Abstract
The Saccharomyces cerevisiae SGS1 gene encodes a RecQ-like DNA helicase, human homologues of which are implicated in the genetic instability disorders, Bloom syndrome (BS), Rothmund-Thomson syndrome (RTS), and Werner syndrome (WS). Telomerase-negative yeast cells can recover from senescence via two recombinational telomere elongation pathways. The “type I” pathway generates telomeres with large blocks of telomeric and subtelomeric sequences and short terminal repeat tracts. The “type II” pathway generates telomeres with extremely long heterogeneous terminal repeat tracts, reminiscent of the long telomeres observed in telomerase-deficient human tumors and tumor-derived cell lines. Here, we report that telomerase-negative (est2) yeast cells lacking SGS1 senesced more rapidly, experienced a higher rate of telomere erosion, and were delayed in the generation of survivors. The est2 sgs1 survivors that were generated grew poorly, arrested in G2/M and possessed exclusively type I telomeres, implying that SGS1 is critical for the type II pathway. The mouse WS gene suppressed the slow growth and G2/M arrest phenotype of est2 sgs1 survivors, arguing that the telomeric function of SGS1 is conserved. Reintroduction of SGS1 into est2 sgs1 survivors restored growth rate and extended terminal tracts by ≈300 bp. Both phenotypes were absolutely dependent on Sgs1 helicase activity. Introduction of an sgs1 carboxyl-terminal truncation allele with helicase activity restored growth rate without extending telomeres in most cases, demonstrating that type II telomeres are not necessary for normal growth in the absence of telomerase.
The sole Saccharomyces cerevisiae RecQ-like helicase, Sgs1, was originally identified by a mutation that suppressed the slow growth phenotype of top3 (topoisomerase III) mutants and cloned by virtue of its interaction with Top3 in a two-hybrid system (1, 2). Deletion of SGS1 causes mitotic hyperrecombination (1, 2), sensitivity to hydroxyurea (3, 4), and a defect in the intra-S phase DNA damage checkpoint (4) but not telomere shortening (2). Sgs1 and the Srs2 helicase have overlapping functions because a double deletion of SGS1 and SRS2, but not the single deletions, inhibits DNA replication (5, 6). The poor growth of sgs1 srs2 double mutants is significantly improved by abolishing homologous recombination, implying that Sgs1 may resolve recombination intermediates during DNA replication (6).
All RecQ helicases can unwind standard duplex DNA with 3′→5′ directionality in vitro (7–10). Interestingly, BLM (BS helicase), WRN (WS helicase), and Sgs1 can also unwind G-DNA (11–13), a highly stable quadruplex structure that has been shown in vitro to form at G-rich sequences such as telomeric DNA (14, 15). WRN unwinds d(CGG)n repeats but not telomeric sequences or IgG switch regions in vitro (13). WRN, BLM, and Sgs1 can also migrate Holliday junctions in vitro (8, 16, 17), and both WRN and Sgs1 appear to localize at sites of stalled DNA replication (4, 17). Together, these findings suggest that eukaryotic RecQ-like helicases promote the resolution of DNA secondary structures at stalled or broken replication forks (6, 13, 18, 19).
Werner syndrome (WS) individuals, and to a lesser extent Rothmund-Thomson syndrome (RTS) individuals, exhibit symptoms that resemble premature aging (20). Fibroblasts cultured from WS individuals senesce more rapidly in culture than those from normal individuals (21, 22). However, the introduction of the telomerase catalytic subunit (hTERT) into WS cells bypasses senescence (23). This result has been viewed as evidence that the premature senescence of WS cells is due to a telomere-related defect (23). To provide a model for the putative function of WRN at telomeres, we have examined whether Sgs1 has a telomeric function in the absence of telomerase, before senescence and during recovery.
Materials and Methods
Strains and Plasmids.
All yeast strains were derivatives of W303-1A. DSY36 (MATα sgs1∷HIS3) was mated to DSY630 (MATa rad52∷TRP1) to generate DSY641. EST2 was disrupted in DSY641 by transformation with BamHI-linearized pVL523 (24) (DSY1167) or by a one-step KANR PCR disruption procedure (DSY1305). YCH1035 (MATa/α est2/EST2 sgs1/SGS1 ADH-mWRN) was generated by mating DSY2001 (MATa est2∷URA3) with DLY190 (MATα sgs1∷HIS3 ADH-mWRN). DLY190 was a gift of D. Lombard (Massachusetts Institute of Technology, Cambridge, MA). DSY454 was generated by integrating PpuMI-linearized pDS35. est2 meiotic segregants were generated from DSY1167, YHC1005, or YHC1035. Haploid strains carrying the Cre-loxP system were generated from DSY1305 carrying pSH47 (25) and pDS381. The rad52∷leu2 disruption was created by using pSM20 (gift of G. Fink, Whitehead Institute, Cambridge, MA). A polylinker was derived by PCR amplification of pRS316 and ligated to pDS116 to generate pDS375. EST2 was excised from pVL657 (gift of V. Lundblad, Baylor College of Medicine, Houston, TX) with NotI/KpnI and inserted into pDS375 cut with the same enzymes to generate pDS381. Plasmids carrying sgs1 alleles were obtained from S. Brill (Rutgers University, Piscataway, NJ) (3).
