Abstract
The development of FCL was motivated by a conundrum: locked plating constructs provide inherently rigid stabilization, yet they should facilitate biological fixation and secondary bone healing that relies on flexible fixation to stimulate callus formation. Recent studies have confirmed that the high stiffness of standard locked plating constructs can suppress interfragmentary motion to a level that is insufficient to reliably promote secondary fracture healing by callus formation. Furthermore, rigid locking screws cause an uneven stress distribution that may lead to stress fracture at the end screw and stress shielding under the plate.
This review summarizes four key features of FCL constructs that have shown to enhance fixation and fracture healing: Flexible fixation, load distribution, progressive stiffening, and parallel interfragmentary motion. Specifically, flexible fixation provided by FCL reduces the stiffness of a locked plating construct by 80–88% to actively promote callus proliferation similar to an external fixator. Load distribution is evenly shared between FCL screws to mitigate stress risers at the end screw. Progressive stiffening occurs by near cortex support of FCL screws and provides additional support under elevated loading. Finally, parallel interfragmentary motion by s-shaped flexion of FCL screws has shown to induce symmetric callus formation.
In combination, these features of FCL constructs have shown to induce more callus and to yield significantly stronger and more consistent healing compared to standard locked plating constructs. As such, FCL constructs function as true internal fixators by replicating the biomechanical behavior and biological healing response of external fixators.
Introduction
The concept of Far Cortical Locking (FCL) was first introduced at the 2005 meeting of the Orthopaedic Research Association, and has since been formally evaluated in biomechanical studies and in vivo to determine the effect of FCL on fracture healing (1–3). The development of FCL was motivated by a conundrum: locked plating constructs provide inherently rigid stabilization (4), yet they should facilitate biological fixation and secondary bone healing that relies on flexible fixation to stimulate callus formation (5, 6). Recent studies have confirmed two clinical concerns arising from the high stiffness of standard locked plating constructs. First, locked plating constructs can suppress interfragmentary motion to a level that is insufficient to reliably promote secondary fracture healing by callus formation (7, 8). Second, fixed-angle stabilization with locking screws causes an uneven stress distribution, whereby stress risers can cause bone fracture at the end screw (1), and stress shielding under the plate can lead to bone resorption (6). In response to these clinical concerns, FCL was developed to enable flexible fixation with locking plates.
This review summarizes four key features of FCL constructs that contribute to fracture healing and durable fixation: Flexible fixation, load distribution, progressive stiffening, and parallel interfragmentary motion. It furthermore illustrates the effect of these features on construct strength and fracture healing. In combination, these features allow an FCL construct to function as a true internal fixator by replicating the biomechanical behavior of external fixators.
Flexible Fixation
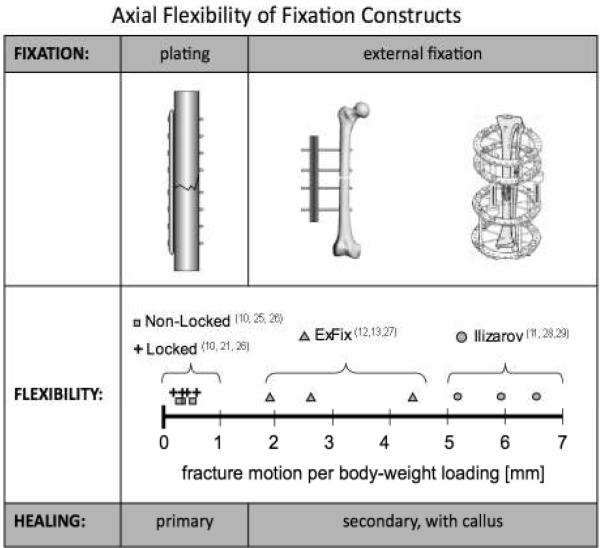
Traditional compression plates were originally designed to provide absolute stability, targeting primary bone healing without callus formation (Figure 1)(9). The axial rigidity of modern locked plating constructs is comparable to that of non-locked plating constructs (10). In contrast, external fixators were designed to provide sufficient interfragmentary motion to stimulate secondary bone healing by callus formation. External fixators can provide over 10 times more interfragmentary motion in response to a given load than rigid fixation with locked or non-locked plates (11–13). Since locked plating relies on secondary rather than on primary bone healing (5, 14), reducing the stiffness of a locked plating construct becomes essential to target secondary bone healing.
Figure 1.
The conundrum: Locked plating constructs are comparably stiff than non-locked constructs that were designed to induce primary bone healing by rigid fixation (10, 21, 25, 26). However, locked bridge plating constructs rely on secondary bone healing with callus formation, which has traditionally been achieved with external fixation constructs that are considerably more flexible than locked plating constructs (12, 13, 27–29).
