Abstract
Background
Nosocomial infections are an important cause of morbidity and mortality in the surgical intensive care unit (SICU). Clinical benefits of glutamine-supplemented parenteral nutrition may occur in hospitalized surgical patients, but efficacy data in different surgical subgroups are lacking. The objective was to determine whether glutamine-supplemented parenteral nutrition differentially affects nosocomial infection rates in selected subgroups of SICU patients.
Methods
This was a double-blind, randomized, controlled study of alanyl-glutamine dipeptide-supplemented parenteral nutrition in SICU patients requiring parenteral nutrition and SICU care after surgery for pancreatic necrosis, cardiac, vascular, or colonic surgery. Subjects (n = 59) received isocaloric/isonitrogenous parenteral nutrition, providing 1.5 g/kg/d standard glutamine-free amino acids (STD-PN) or 1.0 g/kg/d standard amino acids + 0.5 g/kg/d glutamine dipeptide (GLN-PN). Enteral feedings were advanced as tolerated. Nosocomial infections were determined until hospital discharge.
Results
Baseline clinical/metabolic data were similar between groups. Plasma glutamine concentrations were low in all groups and were increased by GLN-PN. GLN-PN did not alter infection rates after pancreatic necrosis surgery (17 STD-PN and 15 GLN-PN patients). In nonpancreatic surgery patients (12 STD-PN and 15 GLN-PN), GLN-PN was associated with significantly decreased total nosocomial infections (STD-PN 36 vs GLN-PN 13, P < .030), bloodstream infections (7 vs 0, P < .01), pneumonias (16 vs 6, P < .05), and infections attributed to Staphylococcus aureus (P < .01), fungi, and enteric Gram-negative bacteria (each P < .05).
Conclusions
Glutamine dipeptide-supplemented parenteral nutrition did not alter infection rates following pancreatic necrosis surgery but significantly decreased infections in SICU patients after cardiac, vascular, and colonic surgery.
Keywords: critical illness, glutamine, hospital infections, parenteral nutrition
Between 10% and 35% of intensive care unit (ICU) patients develop nosocomial infections, which are associated with prolonged hospitalization and high hospital mortality.1,2 Recent epidemiologic data show an annualized increase in incidence rates of hospital sepsis and antimicrobial-resistant organisms from 1979 to 2000.3-6 Patients requiring care in surgical ICU (SICU) settings exhibit the highest risk of hospital infection.6,7 This predisposition may be attributable to immune dysfunction8-10 and gut barrier dysfunction, with infection from gut-derived microorganisms.11-13
Nutrition depletion is common in SICU patients and is associated with immunosuppression, increased infection risk, and poor wound healing.14,15 Parenteral nutrition (PN) is routinely administered to SICU patients who cannot be adequately fed enterally. Unfortunately, use of PN in SICU settings has itself been associated with increased risk of nosocomial infection,16-19 possibly attributable to induction of hyperglycemia.16,20
Glutamine (GLN) is a central amino acid in major metabolic processes21-23 and is used as a major fuel substrate by gut epithelial cells and cells of the immune system.21-24 During catabolic illness, skeletal muscle exports large amounts of GLN into the blood, and GLN-using tissues markedly increase GLN uptake.21,23,25 If stress persists, GLN use exceeds endogenous production, and skeletal muscle and plasma GLN concentrations decline.21,25 Provision of conventional GLN-free PN or standard tube feedings (which typically contain low amounts of GLN) may not adequately support GLN requirements during serious illness.25-29
Free L-GLN is poorly soluble and not heat-stable; thus, GLN has not been a component of standard PN amino acid formulations.22 Heat-stable, soluble GLN dipeptides (alanyl-GLN and glycyl-GLN) are rapidly hydrolyzed to free amino acids in plasma.30 In controlled studies in catabolic patients, PN supplemented with either free GLN or GLN dipeptides has been associated with improved metabolic and clinical outcomes.25,27,31-45 However, several clinical trials have not shown clinical benefits with GLN-supplemented PN,46-52 and routine use remains controversial.53
The primary aim of this pilot study was to evaluate the efficacy of alanyl-GLN-supplemented PN to reduce hospital (nosocomial) infections in adults requiring postoperative SICU care and PN. Secondary aims included evaluation of infection site and presumed causative microorganisms, need for mechanical ventilation, SICU and hospital length of stay (LOS), and organ function. In light of the lack of data on GLN-supplemented PN in specific surgical subgroups, we selected 2 cohorts of patients for inclusion and subgroup analysis, those following major pancreatic surgery (pancreatic surgery cohort) and those following major cardiac, vascular, or colonic surgery (nonpancreatic surgery cohort), who required PN. Such patients are subpopulations with complicated postoperative courses and represent the minority of patients following these operations. We hypothesized that these 2 SICU surgical cohorts would differ in their response to GLN given differences in illness severity and postoperative systemic inflammatory responses. Our data show that GLN dipeptide-supplemented PN did not alter infection rates following pancreatic surgery, but significantly decreased nosocomial infections in patients following cardiac, vascular, and colonic surgery.
Methods
Protocol and Patients
The protocol was a double-blind, randomized, controlled trial approved by the Emory University Institutional Review Board and the U.S. Food and Drug Administration under Investigational New Drug (IND) no. 49,914 (for use of N(2)-l-alanyl-L-glutamine in PN to TRZ). Potential study subjects or their legally authorized representatives were approached for informed consent after the primary physicians had consulted the Emory University Hospital (EUH) Nutrition and Metabolic Support Team (NST) for management of postoperative PN in the SICU setting. Inclusion criteria were (1) signed informed consent; (2) age 18-80 years; (3) SICU care required after 5 types of operation, which were pancreatic necrosis surgery, coronary artery bypass grafting (CABG), cardiac valve surgery, nonneurologic vascular surgery, or colonic surgery; and (4) subject deemed by NST/investigators to likely require PN for at least 7 subsequent days based on NST/investigators’ assessment of underlying medical/surgical conditions (eg, presence of hemodynamic instability requiring high-dose pressor agents or use of an intra-aortic balloon pump to maintain blood pressure; significant GI bleeding, ileus, partial bowel obstruction, and/or intolerance to enteral feeding, manifested by emesis or significant diarrhea, etc). Exclusion criteria were (1) study PN not given for 5 or more consecutive days after initiation of study; (2) evidence of developing acute hepatic failure, based on standard liver function tests and clinical criteria; (3) evidence of acute, uncontrolled infection or history of clinical sepsis in previous 24 hrs; and (4) evidence of active malignancy, significant hepatic dysfunction (total bilirubin >4.0 mg/dL or >5-fold elevation in serum transaminase concentrations), or significant renal dysfunction (evolving acute renal failure or on dialysis). The subjects were block-randomized to 2 groups by the EUH Investigational Drug Service research pharmacist on the basis of type of operation: (1) the control group, whose subjects received standard PN (STD-PN); and (2) the experimental group, whose subjects received isocaloric, isonitrogenous, GLN dipeptide-supplemented PN (GLN-PN).
Nutrition Support
The control PN amino acid formula was a conventional GLN-free amino acid solution (15% Clinisol®, Baxter, Deerfield, IL), given at a dose of 1.5 g/kg/d. The GLN-PN group received PN containing 0.5 g/kg/d alanyl-GLN dipeptide (Dipeptiven®, Fresenius-Kabi, Bad Homburg, Germany) added to 1.0 g/kg/d 15% Clinisol AA solution (total 1.5 g/kg/d). This dose of alanyl-GLN dipeptide was based on results of previous safety and efficacy studies of GLN-supplemented PN.31-35 This dose of GLN dipeptide provides 23 g of L-GLN daily for a 70-kg subject (two-thirds of alanyl-GLN is the GLN moiety). The study amino acid solutions were designed to be essentially isonitrogenous; the STD-PN solution provides 2.35 g of nitrogen and the GLN-PN provides 2.45 g of nitrogen per 100 mL of amino acid solution.
The feeding goals were total daily energy intake (in kilocalories) at 1.3 × basal energy expenditure (predicted by the Harris-Benedict equation) from PN plus enteral intake and total amino acid/protein intake of 1.5 g/kg/d. PN dextrose was designed to comprise 70% of PN non–amino acid kilocalories and intravenous (IV) fat emulsion (20% Intralipid®, Baxter, Deerfield, IL) comprised 30% of PN non–amino acid kilocalories. PN contained electrolytes and standard micronutrients and was infused over a 24-hour period (1800-1800 hours) daily. Fat emulsion was infused over a 12-hour period daily (0600-1800 hours) as a separate infusion. The PN composition was adjusted by the investigators daily as indicated, based on usual NST criteria.