Yeast Media and Viability Assays.
For liquid viability and telomere length assays using the Cre-loxP system, cells were pregrown in glucose-containing medium lacking adenine and uracil. Cells were washed in water and plated on galactose/raffinose (2% wt/vol) medium lacking uracil (400 colonies per plate) to monitor loss of pDS378, as indicated by red colony color. Fewer than 1 in 105 cells failed to convert to Ade−. Approximately 400 red colonies were pooled and resuspended in complete glucose [yeast extract/peptone/dextrose (YPD)] medium at 1 × 105 cells/ml. Cultures were grown until the wild-type culture reached 1.4 × 108/ml and the cell density of each culture was recorded. Fresh YPD cultures were inoculated with 1 × 105 cells/ml, and this cycle was repeated until the cultures ceased dividing (i.e., final density of <1 × 105 cells/ml). For recovery assays, colonies from single spores were serially subcultured between 1 × 105 and 4 × 108 cells/ml in YPD medium. Cell densities were determined by hemocytometer. The loss of pSGS1 from est2 sgs1 survivors was confirmed by PCR and growth on methylmethane sulfonate (0.01% vol/vol) plates.
Southern Blot Analysis.
Yeast genomic DNA (5–10 μg) was digested with XhoI and separated by electrophoresis (1.3 V/cm) in agarose gels (0.9–1.0% wt/vol). DNA was hybridized to a random primed 600-bp KpnI fragment of Y′ or a 270-bp EcoRI fragment of C1–3A (26).
Flow Cytometric Analyses.
Cells were prepared for FACS analysis as previously described (27).
Telomere Length Determination.
Telomere lengths were determined as previously described (28). For each sample, the signal was quantified along a line drawn down the center of each lane using image-quant software (Molecular Dynamics). The distance between the center of the Y′-short peak and the terminal XhoI fragment peak was measured to within 1 mm precision. Six isolates of each genotype were analyzed, and each DNA sample was measured on three separate gels.
Results
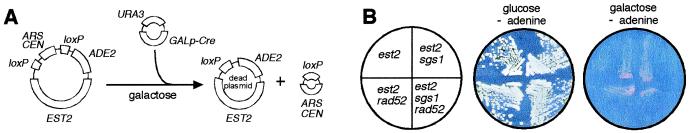
To facilitate our analysis, we developed a Cre-loxP recombination system to conditionally delete EST2, the gene encoding the yeast telomerase catalytic subunit. The system consists of a “destructible” EST2 plasmid in which the ARS/CEN (autonomously replicating sequences/centromeric sequence) cassette is flanked by loxP sites. This plasmid also carries ADE2, a selectable marker required for growth on plates lacking adenine. A second URA3-based plasmid carries the Cre gene under the control of a galactose-inducible promoter. In galactose-containing medium, Cre expression results in the excision of the ARS/CEN sequence, rendering the EST2 plasmid unable to be replicated or efficiently segregated (Fig. 1A). This event can be scored by the conversion of the strain from Ade+ to Ade− (Fig. 1B). The advantages of the system are that it circumvents the need to generate haploid telomerase mutants from heterozygous diploids, greatly reduces the variability between experiments, and permits the analysis of events that occur within the first few generations after loss of telomerase activity.
Figure 1.
A two plasmid Cre-loxP system for the inducible deletion of EST2. (A) One plasmid carries full-length EST2 gene and ADE2, a marker gene required for adenine biosynthesis. In addition, two loxP sites flank the ARS/CEN cassette required for plasmid replication and efficient segregation during mitosis. A second URA3-based plasmid contains the Cre recombinase gene under the control of the GAL10 (galactose-inducible) promoter. (B) Growth of strains on galactose-containing medium resulted in Cre-mediated excision of the ARS/CEN sequence and the loss of EST2 from the strain, as indicated by the conversion of the strains from Ade+ to Ade− and the inability of cells to grow on medium lacking adenine.