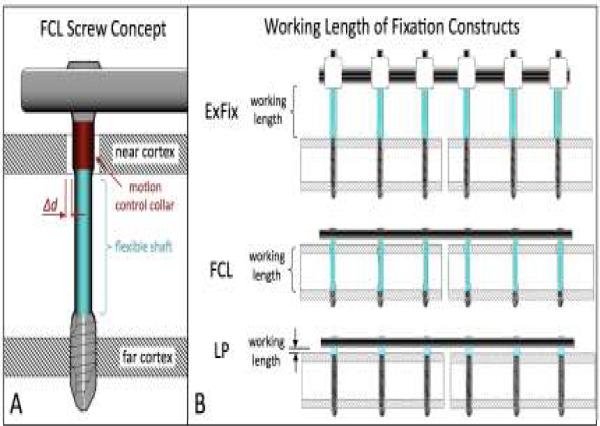
FCL reduces the stiffness of a locked plating construct by means of FCL screws that are fixed in the plate and in the far cortex, while retaining a controlled motion envelope in the near cortex of a diaphysis (Figure 2A). FCL screws have a flexible shaft with a reduced diameter that can elastically deflect within the near cortex motion envelope. The motion envelope is controlled by the diameter of a collar segment adjacent to the FCL screw head. FCL constructs therefore resemble a monolateral external fixator, the bar of which has been applied close to the bone surface, and the pins of which are secured in the far cortex rather than in the near cortex (Figure 2B). Similar to the external fixator, FCL constructs provide fixed-angle yet flexible connections between a bridging member and the bone segments, whereby FCL screws approach the working length of external fixator pins. In contrast, screws of standard locked constructs are rigidly confined between the near and far cortices and therefore have an insufficient working length to enable flexible fixation.
Figure 2.
FCL fixation: A) FCL screws are locked in the plate and in the far cortex, while retaining a controlled motion envelope Δd in the near cortex. B) Similar to the pins of an external fixator, flexible shafts of FCL screws provide a sufficient working length for flexible, fixed-angle connection of a locking plate to a diaphysis.
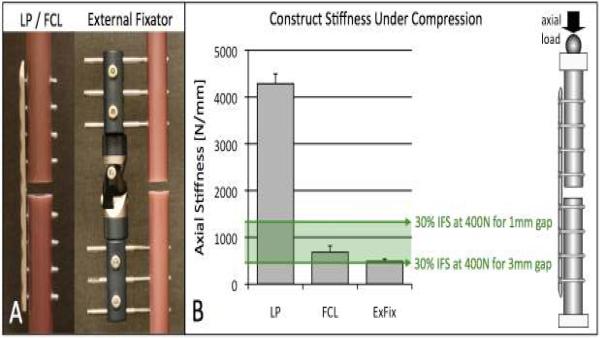
Biomechanical studies demonstrated that FCL screws reduce the axial stiffness of a standard locked plating construct by 88% for bridge plating of the femoral diaphysis (2), by 84% for bridge plating of the tibial diaphysis (3), and by 80% for stabilization of metaphyseal femur fractures (7). For stiffness correlation, three diaphyseal fixation constructs were evaluated under axial compression (Figure 3A): a standard locked plating (LP) construct, using the proximal 9-hole segment of a femoral locking plate applied with standard locking screws (NCB, Zimmer, Warsaw, IN); an FCL construct, using the same NCB plate applied with FCL screws (MotionLoc, Zimmer); and a monolateral external fixator (EBI, Parsippany, NJ). The stiffness of the FCL construct (682 N/mm) was 84% lower than that of the standard locked construct (4,286 N/mm), and approached that of the external fixator (488 N/mm) (Figure 3B). The stiffness of the FCL construct is suitable to generate interfragmentary strain (IFS) in the 30% range known to promote fracture healing by callus formation (15). The actual amount of IFS depends on the applied load and the fracture gap size. For example, under 400 N partial post-operative weight-bearing (16) of a 1–3 mm wide fracture gap, a construct stiffness of 444–1,333 N/mm will be required to induce 30% IFM. Moreover, at 400 N partial post-operative loading the FLC construct generated interfragmentary motion of approximately 0.6 mm, which is within the 0.2 – 1 mm envelope of interfragmentary motion known to stimulate secondary bone healing (17–19). Conversely, the standard locked construct induced less than 0.1 mm motion, and may therefore not reliable promote callus formation.
