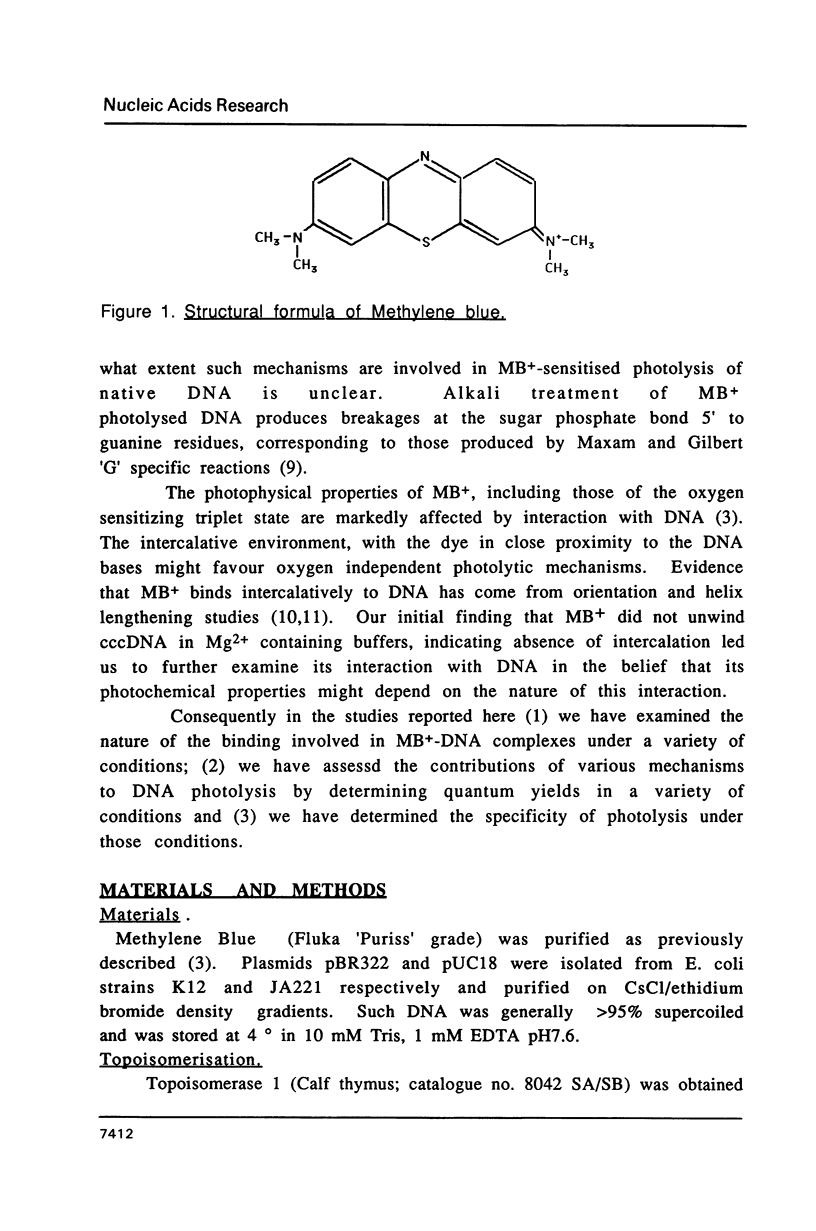
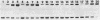Abstract
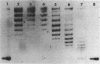
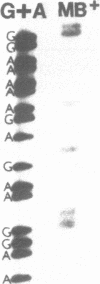
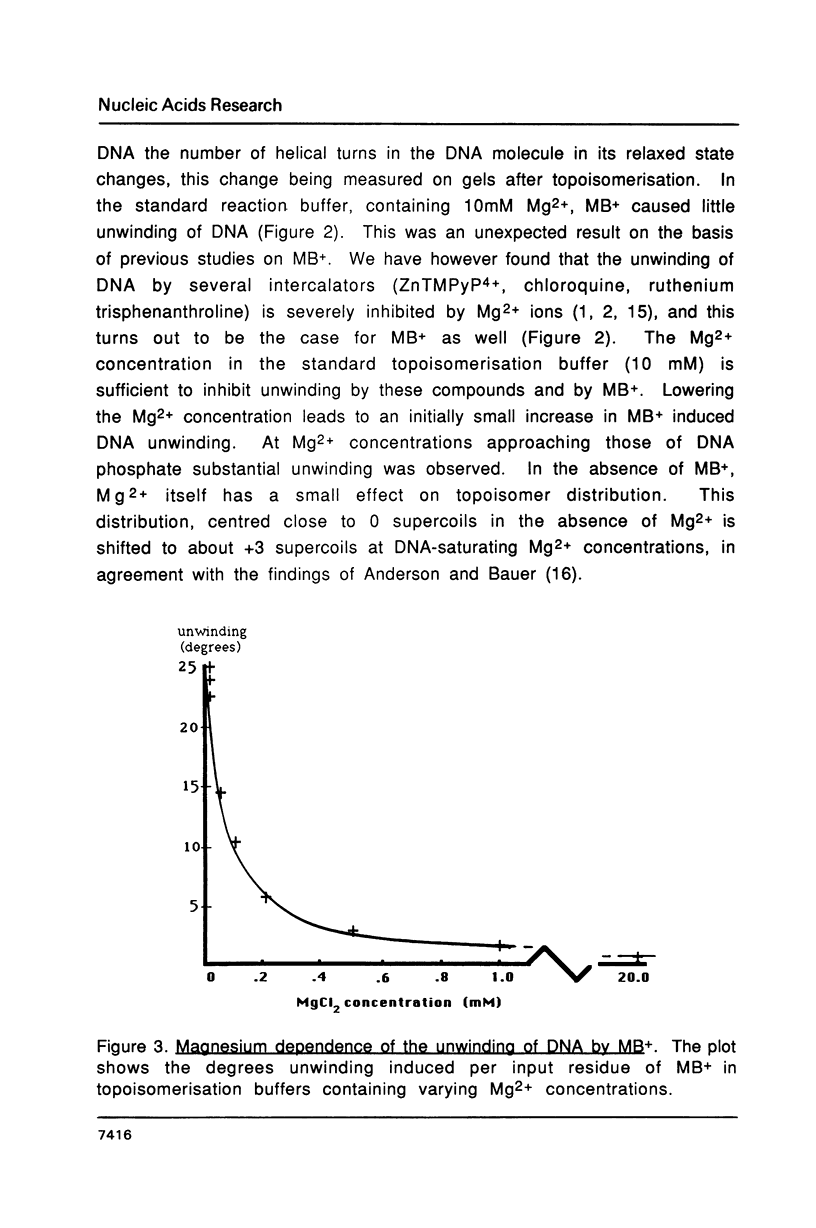
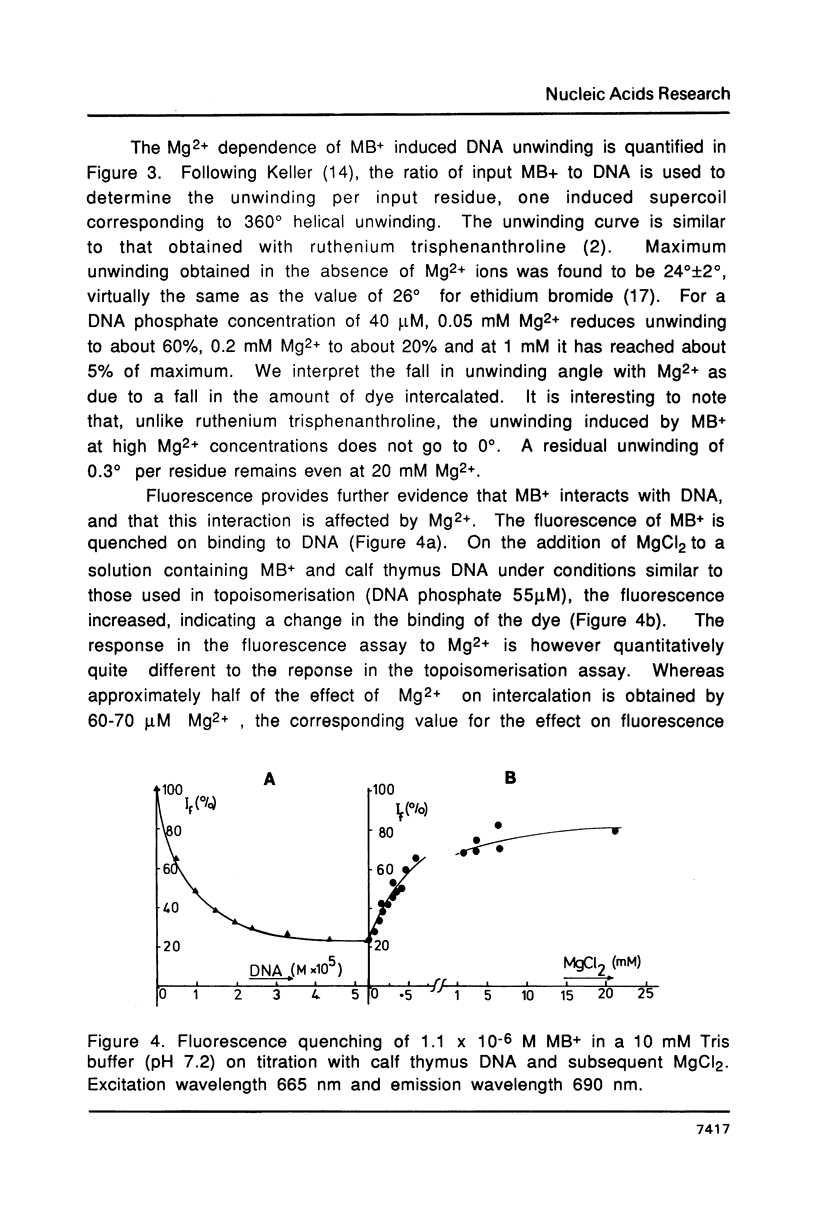
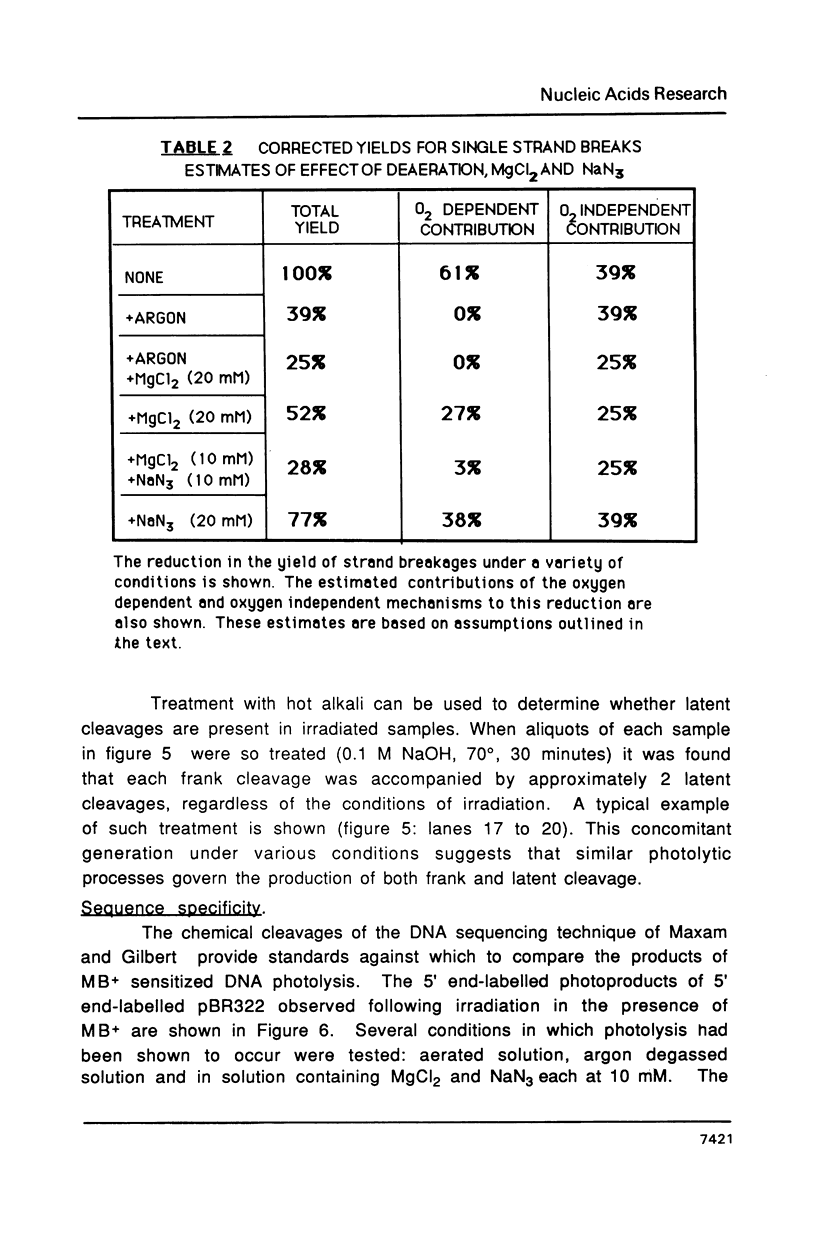
It is shown that methylene blue (MB+) photosensitises DNA in either aerated or deaerated solutions, causing direct cleavage of phosphodiester bonds and rendering additional bonds labile to alkali. Evidence from unwinding and fluorimetric studies indicates that MB+ binds to DNA in at least 2 ways. Intercalation, which optimally induces helical unwinding of 24 degrees +/- 2 degrees per MB+, is markedly reduced upon neutralisation by Mg2+ of the DNA phosphates, while significant non-intercalative binding persists as shown by substantial fluorescence quenching at Mg2+ concentrations where there is little unwinding. MB+ induces photolysis at both low and high Mg2+ concentration - intercalation is apparently not required for photolysis. The quantum yield for strand breakage varies from 1-3 X 10(-7) under different conditions and is oxygen enhanced. The DNA cleavage is guanine specific. The 3' termini of the primary MB+-induced DNA photoproducts, unlike those generated by chemical sequencing retain an alkali labile adduct on the terminal phosphate.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Bauer W. Supercoiling in closed circular DNA: dependence upon ion type and concentration. Biochemistry. 1978 Feb 21;17(4):594–601. doi: 10.1021/bi00597a006. [DOI] [PubMed] [Google Scholar]
