Abstract
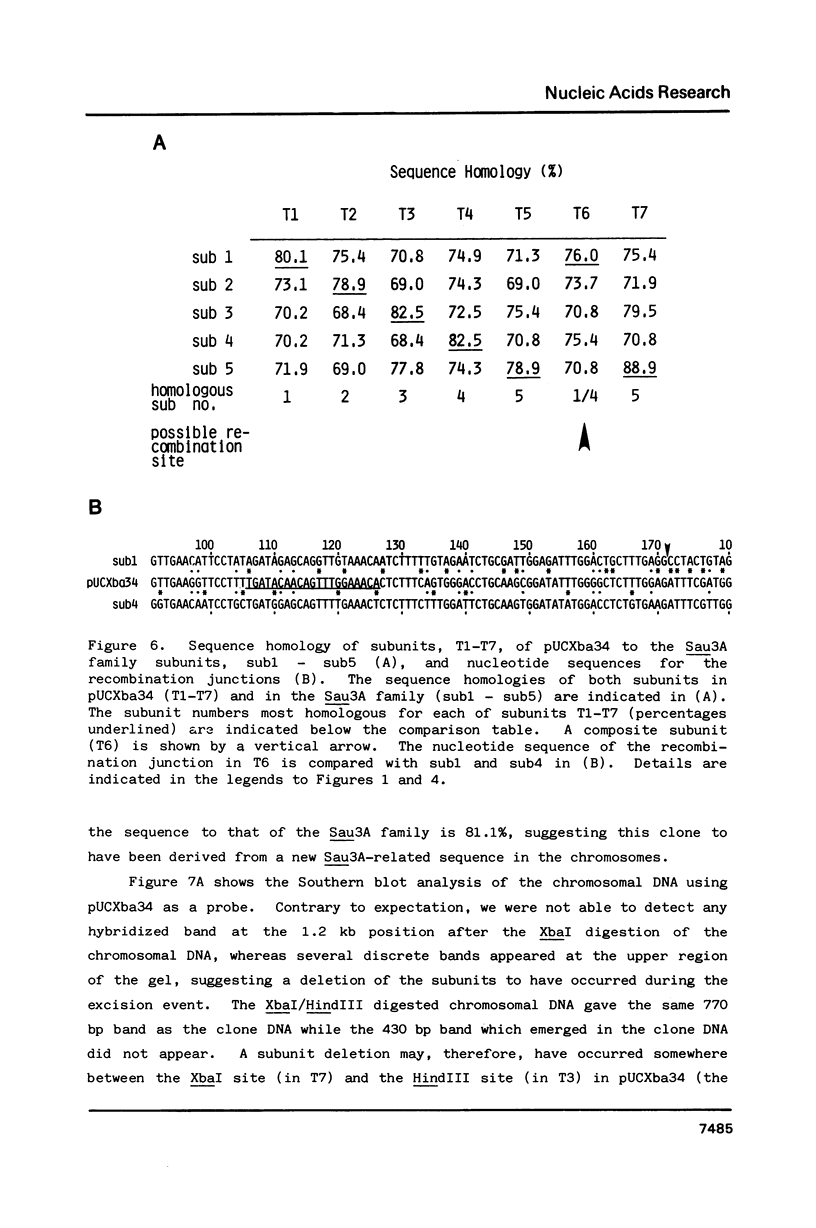
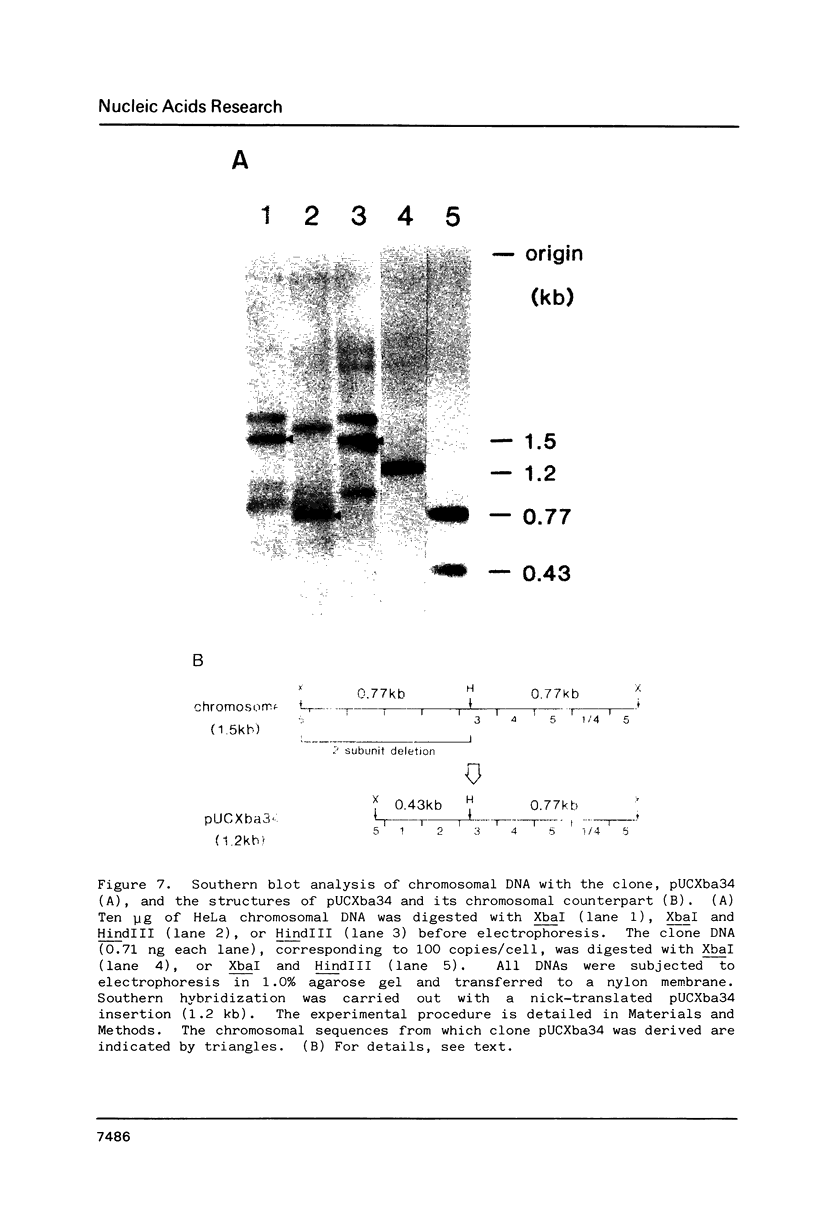
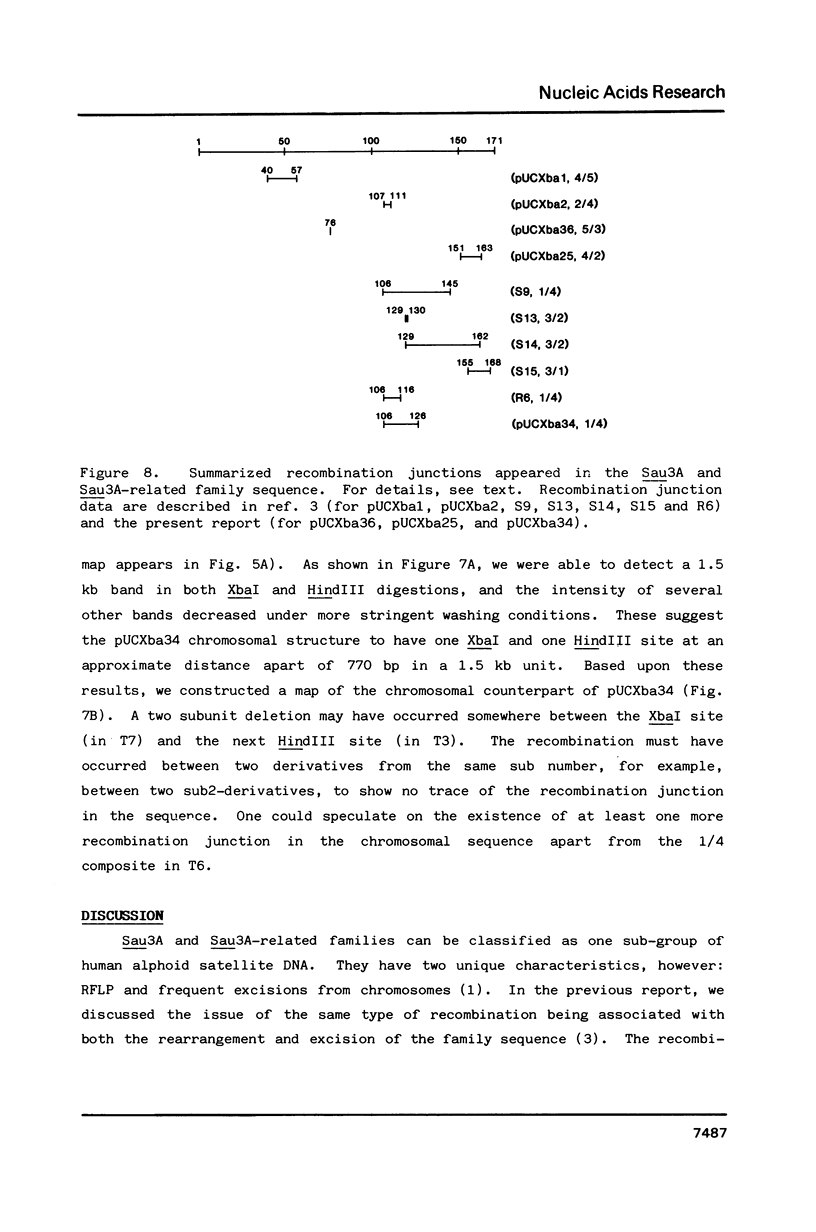
Previously, we reported a recombination-prone human alphoid-like repetitive DNA (Sau3A family) which is characterized by abundance in the extrachromosomal fraction and restriction fragment length polymorphism. We suggested a specific homologous recombination to be responsible for the DNA excision from the chromosomes and also the sequence rearrangement in the chromosomes. In order to investigate the nature of the recombination further, 8 different clones were obtained which hybridized with Sau3A probe among over 1,500 extrachromosomal DNA clones. Restriction mapping and nucleotide sequence analyses showed two to be Sau3A monomers and dimers, four Sau3A recombinants, as observed previously, one a recombinant of the Sau3A-related sequence on chromosome 17, and one a new Sau3A-related sequence. Sequence analyses of the recombination junctions in the recombinant clones indicated a specific homologous recombination also to be responsible for all but one