Abstract
To examine potential mechanisms for the reduced resting membrane potentials (RP) of mature dystrophic (mdx) muscle fibers, the Na+ - K+ pump inhibitor ouabain was added to freshly isolated nondystrophic and mdx fibers. Ouabain produced a 71% smaller depolarization in mdx fibers than in nondystrophic fibers, increased the [Na+]i in nondystrophic fibers by 40%, but had no significant effect on the [Na+]i of mdx fibers, which was approximately double that observed in untreated nondystrophic fibers. Western blots indicated no difference in total and phosphorylated Na+ - K+ ATPase catalytic α1 subunit between nondystrophic and mdx muscle Examination of the effects of the NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC) indicated that direct application of the drug slowly hyperpolarized mdx fibers (7 mV in 90 minutes) but had no effect on nondystrophic fibers. Pretreatment with ouabain abolished this hyperpolarization, and pretreatment with PDTC restored ouabain-induced depolarization and reduced [Na+]i Administration of an NF-κB inhibitor that utilizes a different mechanism for reducing nuclear NF-κB activation, ursodeoxycholic acid (UDCA), also hyperpolarized mdx fibers. These results suggest that in situ Na+ - K+ pump activity is depressed in mature dystrophic fibers by NF-κB dependent modulators, and that this reduced pump activity contributes to the weakness characteristic of dystrophic muscle.
Keywords: Duchenne muscular dystrophy, Mdx mouse, Na+-K+ ATPase pump, Resting Membrane Potential, Ouabain, NF-κB inhibitors, Pyrrolidine Dithiocarbamate, Ursodeoxycholic acid
INTRODUCTION
Our rationale for examining the influence of ouabain and NF-κB inhibitors on the resting potential (RP) of isolated dystrophic (mdx) muscle fibers originated from several studies demonstrating that the RP is reduced in freshly isolated and untreated dystrophic fibers [1–5], and with results showing that long term in vivo treatment with the NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC) increased the RP in mdx muscle fibers [6]. In this laboratory, the mean RP in mouse Ringer solution obtained from mature (6 to 24 months) mdx costal diaphragm fibers (−57. 3) was 4.5 mV less negative (p<0.001) than the RP in mature nondystrophic fibers [5]. In younger mice (5 to 7 weeks), the RP in mdx costal diaphragms (−67.8 mV) was approximately equal to that observed in nondystrophic costal diaphragms [5]. Using freshly excised intercostal fibers from adult patients with limb girdle and facioscapulohumeral dystrophy, Ludin [1] observed an average RP of −71 to −73 mV, which was approximately 8 mV more depolarized than in corresponding nondystrophic biopsies. This investigator further indicated that the dystrophic resting potential at several extracellular K+ concentrations was consistently more positive than that predicted from the Nernst potential. Similarly, Sakakibara et al. [2] reported RP values (approximately - 72.5 mV) in freshly excised external intercostal fibers from Duchenne patients that were about 2 to 3 mV less negative than the lower limit of RPs observed in nondystrophic patients. Nagel et al. [3] indicated an average RP in the mdx costal diaphragm (−60 mV; 2.5 weeks to 9 months of age) that was approximately 3 mV less negative than the corresponding resting potential of nondystrophic fibers. While the individual determinations of RP vary between different muscle preparations and laboratories, these results consistently indicate that, under normal ionic conditions, the RP of freshly isolated intact mature adult dystrophic fibers is approximately 3 to 8 mV less negative than that in age-matched nondystrophic fibers.
Although the reduction in RP characteristic of adult dystrophic muscle fibers may seem small, it could very well contribute to muscle weakness by reducing the electrochemical driving force for Na+ influx and the density of fast Na+ channels available for voltage activation. In fact, recordings from human dystrophic (limb girdle, faciosacapulohumeral dystrophy) intercostal muscle fibers indicated a highly significant 13% reduction in action potential amplitude and a 14% reduction in the rate of rise of action potentials in comparison to nondystrophic controls [7]. These effects on action potential amplitude and rising phase are consistent with what would be expected from a decrease in driving force and an increase in Na+ channel inactivation, and would produce a reduction in sarcoplasmic Ca2+ release and a corresponding reduction in force generation. Such effects would act in series with previously observed reductions in Ca2+ release from the sarcoplasmic reticulum of voltage clamped dissociated mdx fibers [8], and would therefore amplify any impairments in excitation–contraction coupling that may exist in dystrophic fibers. Based on these considerations, the purpose of the present study was to improve our current understanding of the mechanisms responsible for the reduced RP characteristic of dystrophic muscle [1–6].
A slight depolarization of the RP could be the result of an increase in resting inward ionic current such as that produced by resting Ca2+ influx through Ca2+ leakage and nonselective cation channels [9,10]. However, several independent lines of evidence argue against this possibility. First, fluorometric studies of Mn2+ quench rate indicated that resting Ca2+ influx is not increased in adult dystrophic fibers [11,12]. Additional support for this conclusion was obtained from studies showing that concentrations of Gd3+ sufficient to block resting Ca2+ influx through nonselective cation channels and Ca2+ leakage channels had no effect on the resting potential of either nondystrophic or mdx muscle fibers [6]. In the mdx costal diaphragm, the reduced RP was associated with a significant increase in fiber input resistance (Rin; [5]). In contrast, reduced RP’s produced solely by increases in resting Na+ or Ca2+ conductance would be associated with corresponding reductions in Rin. These results from fluorometric and electrophysiological studies are inconsistent with the hypothesis that the reduced RP of dystrophic fibers is due to increases in resting inward current produced by increases in either Ca2+ leakage or nonselective cation channel activity [5, 6, 11, 12]. An alternative possibility for the reduced RP in dystrophic fibers is that in situ Na+-K+ ATPase pump activity is reduced in adult dystrophic fibers. In the present study, this possibility was directly examined by comparing the effects of ouabain exposure on the RP in age-matched mdx and nondystrophic muscle fibers.