When clinically indicated, standard polymeric enteral tube feeds without GLN enrichment (Osmolite®, Ross Products Inc, Columbus OH, or Nutren 1.0, Nestle USA Inc, Deerfield, IL) were initiated as tolerated. PN was proportionally tapered and discontinued as enteral feeds were advanced. Enteral tube feeds were administered per conventional clinical criteria of the EUH NST. This included investigator discussions with the primary critical care attending physicians, physical evaluations by the investigating physicians, and review of medical charts for information on the patient’s clinical course. Advancement of tube feeds was achieved by increasing the ordered rate of administration by 10 mL/h every 8-12 hours until the goal rate was achieved, as tolerated. In the case of oral diet, advancement from clear liquids was progressed to full liquids to solid foods over 2-3 days, as tolerated. Good tolerance of enteral nutrition was evidenced by the absence of nausea, emesis, clinically significant diarrhea, GI bleeding, abdominal pain or distention, and other conventional clinical criteria. Enteral feedings were curtailed or discontinued because of ileus, partial bowel obstruction, GI bleeding, hemodynamic instability and nausea, emesis, and/or significant diarrhea, and resumed on resolution of these. Enteral nutrition support was typically given by nasoenteric tubes, with transition to full oral diet as tolerated.17 PN was proportionally decreased as enteral intake increased to maintain kilocalories and protein goals. If PN was restarted, the patient was maintained on the original blinded PN formula. A maximum of 21 days of study PN was administered; subjects who required PN for more than this period of time were maintained on STD-PN as outlined above.
Study End Points
Predetermined clinical outcome end points were followed during the entire hospitalization (SICU and general surgical ward). These included (1) total number of new nosocomial infections diagnosed during the entire hospitalization after initiation of study PN (primary study end point) and the presumed causative microorganisms; (2) the site of infection (including the total number of new bloodstream infections [BSIs] and pneumonias) and the number of patients affected; (3) number of patients with multiple hospital infections (defined as 2 or more new hospital infections after entry); (4) days on mechanical ventilation after entry; (5) development of acute respiratory distress syndrome (ARDS) after entry; (6) SICU and hospital LOS; (7) hospital mortality; and (8) antibiotic days during the hospitalization (number of antibiotics times the number of days prescribed for each). Regarding the fifth end point, ARDS was defined as (a) a fractional Pao2/Fio2 ratio <200 (regardless of positive end-expiratory pressure); (b) presence of bilateral infiltration on frontal chest x-rays; and (c) pulmonary artery wedge pressure ≤18 mm Hg (when measured) or no clinical evidence of left atrial hypertension (e.g., available central venous pressure readings <12 mm Hg, no physical or echographic evidence of jugular venous distention).54 Infections were considered present if criteria published by the U.S. Centers for Disease Control and Prevention (CDC) were met,55 the primary physicians deemed that a new infection was present, and antibiotics were either added or changed in response to the new infection.
The following secondary metabolic end points were also determined using EUH laboratory methods, except as indicated: (1) organ function tests—blood glucose values at baseline and mean levels during study weeks 1 and 2 and mean daily values for the entire postentry hospitalization; (2) serum creatinine, total bilirubin, alkaline phosphatase, and transaminase concentrations (baseline and mean values during study weeks 1 and 2 and maximal values during PN administration); (3) maximal blood ammonia concentration (baseline, weekly while on PN, and as indicated); (4) change in lymphocyte subset number from the baseline day (day of initiation of study PN) to day 8 (CD-3 [total T-lymphocytes], CD-4 [helper T-cells], and CD-8 [suppressor T-cells] by fluorescence-activated cell-sorting analysis); (5) change in plasma GLN and glutamate (GLU) concentrations from baseline to day 8 (determined by ion exchange chromatography using an amino acid analyzer [Beckman Coulter, Fullerton, CA, System 6300] in the Emory University Medical Genetics Core Laboratory, Atlanta, GA); and (6) D-xylose absorption on day 8 (25 g of D-xylose in 200 mL of water was given enterally, and a 2-hour serum sample and 5-hour urine sample were obtained for D-xylose concentrations, measured commercially by Covance Inc, Princeton, NJ). Serum C-reactive protein concentration, an index of systemic inflammation, was measured at baseline in all subjects. The Acute Physiology and Chronic Health Evaluation (APACHE) II score on the baseline day was determined as an index of illness severity.56
Data Collection and Statistical Analysis
All microbiological, infection-related, and relevant clinical, metabolic, and biochemical data were entered daily during hospitalization into study case report forms by the investigators. All daily PN intake (volume, kilocalories, and amino acid) and all daily enteral intake (as food items, supplements, and/or tube feedings) on days when subjects received any study PN were recorded. Data were analyzed using the χ2 test or Fisher exact test and Poisson regression as appropriate to compare the clinical outcome end points and other dichotomized factors between the control and experimental groups; t tests were used to compare other data (eg, LOS, ventilator days, D-xylose absorption) and continuous factors that were measured only once. Variables measured serially over time were compared between groups using 2-way repeated-measures ANOVA followed by Tukey pairwise comparisons. Baseline characteristics were compared using t test for continuous data and the χ2 and Fisher exact tests for categorical data. The initial tests were done on the entire data set, followed by planned per-protocol analysis performed by stratifying patients into 2 subgroups: pancreatic necrosis and nonpancreatic (cardiac, vascular, and colonic) surgery. Interaction effects between surgery groups and treatment (control or GLN) were tested using Poisson regression or Breslow-Day test as appropriate. Stratified analysis was always done to avoid false-negative conclusions attributable to nonsignificant interactions. The main purpose of stratification was to determine the effectiveness of GLN within surgery groups. An interim analysis was performed in a blinded fashion by the General Clinical Research Center statistician (GAC) on the initial 30 subjects using an O’Brian-Fleming spending function. A power analysis suggested that a final subject number of approximately 60 (≈30 per control and experimental groups) was needed to test the primary end point (total nosocomial infections after entry until hospital discharge). The statistical software package used was SAS version 9.1 (Cary, NC). P values <.05 were considered statistically significant.
Results
Patients
Enrollment of study patients began in May 1996 and was interrupted in November 1998, after 30 initial subjects had been enrolled, because of a lapse in study drug availability. The study was resumed in April 2000 and concluded in April 2003 after a total of 63 subjects were enrolled. Four subjects (2 control STD-PN and 2 GLN-PN-treated) did not receive the required 5 days of study PN. One STD-PN subject died on day 4 due to severe multiple organ failure after which life support was withdrawn; a second control subject was discharged to a nursing home facility on day 4. One GLN-PN subject was discharged to home on day 4 and a second GLN-PN subject was placed on tube feeding and PN was discontinued on day 2. Thus, 29 control STD-PN and 30 GLN-PN subjects comprised the final study group (n = 59).
There were no differences in the primary surgical procedures between the two PN groups (Table 1). All subjects in the pancreatic necrosis surgery subgroup underwent pancreatic debridement for necrotizing pancreatitis, with the exception of 1 subject in each group who underwent excision of benign pancreatic tumors. The STD-PN and GLN-PN patients were not different at baseline for demographic, clinical, and biochemical characteristics as a whole and also within the 2 surgical subgroups (Table 2). There were no significant differences in baseline serum total bilirubin, creatinine levels, or in total lymphocyte, CD-4, or CD-8 counts (not shown). However, compared with patients in the pancreatic necrosis surgery cohort, patients who had undergone cardiac, vascular, or colonic surgery were significantly older, had a higher APACHE II score, were more lymphopenic, and had lower baseline plasma GLN concentrations (Table 3). All of the pancreatic necrosis and colonic surgery patients were managed in the same general surgery SICU of EUH, and all of the cardiac and vascular surgery patients were managed in the same cardiac-vascular SICU of EUH. All patients were managed by their faculty attending surgical teams and also by a site-specific team of faculty critical care specialists.
Table 1.
Primary Surgical Proceduresa
| Pancreatic Necrosis Surgery |
Nonpancreatic Surgeryb |
|||
|---|---|---|---|---|
| STD-PN | GLN-PN | STD-PN | GLN-PN | |
| Total no. of subjects | 17 | 15 | 12 | 15 |
| Pancreatic debridement | 16 | 14 | 0 | 0 |
| Pancreatic tumor excision | 1 | 1 | 0 | 0 |
| CABG | 0 | 0 | 2 | 5 |
| Cardiac valve | 0 | 0 | 3 | 1 |
| Abdominal vascular | 0 | 0 | 5 | 6 |
| Colon | 0 | 0 | 2 | 3 |
STD-PN, standard parenteral nutrition; GLN-PN, glutamine dipeptide-supplemented parenteral nutrition; CABG, coronary artery bypass grafting.
Values are numbers of subjects (N = 59). All comparisons within the pancreatic necrosis and nonpancreatic subgroups are not significantly different (Fisher exact test, not significant).
Nonpancreatic surgical procedures included coronary artery bypass grafting, cardiac valve, abdominal vascular, and colonic operations.
Table 2.
