Abstract
Combined radiation and burn injuries are likely to occur after nuclear events, such as a meltdown accident at a nuclear energy plant or a nuclear attack. Little is known about the mechanisms by which combined injuries result in higher mortality than by either insult alone, and few animal models exist for combined radiation and burn injury. Herein, the authors developed a murine model of radiation and scald burn injury. Mice were given a single dose of 0, 2, 4, 5, 6, or 9 Gray (Gy) alone, followed by a 15% TBSA scald burn. All mice receiving ≤4 Gy of radiation with burn survived combined injury. Higher doses of radiation (5, 6, and 9 Gy) followed by scald injury had a dose-dependent increase in mortality (34, 67, and 100%, respectively). Five Gy was determined to be the ideal dose to use in conjunction with burn injury for this model. There was a decrease in circulating white blood cells in burn, irradiated, and combined injury (5 Gy and burn) mice by 48 hours postinjury compared with sham (49.7, 11.6, and 57.3%, respectively). Circulating interleukin-6 and tumor necrosis factor-α were increased in combined injury at 48 hours postinjury compared with all other treatment groups. Prolonged overproduction of proinflammatory cytokines could contribute to subsequent organ damage. Decreased leukocytes might exacerbate immune impairment and susceptibility to infections. Future studies will determine whether there are long lasting consequences of this early proinflammatory response and extended decrease in leukocytes. (J Burn Care Res 2011;32:317–323)
After nuclear accidents or attacks, radiation exposure is often coupled with other forms of injury, such as burns, blunt trauma, and infectious complications. Projected casualty estimates predict that 65 to 70% of affected people will have some kind of traumatic injury in addition to radiation exposure, whereas only 15 to 20% will be affected by radiation alone.1 In accordance with these estimates, reports from the nuclear explosions in Hiroshima and Nagasaki, and the Chernobyl nuclear accident, showed that more than 60% of radiation victims also sustained a traumatic injury of some kind, most often burn injury.1,2 Clinical data from these incidents show that victims of combined injury suffer worse postinjury complications than those patients with a single type of injury. The pathology of combined injuries is extremely complex; individuals who died shortly after these incidents sustained injuries to nearly every organ system, in addition to infectious complications.3 It is known that combined radiation and burn causes aberrant wound healing and severe hematopoietic impairment.4,5 Little is known about the mechanisms by which combined injuries result in higher morbidity and mortality than either injury alone, and few animal models exist for the combined insult of radiation and burn injury.
To examine the mechanisms of tissue and organ damage and failure after this complex combined insult, we developed a mouse model of radiation and burn injury and examined inflammatory mediators and leukocyte distribution in the blood. These experiments should provide valuable information about the mechanisms that might cause increased tissue damage after the combined insult of radiation exposure and thermal burn injury and give insight into potential therapeutic interventions that might diminish the extensive inflammatory response, organ damage, susceptibility to infections, and other complications associated with this unique type of injury.
METHODS
Animals
Eight- to 10-week-old male C57BL/6 mice were obtained from Harlan Laboratories (Indianapolis, IN) and housed with food and water available ad libitum at the Loyola University Medical Center Animal Facility. In this barrier facility, mice were kept under specific pathogen-free conditions. Temperature (70–72°F), humidity (45–55%), and a 12/12-hour light/dark cycle are controlled. Cages are connected to a ventilation system that provides 60 air changes per hour. All animal studies described here were approved and performed with strict accordance to the guidelines established by the Loyola University Institutional Animal Care and Use Committee.
