient receptor potential) protein and its mammalian homologues are subunits of Ca2+-permeable cation channels that are activated in a variety of cells after stimulation of the phospholipase C pathway by agonists (3–6). However, because channels formed by Trps are not always activated by store depletion alone and they display electrophysiological properties different from those of well-characterized native SOCs, it remains to be proved whether Trps are components of SOCs (6–9).
Recent studies have shown that IP3Rs play a role in activating human Trp3 (hTrp3) (9–11), consistent with a conformational coupling hypothesis proposed for the activation of SOCs (2, 12). Direct interaction between type 3 IP3R (IP3R3) and Trp3 was confirmed in in vitro binding studies, which also led to the identification of two interaction domains in the N-terminal third of the IP3R3 protein and one on the C terminus of Trp3 (9). Expression of IP3R3 or Trp3 sequences containing the respective binding domains in HEK 293 cells altered store-operated Ca2+ influx (9). IP3Rs have also been shown to interact with Trp1 and Trp6 (9, 13, 14), indicating that interaction of IP3R with Trp may be an important gating mechanism for Trp channels.
Other factors have also been shown to affect the activity of heterologously expressed Trp channels. These include the activated Gα11 and Gαq (15), Ca2+ (16–18), polyunsaturated fatty acids (19), and diacylglycerol (20–22). Among these, the effect of Ca2+ remains controversial, with reports suggesting either a stimulatory or an inhibitory effect on Drosophila TrpL (16, 17) and a stimulatory effect on hTrp3 (18). Some effects of Ca2+ may be mediated by calmodulin (CaM), as two CaM-binding sites have been localized on the C terminus of TrpL (23, 24). Although CaM has been implicated in the inactivation of TrpL and proposed to be responsible for the transient nature of the light-activated current in the photoreceptors lacking a functional Trp (25), a direct proof that CaM is involved in regulating the activity of Trp channels is still lacking. On the other hand, Ca2+ and CaM are involved in regulating native SOCs. Ca2+-dependent inactivation has been documented for SOCs found in lymphocytes (26), pancreatic acinar cells (27), basophilic leukemia cells (28, 29), and submandibular gland cells (30). CaM has been shown to inhibit a SOC in bovine vascular endothelial cells by delaying its activation and promoting its inactivation (31).
The present studies were initiated to test the hypothesis that mammalian Trp homologues are regulated by Ca2+/CaM. We report here the presence of a CaM-binding site in the C terminus of Trp3 that overlaps with the one recently shown to interact with two regions of IP3R3 (9). Assays for a functional correlate of the interaction of CaM with Trp3, carried out by patch-clamp recordings from inside-out membrane patches, showed that Trp3 has strong intrinsic activity that is suppressed by CaM. Our data suggest that IP3R-mediated displacement of CaM from the CaM/IP3R binding (CIRB) site is involved in the activation of Trp3. This scenario provides a model for the mechanism of activation of Trp-based ion channels, which may be applicable to both Gq/phospholipase C-activated as well as store-operated Ca2+ entry channels.
Materials and Methods
DNA Constructs and Pull-Down Experiments.
Fragments of IP3R3 and Trp3 were generated by PCR and were subcloned into either pGEX4T-1 (Amersham Pharmacia) for the expression of glutathione S-transferase (GST) fusion proteins or pAGA (32) for in vitro synthesis of 35S-labeled proteins. Mutant IP3R3-F2v was made by PCR through incorporation of the nucleotide changes in the antisense primers. Maltose-binding protein (MBP) cDNA was amplified from pMAL-C2 (New England Biolabs) by PCR, and the coding sequence for its first 322 residues was placed in-frame before the Trp3 C-terminal fragments. Preparation of GST fusion proteins, 35S-labeled Trp3, and MBP fusion proteins and procedures for pull-down experiments are as described (33). The binding buffer used for IP3R3–Trp interaction contains 100 mM KCl, 2 mM MgCl2, 0.5% Lubrol, and 20 mM Tris⋅HCl (pH 7.5). The buffers used for CaM–Trp interaction contain 120 mM KCl, 0.5% Lubrol, 20 mM Tris⋅HCl, 10 mM EGTA or HEDTA, and added CaCl2 to give rise to desired free Ca2+ concentrations according to the maxchelator program (C. Patton, Stanford University). The pH was adjusted to 7.5. The free Ca2+ concentration in each buffer was then determined by ratiometric fluorescence measurement with the use of Fura-2FF (TEF-Labs, Austin, TX).
Peptides and Affinity Measurement for Trp–CaM Interaction.