- Cadet J., Berger M., Decarroz C., Wagner J. R., van Lier J. E., Ginot Y. M., Vigny P. Photosensitized reactions of nucleic acids. Biochimie. 1986 Jun;68(6):813–834. doi: 10.1016/s0300-9084(86)80097-9. [DOI] [PubMed] [Google Scholar]
- Feldberg R. S., Brown C., Carew J. A., Lucas J. L. Probing photodynamic damage in nucleic acids with a damage-specific DNA binding protein: a comparison of the B and Z DNA conformations. Photochem Photobiol. 1983 May;37(5):521–524. doi: 10.1111/j.1751-1097.1983.tb04511.x. [DOI] [PubMed] [Google Scholar]
- Friedmann T., Brown D. M. Base-specific reactions useful for DNA sequencing: methylene blue--sensitized photooxidation of guanine and osmium tetraoxide modification of thymine. Nucleic Acids Res. 1978 Feb;5(2):615–622. doi: 10.1093/nar/5.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henner W. D., Grunberg S. M., Haseltine W. A. Sites and structure of gamma radiation-induced DNA strand breaks. J Biol Chem. 1982 Oct 10;257(19):11750–11754. [PubMed] [Google Scholar]
- Hogan M., Wang J., Austin R. H., Monitto C. L., Hershkowitz S. Molecular motion of DNA as measured by triplet anisotropy decay. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3518–3522. doi: 10.1073/pnas.79.11.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson F. Chemical changes induced in DNA by ionizing radiation. Prog Nucleic Acid Res Mol Biol. 1985;32:115–154. doi: 10.1016/s0079-6603(08)60347-5. [DOI] [PubMed] [Google Scholar]
