Abstract
In rodents, exposure to acute inescapable, but not escapable, stress potentiates morphine conditioned place preference (CPP), an effect that is dependent upon hyperactivation of serotonin (5-HT) neurons in the dorsal raphe nucleus (DRN). Six weeks of voluntary wheel running constrains activation of DRN 5-HT neurons during exposure to inescapable stress. Six weeks of voluntary wheel running before inescapable stress blocked stress-induced potentiation of morphine CPP.
Keywords: exercise, stress, morphine, addiction, serotonin, learned helplessness
Addiction is a behavioral outcome that develops across age, sex, and culture. It is often defined as a condition in which an individual is unable to control their drug intake despite detrimental social and occupational consequences [1]. A large body of research has explored genetic [2] and environmental [3] factors that lead to the development of addiction. Perhaps the most extensively investigated environmental factor that predisposes an organism to increase drug administration is stress. Stressor exposure has been shown to exaggerate drug taking, drug reward, and drug reinstatement using a variety of paradigms [4]. In our own laboratory, uncontrollable stress in the form of inescapable shock (IS), not only decreases social exploration [5], exaggerates fear conditioning [6], and produces failure to escape shock in a shuttlebox [7], but also potentiates morphine conditioned place preference (CPP) [8]. Importantly, potentiated morphine CPP does not occur if the shock is escapable, and so the exaggeration of morphine reward is specific to uncontrollable stress.
The mechanisms involved in IS-potentiation of morphine CPP have been extensively studied. Notably, IS potently activates serotonin (5-HT) neurons in the dorsal raphe nucleus (DRN), rendering them sensitized for a number of days after stressor exposure [9]. This sensitization is thought to occur by a functional desensitization of 5-HT autoreceptors in the DRN [10]. During this period of DRN sensitization, drugs of abuse that stimulate 5-HT release from the DRN, such as morphine [11, 12], potentiate drug reward by activating the sensitized DRN [13]. This is likely because exaggerated 5-HT release at DRN projection sites potentiates drug-induced dopamine release [14].
The work reviewed above suggests a number of manipulations that could block IS-induced potentiation of CPP. Because IS sensitizes 5-HT neurons in the DRN, and sensitization of DRN 5-HT neurons is necessary for potentiation of morphine CPP [13], a manipulation that prevents sensitization of the DRN should block IS-induced effects. For example, 6 weeks of voluntary exercise (provision of a running wheel) increases the expression of 5-HT1A autoreceptors in the DRN [15, 16], an effect that would reduce the ability of IS to activate or desensitize DRN 5-HT neurons. As would be expected, 6 weeks of voluntary exercise before IS exposure prevented the exaggerated fear and failure to escape in a shuttlebox [15] that follows IS. Whether 6 weeks of exercise would block IS-induced potentiation of morphine CPP has not been investigated. If potentiation of morphine CPP depends on a sensitized DRN, then 6 weeks of wheel running prior to IS should block potentiation of morphine CPP. The present experiment tests this possibility.
Rats weighing (320–350 g) (Harlan Laboratories, Indianapolis, IN, USA) were singly housed with rat chow (Harlan) and water available ad libitum in a temperature controlled colony room with a 12:12-hr light/dark cycle (lights on at 06:00 and off at 18:00). All cages contained one running wheel that remained locked during a one week acclimation period. Following acclimation, running wheels were unlocked for half of the subjects and the running distances recorded daily for six weeks. All procedures were approved by the University of Colorado Institutional Animal Care and Use Committee.