Peptides F2v (EYLSIEYSEEEVWLTWTD) and F2vmut (EYLSIEYSEEEVGLTGTD) were synthesized by Research Genetics (Huntsville, AL). Peptide C14 (SFNSILNQPTRYQQIMKRLIKRYVLKAQVD) was synthesized by Waterloo Peptide Synthesis (University of Waterloo, Ontario). All fluorescence measurements were performed on a Perkin–Elmer LS5 Spectrofluorometer at 22°C. For peptide binding with dansyl-CaM, increasing concentrations of C14 or F2v were added to 1 ml of solution containing 100 nM dansyl-CaM, 200 mM 4-morpholinepropanesulfonic acid (pH 7.0), 90 mM KCl, 2 mM EGTA, and 100 μM free Ca2+. Samples were excited at 340 nm, and emission at 490 nm was monitored. Phosphodiesterase (PDE) activity was assayed by monitoring the hydrolysis of fluorescent 2′-methylanthraniloyl derivative of cGMP (Mant-cGMP) (8 μM) (45) in 1 ml of solution containing 200 mM 4-morpholinepropanesulfonic acid (pH 7.0), 90 mM KCl, 3 mM MgCl2, 2 mM EGTA, 10 μM free Ca2+, 25 nM CaM, and the desired amount of peptide. Samples were excited at 330 nM, and emission at 450 nm was measured.
Cell Lines and Transfection.
HEK 293 cells stably expressing hTrp3 (T3–9 cells) and culture conditions were as described (34). For transient transfection of mutant or wild-type CaM, the Trp3 cells were seeded in 35-mm culture dishes at 10,000 cells per dish for 24 h. Two micrograms of pcDNA3 vector containing either the cDNA for rat CaM(EFmut)4 or that for the wild-type CaM was mixed with 0.4 μg pEGFP-N1 (CLONTECH) and transfected into the cells with the use of Lipofectamine Plus reagent (GIBCO), following the manufacturer's protocol. For the expression of mutant Trp3, HEK 293 cells were seeded and Trp3ΔC8 in pcDNA3 was cotransfected with pEGFP-N1. Cells that acquired the expression of green fluorescence were used in electrophysiological studies 48–96 h after transfection.
Recording from Inside-Out Patches.
Cells were bathed in a Ringer's solution containing 140 mM NaCl, 10 mM KCl, 1 mM MgCl2, 2 mM CaCl2, and 10 mM Hepes (pH 7.5). The pipette solution contained 140 mM Na⋅Hepes, 5 mM NaCl, and 2 mM CaCl2 (pH 7.5). Pipettes were pulled from type 7052 borosilicate glass (Garner Glass Company, Claremont, CA) and heat-polished to tip resistances of 9–11 MΩ in the above solution. After we made a tight seal and established the cell-attached configuration, a Ca2+-free intracellular solution containing 140 mM potassium gluconate, 5 mM NaCl, 1 mM MgCl2, 5 mM EGTA, and 10 mM Hepes (pH 7.5) was perfused onto the cells. Patches were excised and continuously perfused with the internal solution. The desired amount of CaCl2 was added to the internal solution to make final concentrations of 0.18, 1.8, and 18 μM free Ca2+; calculated according to the method of Fabiato (35); and confirmed by measurement of free Ca2+ concentration with the use of Fura-2. The stock solutions for calmidazolium (CMZ), RS-20, CaM, and F2v were 10, 5, 0.2, and 1 mM, respectively. They were diluted to the final concentration in the internal solution containing no Ca2+ (for F2v) or 18 μM free Ca2+ (for others) and perfused to the cytoplasmic side of the excised patches. External voltage command control was used to control the holding potentials, and current was recorded at a sampling frequency of 5 kHz and filtered at 1 kHz for generally 15 s for each sweep. Data recorded at −60 mV were analyzed with pclamp8 (Axon Instruments, Foster City, CA) after digital filtering at 500 Hz and baseline adjustment. The mean current was calculated first as the time integral of current flow (i.e., total charge movement) of every 2,000 data points with the use of analysis, a custom-made program (E.S. and F. Bezanilla, unpublished software) and then divided by the duration used for the integration (0.4 s). Channel activities are expressed by time plots of the mean currents in 0.4-s bins for representative patches and by averages ± SEM of the mean currents of 30-s to 150-s recordings from indicated numbers of patches.
Results
CaM and IP3R Bind to an Overlapping Site on Trp3.
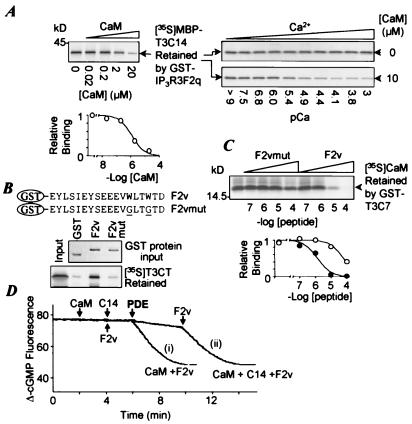
A previous study showed that a GST fusion protein containing the C7 fragment of Trp3 (T3C7, M742-E795, Fig. 1A) interacted with IP3R3 (9). We now find that GST-T3C7 also interacts with 35S-labeled CaM (Fig. 1B). Although a very weak interaction was seen under Ca2+-free conditions with 10 mM EGTA and no added Ca2+, more than 200 times more CaM was retained in the presence of 10 μM free Ca2+. A mutant CaM, CaM(EFmut)4, containing aspartate-to-alanine substitutions in all four of its EF hands (36), interacted weakly with T3C7 in a Ca2+-independent manner (Fig. 1B). In another set of experiments, we tested the interactions between 35S-labeled Trp3 fragments and CaM-Sepharose. The C terminus (N725-E848) but not the N terminus (M1-A304) of Trp3 interacted with CaM-Sepharose (not shown). We tested smaller regions of T3C7 for the interaction with IP3R3 and CaM. MBP fusion proteins were used to facilitate the labeling and separation of the Trp3 fragments. Although MBP interacted very weakly with GST-IP3R3-F2q, a fragment containing the first and stronger of the two Trp-binding sites of IP3R3 (9), and not at all with CaM (Fig. 1C), the fusion protein containing T3C14 (E761-E795) interacted strongly with both GST-IP3R3-F2q and CaM (Fig. 1C). Smaller T3 fragments, C15 and C8, had weaker interactions with IP3R3 and CaM as compared with C14 (Fig. 1C).