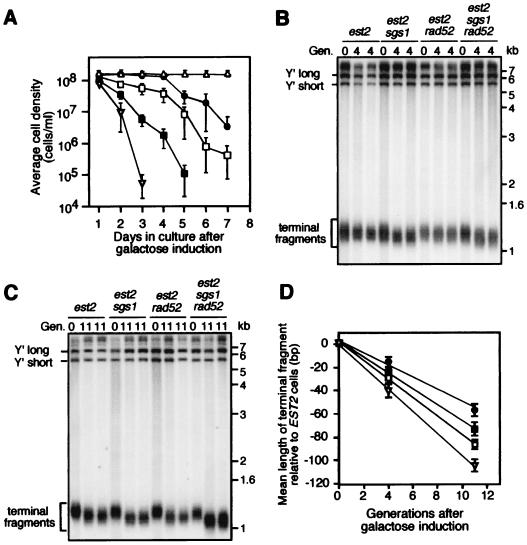
Using the Cre-loxP system, we examined the effect of deleting SGS1, RAD52, or both, on the senescence rate of est2 strains. Telomerase-negative yeast cells lacking RAD52 senesce more rapidly and are unable to recover from senescence because of a defect in homologous recombination (26, 29). Cultures were pregrown in glucose-containing medium, then serially subcultured in galactose-containing medium, with each passage representing 10 generations of the wild-type strain. To score viability, cell densities were recorded daily when the wild-type culture reached 1.4 × 108 cells/ml. As shown in Fig. 2A, est2 strains reached the point of maximum cell death after an average of 7 days in liquid culture, similar to a prior report for tlc1 strains in liquid culture (30). In agreement with other reports of telomerase mutants (24, 30), est2 rad52 strains died earlier than est2 controls. Interestingly, the est2 sgs1 strains died earlier than est2 strain, and the est2 sgs1 rad52 strain died earlier than the est2 rad52 control.
Figure 2.
Senescent phenotypes and telomere loss rates of sgs1, est2, and rad52 mutant combinations. (A) Five cultures of est2 (●), est2 sgs1 (□), est2 rad52 (■), and est2 sgs1 rad52 (▿) and two cultures of wild type (▵) carrying the Cre-loxP EST2 recombination system were pregrown in glucose-containing medium and then serially passaged each day in galactose/raffinose-containing medium. Cultures were inoculated to 1 × 105 cells/ml and grown until the wild-type culture reached a cell density of 1.4 × 108/ml (≈10 generations of the wild-type strain/passage). We estimated that the strains had undergone ≈30 generations before day 1. To determine the rates of telomere loss, glucose-grown cells (generation 0) were resuspended in galactose/raffinose-containing medium and harvested for telomere analysis exactly 4 and 11 generations later (B and C, respectively). XhoI-digested genomic DNA was probed with a Y′ sequence after Southern blotting. Lanes 1–3, est2; lanes 4–6, est2 sgs1; and lanes 7–9, est2 sgs1 rad52. (D) Mean terminal fragment lengths of est2 (●), est2 sgs1 (□), est2 rad52 (■), and est2 sgs1 rad52 (▿) strains after growth in galactose/raffinose-containing medium. For each strain, nine independent cultures were assayed, and each culture was assayed by using three independent blots. Mean terminal fragment lengths (±SD) are shown relative to the glucose-grown control (0 generations).
Next, we investigated whether the increased rates of cell death were due to accelerated telomere erosion. Nine independent cultures of each strain were analyzed for telomere length, 4 and 11 generations after transfer to galactose/raffinose medium. Within this period, a decline in the growth rates of strains was not detected (not shown), indicating that they were all presenescent. S. cerevisiae telomeric DNA consists of ≈350 ± 75 bp of C1–3A/TG1–3 DNA, and internal to the C1–3A/TG1–3 tracts are repetitive DNA elements, called X and Y′ (31). Digestion of genomic DNA with XhoI liberates two large Y′ fragments and a ≈1.3-kb Y′/TG1–3 terminal fragment (Fig. 2 B and C) (29), the latter of which we used to determine terminal repeat length. As shown in Figure 2D, the est2 strains had an average telomere loss rate of 5.1 ± 0.6 bp/generation, whereas the wild-type control strains did not show any significant change in telomere length (not shown). This rate of loss for the est2 strain is in close agreement with the loss of 3–5 bp/generation reported by Marcand et al. (28). The average rate of telomere loss for the est2 sgs1 strain was higher than that of the est2 strain, at 7.5 ± 0.5 bp/generation. In agreement with this finding, we found that seven of eight meiotically derived est2 sgs1 strains had significantly shorter telomeres than their est2 siblings after both had been cultured for exactly 30 generations (data not shown). Consistent with its rapid rate of senescence, the est2 sgs1 rad52 mutant had the highest rate of telomere loss (9.4 ± 0.4 bp/generation), significantly higher than that of the est2 rad52 control. We also detected a slight increase in the rate of telomere loss in an est2 rad52 strain (6.4 ± 0.4 bp/generation) compared with est2 alone. Interestingly, other telomerase mutants, namely tlc1 and est1, reportedly do not show an increase in a rad52 background, despite their higher rate of senescence (24, 30). Investigation is warranted about whether these results reflect differences in the specific functions of the telomerase factors.
