Abstract
Substantial progress has been made in our understanding of the biology of pancreatic cancer, and advances in patients’ management have also taken place. Evidence is beginning to show that screening first-degree relatives of individuals with several family members affected by pancreatic cancer can identify non-invasive precursors of this malignant disease. The incidence of and number of deaths caused by pancreatic tumours have been gradually rising, even as incidence and mortality of other common cancers have been declining. Despite developments in detection and management of pancreatic cancer, only about 4% of patients will live 5 years after diagnosis. Survival is better for those with malignant disease localised to the pancreas, because surgical resection at present offers the only chance of cure. Unfortunately, 80–85% of patients present with advanced unresectable disease. Furthermore, pancreatic cancer responds poorly to most chemotherapeutic agents. Hence, we need to understand the biological mechanisms that contribute to development and progression of pancreatic tumours. In this Seminar we will discuss the most common and deadly form of pancreatic cancer, pancreatic ductal adenocarcinoma.
Epidemiology
Pancreatic cancer is the fourth leading cause of cancer death in the USA and leads to an estimated 227 000 deaths per year worldwide.1 Risk factors for this malignant disease include smoking, family history2 of chronic pancreatitis, advancing age, male sex, diabetes mellitus, obesity, non-O blood group,3,4 occupational exposures, African-American ethnic origin, a high-fat diet, diets high in meat and low in vegetables and folate, and possibly Helicobacter pylori infection and periodontal disease.1 Findings of preliminary studies suggest that metformin could protect against development of pancreatic cancer.5 Coffee intake is not regarded as a risk factor for disease. Although the cause of pancreatic cancer is complex and multifactorial, cigarette smoking and family history are dominant. About 20% of pancreatic tumours are caused by cigarette smoking, and cancers from smokers harbour more genetic mutations than those from non-smokers.6
A family history of pancreatic cancer is an important risk factor for disease;1 about 7–10% of affected individuals have a family history.7 Familial pancreatic cancer is defined in most studies as families in which a pair of first-degree relatives have been diagnosed with pancreatic tumours. Prospective analysis of families with this malignant disease shows that first-degree relatives of individuals with familial pancreatic cancer have a ninefold increased risk of this neoplasm over the general population.2 This risk rises to 32-fold greater in kindreds with three or more first-degree relatives with pancreatic cancer. Furthermore, evidence indicates that the risk of pancreatic cancer is modestly increased in first-degree relatives of patients with sporadic pancreatic cancer compared with the general population.8 Of kindreds with familial pancreatic cancer, risk is highest in those with a case of young-onset pancreatic cancer (age <50 years) in the family compared with those without a young-onset case.8 Patients with familial pancreatic cancer also have more precancerous lesions than those with sporadic pancreatic tumours9 and have an augmented risk of developing extra-pancreatic cancers.10
Panel 1 describes genes known to form the inherited basis of pancreatic cancer. Once an individual’s cancer predisposition gene is identified, family members can undergo genetic testing and, if appropriate, cancer screening and chemoprevention. However, germline genetic testing of patients with pancreatic cancer is probably underused, in large part because of a failure to recognise from the family history the possibility of a familial cancer syndrome. Many clinicians do not record an adequate cancer family history.25 Usually, kindreds affected by pancreatic cancer who have mutated susceptibility genes do not manifest a high penetrance of pancreatic cancer. For this reason, and because much of the inherited sensitivity to pancreatic cancer remains unexplained, consensus guidelines have not been established to steer genetic testing for inherited susceptibility to pancreatic cancer. BRCA2 gene testing should be considered—after appropriate genetic counselling —for patients of Jewish ethnic origin, those with a strong family history of breast cancer, or individuals with many first-degree relatives with pancreatic cancer; germline CDKN2A testing should be done if there is a family history of familial atypical multiple-mole melanoma (panel 1). Even without genetic testing, obtaining a detailed family history of cancer can be used for prediction of clinical risk, and mendelian risk-prediction programs have been evaluated for their use for individuals with familial pancreatic cancer.26
Panel 1: Inherited susceptibility to pancreatic cancer11.
Germline mutations in BRCA2, PALB2, CDKN2A, STK11, and PRSS1 genes, and Lynch syndrome, are associated with a substantially increased risk of pancreatic cancer
Germline BRCA2 gene mutations account for the highest proportion of known causes of inherited pancreatic cancer
Germline BRCA2 gene mutations have been identified in 5–17% of families with familial pancreatic cancer12–14
Some patients with pancreatic cancer and a germline BRCA2 gene mutation do not have a relevant family history of breast, ovarian, or pancreatic cancer to raise suspicion that they carry such mutations15
Germline BRCA2 gene mutations are associated with 10% of unselected, apparently sporadic, pancreatic cancers in the Ashkenazi Jewish population11
PALB2 (partner and localiser of BRCA2) has been identified as a pancreatic cancer susceptibility gene16
Germline PALB2 mutations are recorded in up to 3% of patients with familial pancreatic cancer16–18
Protein products of BRCA2 and PALB2 function in the Fanconi DNA repair pathway
Germline mutations in other genes of the Fanconi DNA repair pathway (FANCC, FANCG) are rare11
Identification of cancers with inactivation of the BRCA2-PALB2-Fanconi DNA repair pathway has therapeutic implications, since these cancers are highly sensitive to PARP inhibitors and alkylating agents19–22
Germline CDKN2A gene mutations are noted generally in families with familial atypical multiple-mole melanoma, germline STK11 mutations in patients with Peutz-Jeghers syndrome, and germline PRSS1 mutations in people with hereditary pancreatitis
Patients with hereditary non-polyposis colon cancer (Lynch syndrome)23 have a modest increased risk of developing pancreatic cancer11
Findings of genome-wide association studies showed an association between non-O blood group and pancreatic cancer,3 confirming data of prospective cohort studies4
Other variants have been implicated as risk factors for pancreatic cancer by findings of genome-wide association studies, including the telomerase subunit locus TERT24
PARP=poly (ADP-ribose) polymerase.
Pathophysiology
Pancreatic ductal adenocarcinomas evolve through noninvasive precursor lesions, most typically pancreatic intraepithelial neoplasias (figure 1, panel 2), acquiring clonally selected genetic and epigenetic alterations along the way (figure 2). Pancreatic cancers can also evolve from intraductal papillary mucinous neoplasms (figure 3) or mucinous cystic neoplasms (panel 2).
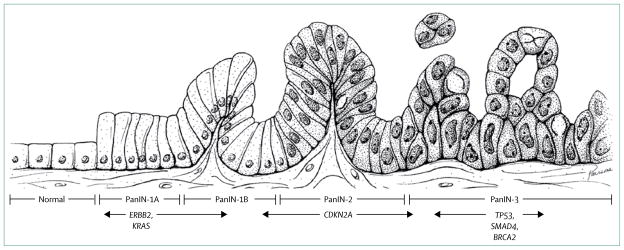
Figure 1. Histological features of pancreatic intraepithelial neoplasia.
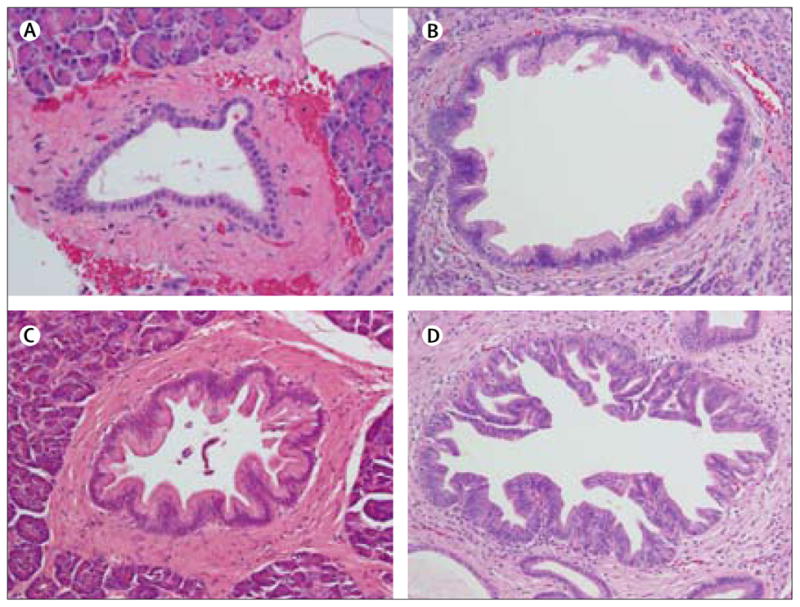
(A–D) Magnification x??.
Panel 2: Non-invasive precursors to pancreatic cancer.