Figure 1.
Colocalization of the IP3R-binding domain and the CaM-interaction site on Trp3. (A) Diagram of hTrp3 and constructs of fusion proteins used for binding assays. Shaded boxes in the full-length hTrp3 indicate locations of transmembrane segments. (B) Interaction of T3C7 with CaM and CaM(EFmut)4. 35S-labeled wild type and the mutant CaM were incubated with GST-T3C7 bound to glutathione-Sepharose in the absence or presence of 10 μM Ca2+. For all binding experiments, input represents 40% of 35S-labeled protein added to the binding assay. (C) Interaction of Trp3 C-terminal fragments with IP3R3-F2q and CaM. 35S-labeled MBP or MBP fusion proteins were incubated with GST-IP3R3-F2q bound to glutathione-Sepharose or with CaM-Sepharose. Autoradiograms show the input 35S-labeled proteins (Top) and those retained by GST-F2q (Middle) or CaM (Bottom). [Ca2+] = 10 μM for testing binding with CaM. (D) Ca2+ dependence of CaM–Trp3 interaction. Binding buffers containing different free Ca2+ concentrations were used in assays to pull down [35S]MBP (▴) or [35S]MBP-T3C14 (○) with CaM-Sepharose. After separation by SDS/PAGE, the radioactivity of [35S]MBP and [35S]MBP-T3C14 retained was quantified by PhosphorImaging. Data, shown as fold increases over the values obtained in 10 mM EGTA with no added Ca2+, are averages ± SEM of three experiments. The curve is a least-squares fit of the Hill equation, I/Imax = Cn/[Cn + (K1/2)n], where I/Imax is the relative binding, C is Ca2+ concentration, n is the Hill coefficient, and K1/2 is the Ca2+ concentration that gives half-maximal binding. The autoradiogram shows a representative experiment. (E) Peptide C14 (○) but not F2v (▴; see Fig. 2C for details) increased the fluorescence emission of dansyl-CaM at 490 nm. Data are averages ± SD of three titrations. (F) Peptide C14 inhibited PDE activity stimulated by 25 nM CaM. Data are averages ± SD of three experiments.
Fig. 1D shows a dose-dependent effect of Ca2+ on T3C14 binding to CaM. Half-maximal binding to CaM occurred at 6.7 μM Ca2+ with a Hill coefficient of 1.5. A synthetic peptide representing the C14 region of Trp3, peptide C14 (S764-D793), dose dependently increased the fluorescence emission of dansyl-CaM, with a half-maximal increase at 58 nM (Fig. 1E). The peptide also inhibited the CaM-activated PDE activity with half-maximal inhibition (IC50) at 0.33 μM (Fig. 1F). The dissociation constant (Kd) for C14 binding to Ca2+/CaM, calculated from the activation curves of PDE by CaM in the absence and the presence of the peptide (37), was 0.1 μM.
Interaction of Trp3 with CaM and That with IP3R3 Are Mutually Exclusive.
We next attempted to determine whether there is direct competition between CaM and IP3R3 for binding to Trp3. Addition of CaM reduced the binding of [35S]MBP-T3C14 to GST-IP3R3-F2q in a dose-dependent manner, with an IC50 of 1.2 μM (Fig. 2A Left). The effect of CaM was Ca2+ dependent. At low Ca2+ concentrations, 10 μM CaM did not significantly inhibit the interaction between T3C14 and IP3R3-F2q, whereas at higher Ca2+ concentrations, it caused a Ca2+ dose-dependent inhibition of the interaction (Fig. 2A Right).
Figure 2.