Figure 3. Flexible fixation.
a) Stiffness comparison of bridge osteosynthesis with standard locking plate (LP), FCL, and external fixation. B) FCL reduced the stiffness of the standard locking constructs by 84%. The FCL and external fixator stiffness was within a range that permits 30% interfragmentary strain (IFS) known to promote fracture healing by callus formation, assuming a fracture gap in the range of 1–3 mm and partial load bearing of 400N.
FCL may therefore be essential to reduce the stiffness of locked plating constructs in order to actively promote callus proliferation in the early healing phase, and to enable load sharing for callus maturation in the late remodeling stages of fracture healing.
Load Distribution
Locking plates transmit load through fixed-angle screws instead of relying on plate-to-bone compression. This focused load transfer induces stress concentrations at the screw-bone interface, particularly at the outermost locking screw, which has shown to increase the fracture risk at the plate end in osteoporotic bone as compared to conventional plates (1). Additionally, these stress concentrations are indicative of an uneven load distribution that will inflict stress shielding in adjacent regions and that may give rise to cortical porosis or delayed bridging (6).
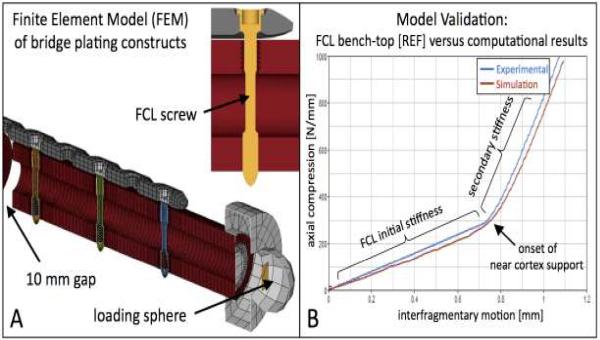
In order to determine the effect of FCL fixation on the load distribution in locked plating constructs, computational models of a standard locked and FCL bridge plating construct were generated by means of the finite element method using ANSYS software (Figure 4A). These numerical models consisted of over 130,000 elements and closely replicated the generic bridge plating constructs of the femoral diaphysis used for bench-top evaluation of FLC constructs (2). Model validation was based on axial compression tests through a proximal sphere, replicating the axial loading scenario of the bench-top test. Model validity was supported by close result correlation between computational simulations and experimental tests that describing the load versus interfragmentary motion behavior (Figure 4B).
Figure 4.
Computational Finite Element Model (FEM) of an FCL bridge plating constructs for calculation of stress and strain distributions. B) Model validation in comparison to bench-test results of FCL constructs demonstrated close correlation between predicated and actual interfragmentary motion results.
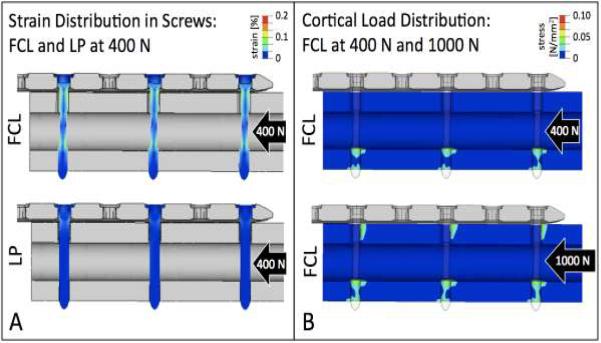
Distribution of strain in screws demonstrated that FCL screws underwent equal amounts of flexion, whereby strain was distributed over the entire working length in all FCL screw shafts (Figure 5A). In contrast, standard locking screws exhibited focused strain adjacent to the near cortex, while the screw segments between the near and far cortex remained functionally latent.
Figure 5. Load distribution.
A) Each FCL screws exhibits equal amounts of flexion, whereby strain is distributed over the entire working length of the FCL screw shaft. In contrast, standard locked plating screws exhibited focused strain adjacent to the near cortex, whereby the screw segment between the near and far cortex remained functionally latent. B) At 400 N loading, FCL screws provide even load distribution in the far cortex. At 1000 N, FCL screws furthermore provide load sharing between the far and near cortices.
Distribution of stress in the diaphysis demonstrated that load transfer in FCL constructs is equally shared between each screw-bone interface in the far cortex (Figure 5B). At elevated loading to 1,000 N, load transfer is furthermore shared between the near and far cortices. Consequently, FCL constructs can suppress stress risers at the outermost screw characteristic for a standard locked plating constructs.