- Kawanishi S., Inoue S., Sano S., Aiba H. Photodynamic guanine modification by hematoporphyrin is specific for single-stranded DNA with singlet oxygen as a mediator. J Biol Chem. 1986 May 5;261(13):6090–6095. [PubMed] [Google Scholar]
- Keller W. Determination of the number of superhelical turns in simian virus 40 DNA by gel electrophoresis. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4876–4880. doi: 10.1073/pnas.72.12.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. M., Murphy M. J., McConnell D. J., OhUigin C. A comparative study of the interaction of 5,10,15,20-tetrakis (N-methylpyridinium-4-yl)porphyrin and its zinc complex with DNA using fluorescence spectroscopy and topoisomerisation. Nucleic Acids Res. 1985 Jan 11;13(1):167–184. doi: 10.1093/nar/13.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. M., Tossi A. B., McConnell D. J., OhUigin C. A study of the interactions of some polypyridylruthenium (II) complexes with DNA using fluorescence spectroscopy, topoisomerisation and thermal denaturation. Nucleic Acids Res. 1985 Sep 11;13(17):6017–6034. doi: 10.1093/nar/13.17.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. M., van der Putten W. J., McConnell D. J. Laser flash spectroscopy of methylene blue with nucleic acids. Photochem Photobiol. 1987 Feb;45(2):167–175. doi: 10.1111/j.1751-1097.1987.tb05360.x. [DOI] [PubMed] [Google Scholar]
- Knowles A. A mechanism for the methylene blue sensitized oxidation of nucleotides. Photochem Photobiol. 1971 Jun;13(6):473–487. doi: 10.1111/j.1751-1097.1971.tb06142.x. [DOI] [PubMed] [Google Scholar]