Direct competition between CaM and IP3R3 for binding to Trp3. (A) (Left) CaM inhibits Trp3 binding to IP3R3. Varying concentrations of CaM were included in the binding reaction with [35S]MBP-T3C14 and GST-F2q in the presence of 10 μM free Ca2+. (Upper) An autoradiogram. (Lower) The results of PhosphorImaging analysis of the relative amount of [35S]MBP-T3C14 retained. Data are averages of three experiments. The curve is the least-squares fit of the equation y = 1/(1 + [P]/IC50), where y is the relative binding, [P] is the CaM concentration, and IC50 is the concentration for 50% inhibition. (Right) Ca2+ dependence of CaM inhibition of Trp3-IP3R3 interaction. Experiments similar to those at Left, except that varying concentrations of Ca2+ were used. The buffers contained either no (Upper) or 10 μM CaM (Lower). (B) Amino acid compositions of the wild-type and mutant IP3R3-F2v. (Lower) The amount of GST, GST-F2v, or GST-F2vmut used in the binding assay as revealed by Coomassie blue staining (Upper box) and [35S]Trp3C terminus (T3CT) retained by the GST and GST fusion proteins as revealed by autoradiography (Lower box). (C) Peptide F2v inhibits binding of CaM to T3C7. Varying concentrations of peptide F2v or F2vmut were included in the binding reaction with [35S]CaM and GST-T3C7 in the presence of 10 μM free Ca2+. (D) Peptide F2v competes with CaM for interaction with the Trp3 CIRB site in the PDE assay. See the text for details.
The last 18 residues of IP3R3-F2q (F2v, E681-D698) were sufficient to bind to the Trp3 C terminus (N725-E848, Fig. 2B). Substitution of the two tryptophans in IP3R3-F2v (W693, W696) with glycines (F2vmut, Fig. 2B), alanine, or arginine (not shown) almost completely eliminated the binding. Synthetic IP3R3-F2v and F2vmut (W693/696G) peptides were used to examine the effect of the Trp-binding domain on the interaction between [35S]CaM and GST-T3C7. Fig. 2C shows that the F2v peptide inhibited the interaction of CaM with T3C7 with an IC50 of 1.4 μM. This inhibition occurred even at a Ca2+ concentration that is optimal for the Trp3–CaM interaction. F2vmut had about 40-fold lower potency (IC50 = 62 μM). The competition of peptide F2v with CaM for binding to the Trp3 CIRB domain was also demonstrated with the PDE assay. When PDE was added in the presence of CaM and peptide F2v, rapid hydrolysis caused a decrease in Mant-cGMP fluorescence [Fig. 2D, curve (i)]. Without F2v, 2.5 μM peptide C14 inhibited PDE activity by 95%. Subsequent addition of 75 μM peptide F2v caused a rapid hydrolysis of cGMP [Fig. 2D, curve (ii)], indicating that the interaction of F2v with C14 released CaM, which then was able to activate PDE.
Ca2+ Inhibits Trp3 Activity.
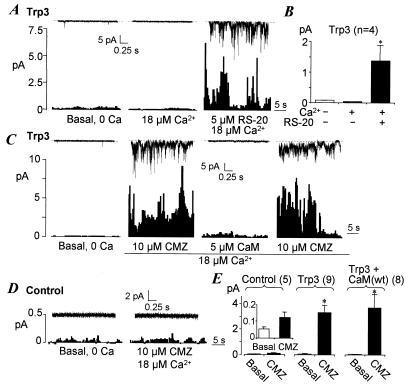
To examine how Ca2+, CaM, and the Trp-binding domain of IP3R3 regulate Trp3 function, we performed single-channel measurements with the use of inside-out membrane patches excised from an HEK 293 cell line expressing hTrp3. This cell line was used in previous studies demonstrating that Trp3 was activated by the N-terminal third of IP3R1 in the presence of IP3 (10, 11). These studies showed that when patches were excised to an intracellular solution containing 100 nM free Ca2+ but no IP3, there was no Trp3 activity. We reasoned that at rest, the CIRB site could bind to either IP3R or CaM. If CaM were bound to the channel, then the basal activity would be sensitive to changes in intracellular Ca2+ concentrations. We thus excised the patches into a Ca2+-free intracellular solution containing 5 mM EGTA and no added Ca2+. Under these conditions, about one-third of inside-out patches from the Trp3 cell line displayed varying degrees of activity with single-channel behaviors similar to those elicited by IP3 (Fig. 3A) (10, 11). The channel had relatively large single-channel amplitudes and a very brief mean open time (Fig. 3 B and C), typical of that formed by Trp3 (10, 11, 18). The open probability for the outward current recorded at the transmembrane potential of +60 mV was more than 10 times higher than that for the inward current recorded at −60 mV. This finding agrees with the observation for single-channel activities of Trp3 activated by agonist or IP3 (10). Because the inward currents carried Ca2+ and Na+ and the outward currents carried only K+, the lower open probability for the inward current suggests that the channel tends to be inhibited by Ca2+ entering through the channel. Indeed, application of 180 nM and 1.8 μM Ca2+ to the cytoplasmic side of the patches inhibited the activity by 83% and 93%, respectively (Fig. 3 D and E). Nontransfected control HEK 293 cells or cells expressing unrelated proteins did not show any Trp3-like single-channel activity whether or not Ca2+ was present at the cytoplasmic side (not shown).
Figure 3.