Following six weeks of wheel running or locked wheels, rats began a five day CPP procedure. The apparatus used for CPP was a black Plexiglas box measuring 72 cm × 30 cm × 30 cm (length, width, height) with 3 compartments differing in visual and tactile features. The conditioning compartments measured 30 cm × 30 cm × 30 cm. One conditioning compartment had alternating horizontal black and white stripes 2 cm wide with a 2 cm wire grid on the floor and the other conditioning compartment contained similar black and white stripes alternating vertically and a 3 mm wire mesh grid on the floor. A third, neutral, non-conditioning compartment measured 12 cm × 30 cm × 30 cm and was located between the two conditioning compartments. The neutral area was painted gray and had no tactile cues. During conditioning sessions, Plexiglas partitions were placed in the apparatus to confine the rat to one compartment. Activity was monitored with a Philips TC352A (Lancaster, PA, USA) video camera that relayed information of the rat’s location to Chromotrack version 4.02 (Prototype Systems Ltd., Boulder, CO, USA) tracking software. A SA-3 Tracker (San Diego Instruments, San Diego, CA, USA) was used to track the time spent and locomotor behavior of the rats in each of the three compartments. One day prior to exposure to the CPP apparatus, rats were handled and fitted loosely with a collar (BAS, West Lafayette, IN, USA) around the neck. Affixed to the collar was a piece of reflective tape to augment video tracking.
Day 1 of CPP was a 20 min pre-exposure to the apparatus to test bias for the conditioning compartments. Rats that spent less than 4 min (20%) in a conditioning environment were eliminated due to strong bias. Two rats were eliminated due to bias. On Day 2, rats received either IS or homecage (HC) control treatment. IS was administered in a distinctly different environment than the CPP environment. Rats were placed in a clear Plexiglas tube measuring 17 cm (length) × 7 cm (diameter) with several air holes. Tails were taped to a Plexiglas rod extending from the rear of the tube and two copper strips augmented with electrode paste were affixed to the midsection of the tail. Shocks were delivered using a Precision-Regulated Animal Shocker with Graphic State 3.0 software (Coulbourn Instruments, Whitehall, PA, USA). The IS session consisted of 100, 5 sec shocks at 1.0 mA, with an average 60 sec inter-trial-interval. Following IS, rats were immediately taken to their homecages. Prior studies indicated that restraint without shock does not potentiate morphine CPP [8], so HC rats were simply left in their cages on Day 2.
On Day 3, rats were weighed and assigned a conditioning environment using a counterbalanced conditioning procedure. Two partitions were inserted in the open sides of the neutral compartment to confine the rats to a single environment during conditioning. Rats received two 45 min conditioning sessions on Day 3, one session with morphine and the other session with saline. In the morning, half of the rats were conditioned with 3 mg/kg subcutaneous morphine sulfate (NIDA) and were subsequently placed in the morphine-conditioned environment. The other half of the subjects were injected with equivolume subcutaneous saline and were immediately placed in the saline-conditioned environment. During the afternoon conditioning session, injections were alternated so rats that received saline in the morning were now conditioned with morphine and rats that were conditioned with morphine in the morning now received saline. Day 4 conditioning was similar to Day 3 except the order that morphine and saline were injected on Day 3 was reversed. The 3 mg/kg dose of morphine was chosen based on previous experiments demonstrating IS-potentiation of CPP at this dose [8]. On Day 5, rats were allowed to explore the entire apparatus for 20 min in a drug-free state and time spent and locomotor activity was recorded for each compartment. Thus, this study used a 2 × 2 experimental design with exercise as one factor and IS as the other factor. A total of 29 rats were used in this study, the wheel running group contained 8 HC and 7 IS subjects while the sedentary group contain 6 IS and 8 HC subjects.
The dependent measure used for preference was the difference in time spent in the conditioning environment before and after drug conditioning. Therefore, a positive value indicates increased preference for the environment after morphine conditioning. Locomotor behavior in the CPP apparatus is expressed as a difference of distance traveled before and after conditioning. The difference in distance traveled before and after conditioning accounts for any effect morphine conditioning and IS exposure may have had on subsequent locomotor behavior. Therefore, a negative value represents decreased locomotor activity following morphine conditioning.