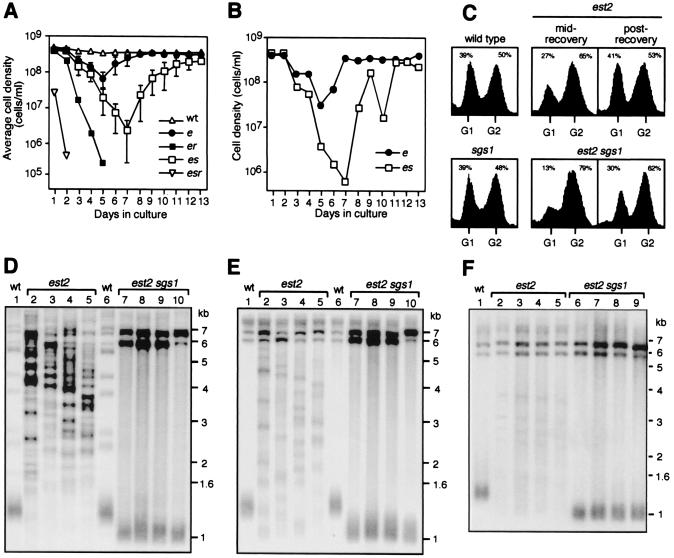
The effect of SGS1 on telomere erosion prompted us to examine whether SGS1 also influences recombination-mediated telomere lengthening, a process that allows telomerase-negative yeast cells to recover from senescence (26, 29, 32). Strains were serially passaged in liquid culture for 13 days, with each passage representing 11 generations of the wild-type strain. As shown in Fig. 3A, the est2 sgs1 cultures persisted in a senescent state for 2 days longer than the est2 strains, reaching cell densities an average of ≈50-fold lower than the est2 strains. The est2 sgs1 survivors that were eventually generated experienced recurring losses of cell viability and failed to recover to wild-type growth rates (Fig. 3 A and B). Senescent est2 rad52 and est2 sgs1 rad52 strains failed to produce survivors, owing to the critical role of homologous recombination in telomerase-independent telomere lengthening (26, 29, 32).
Figure 3.
Analysis of SGS1 function during senescence and recovery. Seventeen spore colonies of est2 (●), est2 sgs1 (□), est2 rad52 (■), and est2 sgs1 rad52 (▿) strains and two cultures of wild type (▵) were subcultured for 13 days. Each culture was inoculated to 1 × 105 cells/ml and grown until the wild-type culture reached a cell density of 4 × 108/ml. Each passage represented 11 generations of the wild-type strain. We estimated that strains had undergone ≈40 generations before day 1. (A) Average daily cell densities for each genotype. Error bars represent standard deviations. (B) Typical time course for individual cultures showing the typical loss of viability of est2 sgs1 survivors. (C) Representative flow cytometric analyses of wild-type, sgs1, est2, and est2 sgs1 cultures, 1 and 11 days after survivors were generated (mid- and post-recovery, respectively). Percentage of cells with a G1 or G2 DNA content are shown. (D) Poly[AC/TG]-probed Southern blot XhoI-digested genomic DNA from est2 (lanes 2–5), est2 sgs1 survivors (lanes 7–10), and wild-type (lanes 1 and 6) cultures on day 13. (E) Equivalent blot as for D but probed for Y′ sequences. (F) Y′-probed Southern blot of XhoI-digested genomic DNA from est2 and est2 sgs1 survivors 1, 2, 3, and 4 days after survivors were first generated. Lane 1, wild type; lanes 2–5, est2; and lanes 6–9, est2 sgs1.
A visual inspection of cell morphologies of six independent est2 sgs1 cultures, 1 day after survivors were first generated, revealed an ≈3-fold higher proportion of large-budded cells relative to an sgs1 culture (data not shown). Flow cytometric analyses of DNA content showed that these cultures had an ≈2.5-fold higher proportion of cells with a G2 content of DNA than the sgs1 control (Fig. 3C, mid-recovery). Even 6 days after the first survivors were generated, there was an ≈1.5-fold higher proportion of G2 cells in the est2 sgs1 cultures (Fig. 3C, postrecovery), and this level was still apparent after 23 days (not shown). Although the est2 survivors had a slightly higher proportion of G2 cells during recovery (Fig. 3C, mid-recovery), they had returned to near wild-type cell cycle distributions 6 days after survivors were generated (Fig. 3C, postrecovery), which was consistent with their wild-type growth rate.
Two distinct types of telomere elongation can occur in the absence of telomerase, and these can be distinguished by their banding pattern on Southern blots (26, 29). We found that all of the est2 survivors (35/35) displayed banding patterns indicative of a type II telomere structure, i.e., greatly elongated and heterogeneous tracts of C1–3A/TG1–3 telomeric repeats (26, 29) (Fig. 3 D and E, lanes 2–5). Although we did not detect any est2 type I survivors, it is possible that type I survivors were generated but these were out-competed by faster growing type II survivors. In contrast to the est2 survivors, all est2 sgs1 survivors (35/35) displayed patterns indicative of a type I telomere structure, i.e., an increase in the number of tandem Y′ sequences and short terminal C1–3A/TG1–3 tracts (26, 29) (Fig. 3 D and E, lanes 7–10). The two types of survivor did not interconvert within the time frame of the experiment (Fig. 3F).