Effects of Ca2+ and CaM mutant on Trp3 activity in inside-out patches. (A–C) Single-channel activities in patches excised from Trp3-expressing HEK 293 cells to a Ca2+-free solution. (A) Representative traces recorded from a patch at a transmembrane potential of +60 or −60 mV. (B and C) Amplitude (B) and open time (C) histograms generated from idealized traces encompassing 90-s recordings. Note the actual open time is very short for Trp3 (<0.2 ms) (18), and thus there are many incompletely resolved events in the records. Because of this, the mean amplitudes obtained from Gaussian fits are underestimations, and the mean open times obtained by fitting with one exponential are overestimations of the real values. (D) Effect of Ca2+ on the basal Trp3 single-channel activity in excised inside-out patches. A patch held at −60 mV was perfused sequentially with intracellular solution containing 5 mM EGTA and no added Ca2+ (0 Ca2+) and then a solution containing 1.8 μM Ca2+. Current recovered in 0 Ca2+. Time plots show mean currents under each condition in 0.4-s bins. Representative traces are shown above the plots. Dashed lines indicate zero current levels. (E) Averages ± SEM (n = 11) of inhibition of Trp3 basal activity by Ca2+. Relative activity was calculated based on NPo, which is defined as mean current/unitary current amplitude and was calculated from idealized traces with the use of pclamp8. (F) Expression of a mutant (Upper) but not wild-type (Lower) CaM increased Trp3 basal activity in excised patches. For cells overexpressing the wild-type CaM, there was no activity under both conditions and therefore the time plots show baseline fluctuations. (G) Average ± SEM of mean currents for numbers of patches shown in parentheses. P < 0.01 different from Trp3 alone (*) or from 0 Ca2+ (**) by Student's t test.
Removing CaM Activates Trp3.
The high sensitivity of Trp3 activity to Ca2+ suggests that CaM may be tethered to the channel complex in excised patches, and this association was not disrupted even though the cytoplasmic side of the membrane was continuously perfused with a Ca2+-free solution. Removing CaM from Trp3 might unblock the channel and hence enhance its activity. We used the CaM mutant CaM(EFmut)4, which interacted weakly with Trp3 in a Ca2+-independent manner (Fig. 1B), to test this possibility. Expression of CaM(EFmut)4 in the Trp3 cell line caused a large increase in basal activity in the inside-out patches excised to the Ca2+-free solution (Fig. 3 F and G). Each patch had multiple open levels that showed mostly flickers of brief openings. In contrast to the cells that expressed Trp3 alone, raising Ca2+ to 1.8 μM at the cytoplasmic side of cells expressing Trp3 plus CaM(EFmut)4, instead of inhibiting the current, enhanced the activity by about 3-fold. This Ca2+-dependent increase in channel activity resembles that observed for Trp3 expressed in Chinese hamster ovary cells by intranuclear cDNA injection (18) and thus is likely to be an effect unrelated to the inhibition by Ca2+/CaM. As a control, Trp3 cells transfected with the wild-type CaM did not show any basal activity in the inside-out patches (n = 10), and increasing Ca2+ at the cytoplasmic side did not cause the activation of Trp3 (Fig. 3 F and G). These data suggest that CaM is closely associated with Trp3 under basal conditions, and displacing CaM with the EF hand mutant results in both spontaneous activation of the channel and its potentiation by Ca2+.
Next, we attempted to determine whether displacing CaM by other means could also activate Trp3. First, a synthetic peptide containing the CaM binding site of chicken smooth muscle myosin light-chain kinase, RS-20, binds to CaM with a Kd of approximately 1 nM in the presence of Ca2+ (38, 39). Such a high affinity might allow RS-20 to compete with Trp3 for CaM binding and displace the CaM bound to Trp3. Because the binding of RS-20 to CaM is Ca2+-dependent, we included 18 μM Ca2+ in the solution. As expected, 18 μM Ca2+ alone only caused the inhibition of Trp3 activity (Fig. 4 A and B). However, when 5 μM RS-20 was applied together with 18 μM Ca2+, a large activity became evident (Fig. 4 A and B). The RS-20-stimulated activity was similar to that obtained in cells expressing CaM(EFmut)4. Second, we used a CaM antagonist, CMZ, to inactivate CaM in the excised patches. Perfusion of 10 μM CMZ to the cytoplasmic side of the patches in the presence of 18 μM Ca2+ also strongly activated Trp3 (Fig. 4 C and E). There was no significant difference in the properties of the channel when it was activated by RS-20 or CMZ. The effect of CMZ was reversed by the addition of 5 μM CaM (≈84% inhibition, n = 5) and recovered upon reapplication of CMZ (Fig. 4C). When treated with 10 μM CMZ, the activities of patches from T3 cells overexpressing the wild-type CaM were similar to those in patches from cells expressing Trp3 alone (Fig. 4E), further substantiating the conclusion that the diminished basal activity in the Trp3 cells transfected with CaM was due to the function of CaM. On the other hand, although CMZ treatment caused in average about a 130% increase in the mean current in patches excised from control HEK 293 cells, with P = 0.13 by Student's t test, the difference was not statistically significant (Fig. 4 D and E). The records for patches from the control cells typically became “noisier” after the CMZ treatment. However, at the single-channel level, we were unable to resolve the activity in control cells, probably because the endogenous SOC has a very small conductance (10). Therefore, it is possible that inactivating CaM also activates the endogenous channels in the HEK cells. However, more sophisticated measurements are needed to verify this possibility.