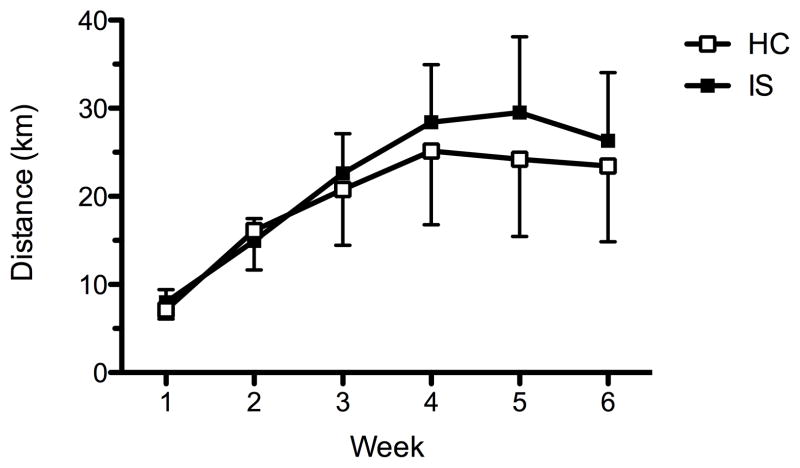
Figure 1 shows the mean weekly distance run for each group (Fig. 1). Running increased across time (F(5, 84) = 10.48; p < 0.0001). There were no significant effects of stress (F(1, 88) = 0.07; p > 0.05) or interaction of stress and time (F(5, 84) = 0.22; p > 0.05). Thus, rats in the HC and IS wheel running groups ran equal amounts during the 6 weeks prior to stressor exposure and morphine conditioning.
Figure 1.
Distance ran during 6 weeks of voluntary wheel running. Each data point represents mean (± SEM) number of kilometers ran for each stress group. No statistical differences (p > 0.05) were found between inescapable stress (IS) and homecage (HC) controls.
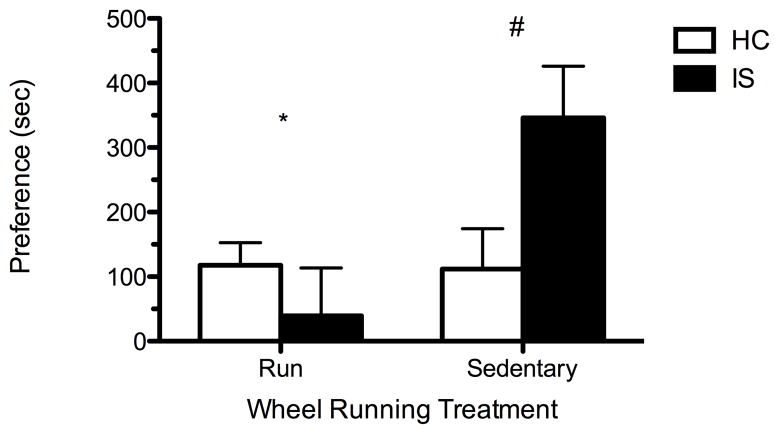
As in prior work, IS potentiated morphine CPP as compared to HC in sedentary rats (Fig. 2). The new finding is that 6 weeks of wheel running prior to stressor exposure blocked IS-induced potentiation of morphine CPP. ANOVA revealed a significant effect of exercise (F(1, 27) = 5.68, p < 0.05) and a significant interaction between stress and exercise (F(1, 27) = 6.14; p < 0.05). However, no significant main effect of stress was observed (F(1, 27) = 1.53; p > 0.05). A subsequent Fisher’s PLSD post hoc analysis (α= 0.05) confirmed a significant difference between IS-sedentary and –wheel running rats. Additionally, Fisher’s PLSD post hoc analysis revealed a significant difference between sedentary IS and HC controls, as is typically observed [17]. An additional two-way ANOVA was conducted to determine if stress or wheel running significantly altered locomotor activity during the test of preference on Day 5, which could produce an apparent decrease of preference. No effect of wheel running (F(1, 27) = 0.00; p > 0.05), stress (F(1, 27) = 3.99; p > 0.05), or interaction of wheel running and stress (F(1, 27) = 0.53; p > 0.05) were observed.
Figure 2.
Six weeks of voluntary wheel running blocks potentiation of morphine conditioned place preference by inescapable stress (IS). Preference is calculated by taking the difference of time, in seconds (sec), spent in the conditioning environment before and after morphine conditioning. Preference is expressed as a group mean (+SEM). # Fisher’s PLDS post hoc revealed a significant difference of preference between sedentary IS and homecage (HC) rats and a significant difference of preference between run and sedentary treatments in IS rats (p < 0.05).