Baseline Demographic and Clinical Characteristicsa
|
All Subjects |
Pancreatic Necrosis Surgery Subgroup |
Nonpancreatic Surgery Subgroup |
||||
|---|---|---|---|---|---|---|
| STD-PN | GLN-PN | STD-PN | GLN-PN | STD-PN | GLN-PN | |
| No. of subjects | 29 | 30 | 17 | 15 | 12 | 15 |
| Age, y | 57 ± 3 | 56 ± 3 | 51 ± 3 | 51 ± 3 | 67 ± 3 | 61 ± 4 |
| Gender, male/female | 21/8 | 19/11 | 14/3 | 10/5 | 7/5 | 9/6 |
| APACHE II score | 13.1 ± 1.2 | 13.4 ± 1.4 | 9.4 ± 1.5 | 11.1 ± 1.5 | 17.7 ± 2.1 | 14.1 ± 1.9 |
| Hospital day at entry | 12 ± 2 | 10 ± 1 | 14 ± 3 | 12 ± 2 | 10 ± 3 | 9 ± 2 |
| Postoperative day at entry | 3.5 ± 0.4 | 4.2 ± 0.6 | 3.2 ± 0.5 | 2.7 ± 0.4 | 3.8 ± 0.6 | 5.7 ± 1.1 |
| Days on STD-PN prior to entry | 4 ± 1 | 6 ± 2 | 6 ± 1 | 8 ± 3 | 2 ± 1 | 5 ± 2 |
| On ventilator at entry, n (%) | 16 (55) | 23 (77) | 7 (41) | 9 (60) | 9 (75) | 14 (93) |
| ARDS at entry, n (%) | 1 (3) | 3 (10) | 1 (6) | 0 (0) | 0 (0) | 3 (20) |
| Body mass index, kg/m2 | 26 ± 1 | 28 ± 1 | 26 ± 1 | 29 ± 2 | 26 ± 2 | 27 ± 2 |
| Serum CRP, mg/dL | 18 ± 2 | 18 ± 1 | 16 ± 2 | 16 ± 2 | 20 ± 3 | 21 ± 3 |
| Plasma GLN, μM | 405 ± 44 | 432 ± 25 | 448 ± 38 | 466 ± 22 | 363 ± 16 | 392 ± 28 |
| Plasma GLU, μM | 60 ± 7 | 61 ± 6 | 73 ± 8 | 76 ± 10 | 42 ± 7 | 49 ± 8 |
| Blood glucose, mg/dL | 156 ± 6 | 151 ± 6 | 160 ± 9 | 155 ± 9 | 150 ± 10 | 145 ± 7 |
| White blood cell count, 103 μL | 14 ± 2 | 16 ± 1 | 15 ± 1 | 17 ± 2 | 13 ± 2 | 15 ± 2 |
STD-PN, standard parenteral nutrition; GLN-PN, glutamine dipeptide-supplemented parenteral nutrition; APACHE, Acute Physiology and Chronic Health Evaluation; ARDS, acute respiratory distress syndrome; CRP, C-reactive protein; GLN, glutamine; GLU, glutamate.
Comparisons of STD-PN vs GLN-PN in all subjects and within surgical subgroups are not significantly different (t test, χ2 test, or Fisher exact test). Data presented as mean ± SEM or n (%).
Table 3.
Comparison of Baseline Demographic and Clinical Characteristics Between Pancreatic and Nonpancreatic Surgical Subgroups
| Parameter | Pancreatic Necrosis Surgery |
Nonpancreatic Surgerya |
|---|---|---|
| n | 32 | 27 |
| Age, y | 51 ± 2 | 63 ± 3b |
| APACHE II score | 9.8 ± 1.1 | 14.8 ± 1.4b |
| Hospital day at entry | 13 ± 3 | 9 ± 2 |
| On ventilator at entry, n (%) | 16 (50) | 23 (85) |
| ARDS at entry, n (%) | 1 (3) | 3 (11) |
| Serum CRP, mg/dL | 16 ± 1 | 20 ± 2 |
| Plasma GLN, μM | 457 ± 22 | 385 ± 20b |
| Total T-lymphocytes, cells | 992 ± 104 | 666 ± 98b |
| Total CD-4 count, cells | 571 ± 59 | 423 ± 66b |
| Total CD-8 count, cells | 389 ± 54 | 221 ± 36b |
APACHE, Acute Physiology and Chronic Health Evaluation; ARDS, acute respiratory distress syndrome; CRP, C-reactive protein; GLN, glutamine; CD-4, helper T-cells; CD-8, suppressor T-cells. Data presented as mean ± SEM or n (%).
Nonpancreatic surgical procedures included coronary artery bypass grafting, cardiac valve, abdominal vascular, and colonic operations.
P < .05 (t test) pancreatic vs nonpancreatic subgroups. All other comparisons are not significantly different (t test, χ2 test, or Fisher exact test).
Nutrient Intake
The average number of days patients received study PN were not different between the pancreatic necrosis and nonpancreatic study groups overall (eg, pancreatic necrosis surgery, 15 ± 1 vs nonpancreatic surgery 12 ± 1 study PN days; not significant [NS]). The average daily study PN kilocalorie intake days were also not different between groups (21 ± 1 vs 23 ± 1 kcal/kg/d; NS). The number of days that study PN was administered, the PN amino acid and kilocalorie intake, and the enteral kilocalorie intake during PN were not different between the STD-GLN and GLN-PN cohorts within each surgical subgroup. Enteral kilocalorie intake during study PN infusion (ie, during transition from PN to enteral feeds) was not different between the control and GLN-treated subjects in the pancreatic necrosis subgroup (STD-PN 558 ± 86 vs GLN-PN 561 ± 118 kcal/d; NS).
In the nonpancreatic surgery patients, the STD-PN group received 12 ± 1 days of study PN, providing amino acid at 1.1 ± 0.09 g/kg/d and 23 ± 2 kcal/kg/d, and the GLN-PN group received 12 ± 1 days of study PN, providing amino acid at 1.2 ± 0.05 g/kg/d and 22 ± 2 kcal/kg/d (all NS). In the GLN-PN group, the study amino acids thus provided 0.4 g of alanyl-GLN dipeptide per day (or approximately 18 g/d GLN in a 70-kg patient, given that two-thirds of the dipeptide moiety is GLN nitrogen). Enteral kilocalorie intake during study PN infusion was not different between the control and GLN-treated subjects (STD-PN 549 ± 108 vs GLN-PN 494 ± 78 kcal/d; NS).
Plasma GLN and GLU Concentrations
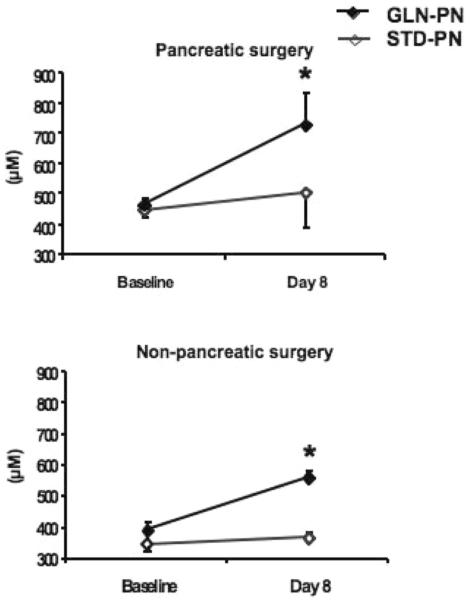
After 1 week of study PN infusion, plasma GLN levels increased significantly with GLN-supplemented PN in both surgical subgroups but did not change with standard, GLN-free PN (Figure 1). In the pancreatic necrosis surgery subgroup, plasma GLN increased by 159 ± 22 μM with GLN-PN (P < .01 compared with baseline) and 57 ± 19 μM with STD-PN (not different compared with baseline). In the nonpancreatic surgery subgroup, plasma GLN levels increased by 142 ± 46 μM with GLN-PN (P < .01 compared with baseline) and 11 ± 15 μM with STD-PN (NS compared with baseline; Figure 1). There were no significant changes in plasma GLU concentrations from baseline to day 8 between groups (all subjects: STD-PN, baseline 60 ± 7 vs day eight 56 ± 6 μM; GLN-PN, baseline 61 ± 6 vs day eight 74 ± 11 μM; NS between groups).
Figure 1.
Plasma glutamine (GLN) concentrations: Patients in the pancreatic necrosis surgery subgroup (n = 32) demonstrated significantly higher mean baseline plasma GLN concentrations than patients in the nonpancreatic (n = 27) surgery subgroup (457 ± 22 vs 385 ± 20; t test P < .05). Plasma GLN levels did not change from baseline to day 8 of study in the subjects in either surgical subgroup who received standard, GLN-free parenteral nutrition (PN). However, subjects who received GLN-PN demonstrated a significant increase of plasma GLN into the normal range. *P < .07.
Safety of GLN-PN
There were no adverse events attributable to the study PN. Medical support was withdrawn in 2 STD-PN subjects with multiple organ failure. No significant changes in serum ammonia occurred within or between STD-PN and GLN-PN subjects in either surgical cohort over time (not shown). The maximal mean serum ammonia value during infusion of study PN was 44 ± 3 mg/dL in the STD-PN group and 45 ± 3 mg/dL in the GLN-PN group (NS) and was also not different between GLN and control groups within the pancreatic necrosis and nonpancreatic surgery cohorts (not shown).
Efficacy of GLN-PN to Decrease Hospital Infections
None of the 59 subjects had an acute, uncontrolled infection or history of clinical sepsis within the previous 24 hours at entry, per inclusion criteria. However, most of the pancreatic necrosis surgery patients had infected pancreatic pseudocysts, which had been surgically debrided and were treated with postoperative intravenous antibiotics at entry (12/17 STD-PN subjects [71%] vs 10/15 AG-PN subjects [67%]; NS). The proportion of patients with a nosocomial infection diagnosed prior to study entry was not different between STD-PN and GLN-PN groups in the nonpancreatic cohort (6/12 STD-PN subjects [50%] vs 6/15 AG-PN subjects [40%]; NS).