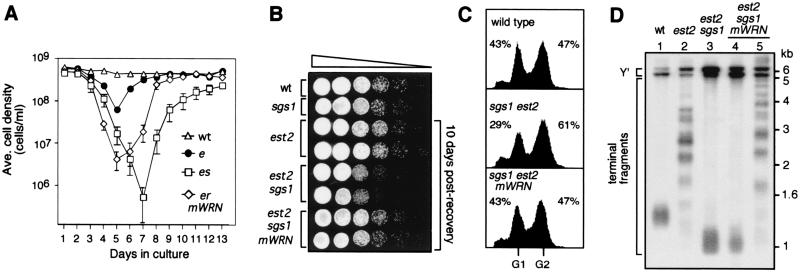
Next, we tested whether mouse WRN could complement any of the sgs1 telomere-related phenotypes. As shown in Fig. 4A, the est2 sgs1 cultures expressing mouse WRN (mWRN) had a slightly higher rate of senescence, but they recovered more rapidly than the est2 sgs1 cultures. Moreover, the mWRN-expressing survivors exhibited growth rates (Fig. 4B) and cell cycle distributions similar to est2 survivors (Fig. 4C). Unlike est2 sgs1 strains that produced only type I survivors, the mWRN-expressing est2 sgs1 strains produced one-third type I and two-thirds type II survivors (Fig. 4D). Thus, mWRN can suppress the slow growth phenotype of est2 sgs1 survivors and partially suppress the defect in the type II pathway.
Figure 4.
Murine WRN can suppress telomere-related phenotypes of est2 sgs1 strains. (A) The average rate of senescence and recovery for six wild-type (▵) est2 (●), est2 sgs1(□), and est2 sgs1 mWRN (⋄) cultures. (B) Survivors from A passaged in liquid complete (YPD) medium for 10 days after survivors were generated. Cells were then spotted to YPD plates in serial 10-fold dilutions and grown for 24 h. (C) Flow cytometric analysis of strains in B. (D) Representative telomere structures of mWRN-expressing survivors in B. Y′-probed Southern blot of XhoI-digested genomic DNA isolated from strains. Lane 1, wild type; lane 2, est2 survivor; lane 3, est2 sgs1 survivor; and lanes 4 and 5, representative est2 sgs1 mWRN survivors.
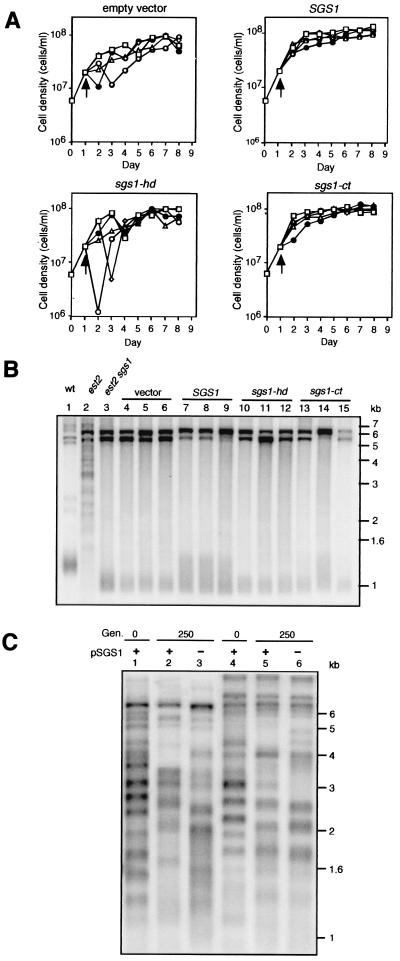
To examine the role that SGS1 might play in the generation of type II survivors, we tested the effect of reintroducing SGS1 on low copy plasmid into three independently derived est2 sgs1 survivors. The reintroduction of SGS1 rapidly restored the growth of the est2 sgs1 survivors (Fig. 5 shows representative survivor) and increased the maximal length of the terminal C1–3A/TG1–3 tracts from ≈50 to ≈350 bp (Fig. 5B, lanes 7–9), similar to the maximal length of wild-type terminal tracts. The lower Y′ bands were also less intense (Fig. 5B, lanes 7–9). This novel telomere type was present after 16 days of culturing (data not shown), implying that the cells were prevented from fully converting to type II.
Figure 5.