The most common neoplastic precursor to invasive adenocarcinoma of the pancreas is known as PanIN (figure 1)
PanINs are microscopic (<5 mm diameter) and are not directly visible by pancreatic imaging
PanINs can harbour the somatic genetic alterations seen in invasive pancreatic cancers, and prevalence of these genetic alterations rises as the amount of cytological and architectural atypia in PanINs increases27,28 (figure 2)
Low-grade PanINs (PanIN 1) are very common with increasing age and high-grade PanINs (PanIN 3) are usually present in pancreata with invasive cancer
Pancreata resected from individuals with a strong family history of pancreatic cancer usually have multifocal PanINs associated with lobulocentric atrophy29–31
Molecular markers are being investigated to see if they can be used to estimate the burden and grade of PanIN32
Molecular imaging has the potential to detect PanIN but further research is needed33
Intraductal papillary mucinous neoplasms are a less frequent precursor to invasive pancreatic cancer; they are large cystic neoplasms (≥5mm)34 diagnosed increasingly because of improvements in pancreatic imaging35,36 (figure 3)
Non-invasive intraductal papillary mucinous neoplasms are classified on the basis of the amount of cytological and architectural dysplasia, as either low-grade, intermediate-grade, or high-grade dysplasia (carcinoma in situ)
Cure rates are very high after resection of intraductal papillary mucinous neoplasms that do not have an associated invasive pancreatic cancer but, if left alone, these lesions can progress to incurable invasive cancers
Intraductal papillary mucinous neoplasms can affect pancreatic branch ducts, main ducts, or both
Most small asymptomatic Intraductal papillary mucinous neoplasms in branch ducts have low malignant potential, so international guidelines have been developed for their management37
If the patient can tolerate surgery, resection of intraductal papillary mucinous neoplasms should be done if the neoplasm is in the main pancreatic duct, if it is associated with symptoms, if it is larger than 3 cm, and if it has a mural nodule
By contrast, most intraductal papillary mucinous neoplasms confined to branches of the main pancreatic duct (branch-duct neoplasms) have low malignant potential but should be followed up with regular pancreatic imaging, with the imaging interval based on lesion size
Branch-duct intraductal papillary mucinous neoplasms of 1–3 cm in size should be assessed by endoscopic ultrasound and magnetic resonance cholangiopancreatography
CT is also appropriate for intraductal papillary mucinous neoplasms, but since many patients need repeated imaging, some prefer alternate MRI and endoscopic ultrasound, thereby avoiding repeated radiation associated with CT
Surgical resection is recommended for intraductal papillary mucinous neoplasms if high-risk stigmata are present
Criteria for surveillance and resection could be different for patients with a strong family history of pancreatic cancer31 because they sometimes have concurrent microscopic PanIN that cannot be followed up accurately by imaging
A less frequent precursor to pancreatic cancer is the mucinous cystic neoplasm, which is composed of mucin-producing epithelial cells and an associated ovarian-type stroma38
Unlike intraductal papillary mucinous neoplasms, mucinous cystic neoplasms do not communicate with pancreatic ducts
Mucinous cystic neoplasms arise predominantly in women; about a third of these neoplastic precursors have an associated invasive carcinoma
PanIN=pancreatic intraepithelial neoplasia.
Figure 2. PanIN progression model, showing genetic alterations.
PanIN=pancreatic intraepithelial neoplasia. Reprinted from ref 27, with permission of the American Association for Cancer Research.
Figure 3. Cross-sectional imaging and analysis of an intraductal papillary mucinous neoplasm.
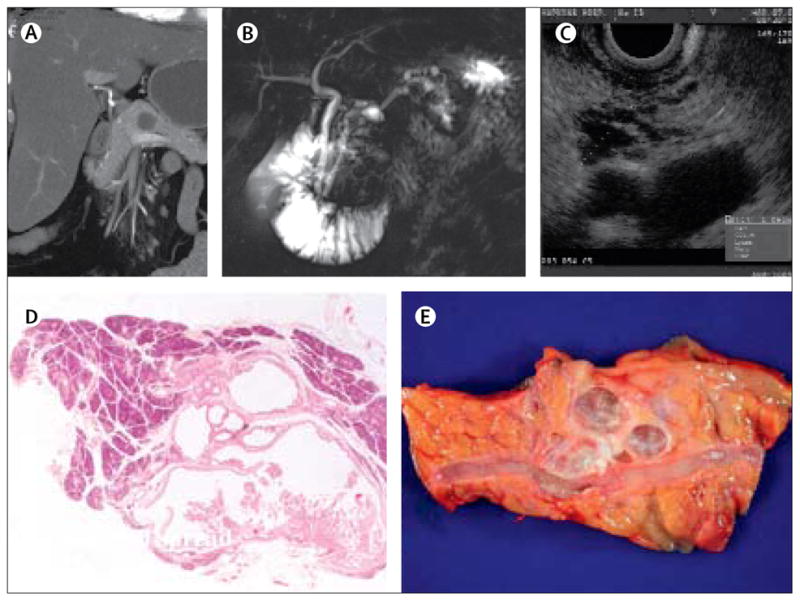
(A) CT. (B) ??. (C) Endoscopic ultrasound. (D) Histological analysis (magnification x??). (E) Resected tumour.
The exomes of 24 pancreatic ductal adenocarcinomas were sequenced to characterise more fully the genes mutated in pancreatic cancers.39 The most frequent genetic abnormalities in invasive pancreatic adenocarcinomas are mutational activation of the KRAS oncogene, inactivation of tumour-suppressor genes including CDKN2A, TP53, SMAD4, and BRCA2,40 widespread chromosomal losses, gene amplifications,39 and telomere shortening.41 KRAS mutations and telomere shortening are the earliest known genetic abnormalities recorded, even in low-grade pancreatic intraepithelial neoplasias,41,42 and telomere shortening is believed to contribute to chromosomal instability, whereas inactivation of TP53, SMAD4, and BRCA2 happens in advanced pancreatic intraepithelial neoplasias and invasive carcinomas.27,28 Genes mutated in a few (<20%) pancreatic cancers include oncogenes such as BRAF, MYB, AKT2, and EGFR, and tumour-suppressor genes such as MAP2K4, STK11, TGFBR1, TGFBR2, ACVR1B, ACVR2A, FBXW7, and EP300.39 Structural analysis of mutated genes implicates PIK3CG, DGKA, STK33, TTK, and PRKCG as low-frequency driver mutations.43
Intraductal papillary mucinous neoplasms harbour many of the genetic alterations recorded in pancreatic intraepithelial neoplasias but with notable differences— eg, intraductal papillary mucinous neoplasms rarely inactivate SMAD4. Genetically engineered mouse models targeting some of the genes most commonly altered in human pancreatic cancer have been developed, and several of these recapitulate the human disease and have been used to study mechanisms and investigate therapeutic agents.44
In addition to the driver genes discussed above, epigenetic changes can also alter gene function in pancreatic cancers.45 Epigenetic dysregulation includes alterations in DNA methylation and histone modifications and non-coding RNAs. Promoter methylation and gene silencing in pancreatic cancers was first reported for the tumour-suppressor gene CDKN2A, of which epigenetic silencing is restricted to neoplasms without genetic inactivation of CDKN2A.46 Only a few classic tumour-suppressor and DNA-repair genes undergo epigenetic silencing in pancreatic cancers—eg, MLH1 and CDH1 are methylated in a small proportion of tumours. Many other genes are frequent targets of aberrant methylation and gene silencing in pancreatic cancers, including CDKN1C, RELN, SPARC, TFPI2, and others.45,47–51 Some of the most commonly aberrantly hypermethylated genes in pancreatic neoplasms have been evaluated for their diagnostic or biological relevance.32,45,52 Promoter hypomethylation of overexpressed genes has also been reported for several genes, such as SFN, MSLN, and S100A4,53 and mucin genes.54
Alterations in microRNA expression seem to contribute to cancer development and progression. Overexpression of several microRNAs in pancreatic cancers—including miR-21, miR-34, miR-155, and miR-200—is thought to contribute to neoplastic progression.55–58 Furthermore, since microRNAs are stable and detectable in human plasma they could be useful diagnostic markers.56
Genetic and epigenetic alterations of pancreatic cancers probably play a part in tumour aggressiveness and patterns of progression. Panel 3 describes major pathways affected by these alterations.
Panel 3: Major signalling pathways and tumour stromal interactions entailed in pancreatic cancer development and progression.
The most important pathways include those targeted for genetic and epigenetic alterations—ie, those that include protein products of KRAS, RB1 and CDKN2A, TP53, and SMAD4 and TGFB1 genes
The hedgehog, NOTCH, AKT1-PI3K-MTOR, and BRCA2-PALB2-Fanconi pathways are being investigated as therapeutic targets39
Suspected downstream members of the RAS signalling cascade include RAF, MEK, MAPK (previously known as ERK), STK33, and PLK1
Melanomas with BRAF mutations respond to BRAF inhibitors and could potentially be of benefit to the few pancreatic cancers (<5%) that harbour BRAF gene mutations
Tumour-stromal interactions contribute to oncogenic signalling, including interactions entailing the hedgehog pathway, cyclo-oxygenases, the extracellular matrix protein SPARC, and NFκB, among others51,62,63
Hedgehog ligands derived from pancreatic cancer cells stimulate non-neoplastic stromal fibroblasts that overexpress the hedgehog pathway receptor called smoothened (SMO), and this paracrine hedgehog signalling stimulates fibroblast-mediated tumour growth;62 this mechanism of activation of the hedgehog pathway is more typical than alterations of the hedgehog pathway in pancreatic cancer cells64
Hedgehog inhibitors effective for patients with basal cell carcinomas65 and medulloblastomas—cancers with mutational activation of the hedgehog pathway—are undergoing testing in clinical trials in patients with pancreatic cancer
The stromal environment could be a physical or pathophysiological barrier preventing chemotherapeutic drugs from reaching pancreatic cancer cells, and elimination of stroma could enhance cancer drug delivery66
Molecular evolution of pancreatic cancers has been estimated using somatic mutations as molecular clocks. From this analysis, an initial precursor neoplastic clone will take roughly more than 10 years to evolve into a malignant clone and several additional years for metastatic subclones to emerge from within the primary cancer.59 Comparison of molecular alterations of a patient’s primary pancreatic cancer and metastases reveals not only that almost all the major driver genes are mutated before development of invasive adenocarcinoma but also that genetic instability continues after cancer dissemination, with some genetic heterogeneity arising in different metastases.60 Although these estimates reflect a range of tumour behaviour in different patients, they indicate that a primary cancer can reside in the pancreas for many years before metastasis, potentially providing opportunities for screening.60,61
Tumour microenvironment
Interactions between cancer-associated fibroblasts—the predominant stromal cell type—and neoplastic cells could contribute to tumour initiation, progression, and metastasis. Some of the best characterised pathways affected by tumour-stromal interactions are described in panel 3.