As shown in Figure 2, the number of new nosocomial infections in the entire group of GLN-PN patients compared with the entire group of STD-PN patients was not different (STD-PN 65 infections vs GLN-PN 38 infections; P = .060). There were also no differences in total new nosocomial infection episodes in STD-PN- vs GLN-PN-treated patients following pancreatic necrosis surgery (29 vs 25; NS). However, GLN-PN reduced nosocomial infections in the nonpancreatic surgery subjects. These individuals demonstrated a >3-fold decrease in the total number of new hospital infections during hospitalization compared with the STD-PN group (Figure 2, Table 4). There were 36 infections in the 12 control patients who received STD-PN compared with 13 infections in the 15 GLN-PN-treated patients (3.0 vs 0.9 new infections per patient; P = .030). Only 2 of 12 STD-PN subjects in this cohort remained free of infection from study entry until hospital discharge (17%). In contrast, 53% of GLN-PN-treated subjects (8 of 15) did not develop a new hospital-acquired infection after entry (NS; P = .107). Patients who underwent pancreatic necrosis surgery demonstrated no differences in the percentage of subjects not developing any new hospital infections (STD-PN, 8/17 subjects [47%] vs GLN-PN, 5/15 subjects [33%]; NS).
Figure 2.
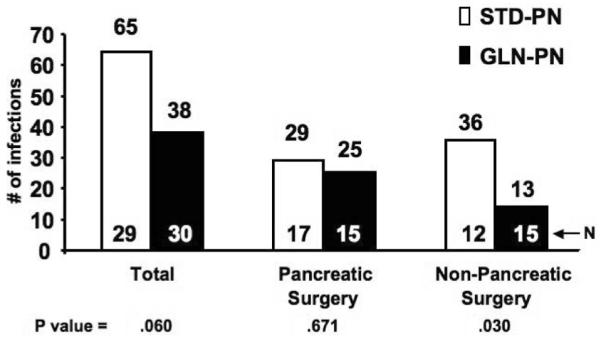
Total nosocomial infections. The total number of hospital-acquired infections was determined from the day of study PN initiation until hospital discharge, as outlined in the methods. The total number of nosocomial infections was unchanged with GLN-PN in patients who underwent pancreatic necrosis surgery but was markedly decreased in patients given GLN-PN after cardiac, abdominal vascular, or colonic surgery compared with patients in this subgroup given STD-PN (3-fold; Poisson regression P value = .030). Interaction P value = .190. Number of patients in each group is shown.
Table 4.
Nosocomial Infections Diagnosed After Initiation of Study Parenteral Nutrition
|
All Subjects |
Pancreatic Necrosis Surgery Subgroup |
Nonpancreatic Surgery Subgroup |
||||
|---|---|---|---|---|---|---|
| STD-PN | GLN-PN | STD-PN | GLN-PN | STD-PN | GLN-PN | |
| Subjects, n Site of infectiona |
29 | 30 | 17 | 15 | 12 | 15 |
| Pneumonia | 23 (16) | 15 (13) | 7 (4) | 9 (7) | 16 (9) | 6 (5)b |
| Bacteremia or fungemia | 12 (10) | 4 (4) | 5 (5) | 4 (4) | 7 (5) | 0 (0)c |
| Urinary tract infection | 11 (9) | 8 (6) | 6 (4) | 5 (3) | 5 (5) | 3 (3) |
| Catheter infection | 7 (6) | 7 (5) | 3 (3) | 5 (5) | 4 (3) | 2 (2) |
| Abscess | 7 (7) | 5 (4) | 5 (5) | 5 (4) | 2 (2) | 0 (0) |
| Cellulitis | 2 (2) | 1 (1) | 1 (1) | 0 (0) | 1 (1) | 1 (1) |
| Wound infection | 2 (2) | 2 (2) | 1 (1) | 1 (1) | 1 (1) | 1 (1) |
STD-PN, standard parenteral nutrition; GLN-PN, glutamine dipeptide-supplemented parenteral nutrition.
Data presented as number of infections, with number of affected patients in parenthesis.
P < .05
P < .01: GLN-PN vs STD-PN in the nonpancreatic surgery subgroup (Fisher exact test). Pneumonia Breslow-Day interaction (P = .012), Bacteremia or fungemia Breslow-Day interaction (P = .031). Other comparisons are not significant.
The number and percentage of patients in the pancreatic necrosis surgery cohort who developed multiple new nosocomial infections after entry (defined as 2 or more new hospital infections), pneumonias (Table 4), and BSIs attributable to bacterial or fungal species (Table 4 and Figure 3) were not different between GLN-PN and STD-PN groups. In contrast, GLN-PN administration in nonpancreatic surgery patients was associated with a significant decrease in the percentage of subjects who developed multiple nosocomial infections compared with the controls (STD-PN 67% vs GLN-PN 27%; P = .034). GLN-PN patients in the nonpancreatic surgery cohort also demonstrated a significant decrease in the number of new nosocomial pneumonias compared with the STD-PN patients in this subgroup (Table 4; P < .05). A total of 5 of 12 STD-PN-treated nonpancreatic surgery patients developed a total of 7 bacterial or fungal BSIs; however, none of the 15 GLN-PN patients in this cohort developed a BSI from study entry until hospital discharge (Table 4 and Figure 3; P < .002).
Figure 3.
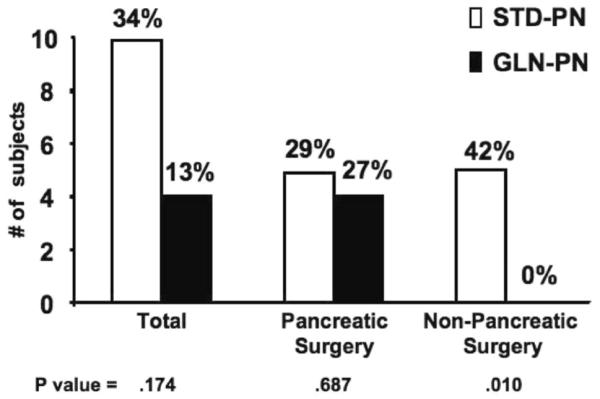
Bloodstream infections (BSIs): Positive blood cultures for bacterial or fungal species during hospitalization did not occur in 15 surgical intensive care unit patients given glutamine-supplemented parenteral nutrition (GLN-PN) after cardiac, abdominal vascular, or colonic surgery compared with 5 of 12 patients (42%) in this subgroup given standard glutamine-free parenteral nutrition (STD-PN), who developed 7 BSIs (Fisher exact test P = .010). There were no differences in the number of BSIs between study groups in the pancreatic surgery cohort (STD-PN 18% [n=17] vs GLN-PN 27% [n=15]; P = .687). Breslow-Day interaction test P = .011.
The number of patients who developed a new Staphylococcus aureus infection was significantly lower in the GLN-PN group vs the STD-PN group (STD-PN 6/12 vs GLN-PN 1/15 subjects; P = .03; Table 5). In this nonpancreatic surgery subgroup, a total of 13 infections attributed to fungal species (primarily Candida albicans) occurred in control STD-PN patients (including 2 fungal BSIs). However, only one patient receiving GLN-PN developed a fungal infection during hospitalization (Table 5; P < .03). In addition, nonpancreatic surgery patients given GLN-PN developed significantly fewer total infections attributed to Gram-negative bacteria (11 such infections in 12 STD-PN subjects [0.93 Gram-negative infections/patient] vs 8 such infections in 15 GLN-PN subjects [0.53 infections/patient]; P = .043; Table 5).
Table 5.
Microbiology of Nosocomial Infections
| Pancreatic Necrosis Surgery |
Nonpancreatic Surgery |
|||
|---|---|---|---|---|
| STD-PN (n = 17) | GLN-PN (n = 15) | STD-PN (n = 12) | GLN-PN (n = 15) | |
| Fungal species | 5 (3) | 7 (4) | 15 (6) | 1 (1)a |
| Gram-positive bacteria | ||||
| Staphylococcus aureus | 5 (1) | 1 (1) | 8 (7) | 1 (1)b |
| Staphylococcus species (coagulase negative) | 4 (4) | 8 (8) | 3 (3) | 3 (2) |
| Enterococcus species | 1 (1) | 2 (2) | 1 (1) | 1 (1) |
| Streptococcus species | 0 | 1 (1) | 0 | 1 (1) |
| Corynebacterium species | 2 (2) | 0 | 0 | 0 |
| Gram-negative bacteria | ||||
| Pseudomonas species | 6 (5) | 8 (4) | 5 (5) | 3 (3) |
| Klebsiella species | 5 (4) | 3 (2) | 2 (2) | 0 |
| Stenotrophomonas maltophilia | 0 | 0 | 3 (3) | 0 |
| Escherichia coli | 0 | 2 (2) | 1 (1) | 2 (1) |
| Miscellaneous Gram-negative bacteria | 5 (5) | 2 (2) | 2 (2) | 2 (2) |
| Viral species | 0 | 0 | 1 (1) | 0 |
STD-PN, standard parenteral nutrition; GLN-PN, glutamine dipeptide-supplemented parenteral nutrition. Values are number of nosocomial infections due to microorganism (number of patients). Presumed causative microorganisms based on microbiological culture results.
P < .03
P < .01: GLN-PN vs STD-PN in the nonpancreatic surgery subgroup (Fisher exact test). Fungal species Breslow-Day interaction (P = .021), Staphylococcus aureus Breslow-Day interaction (P = .069). Other comparisons are not significant.