Previous experiments in this laboratory indicated that long term in vivo administration of pyrrolidine dithiocarbamate (PDTC) substantially increased the resting potential (RP) in mdx triangularis sterni (TS) fibers [6]. Since PDTC is an NF-κB inhibitor [13] that increased cytosolic IκB-α in the mdx costal diaphragm, these results initially suggested that long term inhibition of the NF-κB pathway had beneficial effects on dystrophic (mdx) muscle [6]. Several studies from this and other laboratories now provide substantial evidence that inhibition of the NF-κB pathway has both morphological and functional benefits for dystrophic muscle [6, 14–20].
The influence of PDTC in improving the RP of mdx TS fibers [6] could be due to indirect effects in reducing fiber necrosis or increasing fiber regeneration. Alternatively, the drug may have more direct effects that improve the RP in dystrophic fibers. To examine this possibility, PDTC was administered directly to freshly isolated mdx fibers in vitro. The results indicated that PDTC produced a slow hyperpolarization of mdx muscle fibers, but had no effect on the RP of age-matched nondystrophic fibers[21].
The purpose of the present study was to determine the ouabain sensitivity and the effects of direct exposure to NF-κB inhibitors on mature adult mdx fibers. The results indicate that ouabain produced significantly smaller changes in RP and [Na+]i in mdx TS fibers than in age-matched nondystrophic fibers, that untreated mdx fibers exhibited [Na+]i levels that were approximately double those observed in untreated nondystrophic fibers (cf [22,23]), and that the overall cellular expression and phosphorylation of the Na-K ATPase alpha subunit was not significantly altered in mdx muscle. Pretreatment with ouabain abolished the hyperpolarizing influence of PDTC on the RP of mdx fibers. Conversely, pretreatment with PDTC restored the depolarizing influence of ouabain on the RP of mdx fibers, and reduced the [Na+]i in mdx fibers.
To further evaluate the potential role of the NF-κB pathway, the effect of ursodeoxycholic acid (UDCA) on nuclear p65 activation and RP was examined. UDCA inhibits nuclear activation of NF-κB by a mechanism distinct from that mediated by PDTC, and directly reduces nuclear NF-κB trans-activation by interacting with glucocorticoid receptors [24]. Direct treatment of isolated mdx muscle with UDCA reduced the nuclear activation of p65 and hyperpolarized the RP. In summary, these results provide new evidence that the reduced RP of intact mature adult mdx muscle fibers is associated with negative modulation of resting Na+-K+ pump activity, and that inhibition of the NF-κB pathway increases the RP by reducing or counteracting this negative modulation.
MATERIALS AND METHODS
Intracellular recording
Mdx (C57Bl10SnJ-mdx) and nondystrophic (C57BL/10 SnJ) mice were obtained from Jackson laboratories (Bar Harbour, ME) and bred at local animal facilities under IACUC approved conditions. The left TS muscle was removed as previously described [6, 12, 20] and placed in a muscle chamber containing mouse Ringer solution (in mM: 125 NaCl, 5 KCl, 2 CaCl2, 24 NaHCO3, 1 NaH2PO4, 11 glucose, pH 7.35; room temperature). The solution was continually re-circulated and bubbled with 95% O2/5% CO2. Only one TS muscle was removed from each mouse used in this study.
Conventional glass microelectrode (3M KCl; R = 20–70 MΩ) techniques were used to record RP as previously described [5]. The voltage deflection observed upon impalement and withdrawal of the electrode from each cell was noted, and the maximum of these two values was defined as the RP. The RP’s were obtained at room temperature from the caudal TS region of 7 to 9 month old nondystrophic and mdx mice. Signals were amplified using a Warner Instrument Model IE201 electrometer and displayed on an oscilloscope. Rin was determined using the Wheatstone bridge circuit component of the Warner Instrument Model 1E201 as described in Carlson and Roshek [5]. Fibers with an RP less than −15 mV were not included in the analyses. In general, RP was sampled from individual fibers at a rate of approximately 10 fibers in 20 minutes. The incubation periods that are reported in this study therefore represent the time to the beginning of a sampling interval in which approximately 10 fibers are sampled in 20 minutes.
PDTC was administered in mouse Ringer at a concentration of 100 μM using mouse Ringer (pH 7.35) as a vehicle control, while UDCA was added to a final concentration of 200 μM from a stock solution containing 200 mM UDCA (mouse Ringer plus NaOH, pH 9.8) using the appropriate vehicle (mouse Ringer plus NaOH, pH 9.8) as a control.
Intracellular Na+ concentration
Muscle fibers were loaded at room temperature with the acetylmethoxy ester of benzofuran isophthalate (SBFI-AM; Sigma-Aldrich #129423-53-6) at a final concentration of 10 or 50 μM in HEPES Ringer solution (in mM: 147.5 NaCl, 5 KCl, 2 CaCl2, 11 glucose, 5 HEPES, pH 7.35). After a 2 hour incubation in the dark, the preparation was rinsed several times with HEPES Ringer and mouse Ringer solution before measurements were obtained at room temperature in mouse Ringer solution bubbled with 95% O2/5% CO2. An Olympus IMT2F microscope equipped with an APO UV 20× objective, an IMT2-DMU dichroic mirror, and a PTI Model 104 photometer attached to the side port of the microscope were used. Excitation was provided by the excitation monochromator of an SLM Aminco 8000C spectrofluorometer and a PTI fiber optic cable (Model 060–8003). The aperture setting provided measurements of emission (> 490 nm) from a rectangular area (approximately 40,000 μm2) that included 2 to 4 fibers oriented longitudinally within the plane of focus (cf. [12]). Each experiment used the same settings for aperture, magnification, signal gain, and voltage. Full excitation spectra (300 to 450 nm, 5 nm increments, 4 nm bandwidth) were obtained periodically to monitor the overall signal intensity. Fibers in the caudal region of the TS were sampled exclusively.