The role of the immune system in pancreatic cancer progression has focused on the potential benefit of inhibition of T regulatory lymphocytes (cells that suppress antitumour immune responses) or use of vaccines— including irradiated genetically modified pancreatic cancer cells or immunostimulatory pancreatic cancer antigens, such as overexpressed (eg, mesothelin) or mutated proteins.67,68 Furthermore, the mechanisms of immune evasion by cancer cells and cancer-associated fibroblasts have been studied.66,67
The role of tumour-initiating cells (so-called cancer stem cells) in the development of pancreatic cancer is controversial. Although putative cells have been identified,69 reconciling the notion of tumour-initiating cells with the clonal selection provided to neoplastic cells by tumourigenic mutations acquired during carcinogenesis is difficult.70 One hypothesis is that cancer stem-cell markers identify cells most likely to survive a given cellular stress at any one time, such as the ability to grow in nude mice or survive chemotherapeutic agents.71
Screening to detect curable precursor lesions
The deadly nature of invasive pancreatic cancer, and recognition that most patients present with advanced stage disease,72 has led to efforts to screen individuals with an inherited predisposition for early curable disease— such as pancreatic intraepithelial neoplasias, and noninvasive intraductal papillary mucinous neoplasms and mucinous cystic neoplasms. Family history has been used as a quantitative predictor of pancreatic cancer risk.2,9,29–31,73 Indeed, screening has identified silent pancreatic neoplasia in many individuals with strong family histories of pancreatic cancer.55,56 For example, in the CAPS2 trial (Cancer of the Pancreas Screening 2), about 10% of patients screened (those generally aged 50 years or older, with three blood relatives with pancreatic cancer, including at least one affected first-degree relative) had intraductal papillary mucinous neoplasms detectable by endoscopic ultrasound. However, screening brings with it the risk of over treatment, and the randomised trials needed to ascertain if pancreatic cancer screening can save lives have not been undertaken.
Researchers are doing clinical trials to assess the best screening protocol for individuals at increased risk of pancreatic neoplasia. An ideal screening test for early pancreatic cancer would be a highly accurate blood marker that could be measured fairly non-invasively. Unfortunately, none to date have proven sufficiently specific for diagnosis.74–77 Furthermore, the focus of screening efforts up to now has been to detect preinvasive lesions, rather than early pancreatic cancer, since resection of preinvasive lesions can prevent development of an invasive pancreatic cancer, whereas once an invasive pancreatic cancer develops, its spread beyond the pancreas is probably rapid, restricting use of markers of invasive pancreatic cancer.
Because of its ability to detect small preinvasive lesions (of about 1 cm), endoscopic ultrasound is used widely as a screening test. In the CAPS3 multicentre screening trial (clinicaltrials.gov identifier NCT00438906), pancreatic cystic lesions were detected more frequently with endoscopic ultrasound (93%) and MRI (81%) than with CT (27%).78 Since microscopic pancreatic intraepithelial neoplasias are usually not visible by pancreatic imaging, research is attempting to identify markers in pancreatic fluid that could reliably identify high-grade pancreatic intraepithelial neoplasias.32,75,79 Focal preinvasive lesions evident by endoscopic ultrasound (such as intraductal papillary mucinous neoplasms) are probably most readily sampled with fine-needle aspiration.
Clinical presentation
Early-stage pancreatic cancer is usually clinically silent, and disease only becomes apparent after the tumour invades surrounding tissues or metastasises to distant organs. Most people who present with symptoms attributable to pancreatic cancer have advanced disease.80 Pancreatic cancer patients who have undergone abdominal CT scans for other reasons before their diagnosis are usually noted in retrospect to have had subtle abnormalities suspicious for pancreatic cancer up to 1 year before development of symptoms,81 suggesting a missed opportunity for early detection.
Typical presenting symptoms of pancreatic cancer include abdominal or mid-back pain, obstructive jaundice, and weight loss. Weight loss can arise from anorexia, maldigestion from pancreatic ductal obstruction, and cachexia. Occasionally, pancreatic-duct obstruction could result in attacks of pancreatitis. Deep and superficial venous thrombosis is not unusual and might be a presenting sign of malignant disease. Gastric-outlet obstruction with nausea and vomiting sometimes happens with more advanced disease. Less common manifestations include panniculitis and depression. About 25% of patients with pancreatic cancer have diabetes mellitus at diagnosis and roughly another 40% have impaired glucose tolerance.82,83 The cause of the diabetogenic state is uncertain, but diabetes is sometimes cured by resection of pancreatic cancer. Researchers are investigating whether early-stage pancreatic cancer could be diagnosed in older individuals with new-onset diabetes. Most people with new-onset diabetes, however, do not have pancreatic cancer.84 Apart from weight loss, few clinical clues exist to suspect pancreatic cancer in those with new-onset diabetes. Hence, screening older patients with new-onset diabetes for pancreatic cancer would need new screening tests.85
Diagnosis and staging
Tri-phasic pancreatic-protocol CT is the best initial diagnostic test for pancreatic cancer. It is also best for disease staging, and optimum CT scans— including 3-dimensional reconstruction—provide about 80% accuracy for prediction of resectability. The quality of CT varies, and imaging technology continues to improve. The ability of high-quality pancreatic-protocol CT scans to detect locally advanced and metastatic disease reliably has greatly reduced the number of unnecessary laparotomies and need for staging laparoscopies.86 Endoscopic ultrasound is also highly accurate for diagnosis of pancreatic cancer, and sampling for diagnostic cytology can be undertaken at the time of endoscopic ultrasound. MRI can be used for staging in patients who cannot tolerate intravenous contrast for CT.
Clinical staging classifies patients into resectable, borderline resectable, locally advanced, and metastatic disease (panel 4). It dictates the most appropriate initial treatment. Chest imaging (either chest radiography or CT) is recommended to detect pulmonary metastases. PET CT is currently not part of routine staging but can be helpful if metastases are suspected, such as for indeterminate lesions by CT, and might be better at identification of metastatic disease. Laparoscopy can spot peritoneal metastases but is not undertaken routinely before proceeding with pancreatic resection. Preoperative amounts of carbohydrate antigen 19-9 (CA19-9) of more than 100–200 U/mL predict unresectability and survival.75,87
Panel 4: Staging of pancreatic cancer.
Clinical staging
Local or resectable (about 10%, median survival 17–23 months)
Stage 0 (Tis, N0, M0)
Stage IA (T1, N0, M0)
Stage IB (T2, N0, M0)
Stage IIA (T3, N0, M0)
Stage IIB (T1, N1, M0; T2, N1, M0; T3, N1, M0)
Borderline resectable (10%, median survival up to 20 months)
Stage 3 disease with tumour abutment or <180° circumference of the superior mesenteric artery or coeliac arteries, or a short segment of hepatic artery or the superior mesenteric vein, pulmonary vein, or confluence of these veins
Locally advanced or unresectable (about 30%, median survival 8–14 months)
Stage III (T4, any N, M0)
Tumour encasement >180° circumference of the superior mesenteric artery or coeliac arteries, any unreconstructable venous involvement
Metastatic (about 60%, median survival 4–6 months)
Stage IV (any T, any N, M1)
TNM classification
T=primary tumour
TX: primary tumour cannot be assessed
T0: no evidence of primary tumour
Tis: carcinoma in situ (includes the PanIN 3 classification)
T1: tumour restricted to the pancreas, ≤2 cm greatest dimension
T2: tumour restricted to the pancreas, >2 cm greatest dimension
T3: tumour extends beyond the pancreas, no involvement of coeliac axis or superior mesenteric artery (or extension to the portal vein or superior mesenteric artery, but still resectable)
T4: tumour affects the coeliac axis or superior mesenteric artery (unresectable primary tumour)
N=regional lymph node
NX: regional lymph nodes cannot be assessed
N0: no regional lymph-node metastasis
N1: regional lymph-node metastasis
M=distant metastasis
M0: no distant metastasis
M1: distant metastasis
Although pancreatic cancer can be strongly suspected when imaging reveals a pancreatic mass invading surrounding organs, tissue diagnosis is recommended to confirm the finding and to rule out benign disorders that present with pancreatic enlargement and obstructive jaundice, such as autoimmune pancreatitis.