In contrast to the decreased infection rates in the nonpancreatic surgery cohort, GLN-PN did not alter nosocomial infection rates in the patients who had undergone pancreatic surgery (Figures 2 and 3; Tables 4 and 5). There was a trend for these patients to develop fewer nosocomial infections attributable to S aureus, such that when all 59 study subjects were considered, there was a >5-fold higher incidence of S aureus infection in patients receiving STD-PN vs GLN dipeptide-supplemented PN (STD-PN 11 vs GLN-PN 2 S aureus infections, P < .01; Table 5).
Effect of GLN-PN on Hospital Mortality
The overall in-hospital mortality rate was 17% in the total group of 29 STD-PN controls (5 deaths) compared with 3% (1 death) in the 30 GLN-PN subjects (NS) (Figure 4). However, no deaths occurred in the pancreatic necrosis group, and all hospital mortalities occurred in the nonpancreatic surgical patients. Hospital mortality was decreased by 35% with GLN-PN in the nonpancreatic surgical cohort (Figure 4; P = .060). An additional STD-PN patient died on day 4 of study but was not included in data analysis by pre hoc inclusion criteria mandating at least 5 days of study PN in this non–intent-to-treat trial. All hospital deaths were attributable to infection-associated ARDS and/or multiple organ failure. For example, 3 of the subjects who died in the STD-PN group developed C albicans fungal pneumonia (1 with associated fungemia) and died in the SICU on study days 17, 27, and 37. Another control subject died in the SICU on study day 17, in association with pneumonia attributed to S aureus. The fifth control subject was diagnosed with Klebsiella pneumonia on study day 9, an Escherichia coli urinary tract infection on day 25, and ARDS on day 32 and died on study day 38. One GLN-treated subject died on day 21 after developing ARDS in association with Serratia pneumonia.
Figure 4.
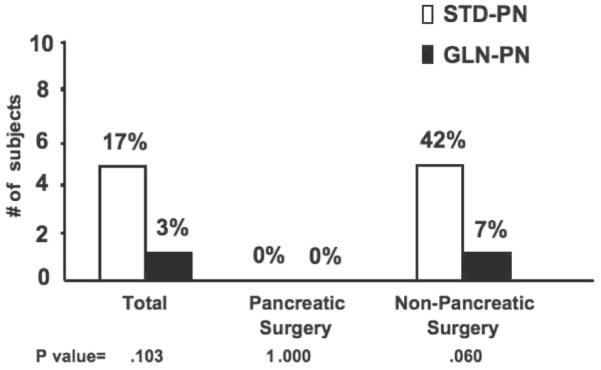
Hospital mortality. There were no hospital deaths after pancreatic surgery: standard glutamine-free parenteral nutrition (STD-PN; n = 17) vs glutamine-supplemented parenteral nutrition (GLN-PN; n = 15). In contrast, in the nonpancreatic surgery cohort, 5 of 12 patients in the STD-PN group developed sepsis-associated acute respiratory distress syndrome and died (42%), compared with only 1 death in 15 patients in this subgroup randomized to GLN-PN (7%; Fisher exact test P = .060). Breslow-Day interaction test was not possible because there were no hospital deaths after pancreatic surgery.
Effect of GLN-PN on Organ Function and Other Clinical Outcomes
All 59 of the subjects experienced a prolonged postoperative SICU stay (>5 days). In the nonpancreatic surgery subgroup (cardiac, vascular, and colonic surgery), those subjects receiving GLN-PN demonstrated a significant improvement in several clinical outcome and organ function indices compared with subjects given STD-PN, con-comitant with the decrease in hospital infection rates (Table 6). Days on mechanical ventilation after entry were significantly decreased with GLN-PN (STD-PN 21 ± 5 vs GLN-PN 9 ± 2 days, P < .025; Table 6). The decrease in SICU LOS (P = .061) approached statistical significance favoring GLN-PN treatment vs STD-PN. There was no difference between the 2 treatment groups in hospital antibiotic usage (STD-PN 40 ± 6 vs GLN-PN 28 ± 5 total antibiotic days; P = .167). Similarly, there were no differences in the average maximum serum ammonia concentrations, change from baseline in plasma GLU levels, or average weekly or maximum serum alkaline phosphatase and transaminase concentrations (not shown). There was no difference between the groups in mean week 1 or week 2 blood glucose concentrations (not shown) or the mean daily blood glucose concentrations during the total hospitalization (STD-PN 150 ± 10 vs GLN-PN 145 ± 7 mg/dL; NS; Table 6).
Table 6.
Clinical and Metabolic Outcomes in Cardiac, Vascular, and Colonic Surgery Patients
| Outcome End Point | STD-PN (n = 12) | GLN-PN (n = 15) |
|---|---|---|
| Patients with no new infections after study entry (%) | 2/12 (17) | 8/15 (53) |
| Days on mechanical ventilation | 21 ± 5 | 9 ± 2 a |
| LOS in the SICU | 23 ± 6 | 12 ± 2 |
| LOS entire hospitalization | 30 ± 6 | 20 ± 2 |
| Maximal serum creatinine, mg/dL | 2.7 ± 0.7 | 1.4 ± 0.2 |
| Mean week 2 serum creatinine, mg/dL | 1.9 ± 0.4 | 1.0 ± 0.1b |
| Maximal serum total bilirubin, mg/dL | 3.9 ± 0.7 | 1.6 ± 0.2c |
| Mean week 2 serum total bilirubin, mg/dL | 2.7 ± 0.8 | 0.7 ± 0.1a |
| Mean daily blood glucose, entire hospitalization, mg/dL | 150 ± 10 | 145 ± 7 |
STD-PN, standard parenteral nutrition; GLN-PN, glutamine dipeptide-supplemented parenteral nutrition; LOS, length of stay; SICU, surgical intensive care unit.
P < .025
P < .05
P < .005, GLN-PN vs STD-PN (t test). Other comparisons are not significant.
The GLN-PN-treated patients in the nonpancreatic surgery cohort demonstrated significantly improved serum hepatic and renal function indices after entry compared with their controls (Table 6). Maximum (P < .08) and average study week 1 serum creatinine levels (not shown) tended to be lower, and average study week 2 serum creatinine values were significantly lower in the GLN-PN group compared with the STD-PN group. Average maximal and study week 2 serum total bilirubin concentrations were significantly lower in the GLN-PN patients compared with their controls (Table 6). There was no significant difference between groups for the change (from day 0 to day 8) in total plasma T-lymphocytes or CD-4 or CD-8 subsets (not shown).
In the pancreatic necrosis surgery patients, there were no differences between groups for days on mechanical ventilation (STD-PN 8 ± 4 vs GLN-PN 6 ± 3 days; NS), SICU LOS (STD-PN 10 ± 4 vs GLN-PN 11 ± 4 days; NS), hospital LOS (STD-PN 31 ± 5 vs GLN-PN 32 ± 4 days; NS), or antibiotic days (STD-PN 37 ± 8 vs GLN-PN 49 ± 10; NS). There were no differences between the groups in mean week 1 or week 2 blood glucose concentrations (not shown) or the mean daily blood glucose concentrations during the total hospitalization (STD-PN 154 ± 5 vs GLN-PN 145 ± 6 mg/dL; NS) or change in hepatic or renal function tests or lymphocyte subsets (not shown).
D-xylose Absorption
D-xylose tests were done in a total of 27 patients: 16 in the pancreatic necrosis surgery subgroup (7 STD-PN, mean APACHE II score 10.3 ± 2.2; 9 GLN-PN, mean APACHE II score 12.4 ± 2.7; NS) and 11 in the nonpancreatic surgery subgroup (5 STD-PN, mean APACHE II score 17.6 ± 3.4; 6 GLN-PN, mean APACHE II score 13.2 ± 2.7; NS). In all 27 subjects studied, the mean 2-hour serum D-xylose concentrations and 5-hour urinary D-xylose excretion after the enteral D-xylose load were significantly below the normal ranges (Figure 5), consistent with SICU-associated gut mucosal dysfunction.11,35 However, the 2-hour serum D-xylose concentration was significantly higher in the total group of 15 GLN-PN subjects than in the 12 STD-PN subjects (P = .033), and the 5-hour urinary excretion tended to be higher in the GLN-PN group (Figure 5; NS). In pancreatic necrosis surgery patients, there were no differences between the 2 study groups for 2-hour serum D-xylose concentrations (STD-PN 15 ± 2 vs GLN-PN 13 ± 2 mg/dL; NS) or 5-hour urinary D-xylose excretion (not shown). In the nonpancreatic surgery patients (Figure 5), 5-hour urinary D-xylose excretion was not different between study groups; however, the 2-hour serum D-xylose concentration was markedly (nearly 5-fold) higher in the GLN-PN group compared with the STD-PN controls.
Figure 5.
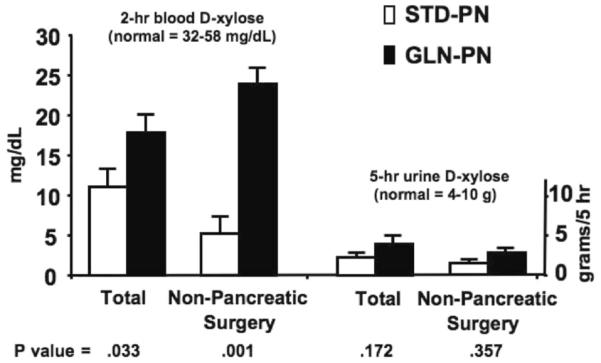
D-xylose absorption studies. D-xylose tests were done in a total of 27 patients: 16 in the pancreatic necrosis surgery subgroup (n = 7 STD-PN and 9 GLN-PN) and 11 in the nonpancreatic surgery subgroup (n =5 STD-PN and 6 GLN-PN). D-xylose absorption indices (2-hour serum value and 5-hour urinary excretion) were below the normal range after a 25-g enteral D-xylose load in the total group of 27 patients studied. Serum values for D-xylose at 2 hours after administration were significantly higher in patients given GLN-PN vs STD-PN (t test P = .033). Pancreatic necrosis patients did not demonstrate differences between the 2 study groups for 2-hour serum D-xylose concentrations or 5-hour urinary D-xylose excretion (not shown). In nonpancreatic surgery patients, 5-hour urinary D-xylose excretion was similar between study groups. However, the 2-hour serum D-xylose concentration was markedly higher with GLN-PN than with STD-PN (P = .001).