Combined Irradiation and Burn Injury
Mice were subjected to a 0-, 2-, 4-, 5-, 6-, or 9-Gy whole-body dose of ionizing radiation by exposure to a 137Cs source in a Gammacell 40 irradiator (MDS Nordion, Ottawa, ON, Canada). The dose rate of irradiation in the Gammacell was 95 cGy per minute. Sham (0 Gy) irradiation mice were placed into the irradiator for matched amounts of time but without exposure to the source. One hour after radiation injury, mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) intra-peritoneally, and their dorsal surfaces were shaved with animal clippers. Mice were then placed into a plastic template with an opening allowing 15% TBSA on their dorsum to be exposed, as calculated by previously described methods.6 Full-thickness scald injury was achieved by immersing the animals in a 95°C water bath for 7 seconds as described previously.7,8 Immediately after exposure to water, animals were dried to prevent any further scalding. Sham animals were anesthetized, shaved, and immersed in room temperature water. To compensate for fluid loss and prevent circulatory shock, all animals received 1.0 ml of warmed 0.9% normal saline intraperitoneally after the burn injury. Body temperature was maintained by placing the cages on heating pads until animals recovered from anesthesia. Some groups of animals were monitored twice daily for survival, and others were killed at early time points of 6, 24, or 48 hours, or late time points of 14 or 28 days postinjury for analysis of various indices of systemic damage.
Peripheral Blood Differential Counts
Differential blood counts were assessed at different time points after injury. Blood was obtained immediately after killing mice by cardiac puncture. After collection into EDTA tubes, blood was analyzed using a Hemavet veterinary blood analyzer (Drew Scientific, Waterbury, CT).
Analysis of Circulating Cytokines
Blood was obtained by cardiac puncture. Blood was allowed to clot, and serum was obtained after centrifugation for 20 minutes at 3000g at 4°C. Interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and granulocyte-macrophage colony-stimulating factor (GM-CSF) were measured in serum using multiplex bead array assay reagents in an Invitrogen Inflammatory Cytokine Mouse 4-plex Panel according to manufacturer’s instructions (Invitrogen, Carlsbad, CA). A Bio-Plex multiplex analyzer system was used to process and read the samples (BioRad, Hercules, CA). The lowest detectable limits of this assay were 8.06 pg/ml (IL-1β), 10.0 pg/ml (IL-6), 10.0 pg/ml (TNF-α), and 14.92 pg/ml (GM-CSF).
Statistical Analysis
Statistical differences were noted among treatment groups and were conducted using analysis of variance followed with a Tukey-Kramer Multiple Comparisons test. Differences in survival between groups were analyzed using Kaplan-Meier Survival Analysis with a log-rank significance test.
RESULTS
Survival
In our laboratory, we have extensively studied the effects of scald injury using a 15% TBSA scald burn.7,9–11 It is rare that scald burn injury alone causes mortality, but it happens occasionally in our model. No mortality was observed with radiation exposure alone at any of the doses except for 9 Gy, in which 30% of the animals died by day 14 postradiation. However, when combined with subsequent 15% TBSA scald injury, increasing the dose of radiation affected survival. Mice given radiation of 4 Gy and lower with burn all survived the combined injury. In contrast, all animals in the 9 Gy and burn injury group died within 3 days postinjury. Mice given radiation of 5 and 6 Gy also resulted in mortality when combined with burn (30 and 50%, respectively) by day 3 postinjury (Figure 1). In addition, mice given the combined insult (5 Gy and burn) have gone as far as 28 days with 50% mortality (data not shown). The majority of the mortality occurred by day 3 postinjury; therefore, mice that were surviving at day 5 and beyond could be used to study various parameters of remote organ injury at later time points. From this series of experiments, we concluded that 5 Gy with burn injury would yield an effective mouse model of severe combined radiation injury. Hence, all further experiments described herein used 5 Gy of radiation.
Figure 1.