In cells loaded with SBFI, the ratio of fluorescent intensities obtained at excitation wavelengths of 340 and 380 nm is correlated with the [Na+]i [25, 26]. Since freshly isolated skeletal muscle fibers exhibit autofluorescence within this range of wavelengths, the fluorescent intensity of unloaded fibers (Fa) was measured at excitation wavelengths of 340 nm and 380 nm in each preparation before loading with SBFI-AM (cf. [12]). The background (or solution) fluorescence at the same plane of focus (Fb) was also measured and subtracted from the corresponding intensity of the unloaded fibers to obtain the autofluorescence corrected for background at 340 and 380 nm excitation wavelengths (F340acb = F340a − F340b; F380acb = F380a − F380b). Fluorescent intensities of the loaded fibers (FS) were similarly corrected for background fluorescence (FSb) to correct for any potential leakage of SBFI (F340Scb = F340S − F340Sb; F380Scb = F380S − F380Sb). For each of the sampled areas, the fluorescence produced by the binding of Na+ to intracellular SBFI (F340Na, F380Na) was determined by subtracting the mean autofluorescence corrected for background (Mean F340acb, Mean F380acb) from the background corrected intensities of the loaded fibers (F340Na = F340Scb − Mean F340acb; F380Na = F380Scb − Mean F380acb). Areas that had F340Scb or F380Scb values that were less than 20% higher than the corresponding autofluorescence determinations (Mean F340acb, Mean F380acb) were not included in the analyses. The [Na+]i for each sampled area was determined from the F340Na/F380Na ratio and a standard curve relating F340Na/F380Na to [Na+]i in situ.
Standard curves were obtained with solutions similar to those used by Diarra et al [25]. Fibers loaded with 10 μM SBFI-AM in HEPES Ringer were exposed to 5, 10, or 20 mM NaCl at room temperature in HEPES (5 mM) buffered saline (pH 7.35 adjusted with KOH) containing 2.0 mM CaCl2 and 11.0 mM glucose. The [KCl] in the [Na+] standard solutions was adjusted to make the sum of the [K+] plus [Na+] equal to the corresponding value in HEPES Ringer solution. Each solution contained 10 μM gramicidin D and 1 mM ouabain to promote Na+ flux across the membrane and inhibit the Na+-K+ ATPase. Additional control experiments to measure autofluorescence in these solutions were used to obtain the F340Na and F380Na values. Since the F340Na and F380Na values from nondystrophic and mdx preparations exposed to standard [Na+] solutions were not significantly different, the data were combined to obtain an empirical standard curve that was used to convert the F340Na/F380Na ratio from each sampled area to a value of [Na+]i. The [Na+]i of mdx fibers loaded with 50 μM SBFI-AM were determined using a separate set of standards at 20, 25, 30, and 40 mM [Na+] (10 μM gramicidin D,1 mM ouabain, [KCl] adjusted such that [KCl] + [NaCl] = 152.5 mM; 2 mM CaCl2, 11 mM glucose, pH 7.35 adjusted with KOH).
Cell Extraction
Whole cell lysates (WCL) and nuclear extracts were obtained using the techniques developed in this laboratory by Singh et al [27]. Briefly, whole cell extracts were prepared by homogenization in isotonic lysis buffer (WCLB: 20 mM Tris-HCl, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, 4μl/ml protease inhibitor cocktail, 10μl/ml phosphate inhibitor, pH 7.5; 15 μl per mg tissue), freeze-thawing (two freeze/thaw cycles; 5 minute freeze on dry ice), vortexing (10 seconds), and centrifugation (6 minutes, 13,000 rpm, 4°C). The supernatant containing the whole cell lysate was stored at −80°C until used in the Western blot determinations of phosphorylated and total Na+-K+ ATPase α1 subunit.
Nuclear extracts were used to assess the efficacy of UDCA in reducing p65 trans-activation in freshly isolated mdx costal diaphragm preparations. Mdx costal diaphragms exposed to UDCA or vehicle in HEPES Ringer solution were flash frozen and homogenized in low salt lysis buffer (LSLB: 10 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, 1.0 mg/ml benzamidine, 0.5 mM phenylmethylsulfonyl fluoride, 4.0 μl/ml protease inhibitor cocktail Sigma # 8340, 10μl/ml phosphatase inhibitor Sigma # P2850, pH 7.9, 1 mg wet weight per 18 μl). Cytosolic extracts were obtained after freeze-thaw lysis on dry ice followed by centrifugation (13,000 rpm, 20 seconds, 4°C). The remaining pellets were washed twice in LSLB (50 μl) to remove any remaining cytosolic components, and re-suspended in ice-cold high-salt lysis buffer (HSLB: 20 mM HEPES (pH 7.9), 420 mM NaCl, 1 mM EDTA, 1 mM EGTA, 25% glycerol, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 1.0 mg/ml benzamidine, 4μl/ml protease inhibitor cocktail, 10μl/ml phosphate inhibitor; 4μl/mg wet weight; 30 min incubation). After mixing vigorously (5 seconds, vortex) several times while incubating on ice (30 minutes), the suspension was centrifuged (13,000 rpm, 6 min, 4°C), and the resulting supernatant containing the nuclear extract was stored at −80°C. Protein concentrations for all cell extracts were determined using the Bradford assay (Bio-Rad 500-0006) in a standard 96 well plate.
Western blot determinations of total cellular α1 Na+-K+ ATPase subunit and α1 subunit phosphorylated at Tyr 11 and Ser 18
Western blots were obtained from whole cell lysates using procedures similar to those described in Singh et al [27]. Muscle proteins (20 μg) were separated by SDS-PAGE under reducing conditions, transferred to PVDF membranes (Bio-Rad), and blocked with 5% non fat dehydrated milk in Tris buffered saline containing Tween 20 (TBST; 25 mM Tris, pH 7.4, 137 mM NaCl, 2.7 mM KCl, 0.1% Tween20; 1 hr, room temperature). The membranes were incubated with primary antibodies in TBST (5% BSA) overnight at 4°C, followed by secondary antibody horseradish peroxidase (HRP) conjugated anti-rabbit IgG (Jackson Laboratory; 111-035-003) in TBST (5% non-fat dehydrated milk or 5% BSA) for 1 hr at room temperature. The immunoblot detection was performed using the ECL detection system (Amersham, UK) according to the instructions of the manufacturer.