Discussion
This randomized, double-blind, controlled clinical trial suggests that supplementation of PN with alanyl-GLN dipeptide was well tolerated, decreased nosocomial infections, and improved organ function indices in adult patients requiring both PN and prolonged SICU care after cardiac, abdominal vascular, and colonic surgery. Such patients are the minority of those undergoing these types of operations. PN as used in this setting supported nutrition status when full enteral feeding was impossible rather than as a method to intervene as a function of the disease or surgical process. In light of our data, it may be of interest to consider studies of intravenously administered GLN as a potential therapy to prevent infectious complications in SICU patients receiving either enteral or parenteral nutrition support. Beneficial effects were not observed in patients who required SICU care and PN after major pancreatic surgery for pancreatic necrosis. Our study did not address effects of GLN-supplemented PN in pancreatic necrosis patients who were not surgical candidates. Several controlled trials in specific subgroups of postsurgical and other types of patients have shown beneficial clinical or metabolic effects when GLN is added to PN.25,31-35,39,41,42 These studies have incorporated L-GLN doses in PN ranging from 0.29 to 0.57 g/kg/d,25,31-35,39 and alanyl-GLN dipeptide doses similar to the dose used in the current trial (0.40-0.50 g/kg/d or equivalent to 0.26-0.33 g of L-GLN/kg/d). However, several trials also show no benefits, possibly attributable to differences in study patient clinical characteristics and degree of GLN depletion within and between studies, as well as the dose of parenteral GLN used.48 To our knowledge, the current study is the first to suggest differential efficacy of GLN-supplemented PN comparing different postsurgical subgroups within the same trial. The efficacy of posttracheotomy ventilator weaning has also been shown to differ among subgroups of critically ill surgical patients.57
A weakness of this trial is that it was performed in small cohorts of pancreatic necrosis, cardiac, vascular, and colonic surgical patients and was a single-center, non–intent-to-treat trial; thus, this study should be considered a pilot clinical study. Only a small percentage of SICU patients typically require PN; nonetheless, those who do require PN have experienced postoperative complications with a high morbidity and mortality.17,44 Also, our study was conducted during 2 distinct time periods given availability of GLN dipeptide for study (1996-1998 and 2000-2003). Analysis within and between these periods showed that there were no differences in response to GLN between time periods, in that the pancreatic necrosis surgery patients had no significant response, whereas the nonpancreatic surgery patients showed decreased infections with GLN-PN vs STD-PN in each period. In the pancreatic surgery cohort, for example, the number of BSIs was similar in the early period (1996-1999) between STD-PN (n = 1) vs GLN-PN patients (n = 1) compared with during the latter period (2000-2003) between STD-PN (n = 4) vs GLN-PN patients (n = 3). Also, the number of patients in the pancreatic surgery cohort without development of new infections was similar between GLN and control patients in the early period (STN-PN n = 4 vs GLN-PN n = 2) and in the latter period (STN-PN n = 3 vs GLN-PN n = 4). In contrast, in the nonpancreatic surgery cohort, the number of BSIs was decreased with GLN-PN patients in the early period (1996-1999; STD-PN [n = 3] vs GLN-PN patients [n = 0]) and during the latter period (2000-2003; STD-PN [n = 4] vs GLN-PN patients [n = 0]). The effect of GLN-PN to decrease the number of new nosocomial infections was evident in the early period (STN-PN n = 24 vs GLN-PN n = 9) and also in the latter period (STN-PN n = 12 vs GLN-PN n = 4).
There were no significant differences in standards of clinical care (eg, prophylactic postoperative antibiotic use) in our SICUs between the 2 study time periods, with the exception that tight blood glucose control modalities were initiated in late 2002 in all SICU units.20 However, there were no differences in mean daily blood glucose levels between control and GLN study groups within our surgical cohorts in the early vs later time periods of study (not shown).
We elected to study patients who had undergone pancreatic debridement for severe necrotic pancreatitis because many of these individuals in our institution require total or partial PN to maintain nutrition status. Patients with severe pancreatitis also exhibit low plasma and muscle GLN levels, which correlate with clinical outcome.26,58 Thus, we hypothesized that this subgroup of patients would benefit from GLN-supplemented PN. Such patients also responded to GLN-PN with increased plasma lymphocyte counts and decreased inflammatory markers.59,60 We studied patients after cardiac, vascular, or colonic surgery because these individuals exhibit significant oxidative stress61,62 and, in those requiring PN, have a high rate of hospital-acquired infection.1,2 These surgical subgroups differ in that pancreatic debridement removes the major focus of inflammation, whereas patients who require PN after cardiac, vascular, and colonic surgery typically have developed significant postsurgical complications and a severe systemic inflammatory response.
There were several differences between our pancreatic and nonpancreatic surgical cohorts that may explain the differential outcomes observed. Average plasma GLN values at entry were significantly lower in the patients who had cardiac, vascular, or colonic surgery compared with the pancreatic surgery patients. No subject in the pancreatic necrosis surgery cohort died during hospitalization; however, hospital mortality in the total nonpancreatic surgery cohort was 22%. Patients in the nonpancreatic surgery subgroup also exhibited significantly higher APACHE II scores and need for mechanical ventilation than the pancreatic surgery patients at entry and were also significantly older and more lymphopenic. Thus, patients in the nonpancreatic surgery cohort were more GLN depleted and had a higher severity of illness than the pancreatic necrosis surgery patients, whose major foci of inflammation were removed at the operation.
In the current trial, GLN supplementation of PN in the nonpancreatic surgical subgroup significantly reduced the rate of nosocomial infection, mainly attributable to pneumonias and BSIs. Our infection data are consistent with previous studies showing a beneficial effect of GLN-supplemented PN to decrease nosocomial infections in other types of critically ill patients.31,39-42 S aureus is responsible for a high percentage of nosocomial infections in postsurgical and ICU patients.1-5 Our data show for the first time that GLN-supplemented PN specifically reduces infections attributed to S aureus, fungi, and enteric Gram-negative bacteria, which were markedly decreased with GLN-PN in the nonpancreatic surgery cohort (Table 4). Gut barrier function (sugar permeability) studies are difficult to perform accurately in critically ill patients and are contraindicated in patients with ileus or other forms of gut failure requiring parenteral feeding, but such studies would have been of interest. The reduction in S aureus and fungal infections suggests a potential effect of GLN on neutrophil function, as has been observed in previous studies.24
We found that GLN supplementation in the nonpancreatic surgical cohort significantly decreased (by an average of 12 days) the number of days patients required mechanical ventilation, probably because of the reduced incidence of pneumonia. Dechelotte et al42 recently demonstrated a significant decrease in the incidence of pneumonia and the average number of infections per patient with GLN-PN in a mixed medical/surgical patient population in 16 French ICUs. Larger trials that are powered to test the hypothesis that GLN-supplemented PN decreases ARDS and overall hospital mortality rates are needed. This study was designed to test whether addition of GLN improves responses to conventional PN only; additional studies are needed to test whether parenteral GLN improves responses to conventional enteral nutrition support.
The underlying mechanisms by which GLN may have decreased nosocomial infections in our study are unclear but potentially could include effects on the number and function of immune cells,24,33,36-38 improvement in gut barrier function and maintenance of the gut mucosa,11,15,34,35,63 protein-anabolic effects,25,27,31 and/or up-regulation of cytoprotective molecules, including the antioxidant glutathione64 and specific heat shock proteins.65 In the current study, we did not detect a significant effect of GLN-PN to up-regulate the total number of circulating T-lymphocytes or CD-4 or CD-8 T-cells, as has been observed in other patient groups.36,37 However, the sampling period of 7 days may have been too short to detect an effect of GLN on these key components of the adaptive immune system. Cells of the intestinal mucosa use GLN as a major fuel.11,23 The higher 2-hour blood D-xylose levels after an enteral D-xylose load with GLN-PN vs the controls in the nonpancreatic surgical group suggest that parenteral GLN may be used by enterocytes during critical illness.
Tight blood glucose control in the medical and surgical ICU setting (to <150 mg/dL) is now recognized to markedly reduce nosocomial infection and overall hospital morbidity and mortality.20,66 We were able to achieve daily blood glucose concentrations between 145 and 154 mg/dL within individual patient subgroups, and these values were not different between the GLN-PN and STD-PN subgroups (Table 2). Thus, differences in blood glucose control between study groups cannot explain the outcomes we observed with GLN-PN.