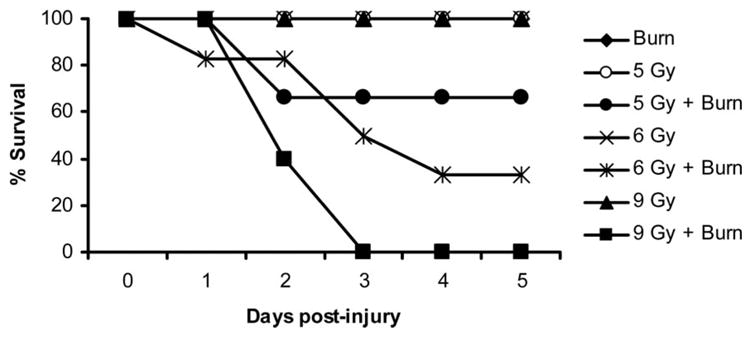
Survival after combined injury. Mice were subjected to a 0-, 2-, 4-, 5-, 6-, or 9-Gy dose of radiation, followed by a 15% TBSA scald burn. Controls were sham irradiated but not burned. Radiation alone was nonlethal at doses of 6 Gy and lower. The addition of radiation to burn injury affected mortality in a dose-responsive manner. Only 5-, 6-, and 9-Gy radiation doses combined with burn injury affected mortality, and all animals that received lower doses of 0 to 4 Gy survived the combined injury. Survival of the 6 Gy + burn group was significantly different compared with all other groups, with the exception of 5 Gy + burn (P < .05). The 9 Gy + burn group was significantly different compared with all other groups (P < .05). Burn alone, 5 Gy, 6 Gy, and 9 Gy symbols overlap because they all had 100% survival through 5 days. This experiment has been repeated twice, with similar results. N = 6 to 12 mice per group.
Leukocyte Distribution in the Blood
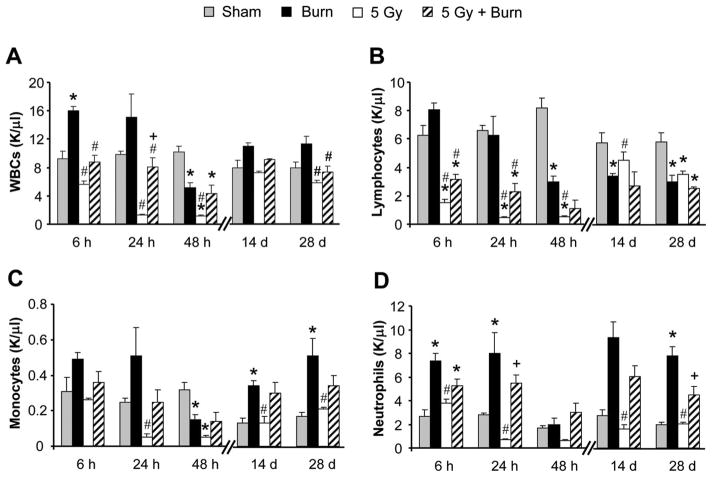
Differential counts were performed to assess the effects of 5 Gy and burn combined injury on circulating white blood cells (WBCs). Total WBCs were reduced over 80% by 24 hours in radiation injury groups when compared with sham. Although counts partially recovered by 28 days postinjury, levels still only reached 73.5% that of sham (Figure 2A, open bars). Burn injury mobilizes leukocytes from the bone marrow and as shown herein yielded an increase in total WBC counts to almost double the levels of sham animals early (6 and 24 hours) after injury. At 48 hours post-burn, burn alone total WBCs decreased 49.7% compared with sham, but later recovered, returning to levels slightly higher than sham by 14 and 28 days postinjury (Figure 2A, black bars). Mice with combined injury had modest WBC decreases at 6 and 24 hours with a 57.3% reduction at 48 hours compared with sham mice (Figure 2A, hatched bars). By 28 days postinjury, leukocyte numbers were comparable with sham.
Figure 2.
Differential counts from peripheral blood. (A) White blood cells, (B) lymphocytes, (C) monocytes, and (D) neutrophils. Blood was collected by cardiac puncture and assayed on a Hemavet veterinary blood analyzer instrument for leukocyte differential counts. Data are shown as the average number of cells for each treatment group in K/μl ± SEM. N + 4 to 8 mice per group. *P < .05 compared with to sham within time point, #P < .05 compared with burn within time point, and #P < .05 compared with 5 Gy within time point.