Two correlational analyses were conducted. The first was designed to examine whether actual distance run predicts the degree of blockade of IS-potentiated CPP. A simple regression analysis revealed no significant correlation between total distance run after 6 weeks of running wheel access and morphine preference (r = −0.23; p > 0.05). Additionally, a possible correlation between locomotor activity in the CPP apparatus and morphine preference was assessed. No significant correlation was found between locomotor activity in the CPP apparatus and morphine preference (r = −0.28; p > 0.05).
The present results indicate that 6 weeks of voluntary wheel running can block stress-induced increases in drug reward. As is typical, morphine CPP in HC controls was modest given the use of a low morphine dosage and only 2 conditioning sessions [17]. Exercise had no effect on morphine CPP per se in HC, consistent with a prior report investigating 3 weeks of voluntary wheel running and morphine CPP [18]. However, a single session of IS potentiated morphine CPP in sedentary rats. Interestingly, a comparison between sedentary and running IS rats revealed that morphine CPP was only potentiated in sedentary rats. It is to be noted that the sedentary subjects were provided with a (locked) running wheel, and so the stress-buffering effect of wheel running is likely specific to exercise and not any environmental enrichment provided by the presence of a wheel.
Potentiation of morphine CPP following IS is thought to occur in two phases, an induction and an expression phase. The induction phase is during exposure to IS. During this phase DRN 5-HT neurons become hyperactivated as indicated by levels of extracellular 5-HT in the DRN [7] and its projection regions [19] and double-labeling of the immediate early gene c-Fos and 5-HT [20]. This large activation of the DRN is thought to produce a downregulation or desensitization of inhibitory 5-HT1A autoreceptors. Indeed, previous reports have demonstrated that increases in extracellular 5-HT desensitizes 5-HT1A autoreceptors [21, 22]. Since agonism of somatodendritic 5-HT1A receptors in the DRN reliably inhibits 5-HT cell firing [23] functional desensitization of 5-HT1A autoreceptors [10] would sensitize DRN 5-HT cells. Following the induction phase, a behavioral or pharmacological manipulation that activates the DRN, such as morphine [11, 12], would now produce exaggerated 5-HT efflux in projection regions of the DRN [9], including regions that are involved in mediated drug reward [24]. This is the expression phase. Sensitized 5-HT release during the expression phase is necessary to observe the behavioral effects of IS [13, 25]. If the DRN is not activated during the induction or expression phases, the behavioral and neurochemical consequences of IS are not observed [5, 25, 26].
The stress-buffering effect of wheel running is thought to occur by interfering with the induction phase of IS. As Greenwood et al. (2003) previously demonstrated, 6 weeks of wheel running produces an increase in 5-HT1A autoreceptor mRNA in the DRN [15]. An increased expression of 5-HT1A autoreceptors would predictably provide increased negative feedback on 5-HT cell activation. As would be expected, 6 weeks of wheel running prior to IS blocks IS-induced activation of the DRN as measured by double-labeling of c-Fos and 5-HT [27]. Consequently, in the present experiment, morphine would not be acting on a sensitized DRN because prior wheel running has prevented the induction phase critical to the expression of IS behaviors.
Exercise has been shown to increase periods of abstinence [28] and decrease drug intake [29] in drug dependent patients. The mechanisms by which exercise may have a beneficial role in mitigating drug relapse and reward is not fully understood. However, it is believed that exercise reduces desire or craving to take a drug rather than providing a cognitive distraction [30]. Drug craving has been linked to 5-HT activity in the medial prefrontal cortex as antagonism of 5-HT receptors here blocks cue-induced cocaine seeking [31]. Perhaps, exercise may have its beneficial effects on reducing drug intake by dampening serotonergic activity necessary for drug seeking, as is suggested in the present experiments.