Equal loading of proteins was evaluated by stripping and developing a blot for GAPDH (Cell Signaling,14C10; # 2118). The primary antibody used for the detection of Na-K ATPase α1 subunit was obtained from Cell Signaling (# 3010; 1:1000). Percent phosphorylated α1 subunit was determined in parallel samples by first developing the membrane for phosphorylated α1 subunit (Tyr10 - Cell Signaling # 3060 or Ser23 - Cell Signaling # 4006 - corresponds to Ser18 of mature α1 subunit; 1:500) to determine the density of phosphorylated signal, and then stripping and exposing the membrane to the Na-K ATPase α1 subunit antibody (Cell Signaling; # 3010, 1:1000). Densitometric analysis to obtain percent phosphorylated α1 subunit and total expression of α1 subunit relative to GAPDH were performed using Image J, Sigma Stat and Sigma Plot. The results were obtained from age (7 to 12 months) and gender matched nondystrophic and mdx mice.
DNA binding activity
DNA binding of p65 was determined in nuclear extracts from freshly isolated mdx costal diaphragms exposed to various concentrations of UDCA or vehicle in HEPES Ringer solution for 2 hours. The TransAM™ NF-κB p65 assay kit (ActiveMotif, Carlsbad, CA) was used in accordance with the manufacturer’s instructions (cf [27]). Sample absorbance was directly compared between individual muscle preparations on individual plates and normalized to the absorbance of a standard positive control (2.5 μg Jurkat nuclear extract).
Statistical Evaluation
The results were analyzed using SAS statistical software 9.0 (ANOVA, linear regression) or SigmaStat v 2.03 (t-test, Mann-Whitney rank sum test) and are presented as the mean ± SEM. Multiple group comparisons were obtained using ANOVA followed by multiple pairwise comparisons (Tukey’s test). Direct comparisons between mdx and nondystrophic preparations were assessed by t test. Significance was defined at p < 0.05.
RESULTS
Differential effect of ouabain on the RP of freshly isolated nondystrophic and mdx muscle fibers
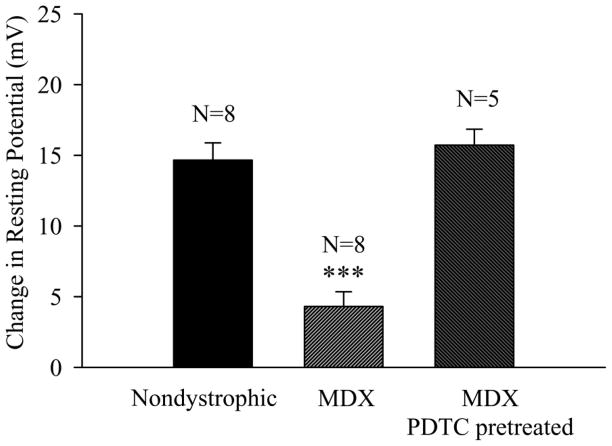
The mean RP of untreated fibers in the caudal region of the severely dystrophic mdx TS muscle [20] at 7 to 9 months of age (−54.8 ± 0.9 mV, N = 208 fibers, 19 muscles) was 4.2 mV less negative (p<0.01, Mann-Whitney Rank Sum Test) than that observed in nondystrophic TS muscles (−59.0 ± 0.7 mV, N= 128 fibers, 12 muscles). Fibers from a cohort of nondystrophic mice (7 to 9 month) were depolarized by 14.7 mV (from −61.1 to −46.4 mV; N= 80 fibers, 8 muscles) following a 30 minute exposure to 1 mM ouabain (Fig. 1). In contrast, the same ouabain exposure produced a significantly (p<0.001) lower depolarization of only 4.3 mV (from −56.3 to −52.0 mV; Fig. 1; N = 160 fibers, 8 muscles) in mdx fibers. These results provide the first evidence that the influence of ouabain-sensitive Na-K+ ATPase pump activity on the RP is greatly diminished in intact, mature adult dystrophic fibers.
Figure 1.
The RP of mdx fibers exhibit reduced ouabain sensitivity that is reversed by exposure to the NF-κB inhibitor PDTC. Shown is the effect of a 30 minute exposure to ouabain (1 mM) on the RP of untreated nondystrophic fibers, untreated mdx fibers, and mdx fibers pretreated and exposed to PDTC. The change in RP was determined for each preparation by subtracting the mean RP obtained after the 30 minute ouabain exposure from that obtained immediately prior to the addition of ouabain. N is the number of TS preparations. *** indicates p<0.001 in comparison to nondystrophic TS.
Intracellular Na+ [Na+]i is elevated and ouabain-sensitivity is reduced in dystrophic muscle fibers
Fluorometric evaluation of [Na+]i provided further evidence that Na+-K+ ATPase pump activity is reduced in freshly isolated intact mdx TS fibers. The mean [Na+]i in untreated mdx TS fibers (22.5 ± 2.3 mM) was significantly (p<0.001; Mann Whitney Rank Sum Test) higher than that observed in nondystrophic TS fibers (11.5 ± 1.0 mM). Nondystrophic TS fibers exposed to ouabain (1 mM) for 30 minutes exhibited significantly (p< 0.01, Mann Whitney Rank Sums test) elevated [Na+]i levels of 16.1 ± 1.9 mM. In contrast, the same treatment of mdx TS fibers had no effect on [Na+]i (Table 1). The elevated [Na+]i and the lack of influence of ouabain on [Na+]i in mdx fibers provides additional confirmatory evidence that ouabain-sensitive Na+-K+ ATPase pump activity is reduced in situ in mature adult dystrophic muscle fibers.
Table 1.