clone. The molecular mechanism and biological significance associated with the recombination are discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Retroviruses and retrotransposons: the role of reverse transcription in shaping the eukaryotic genome. Cell. 1985 Mar;40(3):481–482. doi: 10.1016/0092-8674(85)90190-4. [DOI] [PubMed] [Google Scholar]
- Dunsmuir P., Brorein W. J., Jr, Simon M. A., Rubin G. M. Insertion of the Drosophila transposable element copia generates a 5 base pair duplication. Cell. 1980 Sep;21(2):575–579. doi: 10.1016/0092-8674(80)90495-x. [DOI] [PubMed] [Google Scholar]
- Farabaugh P. J., Fink G. R. Insertion of the eukaryotic transposable element Ty1 creates a 5-base pair duplication. Nature. 1980 Jul 24;286(5771):352–356. doi: 10.1038/286352a0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jones R. S., Potter S. S. L1 sequences in HeLa extrachromosomal circular DNA: evidence for circularization by homologous recombination. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1989–1993. doi: 10.1073/pnas.82.7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyama R., Matsui H., Oishi M. A repetitive DNA family (Sau3A family) in human chromosomes: extrachromosomal DNA and DNA polymorphism. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4665–4669. doi: 10.1073/pnas.83.13.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyama R., Matsui H., Okumura K., Oishi M. A group of repetitive human DNA families that is characterized by extrachromosomal oligomers and restriction-fragment length polymorphism. J Mol Biol. 1987 Feb 20;193(4):591–597. doi: 10.1016/0022-2836(87)90342-1. [DOI] [PubMed] [Google Scholar]
- Riabowol K., Shmookler Reis R. J., Goldstein S. Interspersed repetitive and tandemly repetitive sequences are differentially represented in extrachromosomal covalently closed circular DNA of human diploid fibroblasts. Nucleic Acids Res. 1985 Aug 12;13(15):5563–5584. doi: 10.1093/nar/13.15.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stahl F. W. Special sites in generalized recombination. Annu Rev Genet. 1979;13:7–24. doi: 10.1146/annurev.ge.13.120179.000255. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]