Cytological diagnosis can usually be made with endoscopic ultrasound or CT-guided fine-needle aspiration. Sensitivity of endoscopic ultrasound-guided fine-needle aspiration of pancreatic masses is reported to be about 80%.88 Identification of the cause of biliary or pancreatic-duct strictures might need endoscopic retrograde cholangiopancreatography and brushings for cytological diagnosis. The yield of cells from endoscopic brushings is low (about 20%) because of the small sample and sometimes subtle differences between malignant and non-neoplastic reactive cells. Molecular markers could have a role as an adjunct to brush and fine-needle aspirate cytological diagnosis, but these need further evaluation.52,89 A biopsy specimen is not needed for surgical resection when suspicion of cancer is high; generally, the resection will provide therapeutic benefit, and substantially delaying surgery to confirm a diagnosis could set back commencement of effective treatment.
Principles of management
Patients with pancreatic cancer are best managed by a multidisciplinary team that includes oncologists, surgeons, radiologists, gastroenterologists, radiation oncologists, pathologists, pain management experts, social workers, dieticians, and (when appropriate) palliative care experts.90 Pancreatic cancer is a heterogeneous disease at the molecular, pathological, and clinical level. A patient’s response to treatment and outcome depends on many factors, including the biology of their cancer, their performance status, and their pattern of disease progression.
Surgery
Operative mortality from pancreatic resection is low at most expert centres.91 Findings of several studies show that mortality from pancreaticoduodenectomy is considerably lower in high-volume compared with low-volume centres. For this reason, consensus panels recommend that pancreaticoduodenectomy should be undertaken at institutions doing at least 15–20 of these operations a year. Furthermore, many candidates for curative resection do not undergo surgical resection.92,93 Postoperative complications after resection include pancreatic anastomotic leaks and delayed gastric emptying. Researchers on several randomised trials have attempted to ascertain the best operative approaches to minimise postoperative complications arising from pancreaticoduodenectomy. Findings of these trials have not shown any clear advantage of one technique over another.
Portal or superior mesenteric vein resection and reconstruction is appropriate when it enables an R0 resection and can be done without increased operative morbidity.94 Laparoscopic resection is a viable approach for selected pancreatic-tail resections.95 Endoscopic tattooing can be used to localise small lesions before laparoscopic resection.96
Preoperative biliary drainage is mandatory for patients with cholangitis, those with relevant liver dysfunction, and those who are symptomatic (such as with severe pruritis). Otherwise, routine preoperative biliary drainage might not be necessary, since study findings indicate that outcomes are worse after routine preoperative drainage for individuals with obstructive jaundice from pancreatic cancer versus surgical resection alone.97 On the other hand, in the neoadjuvant setting, obstructive jaundice needs to be corrected before initiation of chemotherapy and radiotherapy. Biliary drainage will also lower non-specific CA19-9 amounts, allowing a more reliable estimate of disease burden.
Pathological findings after surgery
Pathological assessment of the resected pancreatic tumour provides important prognostic information. The pathologist must ascertain if the surgeon has achieved a negative (R0) resection margin. An R1 margin is positive at the microscopic level but not grossly visible, and an R2 resection has grossly visible cancer at the resection margin. Standardised protocols for establishing margin status pathologically are not available, resulting in variability in assessment of margin status. One change in TNM staging (panel 4) of pancreatic cancers is that a neoplasm extending to the portal vein or superior mesenteric vein is now deemed T3 disease if it is still resectable and the venous circulation can be reconstructed successfully.
Pathological assessment includes classification of histological variants of pancreatic ductal adenocarcinoma. Such variants consist of colloid carcinomas (associated with intestinal-type intraductal papillary mucinous neoplasms), medullary cancers (which might have microsatellite instability), and others including adenosquamous tumours, hepatoid carcinoma, signet-ring cell cancer, undifferentiated carcinoma, and undifferentiated carcinoma with osteoclast-like giant cells.38
For patients with surgically resected ductal adenocarcinoma of the pancreatic head, actuarial 5-year overall survival is about 20–25%. The presence of positive resection margins, poor tumour differentiation, a large cancer, and positive lymph nodes all portend a poor prognosis.
Several markers—such as loss of SMAD4 immunolabelling—have been associated with increased risk of development of widespread metastasis98 and poor outcome after surgical resection.99,100 SPARC expression in fibroblasts associated with pancreatic cancer also indicates an adverse outcome.101
Findings of these initial studies suggest that detailed molecular assessment of pancreatic cancers could ultimately improve clinical decision making, although even information provided by complete exome sequencing might not be sufficiently informative to guide therapeutic decision making in most patients. Furthermore, for individuals with inoperable disease, molecular profiling might be limited by what can be done on a Tru-Cut biopsy sample.
Adjuvant and neoadjuvant treatment
Adjuvant therapy
Adjuvant treatment is recommended for individuals who undergo pancreatic resection with curative intent.102 It is generally given once patients have recovered from surgery (1–2 months). Baseline CT scans and CA19-9 concentrations should be obtained before initiation of adjuvant treatment. The benefit of this therapy has been established from findings of randomised controlled trials (GITSG,103 CONKO-001,104 and RTOG-9704)105 and retrospective studies.106–109 In the GITSG trial, fluorouracil-based chemoradiation was superior to observation alone.103 Addition of radiation to adjuvant chemotherapy is unproven and controversial. Researchers on the ESPAC-1 trial compared chemotherapy with chemoradiation and noted that chemoradiation failed to increase survival and was perhaps harmful, although the trial had limitations with respect to its design and variability in radiation delivery.110,111 The RTOG-9704 trial compared gemcitabine with fluorouracil before and after fluorouracil-based chemoradiation. In an updated analysis of this trial, the treatment arms did not differ by much.105 Workers on the ESPAC-3 trial compared adjuvant gemcitabine with fluorouracil and noted no difference in survival.112 The conclusion of the CONKO-001 trial was that gemcitabine was superior to observation alone, with median overall survival of 22·8 versus 20·2 months.104 Since RTOG-9704 and CONKO-001 differed by design (CONKO-001 excluded patients with concentrations of CA19-9 >90 U/mL) and study population (CONKO-001 had fewer participants with node-positive or margin-positive disease) they are not comparable directly, but their results suggest that benefit is similar whether patients receive adjuvant chemotherapy or chemoradiotherapy. These findings, together with results of the ESPAC-1 trial, lead many oncologists to conclude that adjuvant chemotherapy is beneficial and that evidence is scant for a role of conventional adjuvant radiotherapy. Therefore, in many centres (particularly in Europe), patients receive adjuvant chemotherapy without radiotherapy. Some centres, particularly in the USA, still use adjuvant chemoradiotherapy, suspecting that radiotherapy can be helpful in some patients, and clinical trials to date have not ruled out such a benefit.
Individuals most likely to benefit from adjuvant treatment are those who have undergone an R0 resection. Many people with an R1 resection have inferior median survival compared with those with R0 resections (range 8–18 vs 20–25 months). After surgical resection, patients are likely to develop systemic (>70%) and local recurrence (>20%). Worldwide, clinicians are beginning to delay radiotherapy until after an adequate course of chemotherapy, to prevent metastatic disease, so only patients who are free of disease 4–6 months after systemic treatment will receive adjuvant radiotherapy. This approach is being assessed in the international phase III adjuvant trial (EORTC, US Intergroup, RTOG-0848). Although delaying radiotherapy might be adequate for individuals with R0 resections, it is not suitable for patients with R1 resections if a delay will increase the risk of local recurrence. The role of adjuvant treatment after distal pancreatectomy is controversial and based mainly on studies from individual institutions.113
Other adjuvant regimens are under investigation. The combination of adjuvant interferon alfa-2b, cisplatin, and continuous-infusion fluorouracil concurrently with external-beam radiation had unacceptably high toxic effects.114 Adjuvant trials underway include those testing the role of erlotinib, the combination of gemcitabine, docetaxel, and capecitabine (NCT00882310), and the granulocyte-macrophage colony-stimulating factor-secreting vaccine for pancreatic cancer, with or without cyclophosphamide as a T regulatory-depleting agent (NCT00727441). A summary of important, completed, phase III adjuvant trials is outlined in table 1, and other ongoing and completed trials are described in the webappendix (p 1–3).
Neoadjuvant therapy
Is neoadjuvant therapy as effective or superior to adjuvant treatment for pancreatic cancer? Although no data are available from randomised controlled trials to support neoadjuvant over adjuvant therapy, findings of a meta-analysis suggest the proportion of patients who can have resection is similar, whether or not neoadjuvant treatment is given.115 Neoadjuvant therapy does yield partial responses and has the potential to downstage patients with borderline resectable disease,115 and it is usually recommended in this setting.116 Neoadjuvant treatment can detect patients whose disease progresses rapidly and, therefore, it could help to select those who might not benefit from surgical resection. Another potential advantage of neoadjuvant therapy is that postoperative complications do not delay or preclude administration of adjuvant treatment. Conversely, tumour response rates to current neoadjuvant therapies are not high, and delaying surgical resection could also allow disease progression. For this reason, patients undergoing neoadjuvant therapy should be restaged before surgical resection.
The optimum neoadjuvant regimen is not yet known, although combination chemotherapy schedules are usually given.115 Since no advantage has been recorded of neoadjuvant treatment over adjuvant therapy for patients with clearly resectable disease, and strong evidence exists that adjuvant therapy increases survival, most centres use adjuvant treatment, reserving neoadjuvant therapy for patients with borderline resectable disease.