Conclusions
This pilot study suggests that alanyl-GLN dipeptide supplementation of PN reduces infectious complications in surgical patients requiring PN and prolonged SICU care after CABG, cardiac valve, abdominal vascular, and colonic surgery. However, our study did not demonstrate improved outcomes with GLN-PN in SICU patients after major pancreatic debridement to treat necrotizing pancreatitis. Rigorous, multicenter, intent-to-treat phase III trials are needed to confirm the efficacy of GLN-PN in selected patient subgroups in SICU patients; such a trial is now in progress in cardiac, vascular, and intestinal surgery patients requiring PN (ClinicalTrials.gov identifier NCT00248638).
Acknowledgments
We thank the Emory GCRC research staff, the Emory University Hospital SICU nurses, and Susan Rogers of the Emory University Hospital Investigational Drug Service for their help with the protocol. We gratefully acknowledge the support of Ewald Schlotzer, PhD, from Fresenus-Kabi, Bad Homburg, Germany. Authors contributed to study design (CFE, DPG, GAC, DPJ, JRG, TRZ), practical aspects of study performance (CFE, DPG, ML, EES, NB, ND, NMD, GFB, TM, CHB, CEF, LH, JGR, CRA, JRG, TRZ), data analysis (CFE, ML, GAC, TRZ), and manuscript preparation and review (CFE, DPG, ML, GAC, DPJ, JRG, TRZ). TRZ received an investigator-initiated grant to support partial costs of the study and glutamine dipeptide from Fresenius-Kabi. Otherwise, no author has a conflict of interest.
Financial disclosure: supported by National Institutes of Health grant R03 DK54823 (TRZ), Emory General Clinical Research Center (GCRC) grant M01 RR00039 (GAC and TRZ), and an investigator-initiated grant from Fresenius-Kabi (TRZ).
References
- 1.Vincent JL, Bihari DJ, Suter PM, et al. EPIC International Advisory Committee The prevalence of nosocomial infection in intensive care units in Europe: results of the European Prevalence of Infection in Intensive Care (EPIC) Study. JAMA. 1995;274:639–644. [PubMed] [Google Scholar]
- 2.Girou E, Stephan F, Novara A, Safar M, Fagon JY. Risk factors and outcome of nosocomial infections: results of a matched case-control study of ICU patients. Am J Respir Crit Care Med. 1998;157:1151–1158. doi: 10.1164/ajrccm.157.4.9701129. [DOI] [PubMed] [Google Scholar]
- 3.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 4.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21:510–515. doi: 10.1086/501795. [DOI] [PubMed] [Google Scholar]
- 5.Salgado CD, O’Grady N, Farr BM. Prevention and control of antimicrobial-resistant infections in intensive care patients. Crit Care Med. 2005;33:2373–2382. doi: 10.1097/01.ccm.0000181727.04501.f3. [DOI] [PubMed] [Google Scholar]
- 6.Garey KW, Neuhauser MM, Bearden DT, et al. Evaluation of anti-fungals in the surgical intensive care unit: a multi-institutional study. Mycoses. 2006;49:226–233. doi: 10.1111/j.1439-0507.2006.01222.x. [DOI] [PubMed] [Google Scholar]
- 7.Fridkin SK, Jarvis WR. Epidemiology of nosocomial fungal infections. Clin Microbiol Rev. 1996;9:499–511. doi: 10.1128/cmr.9.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park SK, Brody JI, Wallace HA, Blakemore WS. Immunosuppressive effect of surgery. Lancet. 1971;1:53–55. doi: 10.1016/s0140-6736(71)90777-x. [DOI] [PubMed] [Google Scholar]
- 9.Hensler T, Hecker H, Heeg K, et al. Distinct mechanisms of immunosuppression as a consequence of major surgery. Infect Immun. 1997;65:2283–2291. doi: 10.1128/iai.65.6.2283-2291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whelan RL, Franklin M, Holubar SD, et al. Postoperative cell mediated immune response is better preserved after laparoscopic vs open colorectal resection in humans. Surg Endosc. 2003;17:972–978. doi: 10.1007/s00464-001-8263-y. [DOI] [PubMed] [Google Scholar]
- 11.Wilmore DW, Smith RJ, O’Dwyer ST, et al. The gut: a central organ after surgical stress. Surgery. 1988;104:917–923. [PubMed] [Google Scholar]
- 12.Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg. 1996;20:411–417. doi: 10.1007/s002689900065. [DOI] [PubMed] [Google Scholar]
- 13.Fink MP. Intestinal epithelial hyperpermeability: update on the pathogenesis of gut mucosal barrier dysfunction in critical illness. Curr Opin Crit Care. 2003;9:143–151. doi: 10.1097/00075198-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Scrimshaw NS. Historical concepts of interactions, synergism and antagonism between nutrition and infection. J Nutr. 2003;133:316S–321S. doi: 10.1093/jn/133.1.316S. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler TR, Puckett AB, Griffiths DP, Galloway JR. Interactions between nutrients and growth factors in cellular growth and tissue repair. In: Ziegler TR, Pierce GF, Herndon DN, editors. Growth Factors and Wound Healing: Basic Science and Potential Clinical Applications. Springer-Verlag; New York, NY: 1997. pp. 104–150. [Google Scholar]
- 16.The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group Perioperative total parenteral nutrition in surgical patients. N Engl J Med. 2001;325:525–532. doi: 10.1056/NEJM199108223250801. [DOI] [PubMed] [Google Scholar]
- 17.Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P, Canadian Critical Care Clinical Practice Guidelines Committee Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27:355–373. doi: 10.1177/0148607103027005355. [DOI] [PubMed] [Google Scholar]
- 18.Heyland DK, MacDonald S, Keefe L, Drover JW. Total parenteral nutrition in the critically ill patient: a meta-analysis. JAMA. 1998;280:2013–2019. doi: 10.1001/jama.280.23.2013. [DOI] [PubMed] [Google Scholar]
- 19.Blumberg HM, Jarvis WR, Soucie JM, et al. National Epidemiology of Mycoses Survey (NEMIS) Study Group Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. The National Epidemiology of Mycosis Survey. Clin Infect Dis. 2001;33:177–186. doi: 10.1086/321811. [DOI] [PubMed] [Google Scholar]
- 20.Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 21.Wilmore DW. The effect of glutamine supplementation in patients following elective surgery and accidental injury. J Nutr. 2001;131(9 suppl):2543S–2549S. doi: 10.1093/jn/131.9.2543S. [DOI] [PubMed] [Google Scholar]
- 22.Furst P, Ziegler TR. Protein and amino acid metabolism and therapy: what is new and what has been left aside. Curr Opin Clin Nutr Metab Care. 1998;1:59–65. doi: 10.1097/00075197-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Labow BI, Souba WW. Glutamine. World J Surg. 2000;24:1503–1513. doi: 10.1007/s002680010269. [DOI] [PubMed] [Google Scholar]
- 24.Calder PC, Yaqoob P. Glutamine and the immune system. Amino Acids. 1999;17:227–241. doi: 10.1007/BF01366922. [DOI] [PubMed] [Google Scholar]
- 25.Hammarqvist F, Wernerman J, Ali R, von der Decken A, Vinnars E. Addition of glutamine to total parenteral nutrition after elective abdominal surgery spares free glutamine in muscle, counteracts the fall in muscle protein synthesis, and improves nitrogen balance. Ann Surg. 1989;209:455–461. doi: 10.1097/00000658-198904000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth E, Zoch G, Schulz F, et al. Amino acid concentrations in plasma and skeletal muscle of patients with acute hemorrhagic necrotizing pancreatitis. Clin Chem. 1985;31:1305–1309. [PubMed] [Google Scholar]
- 27.Stehle P, Zander J, Mertes N, et al. Effect of parenteral glutamine peptide supplements on muscle glutamine loss and nitrogen balance after major surgery. Lancet. 1989;1:231–233. doi: 10.1016/s0140-6736(89)91254-3. [DOI] [PubMed] [Google Scholar]
- 28.Parry-Billings M, Baigrie RJ, Lamont PM, Morris PJ, Newsholme EA. Effects of major and minor surgery on plasma glutamine and cytokine levels. Arch Surg. 1992;127:1237–1240. doi: 10.1001/archsurg.1992.01420100099017. [DOI] [PubMed] [Google Scholar]
- 29.Oudemans-van Straaten HM, Bosman RJ, Treskes M, van der Spoel HJ, Zandstra DF. Plasma glutamine depletion and patient outcome in acute ICU admissions. Intensive Care Med. 2001;27:84–90. doi: 10.1007/s001340000703. [DOI] [PubMed] [Google Scholar]
- 30.Furst P. Old and new substrates in clinical nutrition. J Nutr. 1998;128:789–796. doi: 10.1093/jn/128.5.789. [DOI] [PubMed] [Google Scholar]
- 31.Ziegler TR, Young LS, Benfell K, et al. Clinical and metabolic efficacy of glutamine-supplemented parenteral nutrition after bone marrow transplantation: a randomized, double-blind, controlled study. Ann Intern Med. 1992;116:821–828. doi: 10.7326/0003-4819-116-10-821. [DOI] [PubMed] [Google Scholar]
- 32.Mertes N, Schulzki C, Goeters C, et al. Cost-containment through L-alanyl-L-glutamine supplemented total parenteral nutrition after major abdominal surgery: a prospective, randomized, double-blind controlled study. Clin Nutr. 2000;19:395–401. doi: 10.1054/clnu.2000.0142. [DOI] [PubMed] [Google Scholar]