Lymphocytes seemed to contribute most to the overall decrease in circulating WBCs in combined injury (Figure 2B). Lymphocytes decreased markedly compared with sham in radiation and combined-injured groups 48 hours after injury (93.6 and 86.2%, respectively). By 28 days, lymphocytes in radiation and combined-injured groups only recovered to 60.2 and 43.4% of sham. Monocytes and neutrophils were most affected by radiation injury alone at early time points but recovered to sham levels (or above) by 14 days postirradiation. In contrast, burn alone and combined injury groups were not as dramatically reduced, and at many time points, burn and combined injury monocytes and neutrophils exceeded the values seen in sham animals (Figure 2C, D, black and hatched bars).
Systemic Inflammatory Response
To further elucidate the effects of this dynamic injury, we measured several key proinflammatory cytokines in the circulation. IL-6 was not detectable in sham mice at any time point tested. However, IL-6 increased to approximately 250 pg/ml in serum by 24 hours postinjury in burn only mice, similar to what our laboratory has reported previously.12 At 24 hours postinjury, combined injury serum IL-6 was also increased to almost 200 pg/ml, whereas radiation alone did not cause any IL-6 production (Table 1). Importantly, at 48 hours postinsult, IL-6 was further increased in combined-injured animals to more than 400 pg/ml, while burn injury concentrations returned to sham levels. Circulating IL-6 was still detectable in combined injury animals at 28 days postinjury, averaging 33.6 ± 18.7 pg/ml, but was no longer detected in the other treatment groups (data not shown). In our mouse model, TNF-α was detectable early after injury (6 hours) in both burn and combined injury groups (Table 2). At 48 hours postinjury, TNF-α was only detectable in the combined injury group but was not detected at any later time points (data not shown). IL-1β and GM-CSF were not detected in any group at any time point past injury (data not shown). This prolonged overproduction of proinflammatory cytokines could contribute to permanent tissue damage to vascular organs such as the lung after combined injury.
Table 1.
Circulating IL-6
| Time Postburn (hr) |
|||
|---|---|---|---|
| 6 | 24 | 48 | |
| Sham | 0 | 0 | 0 |
| Burn | 12,626 ± 4052*† | 270 ± 112 | 11 ± 11 |
| 5 Gy | 125 ± 43 | 0 | 17 ± 17 |
| 5 Gy + burn | 10,961 ± 4547*† | 174 ± 75 | 473 ± 8‡ |
Serum was assayed for circulating IL-6 using multiplex bead analysis (Invitrogen) on a BioRad Bio-Plex analyzer. Data are shown as mean IL-6 in pg/ml ± SEM at 6, 24, and 48 hr postinjury. N = 4–6 mice per group.
P < .05 vs sham at 6 hr.
P < .05 vs 5 Gy at 6 hr.
P < .05 vs all other at 48 hr.
IL, interleukin.
Table 2.
Circulating TNF-α
| Time Postburn (hr) |
|||
|---|---|---|---|
| 6 | 24 | 48 | |
| Sham | 0 | 0 | 0 |
| Burn | 34 ± 11*† | 15 ± 9 | 10 ± 10* |
| 5 Gy | 0 | 0 | 0 |
| 5 Gy 3 burn | 46 ± 32‡ | 0 | 34 ± 4*†‡ |
TNF-α was measured in serum using multiplex bead array reagents (Invitrogen) on a BioRad Bio-Plex analyzer. Data are shown as mean TNF-α in pg/ml ± SEM at 6, 24, and 48 hr postinjury. N = 4–6 mice per group.
P < .05 vs sham within time point.
P < .05 vs 5 Gy within time point.
P < .05 vs burn within time point.
TNF, tumor necrosis factor.