Figure 4.
Removing or inactivating CaM activates Trp3. (A and B) Effect of RS-20, the CaM-binding domain of myosin light chain kinase. (A) Trp3 activity at −60 mV in a representative patch. Treatments are indicated under the mean current time plots. Representative traces are shown on the top. (B) Average ± SEM for four patches excised from Trp3 cells. (C–E) Effect of calmidazolium (CMZ). Currents at −60 mV for representative patches excised from Trp3 and control HEK cells are shown in C and D, respectively. (E) Averages ± SEM of mean currents for control, Trp3, and Trp3 cells overexpressing CaM before and after CMZ treatment. (Inset) The activities in control cells on an enlarged scale. Numbers of cells used are indicated in parentheses. For B and E, *, P < 0.01 different from basal by Student's t test.
A Trp-Binding Peptide Derived from IP3R3 Activates Trp3.
We applied 5 μM peptide F2v to the cytoplasmic side of the excised patches to examine the effect of the Trp-binding domain on Trp3 function. Fig. 5 A and D shows that a channel similar to that activated by removing or inactivating CaM was activated by the peptide. The addition of 5 μM CaM inhibited the Trp3 activity stimulated by peptide F2v by about 92% (n = 3, Fig. 5A), indicating that Ca2+/CaM also mediates the inactivation of Trp3. In contrast to F2v, the mutant peptide F2vmut did not affect Trp3 activity (Fig. 5 B and D). Again, for the control cells there was an average of a 2-fold increase in the mean current after treatment with peptide F2v (Fig. 5 C and D). However, the difference was not statistically significant (P = 0.19), making it difficult to conclude whether a similar gating mechanism also works for the endogenous channels.
Figure 5.
Effect of F2v, a Trp-binding domain of IP3R3, on Trp3 activity. (A) Activation of Trp3 by peptide F2v. The activity was blocked by CaM and recovered upon the readdition of F2v. A representative experiment is shown with the use of the same format as in Fig. 4A. (B and C) Representative experiments for F2vmut applied to patches from Trp3 cells (B) and F2v applied to control cell patches (C). (D) Averages ± SEM of mean currents before and after the application of peptide F2v or F2vmut for Trp3 and control cells. (E and F) Activation of a CaM-insensitive mutant of Trp3 by peptide F2v. (E) Diagram of the Trp3ΔC8 mutant with the deleted region marked. A representative experiment is shown for a patch excised from HEK 293 cells transiently expressing Trp3ΔC8 exposed sequentially to CMZ and to peptide F2v. (F) Averages ± SEM of mean currents for numbers of patches indicated in parentheses. The transmembrane potential was −60 mV. For D and F, *, P < 0.01 different from basal by Student's t test. (G) Diagram of MBP fusion proteins containing Trp3C2 or Trp3C2ΔC8 (Left) and results of a pull-down experiment showing that the ΔC8 mutant was precipitated by GST-IP3R3-F2q but not by Ca2+/CaM (Right). Experimental conditions were as described in Fig. 1C.
The CIRB Site of Trp3 Is Required for Its Regulation by CaM.
CaM interacts with many proteins, including IP3Rs (40). The effect of CMZ, RS-20, or CaM(EFmut)4 on Trp3 activity could be unrelated to the association of CaM at the CIRB site but result from the inhibition of CaM binding to other proteins, which would in turn activate Trp3. To find out whether the CIRB site is required for regulation by CaM, we expressed in HEK cells the ΔC8 form of Trp3, Trp3ΔC8, which lacks the binding to CaM but retains the interaction with IP3R3 (Fig. 5G), and measured its activity in excised patches. No current was elicited by perfusion with the Ca2+-free intracellular solution. Treatment with CMZ increased the activity only slightly, which is similar to the effect of CMZ on patches from control cells. On the other hand, application of 5 μM F2v activated Trp3-like currents (Fig. 5 E and F), indicating that the mutant is functional. Subsequent application of CaM did not inhibit the current (not shown). These results indicate unequivocally that the effect of CaM removal on the activity of wild-type Trp3 is mediated by antagonizing the CaM that is bound to the CIRB site of the channel.
Discussion
The finding that IP3R and Ca2+/CaM compete for a common binding site on Trp3 (here referred to as the CIRB site) suggests that CaM plays an intrinsic role in the activation of Trp3 by IP3Rs. Our functional data indicate that Ca2+/CaM has a general inhibitory effect on Trp3 function, whereas peptide F2v, an 18-aa peptide that represents one of the Trp-binding domains of IP3R3 and competes with CaM for binding to the CIRB site, activates Trp3 in the absence of IP3 and Ca2+. In addition, removing or inactivating CaM caused similar or maybe even stronger activation of Trp3. The data shown in Figs. 3–5 were inward currents for Trp3 recorded at −60 mV. Similar results with outward current activities were obtained at +60 mV (not shown). In aggregate, these data suggest that displacing CaM from the CIRB site by activated IP3Rs is an important step for Trp3 activation. The gating of Trp3 by IP3Rs and CaM is thus another example of channel regulation through competitive association of the regulatory protein to a binding site that is also recognized by CaM. A similar mechanism has been shown for the olfactory cyclic nucleotide-gated channel (41) and the N-methyl-d-aspartate glutamate receptor (42, 43).