[Na+]i in untreated and ouabain–treated (1 mM) nondystrophic and mdx caudal TS muscle fibers loaded with 10 μM SBFI-AM.
| [Na+]i (untreated fibers) | [Na+]i (ouabain treated fibers) | |
|---|---|---|
| Nondystrophic | 11.5 ± 1.0 mM N=62, 7 | 16.1 ± 1.9 mM** N=38, 4 |
| Mdx | 22.5 ± 2.3 mMτττ N=63,7 | 21.4 ± 4.2 mM N=31,9 |
indicates p<0.001 in comparison to untreated nondystrophic fibers.
indicates p<0.01 in comparison to untreated nondystrophic fibers.
N is the number of muscle fibers, number of TS preparations.
The expression and phosphorylation of the α 1 subunit of the Na+-K+ ATPase is unaltered in mdx skeletal muscle
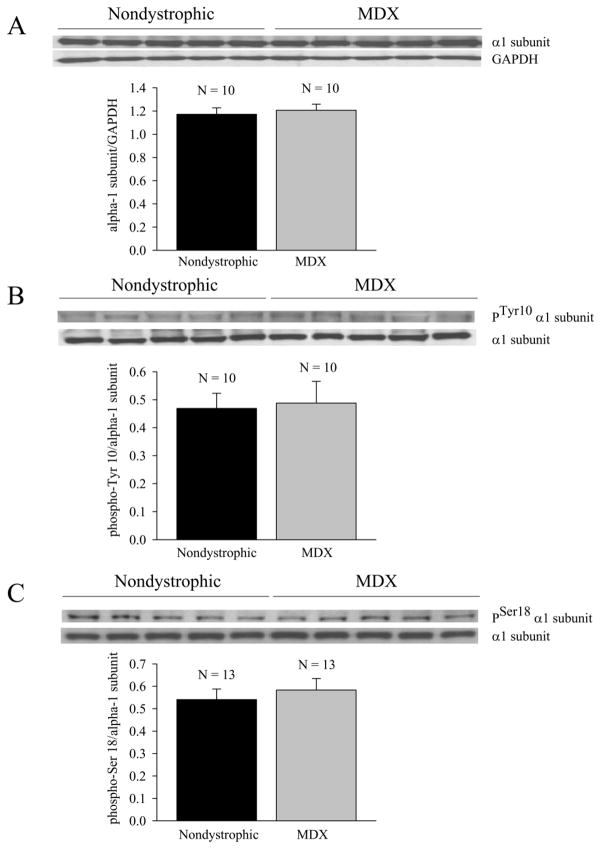
To examine the possibility that the reduced resting potentials, reduced ouabain sensitivity, and elevated Na+ levels were secondary to reduced expression of Na+ - K+ ATPase in mdx muscle, Western blots were obtained from both nondystrophic and mdx muscle to assess the relative expression of the catalytic α subunit, and the phosphorylation of the α subunit at two sites that are known to regulate Na+ - K+ ATPase activity (Tyr10, Ser18; [28–32]). The results indicate equal levels of expression of the catalytic α1 subunit and phosphorylated α1 subunit in mature adult nondystrophic and mdx costal diaphragm (Fig. 2), and suggest that the depressed efficacy of ouabain in intact mdx fibers (Fig. 1, Table 1) is secondary to negative modulation of ouabain-sensitive activity that is independent of the level of phosphorylation at either Tyr10 or Ser18.
Figure 2.
The expression of the α1 catalytic Na+-K+ ATPase subunit and the corresponding degree of phosphorylation at Tyr11 and Ser18 are unaltered in the mdx costal diaphragm. (A) Relative densitometric expression of α1 subunit (α1 subunit/GAPDH). (B) Relative phosphorylation of α1 subunit at Tyr10. (C) Relative phosphorylation at Ser18. Insets show representative blots. N equals the number of costal diaphragm preparations (1 per mouse).
Direct exposure to PDTC increases the RP in mature adult freshly isolated mdx TS muscle fibers
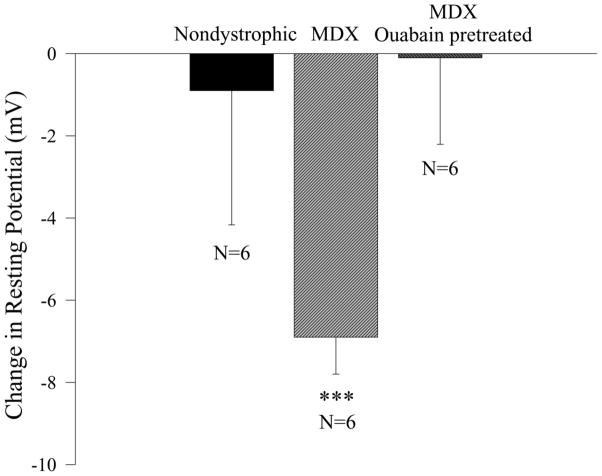
The effect of PDTC on the RP was examined by comparing the effect of a 90 minute exposure to the drug on nondystrophic and mdx TS muscle fibers. In the cohort of preparations used for this study, the RP prior to PDTC administration ranged between −51 and −73 mV, with no significant difference between mdx and nondystrophic preparations (overall mean = −59.7 mV). The addition of PDTC had no effect on the RP in nondystrophic fibers, but significantly (p<0.001) hyperpolarized mdx fibers by approximately 7 mV (p< 0.001; Fig. 3). In a separate group of experiments, the RP was sampled at various time periods after the administration of PDTC. Mdx TS fibers exhibited a slight, but significant (p < 0.05; r2 = 0.0333), positive correlation between PDTC incubation time and RP, while nondystrophic TS fibers exhibited highly stable RPs with no significant correlation over a 2 hour recording period. To examine whether the PDTC-induced increase in RP was associated with a change in resting conductance, a third study was conducted to examine the effect of PDTC on Rin. In this smaller study, the 90 minute treatment with PDTC increased the RP in mdx fibers by 10.6 mV (from a mean of −44.8 to −55.4 mV; p < 0.05, Mann-Whitney Rank Sums test, 2 mdx TS muscles, N = 20 untreated fibers, N = 23 PDTC treated fibers), but had no significant effect on the input resistance (untreated - Rin = 4.4 ± 0.7 MΩ, N = 20 fibers; PDTC treated – Rin = 4.0 ± 1.4 MΩ, N = 20 fibers). These results provide the first evidence that an inhibitor of the NF-κB pathway [6] directly hyperpolarizes the resting plasma membrane of intact, mature adult dystrophic fibers.