Reintroduction of SGS1 into est2 sgs1 survivors restores growth rate and lengthens C1–3A/TG1–3 repeat tracts. The sgs1-hd (helicase-defective) allele carries a single amino acid substitution in the ATP binding domain (3). The sgs1-ct (Δ200) allele is a carboxyl-terminal truncation that removes the conserved RecQ carboxyl-terminal (ct) and RNaseD homology (HRD) domains (3). (A) Low copy plasmids carrying the SGS1, sgs1-hd, or sgs1-ct allele, or no insert, were introduced by transformation into est2 sgs1 survivors, 1 day after they were generated (indicated by arrow). Five random transformants were resuspended in medium lacking leucine and serially passaged each day between 1 × 105 cells/ml and 1.4 × 108 cells/ml. Each passage represented 10 generations of the wild-type strain. (B) Telomere structure analysis of strains in A on day 8. XhoI-digested genomic DNA was probed with a poly[AC/TG] sequence. Identical terminal band sizes were detected by probing blots for Y′ sequences (not shown). Lane 1, wild type; lane 2, est2 survivor; lane 3, est2 sgs1 survivor; lanes 4–6, vector only; lanes 7–9, SGS1; lanes 10–12, sgs1-hd; and lanes 13–15, sgs1-ct. (C) Telomere structure analysis of two independently derived est2 sgs1 type II survivors after the loss of plasmid-borne SGS1 (pSGS1). Lanes 1–3, survivor 1; and lanes 4–6, survivor 2. Lanes 1 and 4, telomeres before loss of pSGS1; lanes 2 and 5, 250 generations with selection for pSGS1; and lanes 3 and 6, 250 generations after loss of pSGS1. Genomic DNA was probed with a poly[AC/TG] sequence as in B.
Prior genetic analyses have provided good evidence that Sgs1 performs two functions: one conferred by the central helicase domain and the other by the carboxyl-terminal third of the protein (3, 33). The introduction of a helicase-defective (sgs1-hd) allele carried by a low copy plasmid into est2 sgs1 survivors had no apparent effect (Fig. 5 A and B, lanes 10–12), demonstrating that the growth restoration and telomere extension requires a functional Sgs1 helicase domain. Interestingly, the introduction of the carboxyl-terminal truncation allele (sgs1-ct) rapidly restored the growth of all cultures to a wild-type rate (Fig. 5A), but only two of five strains experienced an extension of the terminal C1–3A/TG1–3 tract (Fig. 5B, lanes 13–15).
Next, we examined whether SGS1 is required for the viability of type II survivors. To this end, est2 sgs1 survivors carrying SGS1 on a low copy URA3-based plasmid were passaged over 5-fluoroorotic acid (FOA), which negatively selects for Ura+ cells. Strains lacking SGS1 were then streaked ten times (≈250 generations), during which time the strains were analyzed for growth defects and telomere structure (Fig. 5C). Compared with survivors that retained SGS1, there was no significant change in growth rate and only a slight increase in the telomere erosion rate of strains lacking SGS1. Thus, Sgs1 is not critical to the viability of type II survivors or the stability of type II telomeres. This finding is consistent with our observation that SGS1 is expressed at high levels during recovery but expression levels rapidly return to that of wild-type once survivors reach a wild-type growth rate (O. Medvedik and D.A.S., unpublished results).
Discussion
In this study, we describe an inducible Cre-loxP system to delete a telomerase gene simply by transferring cells to galactose-containing medium. The system permits the examination of cells within the first few generations after loss of telomerase and greatly reduces the variability between senescence experiments. Using the Cre-loxP system, we consistently observed that est2 sgs1 strains senesce earlier and lose terminal telomeric DNA more rapidly than est2 strains. DNA secondary structures such as G-DNA may form more readily at telomeres than other sites in the genome (12), owing to the highly repetitive, G-rich composition of telomeric sequences (14, 34). Cells lacking SGS1 may be unable to resolve these DNA secondary structures, resulting in an inability to efficiently replicate telomeric DNA.
Type I and Type II survivors arise via two genetically distinct recombination pathways defined by RAD51 and RAD50/RAD59, respectively (30, 35, 36). Our findings imply that SGS1 functions in the same recombinational pathway as RAD50 and RAD59. Consistent with the purported role of Sgs1 in DNA replication, Sgs1 may resolve recombination intermediates or other DNA secondary structures within the repetitive C1–3A/TG1–3 repeats (14, 15, 34) to facilitate the putative gene conversion events in the type II pathway (26, 37). Whereas our data support the hypotheses that Sgs1 is required for type II recombination, it is also possible that Sgs1 acts to suppress type I recombination. This possibility would be consistent with our observation that there were fewer Y′ amplifications at the telomeres of est2 sgs1 survivors into which SGS1 was introduced.
Although both types of survivor grow poorly when first generated, type II survivors steadily reach a growth rate indistinguishable from wild type whereas type I survivors never reach a wild-type growth rate (26, 29). Here we show that the slow growth phenotype of both types of survivor is due to a propensity for cell cycle arrest in G2/M. These arrest events are likely due to the activation of a cell cycle checkpoint after the breakdown of telomere-capping complex (38, 39) and the recognition of the telomere as a DNA break that requires repair.
Interestingly, the reintroduction of SGS1 into type I survivors did not result in their conversion to type II. This result is consistent with the recent finding of Chen and colleagues (36) that the reintroduction of RAD59 into type I cells did not covert them to type II. The type II pathway is thought to require recombination between C1–3A/TG1–3 repeats on different chromosomes or a rolling circle gene conversion mechanism by using an extrachromosomal circular template (35, 36, 39). An intriguing possibility is that the reintroduction of SGS1 into type I cells restores their ability to perform recombination between two terminal repeat tracts but the cells lack extrachromosomal circular templates to allow the very long type II telomeres to be generated. This model explains why only small increases in terminal tract length are observed after the reintroduction of SGS1 into type I survivors.