Several questions remain on how the competition between IP3Rs and CaM gates the Trp3 channel. First, although IP3Rs contribute to/or trigger channel activation by displacing inhibitory CaM from Trp, this may not be the only contribution of IP3Rs to Trp3 gating. Our data do not exclude the possibility that the full-length IP3R may further increase Trp activity, beyond that obtained by CaM removal. Second, although CaM seems to be important for keeping the channel inactive, it is not clear how CaM is associated with the channel under unstimulated conditions, inasmuch as CaM binding to the isolated CIRB site is Ca2+ dependent. The dominant negative effect of the EF hand mutant suggests that CaM is tethered to full-length Trp3 in a Ca2+-independent manner. Such constitutive association has also been suggested for tethering CaM to the L-type Ca2+ channel (33, 44). However, the exact mechanism remains unknown. Third, it is unclear why Trp3ΔC8, which is incapable of binding to CaM, is not spontaneously active. Perhaps a totally CaM-free Trp3 is less susceptible to spontaneous opening than the channel that has just lost CaM. In this sense, CaM may facilitate the opening of Trp3 by stabilizing the channel in a conformation that has a high tendency toward opening spontaneously, although only in the absence of CaM.
Acknowledgments
We wish to honor Dr. J. D. J. for his encouragement and contribution. We thank Drs. J. Enyeart, S. Herness, D. Kunze, and K. Kiselyov for advice on patch-clamp recordings and data analysis; Drs. J. Oberdick and K. Groschner for critical discussion of the manuscript; Ms. D. Chuang for technical assistance; and Drs. J. P. Adelman and K. Mikoshiba for cDNA of mutant CaM, IP3R1, and IP3R3. The work was supported in part by National Institutes of Health Grants GM54235 to M.X.Z., DK33727 to J.D.J., and HL45198 to L.B.
Abbreviations
- CaM
calmodulin
- CIRB
CaM/IP3 receptor binding
- CMZ
calmidazolium
- GST
glutathione S-transferase
- hTrp3
human Trp3
- IP3
inositol 1,4,5-trisphosphate
- IP3R
IP3 receptor
- MBP
maltose-binding protein
- PDE
phosphodiesterase
- SOC
store-operated channel
- Trp
transient receptor potential
References
- 1.Putney J W., Jr Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 2.Berridge M J. Biochem J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Putney J W, Jr, McKay R R. BioEssays. 1999;21:38–46. doi: 10.1002/(SICI)1521-1878(199901)21:1<38::AID-BIES5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, et al. Proc Natl Acad Sci USA. 1996;93:15195–15202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, Birnbaumer L. Cell. 1996;85:661–671. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann T, Schaefer M, Schultz G, Gudermann T. J Mol Med. 2000;78:14–25. doi: 10.1007/s001099900070. [DOI] [PubMed] [Google Scholar]
- 7.Berridge M J, Lipp P, Bootman M D. Science. 2000;287:1604–1605. doi: 10.1126/science.287.5458.1604. [DOI] [PubMed] [Google Scholar]
- 8.Putney J W., Jr Proc Natl Acad Sci USA. 1999;96:14669–14671. doi: 10.1073/pnas.96.26.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulay G, Brown D, Qin N, Jiang M, Dietrich A, Zhu M X, Chen Z, Birnbaumer M, Mikoshiba K, Birnbaumer L. Proc Natl Acad Sci USA. 1999;96:14955–14960. doi: 10.1073/pnas.96.26.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiselyov K, Xu X, Kuo T H, Mozhayeva G, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Nature (London) 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- 11.Kiselyov K, Mignery GA, Zhu M X, Muallem S. Mol Cell. 1999;4:423–429. doi: 10.1016/s1097-2765(00)80344-5. [DOI] [PubMed] [Google Scholar]
- 12.Irvine R F. FEBS Lett. 1990;263:5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- 13.Lockwich T P, Liu X, Singh B B, Jadlowiec J, Weiland S, Ambudkar I S. J Biol Chem. 2000;275:11934–11942. doi: 10.1074/jbc.275.16.11934. [DOI] [PubMed] [Google Scholar]
- 14.Rosado J A, Sage S O. Biochem J. 2000;350:631–635. [PMC free article] [PubMed] [Google Scholar]
- 15.Obukhov A G, Harteneck C, Zobel A, Harhammer R, Kalkbrenner F, Leopoldt D, Luckhoff A, Nurnberg B, Schultz G. EMBO J. 1996;15:5833–5838. [PMC free article] [PubMed] [Google Scholar]