Figure 3.
PDTC increases the RP of mdx fibers by a mechanism that requires a functional Na+- K + ATPase pump. Shown is the effect of a 90 minute exposure to 100 μM PDTC on the RP of untreated nondystrophic, untreated mdx, and mdx muscle fibers pretreated and exposed to ouabain (1 mM). N is the number of TS muscle preparations. *** indicates p < 0.001 in comparison to nondystrophic TS.
Exposure to ouabain eliminates the hyperpolarizing effect of PDTC on the RP of mdx caudal TS muscle fibers
To examine the possibility that the membrane hyperpolarization induced by PDTC was produced by an increase in ouabain sensitive Na+-K+ ATPase pump activity, mdx TS fibers were exposed to PDTC after first inhibiting the pump with ouabain. Since these experiments involved exposing preparations to PDTC in the presence of ouabain, it was necessary to first determine the effects of ouabain on the RP in the absence of PDTC. The control group, therefore, consisted of preparations that were exposed to ouabain alone for 120 minutes. The RP was determined in these control preparations after both 30 and 120 minutes of ouabain exposure.
In the experimental group, mdx fibers were exposed to 1mM ouabain for 30 minutes before treating the preparation with PDTC (100 μM) in the continued presence of ouabain for another 90 minutes. The RP in the experimental group was obtained after 30 minutes (before PDTC) and 120 minutes (after PDTC) of ouabain exposure. If PDTC had a hyperpolarizing influence on the RP of the ouabain treated preparations, then the actual depolarization in the continued presence of ouabain and PDTC would be smaller than the depolarization in the presence of ouabain and the absence of PDTC. In contrast, the results indicated that the mean change in RP between 30 and 120 minutes of ouabain exposure in the PDTC treated preparations (+19.2 mV) was approximately equal to the corresponding change in the ouabain treated preparations that were not exposed to PDTC (+19.3 mV; Fig. 3; mdx ouabain pretreated). These results indicate that PDTC had no effect on the RP of mdx preparations pretreated and continually exposed to ouabain.
Exposure to PDTC restores the effect of ouabain on the RP and decreases the [Na+]i in mdx caudal TS muscle fibers
To further examine whether PDTC treatment increases ouabain-sensitive Na+-K+ ATPase pump activity in mdx fibers, the effect of ouabain on the RP was determined in mdx fibers that were pretreated with PDTC. Mdx TS preparations were first exposed to 100 μM PDTC for 90 minutes before determining the RP in a sample of 10 fibers per preparation and exposing the preparation to 1 mM ouabain. In contrast to the untreated mdx TS fibers that were depolarized by an average of about 4 mV following 30 minutes of ouabain exposure, the PDTC treated fibers were depolarized by 15.8 mV (from −66.1 to −50.3 mV; Fig. 1, mdx PDTC pretreated), a value that was not significantly different from the ouabain-induced depolarization in nondystrophic fibers (14.7 mV). These results indicate that PDTC hyperpolarizes dystrophic fibers by increasing ouabain-sensitive Na+-K+ATPase pump activity.
The effect of PDTC on [Na+]i was consistent with an action of the drug in increasing ouabain sensitive Na+-K+ ATPase pump activity. Control mdx TS fibers exposed to SBFI-AM in HEPES Ringer for a period of 2 hours exhibited an average [Na+]i of 25.4 ± 1.8 mM when subsequently bathed in mouse Ringer solution (N= 46 fibers, 2 preparations). Experimental fibers treated with SBFI-AM for 30 minutes and 100 μM PDTC in the presence of SBFI-AM for 90 minutes exhibited a significantly reduced average [Na+]i of 17.4 ± 1.1 mM (N=77 fibers, 3 preparations; p<0.001) when subsequently bathed in mouse Ringer solution containing 100 μM PDTC. These effects of PDTC in restoring ouabain sensitivity (Fig. 1) and reducing [Na+]i indicate that the drug increases basal Na+ - K+ ATPase pump activity in mature adult mdx muscle fibers.
Exposure to the NF-κB inhibitor UDCA reduces nuclear p65 activation and hyperpolarizes dystrophic (mdx) muscle fibers
Previous experiments in this laboratory and others indicated that PDTC increased cytosolic IκB-α [6] and reduced nuclear NF-κB activation in mdx limb muscle [14]. The mechanism of action of PDTC does not depend on reductions in intracellular reactive oxygen species (ROS), but instead involves inhibition of the ubiquitin ligase responsible for regulating the subsequent proteasomal degradation of phosphorylated IκB-α [13]. In contrast, UDCA does not increase cytosolic IκB-α, but instead directly reduces the nuclear activation of NF-κB by interfering with DNA binding after interacting with membrane glucocorticoid receptors [24]. To assess whether inhibition of the NF-κB pathway was sufficient to improve the RP in mdx fibers, p65 activation was determined in nuclear extracts from freshly isolated mdx costal diaphragms exposed to various concentrations of UDCA or vehicle for 2 hours.