Clinically, reduction of stress risers at the plate end and equal load transfer through multiple points of fixation makes FCL constructs particularly suitable for fracture fixation in the osteoporotic diaphysis. For fixation in healthy bone, FCL constructs distribute load between all FCL screws as well as between the near and far cortices, which likely will abate stress shielding and porosis.
Progressive Stiffening
FCL constructs exhibit a low initial stiffness, whereby all load is directly transferred from the plate to the far cortex through flexible screw shafts. Under elevated loading, elastic flexion of FCL screws enables additional support at the near cortex, resulting in a six-fold increase in construct stiffness (2). Once FCL screw shafts gain support at the near cortex, any further loading will be supported at the near cortex, similar to a standard locked plating construct. The resulting bi-phasic stiffness profile replicates the non-linear behavior of Ilizarov fixators that become progressively stiffer for increasing loads (11).
Clinically, the low initial stiffness of an FCL construct permits interfragmentary motion under reduced post-operative load bearing in the early healing phase. The amount of interfragmentary motion attainable under the initial FCL stiffness can be controlled by the FCL screw design to fall within the 0.2–1 mm stimulus range of axial interfragmentary motion established for promotion of secondary bone healing (17–19). In case of elevated loading events, near cortex support provided added stability to protect the fracture site from excessive motion. Next to controlling the transition from initial to secondary stiffness, a proper FCL screw design is critical to prevent fatigue of FCL screws, and to protect the far cortex interface from excessive stress. The FCL screw diameter at the near cortex has to be sufficiently large to confine screw shaft flexion within its elastic range, since excessive screw flexion will cause screw fatigue. In addition, the FCL screw diameter of the flexible mid-shaft has to be sufficiently small to limit peak stress in the far cortex.
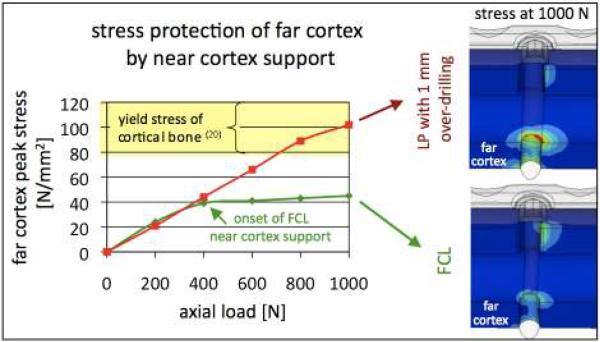
The previously described computational model of an FCL constructs demonstrated that stress in the far cortex increased linearly up to 400 N partial loading (Figure 6). At 400 N, near cortex support caused cortex stress to reach a plateau at approximately half the yield stress tolerated by cortical bone (20). Additional loading up to 1,000 N was supported at the near cortex and caused only a mild increase in far cortex stress. Conversely, over-drilling the near cortex by 1 mm to mimic FCL functionality with standard locking screws (21) overloaded the far cortex due to deficient flexibility of the standard screw. This analysis suggests that while the FCL concept is apparently simple, it does rely on a properly executed FCL design to enable safe and effective functionality. Depending on the screw length and shaft diameter, an optimized FCL screw may require different diameters at the mid-shaft and near cortex to protect the far cortex interface and to prevent FCL screw fatigue.
Figure 6. Progressive stiffening.
Under elevated loading, elastic flexion of FCL screw shafts provides additional support at the near cortex, which increases construct stiffness and protects the far cortex from excessive stress. Conversely, over-drilling the near cortex by 1 mm to mimic FCL function with standard locking screws can overload the far cortex due to deficient flexibility of the screw shaft.
Parallel Interfragmentary Motion
Bridge plating constructs undergo plate flexion (i.e., elastic plate bending) in response to axial loading since plates are offset from the diaphyseal shaft axis. This plate flexion enables interfragmentary motion, whereby the plate acts as a hinge that permits gradually increasing amounts of interfragmentary motion toward the far cortex opposite the plate (Figure 7A). This leads to asymmetric gap closure, whereby motion at the near cortex is suppressed, particularly in locked plating constructs that effectively prevent motion at the plate-bone interface. A recent study demonstrated that asymmetric gap closure with locking plates caused asymmetric callus formation (8), with callus formation decreasing from the far cortex towards the near cortex. Clinically, deficient callus formation is likely underappreciated on planar radiographs since plates obstruct visibility of the near cortex.