Management of borderline resectable disease
Resections are attempted in many patients with borderline resectable cancer (ascertained by clinical staging) if the clinician suspects that an R0 resection can be achieved. Optimum preoperative staging can target individuals who should undergo initial chemoradiotherapy rather than surgery.90,117 The regimens used for adjuvant and neoadjuvant chemoradiotherapy are those typically administered to patients with borderline resectable disease. In an uncontrolled study of individuals with borderline resectable pancreatic cancer, those receiving neoadjuvant therapy and deemed eligible for pancreatic resection had significantly better survival than those who did not have pancreatectomy.118 Patients with borderline resectable disease who require vein resections seem to benefit also from adjuvant treatment.119
Management of advanced disease
Survival is significantly better for patients with locally advanced disease (median survival 9–15 months) than for those with metastatic disease (3–6 months). Chemotherapy is the mainstay of treatment for individuals with advanced disease—provided they have adequate performance status—but is not helpful for those with poor performance status. Gemcitabine is standard for patients with advanced pancreatic cancer: it induces a partial response in a few people and can alleviate symptoms in some with advanced tumours.120–122
Fixed-dose gemcitabine might enable maximum intracellular accumulation of the active triphosphate form of the drug. In one study, fixed dosing resulted in better responses than standard gemcitabine infusion but produced more haematological toxic effects.123 Such a benefit has not been recorded in other studies. Markers have been identified that predict response to gemcitabine (and fluorouracil) but are not sufficiently predictive to affect the decision to use gemcitabine.75,124,125 Unfortunately, most pancreatic cancers do not respond to gemcitabine.
Treatment of locally advanced disease
Chemoradiotherapy downstages about 30% of patients with locally advanced disease to resectable pancreatic cancer, and these individuals go on to achieve median survival similar to that for those who are initially resectable without any preoperative treatment.115 Chemotherapy alone is sometimes used for patients too frail to tolerate radiation. Findings of trials in which attempts have been made to ascertain whether chemotherapy alone is preferable to chemoradiation in patients with locally advanced disease have been inconclusive. The National Comprehensive Cancer Network recommends use of chemoradiation (fluorouracil and 50–60 Gy radiotherapy) followed by systemic chemotherapy (usually 4 months of gemcitabine).126 Fluorouracil or gemcitabine can be used as a radiosensitiser: in the locally advanced setting, chemoradiation regimens containing gemcitabine yield similar results to those with fluorouracil.127,128
Although chemoradiotherapy is usually given before systemic chemotherapy, some evidence suggests that scheduling chemotherapy before chemoradiotherapy might be preferable.129 In patients destined to have rapidly progressive disease, metastases will probably show up during initial chemotherapy and therefore unnecessary local radiotherapy will be avoided.
Radiation therapy
Fractionated radiation therapy is typically delivered as 45–60 Gy over about 6 weeks (1·8–2·0 Gy/day), with fluorouracil or capecitabine—an oral fluoropyrimidine— as a radiosensitiser. In the adjuvant setting, 45 Gy is delivered initially to the tumour bed, surgical anastomosis, and regional lymph nodes. Subsequently, additional radiation (about 5–15 Gy) is directed at the tumour bed to target microscopic extension. Preoperative CT scans (with oral and intravenous contrast) and surgical clips are used to calculate the optimum volume and localisation of radiation. Adjuvant chemoradiotherapy is generally not given to patients who have received neoadjuvant chemoradiotherapy. In the neoadjuvant, borderline, and locally advanced settings, the radiotherapy field targets the tumour bed and adjoining margin with or without adjacent lymph nodes. The radiation volume encompasses the gross tumour volume—ie, a surrounding volume (0·5–2·0 mL) to target microscopic extension (the clinical treatment volume) and an additional 0·5–2·0 mL to account for errors in estimation of tumour size. Smaller margins of radiation are possible when optimum tumour imaging techniques are used and have the advantage of minimising radiation to adjacent normal structures. Advances in radiation techniques such as intensity-modulated radiation therapy and stereotactic body radiation therapy enable dose escalation and sparing of healthy tissues that could improve tumour control with tolerable side-effects, but these modalities need further assessment in prospective clinical trials. The current role of chemoradiotherapy in pancreatic cancer was detailed in a consensus statement.130
Treatment of metastatic disease
Gemcitabine-based combination treatments have been assessed for advanced pancreatic cancer. Since fluorouracil and gemcitabine are both approved for use in patients with pancreatic cancer, the combination of gemcitabine and capecitabine has been evaluated for individuals with advanced pancreatic tumours in phase II and phase III clinical trials. For example, findings of a phase III trial showed that, compared with gemcitabine alone, the combination of gemcitabine and capecitabine yielded a better response and longer progression-free survival, with a trend towards increased overall survival.131 In another study,132 significant overall survival benefits of this combination were reported in the subgroup of patients with good performance status, and data from a metaanalysis indicated a significant overall survival benefit for this combination.131 Conroy and colleagues133 noted, in a phase III trial, that for patients with good performance status, the combination of fluorouracil, folinic acid, irinotecan, and oxaliplatin led to median survival of 11 months (vs 6·8 months for gemcitabine alone).
In phase III clinical trials, the combination of gemcitabine and the epidermal growth factor receptor (EGFR) inhibitor, erlotinib, was modestly superior to gemcitabine alone.134 Erlotinib—a tyrosine kinase inhibitor of the catalytic domain of EGFR—is approved by the US Food and Drug Administration for use in pancreatic cancer. As suspected with colorectal cancer, the benefit of erlotinib might be for patients with pancreatic cancers containing wild-type KRAS, but evidence for this notion was absent in a subgroup analysis.134 The combination of gemcitabine, erlotinib, and bevacizumab versus gemcitabine and erlotinib has been compared in the phase III AVITA trial.135 No difference in the primary endpoint of overall survival was recorded, but progression-free survival was superior in the triple-drug arm. A summary of phase III clinical trials for advanced pancreatic cancer is provided in table 2 and in the webappendix (p 1–3).
Many combination chemotherapy regimens that show initial promise in phase II trials fail to confirm increased survival in phase III studies. Meta-analyses have been used to overcome the disadvantage of small clinical trials. For example, in a meta-analysis of about 3600 patients in phase III trials, overall survival was better when gemcitabine was combined with either a platinum-based drug or a fluoropyrimidine, compared with gemcitabine alone,136,137 especially for individuals with very good performance status.
Another treatment undergoing investigation is nanoparticle-formulated paclitaxel. The combination of gemcitabine (1000 mg/m2) with this formulation of paclitaxel (125 mg/m2) has been reported in abstract form to have good response rates and is now being tested in phase III trials (NCT00844649).
No standard second-line treatment exists for pancreatic cancer: many patients with advanced disease progress too rapidly to tolerate such regimens. Second-line fluoropyrimidine-based therapy is sometimes used if gemcitabine has been given as first-line treatment. In the CONKO-003 trial,138 patients who failed gemcitabine first-line therapy had better overall survival if they received oxaliplatin with fluorouracil and folinic acid than fluorouracil and folinic acid alone.
Measurement of tumour response
CT is the standard method for measurement of tumour burden, and clinical trials usually use RECIST (response evaluation criteria in solid tumors) criteria to gauge tumour response. However, CT-based measurements of tumour size do not always quantify treatment response accurately and are usually only established after two cycles of treatment, which is a long time for patients with low survival. Although not sufficiently accurate for diagnosis, serial CA19-9 concentrations predict treatment response or disease relapse.75 Amounts of mutant DNA in plasma have been shown to represent tumour burden and response to treatment accurately, in patients with colorectal cancer, and it is likely to be useful for individuals with pancreatic and other neoplasms.139 Furthermore, changes in mutant DNA in plasma can be recorded within days of treatment. Moreover, once novel tumour DNA rearrangements are identified in a cancer, their detection in the circulation is feasible.140
Investigational treatments for advanced pancreatic cancer
The success of targeted treatments in other cancers supports the need for further research to identify new targets and better predictors of response to therapy. Several targeted agents are undergoing clinical trials for pancreatic cancer.141,142 Ongoing studies are shown in the webappendix (p 3).
Pancreatic cancer cells with defects in the BRCA2-PALB2-Fanconi DNA repair pathway are sensitive to poly (ADP-ribose) polymerase (PARP) inhibitors.52,53 PARP enzymes add large branched chains of poly (ADP-ribose) on nicked DNA, which cause separation of histones from DNA, to enable DNA repair. In phase I/II clinical trials of patients with a germline BRCA2 gene mutation, response rates of about 40% were recorded with olaparib for recurrent breast and ovarian cancer.143 Clinical trials of PARP inhibitors for patients with pancreatic cancer are currently underway.
The hedgehog pathway inhibitor GDC-0449 (manufacturer, town, country) is under investigation in a phase II clinical trial, in combination with gemcitabine and the nanoparticle formulation of paclitaxel, in patients with metastatic pancreatic adenocarcinoma (NCT01088815). Other therapeutic agents being studied include the multikinase inhibitor, sorafenib, and agents targeting SRC (dasatinib), g secretase, MTOR, TNFSF10 (also known as TRAIL), and IGF1. Endoscopic treatments are under investigation for pancreatic cancer, including endoscopic delivery of chemotherapy, cryotherapy, photodynamic therapy, and radiofrequency ablation, but no evidence exists that these agents are as effective as standard treatment.