Some human cell lines and tumors that lack telomerase activity have very long and heterogeneous telomeres (40–42) that are reminiscent of yeast type II telomeres. The pathway that generates these long human telomeres has been termed ALT (for alternative lengthening of telomeres; ref. 41). Although the proteins that mediate ALT are not known, recent evidence suggests that ALT is mediated by gene-conversion (43), the same process thought to generate type II telomeres in yeast. This result, combined with our finding that mouse WRN can suppress most of the est2 sgs1 telomere-related phenotypes, raises the possibility that human WRN, or another human RecQ helicase, is an ALT factor. Applying these findings to mammals may help elucidate the in vivo function of the mammalian RecQ-helicases as well as the mechanisms by which mammalian cells proliferate in the absence of telomerase.
Acknowledgments
We thank members of the Sinclair lab, V. Lundblad, K. Münger, A. Rizki, and J. Teng for advice. Thanks also to S. Brill, V. Zakian, and V. Lundblad for plasmids and strains. This work was supported by the Leukemia and Lymphoma Society of America and the American Federation for Aging Research. D.A.S. is a Ludwig Scholar supported by the Ludwig Foundation. H.C. is supported by a John Taplin Postdoctoral Fellowship.
Abbreviations
- WS
Werner syndrome
- WRN
WS helicase
- YPD
yeast extract/peptone/dextrose
- ARS
autonomously replicating sequence
- CEN
centromeric sequence
- ALT
alternative lengthening of telomeres
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
*To whom reprint requests should be addressed. E-mail: david_sinclair@hms.harvard.edu.
References
- 1.Gangloff S, McDonald J P, Bendixen C, Arthur L, Rothstein R. Mol Cell Biol. 1994;14:8391–8398. doi: 10.1128/mcb.14.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watt P M, Hickson I D, Borts R H, Louis E J. Genetics. 1996;144:935–945. doi: 10.1093/genetics/144.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullen J R, Kaliraman V, Brill S J. Genetics. 2000;154:1101–1114. doi: 10.1093/genetics/154.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frei C, Gasser S M. Genes Dev. 2000;14:81–96. [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S K, Johnson R E, Yu S L, Prakash L, Prakash S. Science. 1999;286:2339–2342. doi: 10.1126/science.286.5448.2339. [DOI] [PubMed] [Google Scholar]
- 6.Gangloff S, Soustelle C, Fabre F. Nat Genet. 2000;25:192–194. doi: 10.1038/76055. [DOI] [PubMed] [Google Scholar]
- 7.Bennett R J, Sharp J A, Wang J C. J Biol Chem. 1998;273:9644–9650. doi: 10.1074/jbc.273.16.9644. [DOI] [PubMed] [Google Scholar]
- 8.Bennett R J, Keck J L, Wang J C. J Mol Biol. 1999;289:235–248. doi: 10.1006/jmbi.1999.2739. [DOI] [PubMed] [Google Scholar]
- 9.Shen J C, Gray M D, Oshima J, Loeb L A. Nucleic Acids Res. 1998;26:2879–2885. doi: 10.1093/nar/26.12.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki N, Shimamoto A, Imamura O, Kuromitsu J, Kitao S, Goto M, Furuichi Y. Nucleic Acids Res. 1997;25:2973–2978. doi: 10.1093/nar/25.15.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun H, Karow J K, Hickson I D, Maizels N. J Biol Chem. 1998;273:27587–27592. doi: 10.1074/jbc.273.42.27587. [DOI] [PubMed] [Google Scholar]
- 12.Sun H, Bennett R J, Maizels N. Nucleic Acids Res. 1999;27:1978–1984. doi: 10.1093/nar/27.9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fry M, Loeb L A. J Biol Chem. 1999;274:12797–12802. doi: 10.1074/jbc.274.18.12797. [DOI] [PubMed] [Google Scholar]
- 14.Venczel E A, Sen D. Biochemistry. 1993;32:6220–6228. doi: 10.1021/bi00075a015. [DOI] [PubMed] [Google Scholar]
- 15.Smith F W, Feigon J. Biochemistry. 1993;32:8682–8692. doi: 10.1021/bi00084a040. [DOI] [PubMed] [Google Scholar]