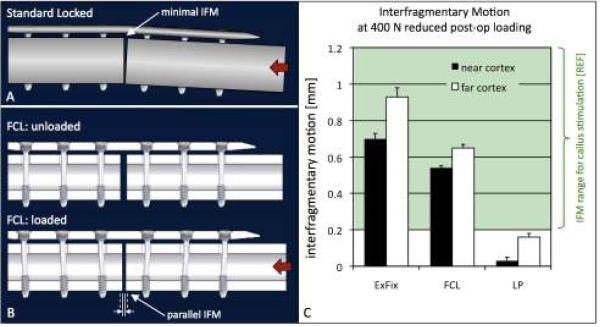
Figure 7. Parallel interfragmentary motion.
A) Standard locked constructs (LP) exhibit asymmetric gap closure, whereby motion at the near cortex is minimal. B) FCL constructs induce symmetric interfragmentary motion by cantilever bending of FCL screws. C) FCL and external fixation constructs delivered substantially parallel gap motion. The LP construct induced over five time less motion at the near cortex than at the far cortex. Interfragmentary motion in FCL and external fixator constructs in response to partial weight-bearing was within the 0.2–1 mm range known to stimulate callus formation.
Several approaches have been proposed to increase interfragmentary motion required for stimulation of callus formation, including long bridge spans (22) and the use of flexible titanium plates in place of more rigid stainless steel plates (8). These approaches increase plate flexion, which improves interfragmentary motion at the far cortex, but near cortex motion likely remains deficient due to asymmetric gap closure. Most recently, Dynamic Locking Screws (DLS) have been proposed to increase motion at the near and far cortices (23). However, these DLS screws reduced the stiffness of a standard locked construct by only 16%, which is considerably less than the over 80% stiffness reduction provided by FCL constructs.
FCL constructs induce nearly parallel interfragmentary motion, whereby the flexible shafts of FCL screws act as cantilever beams that undergo s-shaped flexion (Figure 7B). Nearly parallel interfragmentary motion with FCL screws has been demonstrated for diaphyseal and periarticular plating constructs (2, 7). In the previously described comparison between standard locked (LP), FCL (MotionLoc), and external fixator constructs, the FCL construct induced nearly parallel motion at the near cortex (0.54 mm) and far cortex (0.65 mm) in response to 400 N partial loading (Figure 7C). Similarly, the external fixator construct induced comparable amounts of motion at the near cortex (0.70 mm) and far cortex (0.93 mm). In contrast, the LP construct induced over five times less motion at the near cortex (0.03 mm) than at the far cortex (0.16 mm). This LP motion was furthermore below the 0.2–1 mm motion envelope for callus stimulation (17–19).
Clinically, the nearly parallel interfragmentary motion provided by FCL constructs should contribute to symmetric callus formation across the entire fracture site.
Construct Strength
Evaluating the strength and failure mode of fixation constructs is time and cost intensive, as it requires a considerable number of specimens for testing to failure. Strength evaluation is furthermore complicated by the fact that it is highly affected by bone quality and by the loading mode. For a comprehensive strength and failure mode assessment, FCL constructs were tested to failure in the three principal loading modes (axial compression, bending, torsion) in diaphyseal surrogates representative of osteoporotic bone and healthy bone (2). In addition, FCL constructs were tested to failure in human cadaveric femurs under dynamic quasi-physiologic loading (7). For strength correlation, failure tests were also conducted on standard bi-cortical and unicortical locked plating constructs.
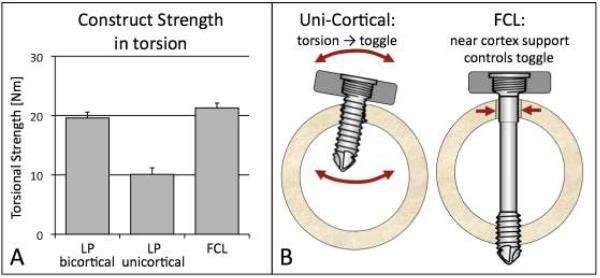
Compared to bi-cortical locked constructs, the strength of FCL constructs in the healthy diaphysis was 7% lower in compression, 54% higher in torsion, and 21% higher in bending (2). In the osteoporotic diaphysis, the strength of FCL constructs was 16% lower in compression, 9% higher in torsion, and 20% higher in bending (2). Compared to uni-cortical locked constructs, the strength of FCL constructs in the osteoporotic diaphysis was 8% lower in compression, 120% higher in torsion (Figure 8A), and 35% higher in bending. In human cadaveric femurs, diaphyseal fixation of periarticular plating with FLC screws did not decrease construct durability or strength (7).
Figure 8. Construct Strength.
A) FCL constructs were comparable in strength to standard locked plating constructs (LP) and did not observe the characteristic weakness of unicortical fixation in torsion. B) In contrast to uni-cortical locking screws, the controlled motion envelope of FCL screws in the near cortex prevents excessive toggle.