DISCUSSION
To further investigate the effects of combined injury, we developed a murine model of radiation injury and scald burn. In this study, there was a dose-dependent increase in mortality in animals that received combined injury. Clearly, higher levels of radiation exposure further impaired the ability of the animal to survive a burn injury. Although limited data exist from animal models, several other studies also demonstrate that traumatic burn injuries increase mortality from irradiation. A canine study showed that a nonlethal thermal burn injury coupled with 1 Gy of radiation exposure increased mortality to 75%, whereas mortality from radiation alone was only 12%.13 Similarly, a rat model of 31 to 35% TBSA burn with a sublethal dose of 2.5 Gy increased mortality to almost 100%, whereas mortality from the burn injury alone was 50%.14 Other types of injuries also exacerbate mortality from radiation in mice. Kiang et al15 found that excisional wound trauma increased radiation-induced mortality. The data presented herein suggest that the dose of 5 Gy combined with a 15% TBSA scald injury will yield an effective mouse model that is severe enough to cause some mortality, yet leaves enough animals to further study the inflammatory and immune effects of combined injury.
Depression in peripheral leukocyte counts was found to correlate highly with mortality after the atomic bombings of Japan.16 Many patients died after the Chernobyl accident during a period of profound leukopenia between 14 and 34 days after exposure.17 Because ionizing radiation kills hematopoietic stem and progenitor cells, we first examined differential blood counts in mice at various time points after injury to determine changes in leukocyte distribution in circulation in our model. Because total WBCs only recovered to approximately 80% of sham levels by 28 days postinjury, further time points postradiation exposure need to be examined to determine if and when WBC counts return to normal levels. Garg et al18 reported a similar pattern of diminished leukocyte counts after total body irradiation of CD2F1 mice, in which WBC counts had significant reductions (>75% decrease) within 4 hours of radiation exposure, with only a modest recovery by 30 days. Although total leukocytes were comparable with sham by 28 days postinjury in our model, lymphocytes only recovered to approximately 40% of normal after combined injury. Others have described similar trends in lymphocyte decreases after radiation injury in CD2F1 mice, which only recovered to approximately 25% of normal by 28 days.18,19 This persistent depression of lymphocyte numbers could lead to significant immune impairment and increased susceptibility to infection after combined injury. The effects of total body irradiation on WBC counts in our model are further confounded by the burn injury, as burn injury lymphocyte counts also remained depressed at 28 days postburn.
Several studies have described the overwhelming inflammatory response resulting from burn injury in animals and humans.20–23 The increased production of TNF-α, IL-1β, and IL-6 all contribute to the aberrant immunologic response experienced after burn injury. Our laboratory has reported increased circulating IL-6 after the combined insult of ethanol exposure and burn injury. Serum IL-6 concentrations were higher in mice that received ethanol and burn compared with either insult alone at 24 and 48 hours postinsult.10,24,25 However, the inflammatory and immunologic changes that occur after combined radiation injury are not nearly as well understood. After burn injury in mice and rats, TNF-α and IL-6 levels increase quickly within 12 hours and then subside by 48 hours.26,27 Here, we found drastically increased serum IL-6 early (6 hours) in both burn and combined injury. At 48 hours postinjury, IL-6 remained increased in the combined injured animals and was virtually nonexistent in the other treatment groups, suggesting a synergistic effect of burn and irradiation on increased circulating levels of proinflammatory cytokines. Serum TNF-α had a modest increase within 6 hours postinjury in both burn and combined injured groups. TNF-α was not detected at 24 hours in combined injury. This was not surprising, as previous data from our burn injury model have shown serum TNF-α to peak within 90 to 120 minutes of injury, and by 24 hours, concentrations are similar to sham.26,28 Interestingly, in our combined injury model, TNF-α was increased compared with all other treatment groups at 48 hours. It is possible that there may be another burst of TNF-α later in surviving cells, as additional bone marrow-derived cells enter circulation. Similarly, Ran et al4 reported significantly increased serum concentrations of IL-6 and TNF-α in rats 24 hours after 12 Gy irradiation followed with a 30% TBSA burn. Excisional dermal wounds coupled with radiation also increased proinflammatory cytokines in mice. Serum IL-6 was increased in both wounded and combined injured animals (9.75 Gy with wounding) at 48 hours, but by 7 days postinjury, combined injury IL-6 concentrations further increased, whereas wound only returned to sham levels.15 In our experiments, IL-6 was still detectable in the circulation of combined injury mice at 28 days postinsult, suggesting a prolonged inflammatory response that could be detrimental over time.29 In our model, circulating IL-1β was not detected after injury, so in this case, it is probably not playing much of a role in the exaggerated early proinflammatory response. However, others have found increased IL-1β after higher doses of radiation exposure in their models and implicated it in the early immune dysfunction observed after combined injury.15,30,31 There is also evidence that IL-1β can have protective effects if administered before radiation exposure.32,33 GM-CSF was also unchanged by the combined injury in our studies, which is similar to results from another mouse model in which there was no increase in GM-CSF in peripheral blood after combined radiation and thermal burn.34 The consequences of the systemic inflammatory response on other tissues and organ systems will need to be investigated further. Additional cytokines and chemokines will also be measured in future studies to expand on these findings and further characterize this murine combined injury model.