- Lee P. C., Rodgers M. A. Laser flash photokinetic studies of rose bengal sensitized photodynamic interactions of nucleotides and DNA. Photochem Photobiol. 1987 Jan;45(1):79–86. doi: 10.1111/j.1751-1097.1987.tb08407.x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Müller W., Crothers D. M. Interactions of heteroaromatic compounds with nucleic acids. 1. The influence of heteroatoms and polarizability on the base specificity of intercalating ligands. Eur J Biochem. 1975 May;54(1):267–277. doi: 10.1111/j.1432-1033.1975.tb04137.x. [DOI] [PubMed] [Google Scholar]
- Nieuwint A. W., Aubry J. M., Arwert F., Kortbeek H., Herzberg S., Joenje H. Inability of chemically generated singlet oxygen to break the DNA backbone. Free Radic Res Commun. 1985;1(1):1–9. doi: 10.3109/10715768509056532. [DOI] [PubMed] [Google Scholar]
- Nordén B., Tjerneld F. Structure of methylene blue-DNA complexes studied by linear and circular dichroism spectroscopy. Biopolymers. 1982 Sep;21(9):1713–1734. doi: 10.1002/bip.360210904. [DOI] [PubMed] [Google Scholar]
- Pathak M. A., Joshi P. C. Production of active oxygen species (1O2 and O2-.) by psoralens and ultraviolet radiation (320-400 nm). Biochim Biophys Acta. 1984 Mar 22;798(1):115–126. doi: 10.1016/0304-4165(84)90018-7. [DOI] [PubMed] [Google Scholar]
- SIMON M. I., VAN VUNAKIS H. The photodynamic reaction of methylene blue with deoxyribonucleic acid. J Mol Biol. 1962 Jun;4:488–499. doi: 10.1016/s0022-2836(62)80104-1. [DOI] [PubMed] [Google Scholar]