- 16.Karow J K, Constantinou A, Li J L, West S C, Hickson I D. Proc Natl Acad Sci USA. 2000;97:6504–6508. doi: 10.1073/pnas.100448097. . (First Published May 23, 2000; 10.1073/pnas.100448097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Constantinou A, Tarsounas M, Karow J K, Brosh R M, Bohr V A, Hickson I D, West S C. EMBO Reports. 2000;1:80–84. doi: 10.1093/embo-reports/kvd004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han H, Bennett R J, Hurley L H. Biochemistry. 2000;39:9311–9316. doi: 10.1021/bi000482r. [DOI] [PubMed] [Google Scholar]
- 19.Karow J K, Wu L, Hickson I D. Curr Opin Genet Dev. 2000;10:32–38. doi: 10.1016/s0959-437x(99)00039-8. [DOI] [PubMed] [Google Scholar]
- 20.Lindor N M, Furuichi Y, Kitao S, Shimamoto A, Arndt C, Jalal S. Am J Med Genet. 2000;90:223–228. doi: 10.1002/(sici)1096-8628(20000131)90:3<223::aid-ajmg7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 21.Salk D, Au K, Hoehn H, Stenchever M R, Martin G M. Cytogenet Cell Genet. 1981;30:108–117. doi: 10.1159/000131597. [DOI] [PubMed] [Google Scholar]
- 22.Holliday R, Porterfield J S, Gibbs D D. Nature (London) 1974;248:762–763. doi: 10.1038/248762a0. [DOI] [PubMed] [Google Scholar]
- 23.Wyllie F S, Jones C J, Skinner J W, Haughton M F, Wallis C, Wynford-Thomas D, Faragher R G, Kipling D. Nat Genet. 2000;24:16–17. doi: 10.1038/71630. [DOI] [PubMed] [Google Scholar]
- 24.Lendvay T S, Morris D K, Sah J, Balasubramanian B, Lundblad V. Genetics. 1996;144:1399–1412. doi: 10.1093/genetics/144.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guldener U, Heck S, Fielder T, Beinhauer J, Hegemann J H. Nucleic Acids Res. 1996;24:2519–2524. doi: 10.1093/nar/24.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teng S C, Zakian V A. Mol Cell Biol. 1999;19:8083–8093. doi: 10.1128/mcb.19.12.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills K D, Sinclair D A, Guarente L. Cell. 1999;97:609–620. doi: 10.1016/s0092-8674(00)80772-2. [DOI] [PubMed] [Google Scholar]
- 28.Marcand S, Brevet V, Gilson E. EMBO J. 1999;18:3509–3519. doi: 10.1093/emboj/18.12.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lundblad V, Blackburn E H. Cell. 1993;73:347–360. doi: 10.1016/0092-8674(93)90234-h. [DOI] [PubMed] [Google Scholar]
- 30.Le S, Moore J K, Haber J E, Greider C W. Genetics. 1999;152:143–152. doi: 10.1093/genetics/152.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zakian V A. Annu Rev Genet. 1996;30:141–172. doi: 10.1146/annurev.genet.30.1.141. [DOI] [PubMed] [Google Scholar]
- 32.McEachern M J, Blackburn E H. Genes Dev. 1996;10:1822–1834. doi: 10.1101/gad.10.14.1822. [DOI] [PubMed] [Google Scholar]
- 33.Lu J, Mullen J R, Brill S J, Kleff S, Romeo A M, Sternglanz R. Nature (London) 1996;383:678–679. doi: 10.1038/383678a0. [DOI] [PubMed] [Google Scholar]
- 34.Sundquist W I, Klug A. Nature (London) 1989;342:825–829. doi: 10.1038/342825a0. [DOI] [PubMed] [Google Scholar]
- 35.Teng S-C, Chang J, McCowan B, Zakian V A. Mol Cell. 2000;6:947–952. doi: 10.1016/s1097-2765(05)00094-8. [DOI] [PubMed] [Google Scholar]
- 36.Chen, Q. & Greider, C. W. (2001) Mol. Cell. Biol., in press. [DOI] [PMC free article] [PubMed]
- 37.Kass-Eisler A, Greider C W. Trends Biochem Sci. 2000;25:200–204. doi: 10.1016/s0968-0004(00)01557-7. [DOI] [PubMed] [Google Scholar]
- 38.Gasser S M. Science. 2000;288:1377–1379. doi: 10.1126/science.288.5470.1377. [DOI] [PubMed] [Google Scholar]
- 39.McEachern M J, Krauskopf A, Blackburn E H. Annu Rev Genet. 2000;34:331–358. doi: 10.1146/annurev.genet.34.1.331. [DOI] [PubMed] [Google Scholar]
- 40.Bryan T M, Englezou A, Gupta J, Bacchetti S, Reddel R R. EMBO J. 1995;14:4240–4248. doi: 10.1002/j.1460-2075.1995.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bryan T M, Englezou A, Dalla-Pozza L, Dunham M A, Reddel R R. Nat Med. 1997;3:1271–1274. doi: 10.1038/nm1197-1271. [DOI] [PubMed] [Google Scholar]
- 42.Murnane J P, Sabatier L, Marder B A, Morgan W F. EMBO J. 1994;13:4953–4962. doi: 10.1002/j.1460-2075.1994.tb06822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunham M A, Neumann A A, Fasching C L, Reddel R R. Nat Genet. 2000;26:447–450. doi: 10.1038/82586. [DOI] [PubMed] [Google Scholar]