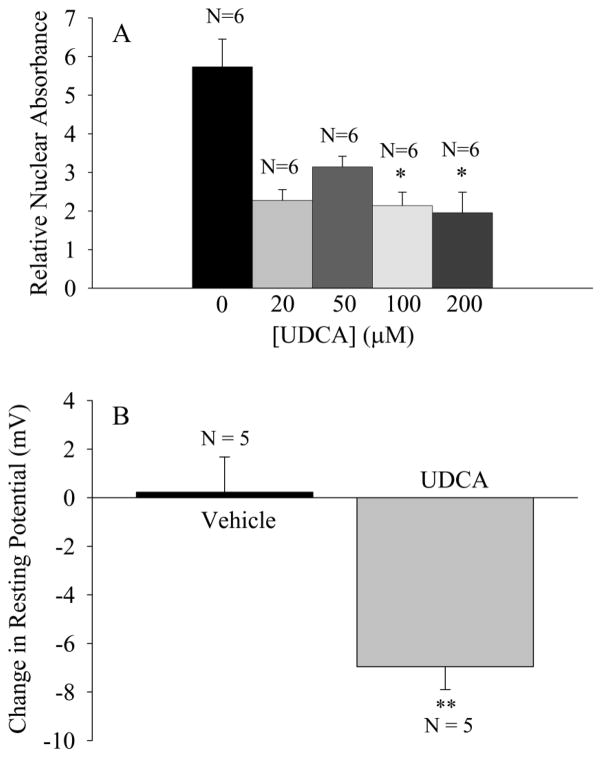
The results indicated that a concentration of 200 μM UDCA, which significantly (p<0.05) reduced nuclear p65 activation to approximately 35% of the levels observed in vehicle treated preparations (Fig. 4A), produced a significant (p<0.01), 7 mV increase in the RP (from −50.5 to −57.5 mV) that was not observed after exposure to the vehicle solution (RP = −50.1 mV before vehicle and −49.9 mV after vehicle; Fig. 4B). These results indicate that two chemically distinct NF-κB inhibitors that act by independent mechanisms to reduce nuclear NF-κB activation produce similar effects in hyperpolarizing the resting plasma membrane of intact, mature adult dystrophic muscle fibers.
Figure 4.
UDCA decreases nuclear p65 activation and hyperpolarizes mdx TS fibers. (A) Nuclear activation of p65 in mdx costal diaphragms exposed to vehicle or UDCA for 2 hours. Relative nuclear absorbance is the absorbance of the sample normalized to that of a positive control consisting of 2.5 μg Jurkat nuclear extract. ANOVA indicated a significant (p<0.01) effect of UDCA dose on relative nuclear absorbance. * indicates p<0.05 in comparison to vehicle control (Tukey). (B) The effect of 200 μM UDCA (90 minute exposure) on the resting potential of mdx TS muscle fibers. ** indicates p<0.01 in comparison to vehicle treated preparations. N is the number of TS muscles (1 per mouse).
DISCUSSION
Mature mdx fibers exhibit substantially reduced sensitivity to ouabain in the presence of normal Na+-K+ ATPase α1 subunit expression
The results indicate that freshly isolated, intact, mature adult dystrophic (mdx) fibers which exhibit elevated nuclear NF-κB activation [27] also exhibit reduced RPs, elevated [Na+]i (Table 1; cf. [22,23]), and reduced sensitivity to ouabain (Fig. 1; Table 1), but have normal cellular expression and phosphorylation (Tyr10, Ser18) of the α1 Na+-K+ ATPase catalytic subunit (Fig. 2). Previous studies had indicated increased Na+-K+ ATPase activity in isolated plasma membrane fractions from mdx muscle [33], and increases in the number of ouabain binding sites in younger 5 to 6 month old mdx muscle fractions [34]. The present study provides the first direct immunochemical evidence in mdx muscle regarding the total cellular expression of the α1 subunit of the Na+-K+ ATPase, and indicates that total cellular expression is unaltered in mature adult mdx costal diaphragm (Figure 2A). Since the earlier investigation examining total ouabain binding was conducted on whole muscle homogenates from back and leg muscles [34], it is possible that differences in muscle preparation may account for these apparent differences in Na+ - K+ ATPase expression. Nevertheless, the present results clearly indicate that the reduced RP, elevated [Na]i, and reduced sensitivity to ouabain are each produced by a reduction in ouabain sensitive Na+ - K+ pump activity in the presence of normal cellular expression of the catalytic α1 subunit (Fig. 2A).
The activity of Na+-K+ ATPase in situ is regulated by increases or decreases in the translocation of the enzyme to the plasma membrane, increases in the synthesis and assembly of Na+-K+ ATPase molecules in a readily available store that can be rapidly transported to the plasma membrane, or by more direct alterations to the activity of existing Na+-K+ ATPase molecules in the plasma membrane [35,36]. Two prominent sites on the catalytic subunit have been implicated in regulating activity. Insulin increases phosphorylation on Tyr10 [30] and, in skeletal muscle, increases Na+-K+ ATPase activity by enhancing the translocation of active enzyme to the plasma membrane [31]. In contrast, dopamine increases phosphorylation on Ser18 and decreases activity by promoting endocytosis of active enzyme [29] and reducing K+ affinity [28].
To examine whether the reduced ouabain sensitivity of dystrophic muscle was secondary to changes in the basal rate of phosphorylation at either of these sites (Tyr10, Ser18), the proportion of phosphorylated α1 subunit at each site was examined in nondystrophic and mdx costal diaphragm. The results indicate that untreated nondystrophic and mdx muscle exhibit equivalent degrees of phosphorylation at both the Tyr10 and Ser18 sites (Fig. 2B, C), and that the in situ reductions of ouabain sensitivity in dystrophic muscle are therefore secondary to reductions in activity that are independent of phosphorylation at these sites.
Alternative explanations for the reduced ouabain sensitivity in intact mature dystrophic muscle include a wide array of possibilities ranging from reductions in available intracellular ATP, oxidative stress, differential expression and assembly of various α and β isoforms [35], altered cytoskeletal interactions, conformational alterations that reduce ouabain binding, increases in the levels of endogenous ouabain [37], and chronic effects of prolonged cytokine exposure and subsequent withdrawal. The reduced sensitivity to ouabain observed in the present study is, however, consistent with previous results indicating that red cell ghosts from patients with Duchenne muscular dystrophy had reduced Na+-K+ ATPase activity and an abnormal positive response to ouabain [38–40] that was mediated by a modulator present in the dystrophic circulation [40]. The results of the present study showing that a brief period of ouabain exposure failed to alter the [Na+]i of mdx fibers (Table 1) are generally consistent with these previous results showing reduced hydrolysis of ATP in dystrophic red cell membrane preparations [38–40]. Nevertheless, mdx fibers must have residual ouabain sensitive Na+-K+ pump activity in order to maintain electrochemical gradients for Na+ and K+ or, alternatively, a store of relatively inactive Na+ - K+ ATPase molecules that are insensitive to exogenous ouabain. Although additional experiments are required to determine the mechanism(s) responsible for reduced ouabain sensitivity in mdx skeletal muscle (Fig. 1, Table 1), the present results provide strong evidence for the novel hypothesis that basal Na+ - K+ pump activity in dystrophic muscle is reduced by a mechanism that does not involve alterations in the cellular expression of the catalytic α1 subunit or phosphorylation at either Tyr11 or Ser18 (Fig. 2).