In summary, compared to bi-cortical locked plating, the strength of FCL constructs was modestly lower in axial compression and considerably stronger in bending and torsion. This finding suggests that under combined loading modes in vivo, FCL constructs are comparable in strength to standard locked constructs. This suggestion has been confirmed by the strength evaluation of periarticular plating constructs under combined physiologic loading, which yielded no difference in strength between FCL and standard locked plating constructs (7).
Furthermore, FCL constructs did not exhibit the torsional weakness characteristic of uni-cortical locked constructs. Uni-cortical locking screws are prone to toggle and break loose in the near cortex (Figure 8B). In contrast, near cortex support of FCL screws effectively prevents excessive toggle and shields the far cortex interface from excessive loading.
Clinically, the incidence of fixation and implant failure does not only depend on construct strength, but also on progression of fracture healing. A review of eight studies that report the timing of implant failure with locking plates revealed that 50% of failures occurred greater than 6 months after the index procedure (7), indicating that the failure was likely the result of implant fatigue in the presence of an established nonunion. Give that FCL constructs are comparable in strength to standard locked constructs, any improvement in fracture healing provided by FCL will likely decrease the incidence of fixation and implant failure.
Effect of FCL on Fracture Healing
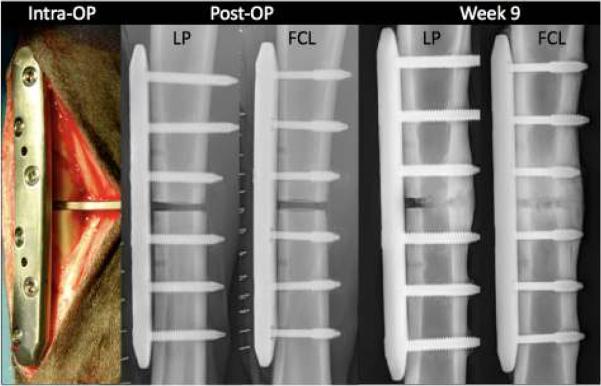
Analyzing fracture healing in clinical studies is complicated by the inherent variability in fracture patterns, quality of reduction, associated soft tissue injuries, and by the limited tools to measure the progression of fracture healing in patients (24). Therefore, the effect of FCL on fracture healing in vivo was assessed using an established ovine tibial osteotomy model (3). Six standard locked plating (LP) constructs and six FCL constructs were used to stabilized tibial osteotomies with a 3 mm gap (Figure 9). Compared to the LP construct, FCL constructs had an 84% lower initial stiffness and provided parallel interfragmentary motion. Progression of fracture healing was monitored on weekly radiographs. After sacrifice at week 9, implants were removed and callus volume and density was measured with quantitative computed tomography (QCT). The mechanical strength of healed tibiae was assessed by torsion testing to failure in a material test system. Finally, bridging was analyzed on histological mid-sagittal cross-sections.
Figure 9.
Comparison of fracture healing between standard locked plating and FCL constructs in an ovine tibia gap osteotomy model.
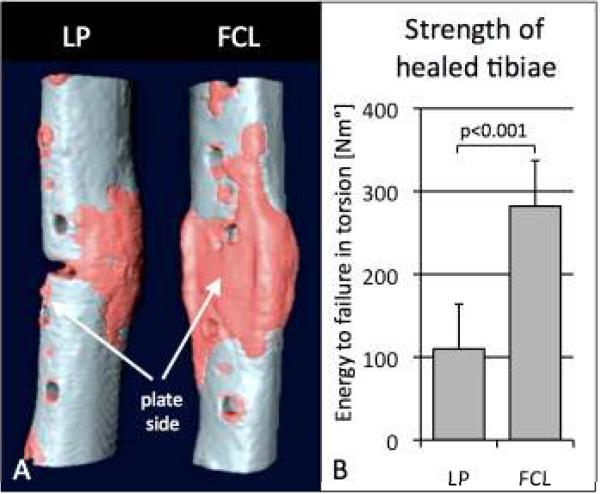
FCL constructs demonstrated significantly more callus on weekly radiographs than LP constructs from weeks 4 – 9 (p = 0.004). At week 9, FCL constructs had 44% more bone mineral content than LP constructs (p = 0.01). Callus formed symmetrically in the FCL group, with FCL constructs having a comparable BMC at the near and far cortex (p=0.91) (Figure 10a). In the LP group, callus formed asymmetrically, having 49% less BMC at the near cortex than at the far cortex (p = 0.003). Loading the healed tibiae to failure demonstrated that FCL specimens tolerated 156% more energy until failure (p < 0.001) than LP specimens (Figure 10B). Histological sections depicted `partial non-unions' in three of six LP constructs, whereby bridging callus did not form at the near cortex. In the FCL group, bridging callus consistently formed in all specimens at the near and far cortices.