- 16.Estacion M, Sinkins W G, Schilling W P. Biochem J. 1999;341:41–49. [PMC free article] [PubMed] [Google Scholar]
- 17.Obukhov A G, Schultz G, Luckhoff A. Neuroscience. 1998;85:487–495. doi: 10.1016/s0306-4522(97)00616-7. [DOI] [PubMed] [Google Scholar]
- 18.Zitt C, Obukhov A G, Strubing C, Zobel A, Kalkbrenner F, Luckhoff A, Schultz G. J Cell Biol. 1997;138:1333–1341. doi: 10.1083/jcb.138.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chyb S, Raghu P, Hardie R C. Nature (London) 1999;397:255–259. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann T, Obukhov A G, Schaefer M, Harteneck C, Gudermann T, Schultz G. Nature (London) 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 21.Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imoto K, et al. J Biol Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- 22.Lintschinger B, Balzer M, Romanin C, Zhu M X, Groschner K. J Biol Chem. 2000;275:27799–27805. doi: 10.1074/jbc.M002705200. [DOI] [PubMed] [Google Scholar]
- 23.Warr C G, Kelly L E. Biochem J. 1996;314:497–503. doi: 10.1042/bj3140497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trost C, Marquart A, Zimmer S, Philipp S, Cavalie A, Flockerzi V. FEBS Lett. 1999;451:257–263. doi: 10.1016/s0014-5793(99)00588-8. [DOI] [PubMed] [Google Scholar]
- 25.Scott K, Sun Y, Beckingham K, Zuker C S. Cell. 1997;91:375–383. doi: 10.1016/s0092-8674(00)80421-3. [DOI] [PubMed] [Google Scholar]
- 26.Zweifach A, Lewis R S. J Gen Physiol. 1995;105:209–226. doi: 10.1085/jgp.105.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louzao M C, Ribeiro C M P, Bird G S J, Putney J W., Jr J Biol Chem. 1996;271:14807–14813. doi: 10.1074/jbc.271.25.14807. [DOI] [PubMed] [Google Scholar]
- 28.Parekh A B. J Biol Chem. 1998;273:14925–14932. doi: 10.1074/jbc.273.24.14925. [DOI] [PubMed] [Google Scholar]
- 29.Fierro L, Parekh A B. J Membr Biol. 1999;168:9–17. doi: 10.1007/s002329900493. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, O'Connell A, Ambudkar I S. J Biol Chem. 1998;273:33295–33304. doi: 10.1074/jbc.273.50.33295. [DOI] [PubMed] [Google Scholar]
- 31.Vaca L. FEBS Lett. 1996;390:289–293. doi: 10.1016/0014-5793(96)00675-8. [DOI] [PubMed] [Google Scholar]
- 32.Sanford J, Codina J, Birnbaumer L. J Biol Chem. 1991;266:9570–9579. [PubMed] [Google Scholar]
- 33.Qin N, Olcese R, Bransby M, Lin T, Birnbaumer L. Proc Natl Acad Sci USA. 1999;96:2435–2438. doi: 10.1073/pnas.96.5.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu X, Jiang M, Birnbaumer L. J Biol Chem. 1998;273:133–142. doi: 10.1074/jbc.273.1.133. [DOI] [PubMed] [Google Scholar]
- 35.Fabiato A. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- 36.Xia X M, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen J E, Ishii T, Hirschberg B, Bond C T, Lutsenko S, et al. Nature (London) 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- 37.Erickson-Viitanen S, DeGrado W F. Methods Enzymol. 1987;139:455–478. doi: 10.1016/0076-6879(87)39106-2. [DOI] [PubMed] [Google Scholar]
- 38.Lukas T J, Burgess W H, Prendergast F G, Lau W, Watterson D M. Biochemistry. 1986;25:1458–1464. doi: 10.1021/bi00354a041. [DOI] [PubMed] [Google Scholar]
- 39.Johnson J D, Snyder C, Walsh M, Flynn M. J Biol Chem. 1996;271:761–767. doi: 10.1074/jbc.271.2.761. [DOI] [PubMed] [Google Scholar]
- 40.Michikawa T, Hirota J, Kawano S, Hiraoka M, Yamada M, Furuichi T, Mikoshiba K. Neuron. 1999;23:799–808. doi: 10.1016/s0896-6273(01)80037-4. [DOI] [PubMed] [Google Scholar]
- 41.Varnum M D, Zagotta W N. Science. 1997;278:110–113. doi: 10.1126/science.278.5335.110. [DOI] [PubMed] [Google Scholar]
- 42.Zhang S, Ehlers M D, Bernhardt J P, Su C T, Huganir R L. Neuron. 1998;21:443–453. doi: 10.1016/s0896-6273(00)80553-x. [DOI] [PubMed] [Google Scholar]
- 43.Krupp J J, Vissel B, Thomas C G, Heinemann S F, Westbrook G L. J Neurosci. 1999;19:1165–1178. doi: 10.1523/JNEUROSCI.19-04-01165.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peterson B Z, DeMaria C D, Yue D T. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- 45.Johnson J D, Walters J D, Mills J S. Anal Biochem. 1987;162:291–295. doi: 10.1016/0003-2697(87)90039-x. [DOI] [PubMed] [Google Scholar]