Clinical trial design is always important when investigating new agents but especially so in pancreatic cancer, for which mortality is high and therapeutic benefits are typically modest. Many clinical trials in pancreatic cancer have to overcome obstacles such as enrolment factors (patients with heterogeneous outcomes arising from poor performance status or different patterns of advanced disease), trial design difficulties (underpowered trials), and the scarcity of predictive markers (and adequate tumour tissue) to identify subgroups who will respond to treatment.144
Supportive care
Findings show that palliative care can help patients even as they are undergoing treatment for advanced disease.145 Supportive care begins with provision of support and information from the time of diagnosis and the appropriate amount of hope for the patient’s stage of disease.146
Pain management is an important component of treatment. Identification of the cause of pain can guide effective therapy. Pain from coeliac plexus infiltration can be treated effectively with endoscopic ultrasound or CT-guided ablation of the plexus. Radiation can relieve pain from locally advanced disease. Most patients with pancreatic-head cancers will develop obstructive jaundice and benefit from biliary stenting. Metal stents remain patent for longer than do plastic ones.147 About 20% of individuals develop gastric-outlet obstruction and benefit from duodenal wall stents or PEG (percutaneous endoscopic gastrostomy) placement for decompression. Owing to the effectiveness of endoscopic wall stents, surgical management of obstructive jaundice and gastric-outlet obstruction is usually not necessary, although it could provide better palliation for individuals with long life expectancy.
Since patients with pancreatic cancer frequently develop venous thromboembolism, prophylaxis is recommended. Because of the nature of the hypercoagulable state, findings of several randomised trials indicate that low-molecular-weight heparin provides better prophylaxis than warfarin.148
Pancreatic enzyme therapy is sometimes needed because of pancreatic-duct blockage or sparse pancreatic-gland tissue. The Johns Hopkins Pancreatic Cancer website and support groups such as the Pancreatic Cancer Action Network and Pancreatic Cancer UK provide invaluable information and support for patients and their families.
Acknowledgments
This work was supported by the National Cancer Institute grants (CA62924, CA120432, RC2CA148346), and the Michael Rolfe Foundation.
Footnotes
Contributors
All authors contributed to writing of the paper. AV prepared the tables.
Conflicts of interest
RHH and MG have a licensing agreement with Myriad Genetics for genetic testing of PALB2 as a pancreatic cancer susceptibility gene. All other authors declare that they have no conflicts of interest.
References
- 1.Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol. 2009;6:699–708. doi: 10.1038/nrgastro.2009.177. [DOI] [PubMed] [Google Scholar]
- 2.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–38. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 3.Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet. 2009;41:986–90. doi: 10.1038/ng.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolpin BM, Chan AT, Hartge P, et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2009;101:424–31. doi: 10.1093/jnci/djp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–88. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackford A, Parmigiani G, Kensler TW, et al. Genetic mutations associated with cigarette smoking in pancreatic cancer. Cancer Res. 2009;69:3681–88. doi: 10.1158/0008-5472.CAN-09-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen GM, de Andrade M, Goggins M, et al. Pancreatic cancer genetic epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2006;15:704–10. doi: 10.1158/1055-9965.EPI-05-0734. [DOI] [PubMed] [Google Scholar]
- 8.Brune KA, Lau B, Palmisano E, et al. Importance of age of onset in pancreatic cancer kindreds. J Natl Cancer Inst. 2010;102:119–26. doi: 10.1093/jnci/djp466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi C, Klein AP, Goggins M, et al. Increased prevalence of precursor lesions in familial pancreatic cancer patients. Clin Cancer Res. 2009;15:7737–43. doi: 10.1158/1078-0432.CCR-09-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Brune KA, Visvanathan K, et al. Elevated cancer mortality in the relatives of patients with pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:2829–34. doi: 10.1158/1055-9965.EPI-09-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi C, Hruban RH, Klein AP. Familial pancreatic cancer. Arch Pathol Lab Med. 2009;133:365–74. doi: 10.5858/133.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy KM, Brune KA, Griffin C, et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17% Cancer Res. 2002;62:3789–93. [PubMed] [Google Scholar]
- 13.Hahn SA, Greenhalf B, Ellis I, et al. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst. 2003;95:214–21. doi: 10.1093/jnci/95.3.214. [DOI] [PubMed] [Google Scholar]
- 14.Couch FJ, Johnson MR, Rabe KG, et al. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:342–46. doi: 10.1158/1055-9965.EPI-06-0783. [DOI] [PubMed] [Google Scholar]
- 15.Goggins M, Schutte M, Lu J, et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 1996;56:5360–64. [PubMed] [Google Scholar]
- 16.Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, Parsons DW, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217. doi: 10.1126/science.1171202. Epub 2009 Mar 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slater EP, Langer P, Niemczyk E, et al. PALB2 mutations in European familial pancreatic cancer families. Clin Genet. 2010;78:490–94. doi: 10.1111/j.1399-0004.2010.01425.x. [DOI] [PubMed] [Google Scholar]
- 18.Tischkowitz MD, Sabbaghian N, Hamel N, et al. Analysis of the gene coding for the BRCA2-interacting protein PALB2 in familial and sporadic pancreatic cancer. Gastroenterology. 2009;137:1183–86. doi: 10.1053/j.gastro.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 20.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–17. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 21.van der Heijden MS, Brody JR, Dezentje DA, et al. In vivo therapeutic responses contingent on Fanconi anemia/BRCA2 status of the tumor. Clin Cancer Res. 2005;11:7508–15. doi: 10.1158/1078-0432.CCR-05-1048. [DOI] [PubMed] [Google Scholar]
- 22.Villarroel MC, Rajeshkumar NV, Garrido-Laguna I, et al. Personalizing cancer treatment in the age of global genomic analyses: PALB2 gene mutations and the response to DNA damaging agents in pancreatic cancer. Mol Cancer Ther. 2011;10:3–8. doi: 10.1158/1535-7163.MCT-10-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kastrinos F, Mukherjee B, Tayob N, et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA. 2009;302:1790–95. doi: 10.1001/jama.2009.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen GM, Amundadottir L, Fuchs CS, et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet. 2010;42:224–28. doi: 10.1038/ng.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grover S, Stoffel EM, Bussone L, Tschoegl E, Syngal S. Physician assessment of family cancer history and referral for genetic evaluation in colorectal cancer patients. Clin Gastroenterol Hepatol. 2004;2:813–19. doi: 10.1016/s1542-3565(04)00352-0. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Chen S, Brune KA, Hruban RH, Parmigiani G, Klein AP. PancPRO: risk assessment for individuals with a family history of pancreatic cancer. J Clin Oncol. 2007;25:1417–22. doi: 10.1200/JCO.2006.09.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–72. [PubMed] [Google Scholar]
- 28.Hruban RH, Maitra A, Goggins M. Update on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol. 2008;1:306–16. [PMC free article] [PubMed] [Google Scholar]
- 29.Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high risk individuals. Clin Gastro Hepatol. 2004;2:606–21. doi: 10.1016/s1542-3565(04)00244-7. [DOI] [PubMed] [Google Scholar]
- 30.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–81. doi: 10.1016/j.cgh.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Brune K, Goggins M, O’Mailey L, et al. Detailed pathologic evaluation of non-invasive precursor lesions of the pancreas in patients with a strong family history of pancreatic cancer. Am J Surg Pathol. 2006;30:1067–76. [PMC free article] [PubMed] [Google Scholar]
- 32.Matsubayashi H, Canto M, Sato N, et al. DNA methylation alterations in the pancreatic juice of patients with suspected pancreatic disease. Cancer Res. 2006;66:1208–17. doi: 10.1158/0008-5472.CAN-05-2664. [DOI] [PubMed] [Google Scholar]
- 33.Kelly KA, Bardeesy N, Anbazhagan R, et al. Targeted nanoparticles for imaging incipient pancreatic ductal adenocarcinoma. PLoS Med. 2008;5:e85. doi: 10.1371/journal.pmed.0050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–87. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 35.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. Am J Radiol. 2008;191:802–07. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806–11. doi: 10.1016/j.cgh.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 38.Hruban RH, Klimstra DS, Pitman MB. Tumors of the pancreas. Washington, DC: Armed Forces Institute of Pathology; 2006. [Google Scholar]
- 39.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–06. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rozenblum E, Schutte M, Goggins M, et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997;57:1731–34. [PubMed] [Google Scholar]