Figure 10.
Fracture healing: A) Deficient interfragmentary motion at the near cortex of standard locked plating (LP) constructs caused partial non-unions at the near cortex. Flexible fixation and parallel motion provided by FCL constructs yielded consistent and symmetric healing and increased bone mineral content by 44%. B) Healed tibiae of the FCL group tolerated 156% more energy to failure.
This study furthermore confirmed that FCL constructs actively promote fracture healing by providing flexible fixation and parallel interfragmentary motion. Compared to LP constructs, FCL constructs formed more callus, healed stronger, and effectively prevented `partial non-unions' seen with locked plating constructs. Therefore, FCL fixation may be advisable for stiffness reduction of locked plating constructs to improve fracture healing.
In conclusion, by providing flexible fixation, load distribution, progressive stiffening, and nearly parallel interfragmentary motion, FCL constructs retain the strength of a standard locked plating construct and actively promote fracture healing by callus formation. Therefore, FCL constructs resemble true internal fixators by replication the biomechanical function of external fixators. The present data are limited to bridge plating constructs, whereby FCL screws can be applied in a standard manner for either periarticular or diaphyseal bridge plating. However, FCL screws cannot be mixed with compression or standard locking screws in the same bone segment, since these screws would disable FCL functionality. Despite the considerable data base on FCL fixation obtained in bench-top and in vivo testing, a prospective comparative clinical study will be required to assess the benefits of FCL fixation on fracture healing compared to standard locked plating.
Acknowledgments
The institution of the authors has received funding from the NIH / NIAMS (AR053611) and from Zimmer (Warsaw, IN) for the conduct of this research. One author (MB) has a licensing agreement with Zimmer and receives payments from Zimmer related to Far Cortical Locking technology.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bottlang M, Doornink J, Byrd GD, et al. A nonlocking end screw can decrease fracture risk caused by locked plating in the osteoporotic diaphysis. The Journal of bone and joint surgery. 2009;91:620–627. doi: 10.2106/JBJS.H.00408. [DOI] [PubMed] [Google Scholar]
- 2.Bottlang M, Doornink J, Fitzpatrick DC, et al. Far Cortical Locking can reduce the stiffness of locked plating constructs while retaining construct strength. The Journal of bone and joint surgery. 2009;92 doi: 10.2106/JBJS.H.01038. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bottlang M, Lesser M, Koerber J, et al. Far cortical locking can improve healing of fractures stabilized with locking plates. The Journal of bone and joint surgery. 2010;92:1652–1660. doi: 10.2106/JBJS.I.01111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubiak EN, Fulkerson E, Strauss E, et al. The evolution of locked plates. The Journal of bone and joint surgery. 2006;88(Suppl 4):189–200. doi: 10.2106/JBJS.F.00703. [DOI] [PubMed] [Google Scholar]
- 5.Perren SM. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Joint Surg Br. 2002;84:1093–1110. doi: 10.1302/0301-620x.84b8.13752. [DOI] [PubMed] [Google Scholar]
- 6.Uhthoff HK, Poitras P, Backman DS. Internal plate fixation of fractures: short history and recent developments. J Orthop Sci. 2006;11:118–126. doi: 10.1007/s00776-005-0984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bottlang M, Doornink J, Lujan TJ, et al. Effects of construct stiffness on healing of fractures stablized with locking plates. The Journal of bone and joint surgery. 2010 doi: 10.2106/JBJS.J.00780. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lujan TJ, Henderson CE, Madey SM, et al. Locked plating of distal femur fractures leads to inconsistent and asymmetric callus formation. J Orthop Trauma. 2010;24:156–162. doi: 10.1097/BOT.0b013e3181be6720. [DOI] [PubMed] [Google Scholar]
- 9.Perren SM. Backgrounds of the technology of internal fixators. Injury. 2003;34(Suppl 2):B1–3. doi: 10.1016/j.injury.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick DC, Doornink J, Madey SM, et al. Relative stability of conventional and locked plating fixation in a model of the osteoporotic femoral diaphysis. Clinical biomechanics (Bristol, Avon) 2009;24:203–209. doi: 10.1016/j.clinbiomech.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caja V, Kim W, Larsson S, et al. Comparison of the mechanical performance of three types of external fixators: linear, circular and hybrid. Clinical biomechanics (Bristol, Avon) 1995;10:401–406. doi: 10.1016/0268-0033(95)00014-3. [DOI] [PubMed] [Google Scholar]