- Saito I., Inoue K., Matsuura T. Occurrence of the singlet-oxygen mechanism in photodynamic oxidations of guanosine. Photochem Photobiol. 1975 Jan;21(1):27–30. doi: 10.1111/j.1751-1097.1975.tb06625.x. [DOI] [PubMed] [Google Scholar]
- Saito I., Sugiyama H., Matsuura T., Ueda K., Komano T. A new procedure for determining thymine residues in DNA sequencing. Photoinduced cleavage of DNA fragments in the presence of spermine. Nucleic Acids Res. 1984 Mar 26;12(6):2879–2885. doi: 10.1093/nar/12.6.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Frohlinde D., Opitz J., Görner H., Bothe E. Model studies for the direct effect of high-energy irradiation on DNA. Mechanism of strand break formation induced by laser photoionization of poly U in aqueous solution. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Sep;48(3):397–408. doi: 10.1080/09553008514551401. [DOI] [PubMed] [Google Scholar]
- Wang J. C. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974 Nov 15;89(4):783–801. doi: 10.1016/0022-2836(74)90053-9. [DOI] [PubMed] [Google Scholar]
- Wang J., Hogan M., Austin R. H. DNA motions in the nucleosome core particle. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5896–5900. doi: 10.1073/pnas.79.19.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskell L. A., Sastry K. S., Gordon M. P. Studies on the photosensitized breakdown of guanosine by methylene blue. Biochim Biophys Acta. 1966 Oct 24;129(1):49–53. doi: 10.1016/0005-2787(66)90007-4. [DOI] [PubMed] [Google Scholar]