Taken together, these data highlight the complexity of severe combined injuries. More investigation needs to be done to begin to understand how the collective effects of each injury synergize to increase morbidity and mortality. At this time, we can only speculate about the cause of mortality of the mice in our model. It is generally accepted that death from radiation injury itself usually occurs as a result of injury to the bone marrow or gastrointestinal tract because both contain populations of rapidly proliferating progenitor cells.35–37 Herein, most of the mice succumb to their injuries between days 2 and 3 postinjury, which is likely too early for gastrointestinal effects to be responsible. The combination of severe leukopenia and increased circulating proinflammatory cytokines within 48 hours of combined injury could overwhelm multiple organ systems in the animals and contribute to mortality in this case.
Future studies will focus on the enhanced degree of inflammation and resulting organ damage (such as lung, ileum, and skin) observed in patients with combined injury using this mouse model. These experiments should provide valuable information about the mechanisms responsible for the extensive inflammatory response after combined insult and potential therapeutic interventions that might diminish the subsequent tissue damage associated with this unique type of injury.
Acknowledgments
Supported by the National Institutes of Health R21 AI080528 (to E.J.K.), U19 AI67798 (to M.H.-J.), Illinois Excellence in Academic Medicine Grant (to E.J.K.), and Ralph and Marian C. Falk Research Trust (to E.J.K.).
We thank Dr. Pamela L. Witte for critical review of the manuscript; Dr. Richard H. Kennedy for thoughtful discussions on this project; and Bart Posnik and Luis Ramirez for technical assistance with the burn injury protocol.
References
- 1.Pellmar TC, Ledney GD. Combined injury: radiation in combination with trauma, infectious disease, or chemical exposures. NATO RTG. 2005;099:1–9. [Google Scholar]
- 2.Johnson AM. Pulmonary effects of combined the blast injury and radiation poisoning. J R Army Med Corps. 2004;150(3 Suppl 1):22– 6. [PubMed] [Google Scholar]
- 3.Manthous CA, Jackson WL., Jr The 9–11 Commission’s invitation to imagine: a pathophysiology-based approach to critical care of nuclear explosion victims. Crit Care Med. 2007;35:716–23. doi: 10.1097/01.CCM.0000257328.31668.22. [DOI] [PubMed] [Google Scholar]
- 4.Ran XZ, Su YP, Zong ZW, et al. Effects of serum from rats with combined radiation-burn injury on the growth of hematopoietic progenitor cells. J Trauma. 2007;62:193–8. doi: 10.1097/01.ta.0000215434.24726.72. [DOI] [PubMed] [Google Scholar]
- 5.Ran X, Cheng T, Shi C, et al. The effects of total-body irradiation on the survival and skin wound healing of rats with combined radiation-wound injury. J Trauma. 2004;57:1087–93. doi: 10.1097/01.ta.0000141885.72033.c7. [DOI] [PubMed] [Google Scholar]
- 6.Spector W. Handbook of biological data. Philadelphia, PA: Saunders; 1956. [Google Scholar]
- 7.Faunce DE, Gregory MS, Kovacs EJ. Effects of acute ethanol exposure on cellular immune responses in a murine model of thermal injury. J Leukoc Biol. 1997;62:733–40. doi: 10.1002/jlb.62.6.733. [DOI] [PubMed] [Google Scholar]
- 8.Walker HL, Mason AD., Jr A standard animal burn. J Trauma. 1968;8:1049–51. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Messingham KA, Faunce DE, Kovacs EJ. Alcohol, injury, and cellular immunity. Alcohol. 2002;28:137–49. doi: 10.1016/s0741-8329(02)00278-1. [DOI] [PubMed] [Google Scholar]