The present data suggest a potential role for exercise as a stress-resistance or resilience factor. Although 6 weeks of prior wheel running prevents several behavioral outcomes following IS, the functional core circuit involved in all of the IS-behaviors is the sensitized DRN. The mechanism by which wheel running prevents IS-induced behaviors could be enhanced negative feedback of serotonergic cell firing in the DRN. For this reason, a general stress-buffering effect of exercise should be interpreted with caution. Further exploration of additional alterations of receptor expression across brain regions may provide novel hypotheses for exercise-induced resistance to other stressors that increase drug reward. Additional experiments exploring whether exercise can block other drug reward behaviors such as locomotor sensitization and drug self-administration are also warranted.
Acknowledgments
This research was supported by National Institutes of Health grants DA023329 (RRR), MH068283(MF), and MH050479 (SFM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Le Moal M, Koob GF. Drug addiction: pathways to the disease and pathophysiological perspectives. Eur Neuropsychopharmacol. 2007;17:377–93. doi: 10.1016/j.euroneuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Yuferov V, Levran O, Proudnikov D, Nielsen DA, Kreek MJ. Search for genetic markers and functional variants involved in the development of opiate and cocaine addiction and treatment. Ann N Y Acad Sci. 2010;1187:184–207. doi: 10.1111/j.1749-6632.2009.05275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uhart M, Wand GS. Stress, alcohol and drug interaction: an update of human research. Addict Biol. 2009;14:43–64. doi: 10.1111/j.1369-1600.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–91. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 5.Christianson JP, Paul ED, Irani M, Thompson BM, Kubala KH, Yirmiya R, et al. The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behav Brain Res. 2008;193:87–93. doi: 10.1016/j.bbr.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, et al. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience. 2007;146:1495–503. doi: 10.1016/j.neuroscience.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–71. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 8.Will MJ, Watkins LR, Maier SF. Uncontrollable stress potentiates morphine’s rewarding properties. Pharmacol Biochem Behav. 1998;60:655–64. doi: 10.1016/s0091-3057(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 9.Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998;812:113–20. doi: 10.1016/s0006-8993(98)00960-3. [DOI] [PubMed] [Google Scholar]
- 10.Rozeske RR, Evans AK, Watkins LR, Lowry CA, Maier SF. Serotonin differentially inhibits in vitro neuronal firing rates in the dorsal raphe nucleus following escapable and inescapable stress. Poster presentation at Society for Neuroscience; Chicago, USA. 2009. [Google Scholar]
- 11.Tao R, Auerbach SB. GABAergic and glutamatergic afferents in the dorsal raphe nucleus mediate morphine-induced increases in serotonin efflux in the rat central nervous system. J Pharmacol Exp Ther. 2002;303:704–10. doi: 10.1124/jpet.102.038133. [DOI] [PubMed] [Google Scholar]
- 12.Tao R, Auerbach SB. Opioid receptor subtypes differentially modulate serotonin efflux in the rat central nervous system. J Pharmacol Exp Ther. 2002;303:549–56. doi: 10.1124/jpet.102.037861. [DOI] [PubMed] [Google Scholar]
- 13.Will MJ, Der-Avakian A, Bland ST, Grahn RE, Hammack SE, Sparks PD, et al. Electrolytic lesions and pharmacological inhibition of the dorsal raphe nucleus prevent stressor potentiation of morphine conditioned place preference in rats. Psychopharmacology (Berl) 2004;171:191–8. doi: 10.1007/s00213-003-1572-1. [DOI] [PubMed] [Google Scholar]
- 14.Bland ST, Schmid MJ, Watkins LR, Maier SF. Prefrontal cortex serotonin, stress, and morphine-induced nucleus accumbens dopamine. Neuroreport. 2004;15:2637–41. doi: 10.1097/00001756-200412030-00016. [DOI] [PubMed] [Google Scholar]