- 12.Kowalski MJ, Schemitsch EH, Harrington RM, et al. A comparative biomechanical evaluation of a noncontacting plate and currently used devices for tibial fixation. J Trauma. 1996;40:5–9. doi: 10.1097/00005373-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Moroz TK, Finlay JB, Rorabeck CH, et al. External skeletal fixation: choosing a system based on biomechanical stability. J Orthop Trauma. 1988;2:284–296. [PubMed] [Google Scholar]
- 14.Egol KA, Kubiak EN, Fulkerson E, et al. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18:488–493. doi: 10.1097/00005131-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Claes LE, Heigele CA. Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J Biomech. 1999;32:255–266. doi: 10.1016/s0021-9290(98)00153-5. [DOI] [PubMed] [Google Scholar]
- 16.Ebert JR, Ackland TR, Lloyd DG, et al. Accuracy of partial weight bearing after autologous chondrocyte implantation. Arch Phys Med Rehabil. 2008;89:1528–1534. doi: 10.1016/j.apmr.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 17.Claes LE, Heigele CA, Neidlinger-Wilke C, et al. Effects of mechanical factors on the fracture healing process. Clinical orthopaedics and related research. 1998:S132–147. doi: 10.1097/00003086-199810001-00015. [DOI] [PubMed] [Google Scholar]
- 18.Goodship AE, Kenwright J. The influence of induced micromovement upon the healing of experimental tibial fractures. J Bone Joint Surg Br. 1985;67:650–655. doi: 10.1302/0301-620X.67B4.4030869. [DOI] [PubMed] [Google Scholar]
- 19.Kenwright J, Richardson JB, Cunningham JL, et al. Axial movement and tibial fractures. A controlled randomised trial of treatment. J Bone Joint Surg Br. 1991;73:654–659. doi: 10.1302/0301-620X.73B4.2071654. [DOI] [PubMed] [Google Scholar]
- 20.Reilly DT, Burstein AH. Review article. The mechanical properties of cortical bone. The Journal of bone and joint surgery. 1974;56:1001–1022. [PubMed] [Google Scholar]
- 21.Gardner MJ, Nork SE, Huber P, et al. Less rigid stable fracture fixation in osteoporotic bone using locked plates with near cortical slots. Injury. 2010;41:652–656. doi: 10.1016/j.injury.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Stoffel K, Dieter U, Stachowiak G, et al. Biomechanical testing of the LCP--how can stability in locked internal fixators be controlled? Injury. 2003;34(Suppl 2):B11–19. doi: 10.1016/j.injury.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Dobele S, Horn C, Eichhorn S, et al. The dynamic locking screw (DLS) can increase interfragmentary motion on the near cortex of locked plating constructs by reducing the axial stiffness. Langenbeck's archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2010;395:421–428. doi: 10.1007/s00423-010-0636-z. [DOI] [PubMed] [Google Scholar]
- 24.Lujan TJ, Madey SM, Fitzpatrick DC, et al. A computational technique to measure fracture callus in radiographs. J Biomech. 2010;43:792–795. doi: 10.1016/j.jbiomech.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Peindl RD, Zura RD, Vincent A, et al. Unstable proximal extraarticular tibia fractures: a biomechanical evaluation of four methods of fixation. J Orthop Trauma. 2004;18:540–545. doi: 10.1097/00005131-200409000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Stoffel K, Lorenz KU, Kuster MS. Biomechanical considerations in plate osteosynthesis: the effect of plate-to-bone compression with and without angular screw stability. J Orthop Trauma. 2007;21:362–368. doi: 10.1097/BOT.0b013e31806dd921. [DOI] [PubMed] [Google Scholar]
- 27.Finlay JB, Moroz TK, Rorabeck CH, et al. Stability of ten configurations of the Hoffmann external-fixation frame. The Journal of bone and joint surgery. 1987;69:734–744. [PubMed] [Google Scholar]
- 28.Orbay GL, Frankel VH, Kummer FJ. The effect of wire configuration on the stability of the Ilizarov external fixator. Clinical orthopaedics and related research. 1992:299–302. [PubMed] [Google Scholar]
- 29.Yilmaz E, Belhan O, Karakurt L, et al. Mechanical performance of hybrid Ilizarov external fixator in comparison with Ilizarov circular external fixator. Clinical biomechanics (Bristol, Avon) 2003;18:518–522. doi: 10.1016/s0268-0033(03)00073-1. [DOI] [PubMed] [Google Scholar]