- 10.Messingham KA, Fontanilla CV, Colantoni A, Duffner LA, Kovacs EJ. Cellular immunity after ethanol exposure and burn injury: dose and time dependence. Alcohol. 2000;22:35–44. doi: 10.1016/s0741-8329(00)00100-2. [DOI] [PubMed] [Google Scholar]
- 11.Murdoch EL, Brown HG, Gamelli RL, Kovacs EJ. Effects of ethanol on pulmonary inflammation in postburn intratracheal infection. J Burn Care Res. 2008;29:323–30. doi: 10.1097/BCR.0b013e3181667599. [DOI] [PubMed] [Google Scholar]
- 12.Faunce DE, Gregory MS, Kovacs EJ. Glucocorticoids protect against suppression of T cell responses in a murine model of acute ethanol exposure and thermal injury by regulating IL-6. J Leukoc Biol. 1998;64:724–32. doi: 10.1002/jlb.64.6.724. [DOI] [PubMed] [Google Scholar]
- 13.Brooks JW, Evans EI, Ham WT, Jr, Reid JD. The influence of external body radiation on mortality from thermal burns. Ann Surg. 1952;136:533–45. doi: 10.1097/00000658-195209000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alpen EL, Sheline GE. The combined effects of thermal burns and whole body X irradiation on survival time and mortality. Ann Surg. 1954;140:113–8. doi: 10.1097/00000658-195407000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiang JG, Jiao W, Cary LH, et al. Wound trauma increases radiation-induced mortality by activation of iNOS pathway and elevation of cytokine concentrations and bacterial infection. Radiat Res. 2010;173:319–32. doi: 10.1667/RR1892.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs GJ, Lynch FX, Cronkite EP, Bond VP. Human radiation injury—a correlation of leukocyte depression with mortality in the Japanese exposed to the atomic bombs. Mil Med. 1963;128:732–9. [PubMed] [Google Scholar]
- 17.DiCarlo AL, Hatchett RJ, Kaminski JM, et al. Medical counter-measures for radiation combined injury: radiation with burn, blast, trauma and/or sepsis. Report of an NIAID Workshop, March 26–27, 2007. Radiat Res. 2008;169:712–21. doi: 10.1667/RR1295.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg S, Boerma M, Wang J, et al. Influence of sublethal total-body irradiation on immune cell populations in the intestinal mucosa. Radiat Res. 2010;173:469–78. doi: 10.1667/RR1742.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu Q, Berbée M, Boerma M, Wang J, Schmid HA, Hauer-Jensen M. The somatostatin analog SOM230 (pasireotide) ameliorates injury of the intestinal mucosa and increases survival after total-body irradiation by inhibiting exocrine pancreatic secretion. Radiat Res. 2009;171:698–707. doi: 10.1667/RR1685.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drost AC, Burleson DG, Cioffi WG, Jr, Jordan BS, Mason AD, Jr, Pruitt BA., Jr Plasma cytokines following thermal injury and their relationship with patient mortality, burn size, and time postburn. J Trauma. 1993;35:335–9. doi: 10.1097/00005373-199309000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Kowal-Vern A, Walenga JM, Hoppensteadt D, Sharp-Pucci M, Gamelli RL. Interleukin-2 and interleukin-6 in relation to burn wound size in the acute phase of thermal injury. J Am Coll Surg. 1994;178:357–62. [PubMed] [Google Scholar]
- 22.Mester M, Carter EA, Tompkins RG, et al. Thermal injury induces very early production of interleukin-1 alpha in the rat by mechanisms other than endotoxemia. Surgery. 1994;115:588–96. [PubMed] [Google Scholar]