- 15.Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, et al. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–98. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenwood BN, Foley TE, Day HE, Burhans D, Brooks L, Campeau S, et al. Wheel running alters serotonin (5-HT) transporter, 5-HT1A, 5-HT1B, and alpha 1b-adrenergic receptor mRNA in the rat raphe nuclei. Biol Psychiatry. 2005;57:559–68. doi: 10.1016/j.biopsych.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 17.Rozeske RR, Der-Avakian A, Bland ST, Beckley JT, Watkins LR, Maier SF. The medial prefrontal cortex regulates the differential expression of morphine-conditioned place preference following a single exposure to controllable or uncontrollable stress. Neuropsychopharmacology. 2009;34:834–43. doi: 10.1038/npp.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenstein SA, Holmes PV. Chronic and voluntary exercise enhances learning of conditioned place preference to morphine in rats. Pharmacol Biochem Behav. 2007;86:607–15. doi: 10.1016/j.pbb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Bland ST, Twining C, Watkins LR, Maier SF. Stressor controllability modulates stress-induced serotonin but not dopamine efflux in the nucleus accumbens shell. Synapse. 2003;49:206–8. doi: 10.1002/syn.10229. [DOI] [PubMed] [Google Scholar]
- 20.Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, et al. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- 21.Hervas I, Vilaro MT, Romero L, Scorza MC, Mengod G, Artigas F. Desensitization of 5-HT(1A) autoreceptors by a low chronic fluoxetine dose effect of the concurrent administration of WAY-100635. Neuropsychopharmacology. 2001;24:11–20. doi: 10.1016/S0893-133X(00)00175-5. [DOI] [PubMed] [Google Scholar]
- 22.Le Poul E, Laaris N, Doucet E, Laporte AM, Hamon M, Lanfumey L. Early desensitization of somato-dendritic 5-HT1A autoreceptors in rats treated with fluoxetine or paroxetine. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:141–8. doi: 10.1007/BF00176767. [DOI] [PubMed] [Google Scholar]
- 23.Sprouse JS, Aghajanian GK. Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse. 1987;1:3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- 24.Bland ST, Hargrave D, Pepin JL, Amat J, Watkins LR, Maier SF. Stressor controllability modulates stress-induced dopamine and serotonin efflux and morphine-induced serotonin efflux in the medial prefrontal cortex. Neuropsychopharmacology. 2003;28:1589–96. doi: 10.1038/sj.npp.1300206. [DOI] [PubMed] [Google Scholar]
- 25.Maier SF, Grahn RE, Watkins LR. 8-OH-DPAT microinjected in the region of the dorsal raphe nucleus blocks and reverses the enhancement of fear conditioning and interference with escape produced by exposure to inescapable shock. Behav Neurosci. 1995;109:404–12. doi: 10.1037//0735-7044.109.3.404. [DOI] [PubMed] [Google Scholar]
- 26.Bland ST, Twining C, Schmid MJ, Der-Avakian A, Watkins LR, Maier SF. Stress potentiation of morphine-induced dopamine efflux in the nucleus accumbens shell is dependent upon stressor uncontrollability and is mediated by the dorsal raphe nucleus. Neuroscience. 2004;126:705–15. doi: 10.1016/j.neuroscience.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 27.Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Res. 2005;1033:164–78. doi: 10.1016/j.brainres.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 28.Brown RA, Abrantes AM, Read JP, Marcus BH, Jakicic J, Strong DR, et al. A Pilot Study of Aerobic Exercise as an Adjunctive Treatment for Drug Dependence. Ment Health Phys Act. 2010;3:27–34. doi: 10.1016/j.mhpa.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roessler KK. Exercise treatment for drug abuse--a Danish pilot study. Scand J Public Health. 2010;38:664–9. doi: 10.1177/1403494810371249. [DOI] [PubMed] [Google Scholar]
- 30.Daniel JZ, Cropley M, Fife-Schaw C. The effect of exercise in reducing desire to smoke and cigarette withdrawal symptoms is not caused by distraction. Addiction. 2006;101:1187–92. doi: 10.1111/j.1360-0443.2006.01457.x. [DOI] [PubMed] [Google Scholar]
- 31.Nic Dhonnchadha BA, Fox RG, Stutz SJ, Rice KC, Cunningham KA. Blockade of the serotonin 5-HT2A receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior in a rat self-administration model. Behav Neurosci. 2009;123:382–96. doi: 10.1037/a0014592. [DOI] [PMC free article] [PubMed] [Google Scholar]