- 41.van Heek NT, Meeker AK, Kern SE, et al. Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am J Pathol. 2002;161:1541–47. doi: 10.1016/S0002-9440(10)64432-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong SM, Heaphy CM, Shi C, et al. Telomeres are shortened in acinar-to-ductal metaplasia lesions associated with pancreatic intraepithelial neoplasia but not in isolated acinar-to-ductal metaplasias. Mod Pathol. doi: 10.1038/modpathol.2010.181. (published online Sept 24, 2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carter H, Samayoa J, Hruban RH, Karchin R. Prioritization of driver mutations in pancreatic cancer using cancer-specific high-throughput annotation of somatic mutations (CHASM) Cancer Biol Ther. 2010;10:582–87. doi: 10.4161/cbt.10.6.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hingorani SR, Wang LF, Multani AS, et al. Trp53(R172H) and KraS(G12D) cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 45.Omura N, Goggins M. Epigenetics and epigenetic alterations in pancreatic cancer. Int J Clin Exp Pathol. 2009;2:310–26. [PMC free article] [PubMed] [Google Scholar]
- 46.Schutte M, Hruban RH, Geradts J, et al. Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res. 1997;57:3126–30. [PubMed] [Google Scholar]
- 47.Sato N, Matsubayashi H, Abe T, Fukushima N, Goggins M. Epigenetic down-regulation of CDKN1C/p57KIP2 in pancreatic ductal neoplasms identified by gene expression profiling. Clin Cancer Res. 2005;11:4681–88. doi: 10.1158/1078-0432.CCR-04-2471. [DOI] [PubMed] [Google Scholar]
- 48.Sato N, Fukushima N, Hruban RH, Goggins M. CpG island methylation profile of pancreatic intraepithelial neoplasia. Mod Pathol. 2008;21:238–44. doi: 10.1038/modpathol.3800991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sato N, Fukushima N, Maitra A, et al. Discovery of novel targets for aberrant methylation in pancreatic carcinoma using high-throughput microarrays. Cancer Res. 2003;63:3735–42. [PubMed] [Google Scholar]
- 50.Sato N, Fukushima N, Chang R, Matsubayashi H, Goggins M. Differential and epigenetic gene expression profiling identifies frequent disruption of the RELN pathway in pancreatic cancers. Gastroenterology. 2006;130:548–65. doi: 10.1053/j.gastro.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 51.Sato N, Fukushima N, Maehara N, et al. SPARC/osteonectin is a frequent target for aberrant methylation in pancreatic adenocarcinoma and a mediator of tumor-stromal interactions. Oncogene. 2003;22:5021–30. doi: 10.1038/sj.onc.1206807. [DOI] [PubMed] [Google Scholar]
- 52.Parsi MA, Li A, Li CP, Goggins M. DNA methylation alterations in endoscopic retrograde cholangiopancreatography brush samples of patients with suspected pancreaticobiliary disease. Clin Gastroenterol Hepatol. 2008;6:1270–78. doi: 10.1016/j.cgh.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sato N, Maitra A, Fukushima N, et al. Frequent hypomethylation of multiple genes overexpressed in pancreatic ductal adenocarcinoma. Cancer Res. 2003;63:4158–66. [PubMed] [Google Scholar]
- 54.Vincent A, Ducourouble MP, Van Seuningen I. Epigenetic regulation of the human mucin gene MUC4 in epithelial cancer cell lines involves both DNA methylation and histone modifications mediated by DNA methyltransferases and histone deacetylases. FASEB J. 2008;22:3035–45. doi: 10.1096/fj.07-103390. [DOI] [PubMed] [Google Scholar]
- 55.Szafranska AE, Doleshal M, Edmunds HS, et al. Analysis of microRNAs in pancreatic fine-needle aspirates can classify benign and malignant tissues. Clin Chem. 2008;54:1716–24. doi: 10.1373/clinchem.2008.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li A, Omura N, Hong SM, et al. Pancreatic cancers epigenetically silence SIP1 and hypomethylate and overexpress miR-200a/200b in association with elevated circulating miR-200a and miR-200b levels. Cancer Res. 2010;70:5226–37. doi: 10.1158/0008-5472.CAN-09-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bloomston M, Frankel WL, Petrocca F, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–08. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 58.Kent OA, Mullendore M, Wentzel EA, et al. A resource for analysis of microRNA expression and function in pancreatic ductal adenocarcinoma cells. Cancer Biol Ther. 2009;8:2013–24. doi: 10.4161/cbt.8.21.9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–17. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campbell PJ, Yachida S, Mudie LJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–13. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scharpf RB, Iacobuzio-Donahue CA, Cope L, et al. Cross-platform comparison of two pancreatic cancer phenotypes. Cancer Inform. 2010;9:257–64. doi: 10.4137/CIN.S5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walter K, Omura N, Hong SM, et al. Overexpression of Smoothened activates the Sonic Hedgehog signaling pathway in pancreatic cancer associated fibroblasts. Clin Cancer Res. 2010;16:1781–89. doi: 10.1158/1078-0432.CCR-09-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Omura N, Griffith M, Vincent A, et al. Cyclooxygenase-deficient pancreatic cancer cells use exogenous sources of prostaglandins. Mol Cancer Res. 2010;8:821–32. doi: 10.1158/1541-7786.MCR-09-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hidalgo M, Maitra A. The hedgehog pathway and pancreatic cancer. N Engl J Med. 2009;361:2094–96. doi: 10.1056/NEJMcibr0905857. [DOI] [PubMed] [Google Scholar]
- 65.Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–72. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 66.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Disis ML, Bernhard H, Jaffee EM. Use of tumour-responsive T cells as cancer treatment. Lancet. 2009;373:673–83. doi: 10.1016/S0140-6736(09)60404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lutz E, Yeo CJ, Lillemoe KD, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma: a phase II trial of safety, efficacy, and immune activation. Ann Surg. doi: 10.1097/SLA.0b013e3181fd271c. (published online Jan 6, 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee CJ, Dosch J, Simeone DM. Pancreatic cancer stem cells. J Clin Oncol. 2008;26:2806–12. doi: 10.1200/JCO.2008.16.6702. [DOI] [PubMed] [Google Scholar]
- 70.Kern SE, Shibata D. The fuzzy math of solid tumor stem cells: a perspective. Cancer Res. 2007;67:8985–88. doi: 10.1158/0008-5472.CAN-07-1971. [DOI] [PubMed] [Google Scholar]
- 71.Caldwell ME, Tuveson DA. Finding and killing the CRABs of pancreatic cancer. Gastroenterology. 2009;137:782–85. doi: 10.1053/j.gastro.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 72.Niederhuber JE, Brennan MF, Menck HR. The National Cancer Data Base report on pancreatic cancer. Cancer. 1995;76:1671–77. doi: 10.1002/1097-0142(19951101)76:9<1671::aid-cncr2820760926>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 73.Brentnall TA, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med. 1999;131:247–55. doi: 10.7326/0003-4819-131-4-199908170-00003. [DOI] [PubMed] [Google Scholar]
- 74.Harsha HC, Kandasamy K, Ranganathan P, et al. A compendium of potential biomarkers of pancreatic cancer. PLoS Med. 2009;6:e1000046. doi: 10.1371/journal.pmed.1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lennon AM, Goggins M. Diagnostic and therapeutic response markers. In: Neoptolemos JP, Urrutia RA, Abbruzzese J, Büchler MW, editors. Pancreatic cancer. Berlin: Springer; 2010. pp. 675–701. [Google Scholar]
- 76.Koopmann J, Rosenweig CN, Zhang Z, et al. Serum markers in patients with resectable pancreatic adenocarcinoma: MIC-1 vs. CA19-9. Clin Cancer Res. 2006;15:442–46. doi: 10.1158/1078-0432.CCR-05-0564. [DOI] [PubMed] [Google Scholar]
- 77.Gold DV, Goggins M, Modrak DE, et al. Detection of early-stage pancreatic adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2010;19:2786–94. doi: 10.1158/1055-9965.EPI-10-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Canto MI, Schulick RD, Kamel IR, et al. Screening for familial pancreatic neoplasia: a prospective, multicenter blinded study of EUS, CT, and Secretin-MRCP. Presented at Digestive Disease Week DDW; New Orleans, LA, USA. May 1–5, 2010; 2010. Abstract 415g. [Google Scholar]
- 79.Goggins M. Development of novel pancreatic tumor biomarkers. In: Neoptolemos JP, Urrutia RA, Abbruzzese J, Büchler MW, editors. Pancreatic cancer. Berlin: Springer; 2010. pp. 1176–201. [Google Scholar]
- 80.Kelsen DP, Portenoy R, Thaler H, Tao Y, Brennan M. Pain as a predictor of outcome in patients with operable pancreatic carcinoma. Surgery. 1997;122:53–59. doi: 10.1016/s0039-6060(97)90264-6. [DOI] [PubMed] [Google Scholar]
- 81.Pelaez-Luna M, Takahashi N, Fletcher JG, Chari ST. Resectability of presymptomatic pancreatic cancer and its relationship to onset of diabetes: a retrospective review of CT scans and fasting glucose values prior to diagnosis. Am J Gastroenterol. 2007;102:2157–63. doi: 10.1111/j.1572-0241.2007.01480.x. [DOI] [PubMed] [Google Scholar]
- 82.Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134:981–87. doi: 10.1053/j.gastro.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134:95–101. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129:504–11. doi: 10.1053/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10:88–95. doi: 10.1016/S1470-2045(08)70337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.White R, Winston C, Gonen M, et al. Current utility of staging laparoscopy for pancreatic and peripancreatic neoplasms. J Am Coll Surg. 2008;206:445–50. doi: 10.1016/j.jamcollsurg.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 87.Maithel SK, Maloney S, Winston C, et al. Preoperative CA 19-9 and the yield of staging laparoscopy in patients with radiographically resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2008;15:3512–20. doi: 10.1245/s10434-008-0134-5. [DOI] [PubMed] [Google Scholar]
- 88.Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386–91. doi: 10.1111/j.1572-0241.2002.05777.x. [DOI] [PubMed] [Google Scholar]