- 23.Ogle CK, Mao JX, Wu JZ, Ogle JD, Alexander JW. The production of tumor necrosis factor, interleukin-1, interleukin-6, and prostaglandin E2 by isolated enterocytes and gut macrophages: effect of lipopolysaccharide and thermal injury. J Burn Care Rehabil. 1994;15:470–7. [PubMed] [Google Scholar]
- 24.Faunce DE, Gregory MS, Kovacs EJ. Acute ethanol exposure prior to thermal injury results in decreased T-cell responses mediated in part by increased production of IL-6. Shock. 1998;10:135–40. doi: 10.1097/00024382-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Fontanilla CV, Faunce DE, Gregory MS, et al. Anti-interleukin-6 antibody treatment restores cell-mediated immune function in mice with acute ethanol exposure before burn trauma. Alcohol Clin Exp Res. 2000;24:1392–9. [PubMed] [Google Scholar]
- 26.Plackett TP, Colantoni A, Heinrich SA, Messingham KA, Gamelli RL, Kovacs EJ. The early acute phase response after burn injury in mice. J Burn Care Res. 2007;28:167–72. doi: 10.1097/BCR.0b013E31802CB84F. [DOI] [PubMed] [Google Scholar]
- 27.Kataranovski M, Magić Z, Pejnović N. Early inflammatory cytokine and acute phase protein response under the stress of thermal injury in rats. Physiol Res. 1999;48:473–82. [PubMed] [Google Scholar]
- 28.Heinrich SA, Messingham KA, Gregory MS, et al. Elevated monocyte chemoattractant protein-1 levels following thermal injury precede monocyte recruitment to the wound site and are controlled, in part, by tumor necrosis factor-alpha. Wound Repair Regen. 2003;11:110–9. doi: 10.1046/j.1524-475x.2003.11206.x. [DOI] [PubMed] [Google Scholar]
- 29.Biffl WL, Moore EE, Moore FA, Peterson VM. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg. 1996;224:647–64. doi: 10.1097/00000658-199611000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W, Ding I, Chen K, et al. Interleukin 1beta (IL1B) signaling is a critical component of radiation-induced skin fibrosis. Radiat Res. 2006;165:181–91. doi: 10.1667/rr3478.1. [DOI] [PubMed] [Google Scholar]
- 31.Okunieff P, Xu J, Hu D, et al. Curcumin protects against radiation-induced acute and chronic cutaneous toxicity in mice and decreases mRNA expression of inflammatory and fibrogenic cytokines. Int J Radiat Oncol Biol Phys. 2006;65:890–8. doi: 10.1016/j.ijrobp.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 32.Neta R, Douches S, Oppenheim JJ. Interleukin 1 is a radio-protector. J Immunol. 1986;136:2483–5. [PubMed] [Google Scholar]
- 33.Neta R, Oppenheim JJ. Radioprotection with cytokines—learning from nature to cope with radiation damage. Cancer Cells. 1991;3:391–6. [PubMed] [Google Scholar]
- 34.Budagov RS, Ul’ianova LP. Role of interleukin-6 (IL-6) in the pathogenesis of combined radiation/thermal injuries. Radiats Biol Radioecol. 2004;44:398–402. [PubMed] [Google Scholar]
- 35.Rotolo JA, Kolesnick R, Fuks Z. Timing of lethality from gastrointestinal syndrome in mice revisited. Int J Radiat Oncol Biol Phys. 2009;73:6–8. doi: 10.1016/j.ijrobp.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Hauer-Jensen M, Kumar KS, Wang J, Berbee M, Fu Q, Boerma M. Intestinal toxicity in radiation- and combined injury: significance, mechanisms, and countermeasures. Hauppauge, NY: Nova Science Publishers; 2008. [Google Scholar]
- 37.Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–7. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]