- 33.Karwowska KA, Dworacki G, Trybus M, Zeromski J, Szulc R. Influence of glutamine-enriched parenteral nutrition on nitrogen balance and immunologic status in patients undergoing elective aortic aneurysm repair. Nutrition. 2001;17:475–478. doi: 10.1016/s0899-9007(01)00537-8. [DOI] [PubMed] [Google Scholar]
- 34.van der Hulst RR, van Kreel BK, von Meyenfeldt MF, et al. Glutamine and the preservation of gut integrity. Lancet. 1993;341:1363–1365. doi: 10.1016/0140-6736(93)90939-e. [DOI] [PubMed] [Google Scholar]
- 35.Tremel H, Kienle B, Weilemann LS, Stehle P, Furst P. Glutamine dipeptide-supplemented parenteral nutrition maintains intestinal function in the critically ill. Gastroenterology. 1994;107:1595–1601. doi: 10.1016/0016-5085(94)90797-8. [DOI] [PubMed] [Google Scholar]
- 36.O’Riordain MG, Fearon KCH, Ross JA, et al. Glutamine-supplemented total parenteral nutrition enhances T-lymphocyte response in surgical patients undergoing colorectal resection. Ann Surg. 1994;220:212–221. doi: 10.1097/00000658-199408000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziegler TR, Bye RL, Persinger RL, et al. Effects of glutamine supplementation on circulating lymphocytes after bone marrow transplantation: a pilot study. Am J Med Sci. 1998;315:4–10. doi: 10.1097/00000441-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Exner R, Tamandl D, Goetzinger P, et al. Perioperative GLY-GLN infusion diminishes the surgery-induced period of immunosuppression: accelerated restoration of the lipopolysaccharide-stimulated tumor necrosis factor-alpha response. Ann Surg. 2003;237:110–115. doi: 10.1097/00000658-200301000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wischmeyer PE, Lynch J, Liedel J, et al. Glutamine administration reduces Gram-negative bacteremia in severely burned patients: a prospective, randomized, double-blind trial versus isonitrogenous control. Crit Care Med. 2001;29:2075–2080. doi: 10.1097/00003246-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Griffiths RD, Allen KD, Andrews FJ, Jones C. Infection, multiple organ failure, and survival in the intensive care unit: influence of glutamine-supplemented parenteral nutrition on acquired infection. Nutrition. 2002;18:546–552. doi: 10.1016/s0899-9007(02)00817-1. [DOI] [PubMed] [Google Scholar]
- 41.Fuentes-Orozco C, Anaya-Prado R, Gonzalez-Ojeda A, et al. L-alanyl-L-glutamine-supplemented parenteral nutrition improves infectious morbidity in secondary peritonitis. Clin Nutr. 2004;23:13–21. doi: 10.1016/s0261-5614(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 42.Dechelotte P, Hasselmann M, Cynober L, et al. L-alanyl-L-glutamine dipeptide-supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: the French controlled, randomized, double-blind, multicenter study. Crit Care Med. 2006;34:598–604. doi: 10.1097/01.CCM.0000201004.30750.D1. [DOI] [PubMed] [Google Scholar]
- 43.Griffiths RD, Jones C, Palmer TE. Six-month outcome of critically ill patients given glutamine-supplemented parenteral nutrition. Nutrition. 1997;13:295–302. [PubMed] [Google Scholar]
- 44.Goeters C, Wenn A, Mertes N, et al. Parenteral L-alanyl-L-glutamine improves 6-month outcome in critically ill patients. Crit Care Med. 2002;30:2032–2037. doi: 10.1097/00003246-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Novak F, Heyland DK, Avenell A, Drover JW, Su X. Glutamine supplementation in serious illness: a systematic review of the evidence. Crit Care Med. 2002;30:2022–2029. doi: 10.1097/00003246-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 46.van Zaanen HC, van der Lelie H, Timmer JG, Furst P, Sauerwein HP. Parenteral glutamine dipeptide supplementation does not ameliorate chemotherapy-induced toxicity. Cancer. 1994;74:2879–2884. doi: 10.1002/1097-0142(19941115)74:10<2879::aid-cncr2820741022>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 47.Schloerb PR, Skikne BS. Oral and parenteral glutamine in bone marrow transplantation: a randomized, double-blind study. JPEN J Parenter Enteral Nutr. 1999;23:117–122. doi: 10.1177/0148607199023003117. [DOI] [PubMed] [Google Scholar]
- 48.Powell-Tuck J, Jamieson CP, Bettany GE, et al. A double blind, randomised, controlled trial of glutamine supplementation in parenteral nutrition. Gut. 1999;45:82–88. doi: 10.1136/gut.45.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pytlik R, Benes P, Patorkova M, et al. Standardized parenteral alanyl-glutamine dipeptide supplementation is not beneficial in autologous transplant patients: a randomized, double-blind, placebo controlled study. Bone Marrow Transpl. 2002;30:953–961. doi: 10.1038/sj.bmt.1703759. [DOI] [PubMed] [Google Scholar]
- 50.Poindexter BB, Ehrenkranz RA, Stoll BJ, et al. National Institute of Child Health and Human Development Neonatal Research Network Parenteral glutamine supplementation does not reduce the risk of mortality or late-onset sepsis in extremely low birth weight infants. Pediatrics. 2004;113:1209–1215. doi: 10.1542/peds.113.5.1209. [DOI] [PubMed] [Google Scholar]
- 51.Ockenga J, Borchert K, Stuber E, et al. Glutamine-enriched total parenteral nutrition in patients with inflammatory bowel disease. Eur J Clin Nutr. 2005;59:1302–1309. doi: 10.1038/sj.ejcn.1602243. [DOI] [PubMed] [Google Scholar]
- 52.Albers MJ, Steyerberg EW, Hazebroek FW, et al. Glutamine supplementation of parenteral nutrition does not improve intestinal permeability, nitrogen balance, or outcome in newborns and infants undergoing digestive-tract surgery: results from a double-blind, randomized, controlled trial. Ann Surg. 2005;241:599–606. doi: 10.1097/01.sla.0000157270.24991.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alpers DH. Glutamine: do the data support the cause for glutamine supplementation in humans? Gastroenterology. 2006;130(2 suppl 1):S106–S116. doi: 10.1053/j.gastro.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 54.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 55.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 56.Knaus WA. APACHE 1978-2001: the development of a quality assurance system based on prognosis: milestones and personal reflections. Arch Surg. 2002;137:37–41. doi: 10.1001/archsurg.137.1.37. [DOI] [PubMed] [Google Scholar]
- 57.van der Lely AJ, Veelo DP, Dongelmans DA, et al. Time to wean after tracheotomy differs among subgroups of critically ill patients: retrospective analysis in a mixed medical/surgical intensive care unit. Respir Care. 2006;51:1408–1415. [PubMed] [Google Scholar]
- 58.Steininger R, Karner J, Roth E, Langer K. Infusion of dipeptides as nutritional substrates for glutamine, tyrosine, and branched-chain amino acids in patients with acute pancreatitis. Metabolism. 1989;38:78–81. doi: 10.1016/0026-0495(89)90147-9. [DOI] [PubMed] [Google Scholar]
- 59.de Beaux AC, O’Riordain MG, Ross JA, et al. Glutamine-supplemented total parenteral nutrition reduces blood mononuclear cell inter-leukin-8 release in severe acute pancreatitis. Nutrition. 1998;14:261–265. doi: 10.1016/s0899-9007(97)00477-2. [DOI] [PubMed] [Google Scholar]
- 60.Ockenga J, Borchert K, Rifai K, Manns MP, Bischoff SC. Effect of glutamine-enriched total parenteral nutrition in patients with acute pancreatitis. Clin Nutr. 2002;21:409–416. doi: 10.1054/clnu.2002.0569. [DOI] [PubMed] [Google Scholar]
- 61.Nathens AB, Neff MJ, Jurkovich GJ, et al. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg. 2002;236:814–822. doi: 10.1097/00000658-200212000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alonso de Vega JM, Diaz J, Serrano E, Carbonell LF. Oxidative stress in critically ill patients with systemic inflammatory response syndrome. Crit Care Med. 2002;30:1782–1786. doi: 10.1097/00003246-200208000-00018. [DOI] [PubMed] [Google Scholar]
- 63.Evans ME, Jones DP, Ziegler TR. Glutamine prevents cytokine-induced apoptosis in human colonic epithelial cells. J Nutr. 2003;133:3065–3071. doi: 10.1093/jn/133.10.3065. [DOI] [PubMed] [Google Scholar]
- 64.Flaring UB, Rooyackers OE, Wernerman J, Hammarqvist F. Glutamine attenuates post-traumatic glutathione depletion in human muscle. Clin Sci (Lond) 2003;104:275–282. doi: 10.1042/CS20020198. [DOI] [PubMed] [Google Scholar]
- 65.Ziegler TR, Ogden LG, Singleton KD, et al. Parenteral glutamine increases serum heat shock protein-70 in critically ill patients. Intensive Care Med. 2005;31:1079–1086. doi: 10.1007/s00134-005-2690-5. [DOI] [PubMed] [Google Scholar]
- 66.Finney SJ, Zekveld C, Elia A, Evans TW. Glucose control and mortality in critically ill patients. JAMA. 2003;290:2041–2047. doi: 10.1001/jama.290.15.2041. [DOI] [PubMed] [Google Scholar]