- 89.McCarthy DM, Maitra A, Argani P, et al. Novel markers of pancreatic adenocarcinoma in fine-needle aspiration: mesothelin and prostate stem cell antigen labeling increases accuracy in cytologically borderline cases. Appl Immunohistochem Mol Morphol. 2003;11:238–43. doi: 10.1097/00129039-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 90.Pawlik TM, Laheru D, Hruban RH, et al. Evaluating the impact of a single-day multidisciplinary clinic on the management of pancreatic cancer. Ann Surg Oncol. 2008;15:2081–88. doi: 10.1245/s10434-008-9929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bilimoria KY, Bentrem DJ, Lillemoe KD, Talamonti MS, Ko CY. Assessment of pancreatic cancer care in the United States based on formally developed quality indicators. J Natl Cancer Inst. 2009;101:848–59. doi: 10.1093/jnci/djp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173–80. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sato N, Goggins M. Epigenetic alterations in intraductal papillary mucinous neoplasms of the pancreas. J Hepatobiliary Pancreat Surg. 2006;13:280–85. doi: 10.1007/s00534-005-1056-2. [DOI] [PubMed] [Google Scholar]
- 95.Kooby DA, Hawkins WG, Schmidt CM, et al. A multicenter analysis of distal pancreatectomy for adenocarcinoma: is laparoscopic resection appropriate? J Am Coll Surg. 2010;210:779–87. doi: 10.1016/j.jamcollsurg.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 96.Newman NA, Lennon AM, Edil BH, et al. Preoperative endoscopic tattooing of pancreatic body and tail lesions decreases operative time for laparoscopic distal pancreatectomy. Surgery. 2010;148:371–77. doi: 10.1016/j.surg.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 97.van der Gaag NA, Rauws EA, van Eijck CH, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129–37. doi: 10.1056/NEJMoa0903230. [DOI] [PubMed] [Google Scholar]
- 98.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–13. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blackford A, Serrano OK, Wolfgang CL, et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res. 2009;15:4674–79. doi: 10.1158/1078-0432.CCR-09-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tascilar M, Skinner HG, Rosty C, et al. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2001;7:4115–21. [PubMed] [Google Scholar]
- 101.Infante JR, Matsubayashi H, Sato N, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–25. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 102.O’Reilly EM. Refinement of adjuvant therapy for pancreatic cancer. JAMA. 2010;304:1124–25. doi: 10.1001/jama.2010.1302. [DOI] [PubMed] [Google Scholar]
- 103.Kalser MH, Ellenberg SS. Pancreatic cancer: adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 104.Neuhaus P, Riess H, Post S, et al. CONKO-001: Final results of the randomized, prospective, multicenter phase III trial of adjuvant chemotherapy with gemcitabine versus observation in patients with resected pancreatic cancer (PC). Presented at the 2008 ASCO Annual Meeting; Chicago, IL, USA. May 30–June 3, 2008; Abstract LBA4504. [Google Scholar]
- 105.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019–26. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 106.Corsini MM, Miller RC, Haddock MG, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: the Mayo Clinic experience (1975–2005) J Clin Oncol. 2008;26:3511–16. doi: 10.1200/JCO.2007.15.8782. [DOI] [PubMed] [Google Scholar]
- 107.Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26:3503–10. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17:981–90. doi: 10.1245/s10434-009-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hattangadi JA, Hong TS, Yeap BY, Mamon HJ. Results and patterns of failure in patients treated with adjuvant combined chemoradiation therapy for resected pancreatic adenocarcinoma. Cancer. 2009;115:3640–50. doi: 10.1002/cncr.24410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–10. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 111.Koshy MC, Landry JC, Cavanaugh SX, et al. A challenge to the therapeutic nihilism of ESPAC-1. Int J Radiat Oncol Biol Phys. 2005;61:965–66. doi: 10.1016/j.ijrobp.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 112.Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073–81. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 113.Redmond KJ, Wolfgang CL, Sugar EA, et al. Adjuvant chemoradiation therapy for adenocarcinoma of the distal pancreas. Ann Surg Oncol. 2010;17:3112–19. doi: 10.1245/s10434-010-1200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Picozzi VJ, Abrams RA, Decker PA, et al. for the American College of Surgeons Oncology Group. Multicenter phase II trial of adjuvant therapy for resected pancreatic cancer using cisplatin, 5-fluorouracil, and interferon-alfa-2b-based chemoradiation: ACOSOG Trial Z05031. Ann Oncol. doi: 10.1093/annonc/mdq384. (published online Aug 9, 2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gillen S, Schuster T, Meyer zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Callery MP, Chang KJ, Fishman EK, Talamonti MS, Traverso LW, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–33. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 117.Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–47. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–46. doi: 10.1016/j.jamcollsurg.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hristov B, Reddy S, Lin SH, et al. Outcomes of adjuvant chemoradiation after pancreaticoduodenectomy with mesenterico-portal vein resection for adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 2010;76:176–80. doi: 10.1016/j.ijrobp.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 120.Rothenberg ML, Moore MJ, Cripps MC, et al. A phase II trial of gemcitabine in patients with 5-FU-refractory pancreas cancer. Ann Oncol. 1996;7:347–53. doi: 10.1093/oxfordjournals.annonc.a010600. [DOI] [PubMed] [Google Scholar]
- 121.Burris HA, III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 122.Storniolo AM, Enas NH, Brown CA, Voi M, Rothenberg ML, Schilsky R. An investigational new drug treatment program for patients with gemcitabine: results for over 3000 patients with pancreatic carcinoma. Cancer. 1999;85:1261–68. [PubMed] [Google Scholar]
- 123.Tempero M, Plunkett W, Ruiz Van Haperen V, et al. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21:3402–08. doi: 10.1200/JCO.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 124.Farrell JJ, Elsaleh H, Garcia M, et al. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology. 2009;136:187–95. doi: 10.1053/j.gastro.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 125.Brody JR, Hucl T, Costantino CL, et al. Limits to thymidylate synthase and TP53 genes as predictive determinants for fluoropyrimidine sensitivity and further evidence for RNA-based toxicity as a major influence. Cancer Res. 2009;69:984–91. doi: 10.1158/0008-5472.CAN-08-3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.National Comprehensive Cancer Network. NCCN Guidelines. [accessed Jan 14, 2011]. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PubMed]
- 127.Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487–95. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 128.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 129.Huguet F, Andre T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–31. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 130.Abrams RA, Lowy AM, O’Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1751–56. doi: 10.1245/s10434-009-0413-9. [DOI] [PubMed] [Google Scholar]
- 131.Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513–18. doi: 10.1200/JCO.2009.24.2446. [DOI] [PubMed] [Google Scholar]
- 132.Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25:2212–17. doi: 10.1200/JCO.2006.09.0886. [DOI] [PubMed] [Google Scholar]
- 133.Conroy T, Desseigne F, Ychou M, et al. Randomized phase III trial comparing FOLFIRINOX (F: 5FU/leucovorin [LV], irinotecan [I], and oxaliplatin [O]) versus gemcitabine (G) as first-line treatment for metastatic pancreatic adenocarcinoma (MPA): preplanned interim analysis results of the PRODIGE 4/ACCORD 11 trial. Presented at the 2010 ASCO Annual Meeting; Chicago, IL, USA. June 4–8, 2010; Abstract 4010. [Google Scholar]
- 134.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada clinical trials group. J Clin Oncol. 2007;25:1960–66. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 135.Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–37. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 136.Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C. Meta-analysis of randomized trials: Evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer. 2008;8:82. doi: 10.1186/1471-2407-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946–52. doi: 10.1200/JCO.2005.05.1490. [DOI] [PubMed] [Google Scholar]
- 138.Pelzer U, Kubica K, Stieler J, et al. A randomized trial in patients with gemcitabine refractory pancreatic cancer: final results of the CONKO 003 study. Presented at the 2008 ASCO Annual Meeting; Chicago, IL, USA. May 30–June 3, 2008; Abstract 4508. [Google Scholar]
- 139.Diehl F, Schmidt K, Choti MA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–90. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Leary RJ, Kinde I, Diehl F, et al. Development of personalized tumor biomarkers using massively parallel sequencing. Sci Transl Med. 2010;2:20ra14. doi: 10.1126/scitranslmed.3000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–17. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 142.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–72. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 143.Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–51. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 144.Philip PA, Mooney M, Jaffe D, et al. Consensus report of the national cancer institute clinical trials planning meeting on pancreas cancer treatment. J Clin Oncol. 2009;27:5660–69. doi: 10.1200/JCO.2009.21.9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–42. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 146.Feudtner C. The breadth of hopes. N Engl J Med. 2009;361:2306–07. doi: 10.1056/NEJMp0906516. [DOI] [PubMed] [Google Scholar]
- 147.Moss AC, Morris E, MacMathuna P. Palliative biliary stents for obstructing pancreatic carcinoma. Cochrane Database Syst Rev. 2006;2:CD004200. doi: 10.1002/14651858.CD004200.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. Lancet Oncol. 2004;5:655–63. doi: 10.1016/S1470-2045(04)01606-7. [DOI] [PubMed] [Google Scholar]