Inhibitors of the NF-κB signaling pathway increase the resting potential, reduce [Na+]i, and enhance ouabain sensitivity in mature adult dystrophic (mdx) fibers
The initial results showing that long term treatment with PDTC substantially improved the RP in the mdx TS muscle [6] were further examined by exposing freshly isolated nondystrophic and mdx TS muscles to PDTC in vitro. The results indicate that PDTC produced a significant hyperpolarization of the plasma membrane in mdx TS fibers over an interval of 90 minutes (Fig. 3). To determine whether the increase in RP may have been produced by a reduction in the resting conductance to Na+, Ca2+, or Cl−, Rin was determined before and after PDTC exposure. A reduction in resting conductance to these ions would produce an increase in Rin. In contrast, fibers exposed to PDTC exhibited a slightly reduced Rin that was not significantly different from the value in untreated mdx fibers. The hyperpolarizing influence of PDTC was, however, associated with a reduction in [Na+]i and the restoration of normal ouabain sensitivity (Fig. 1), and was abolished by pretreatment with ouabain (Fig. 3). These results indicate that PDTC improves the RP in dystrophic fibers by increasing resting, ouabain-sensitive, Na+-K+ pump activity.
PDTC inhibits the NF-κB signaling pathway by stabilizing cytosolic IκB-α [6,13,14], and improves mdx limb tension generation by about 12% when administered in vivo to mature mdx mice [19]. Results from this laboratory indicate that UDCA is more efficacious than PDTC in reducing nuclear p65 activation in the mdx costal diaphragm [19], which exhibits substantially higher proportional increases in nuclear p65 activation than mdx limb muscle [27]. UDCA produced a 21% increase in limb tension after 30 days of treatment [19]. The mechanism of action for UDCA does not involve increases in cytosolic IκB-α, but instead involves more direct inhibition of DNA binding and trans-activation [24]. In the present study, UDCA (100 to 200 μM; 2 hours) inhibited nuclear p65 activation in the mdx costal diaphragm to levels of approximately 35% of vehicle-treated levels (Fig. 4A). Exposure to 200 μM UDCA for 90 minutes produced a significant (p<0.01) hyperpolarization of the RP in mdx TS muscle fibers (Fig. 4B).
Both of the NF-κB inhibitors used in this study have been shown to exert direct anti-oxidant effects in reducing reactive oxygen species (ROS) and it is therefore possible that the restorative effects of these agents on the RP may be partly mediated by reductions in intracellular ROS. However, a study directly examining the efficacy of UDCA in scavenging O2 using the nitroblue tetrazolium reduction (NBT) assay indicated small (11%) decreases in NBT reduction at a concentrations of 500 μM UDCA and no effect on NBT reduction at 100 μM [41]. Interpolating these results [41], one would expect that a concentration of 200 μM UDCA would decrease the rate of NBT reduction by approximately 3%. Since 100 to 200 μM produced roughly 65% inhibition of nuclear p65 activation in the present study (Fig. 4A) and was relatively ineffective at reducing NBT reduction [41], it is quite unlikely that reductions in ROS played an important role in improving the RP in the present study. Although the precise role of nuclear NF-κB activation in modulating Na+-K+ ATPase activity in dystrophic muscle will require additional studies using a broad array of NF-κB inhibitors, the present results showing similar hyperpolarizing effects of two NF-κB inhibitors that have distinct mechanisms of action strongly suggest that the induced hyperpolarization is secondary to inhibition of the NF-κB signaling pathway.
In summary, the results indicate that ouabain sensitive Na+-K+ pump activity and the RP are reduced in freshly isolated mature mdx muscle fibers, and are enhanced by two distinct inhibitors of the NF-κB signaling pathway. Although the results suggest that an NF-κB dependent modulator suppresses ouabain sensitive Na+-K+ ATPase pump activity in dystrophic fibers (cf. [42,43]), it is possible that the positive effects of inhibiting the pathway in dystrophic muscle are independent of the negative modulation of ouabain sensitive activity in untreated dystrophic fibers. More importantly, however, these results indicate that ouabain sensitive Na+-K+ ATPase pump activity in mature dystrophic fibers is labile, and may be rapidly modulated by agents that affect cell signaling.
Since small reductions in RP could be responsible for reductions in the amplitude and time course of action potentials in dystrophic muscle [7] that would in turn reduce the quantity of Ca2+ released from the sarcoplasmic reticulum, the reduction in RP and ouabain sensitivity of dystrophic fibers likely contributes to the muscle weakness characteristic of dystrophic muscle. Previous results from voltage-clamped, dissociated mdx flexor digitorum fibers indicated defects in the sarcoplasmic release of Ca2+ [8] that would act in series with a reduced action potential to produce reductions in active tension development. The reduced RP and elevated [Na]i seen in the present study would produce reductions in action potential amplitude that would therefore have a multiplicative effect in further reducing sarcoplasmic Ca2+ release in intact mature adult mdx fibers, and would thus contribute to weakness in intact dystrophic muscle. Additional studies to elucidate the signaling pathways that influence the Na+-K+ ATPase pump activity of dystrophic muscle would therefore be quite useful in designing new treatments to improve muscle function in Duchenne and related muscular dystrophies.
Acknowledgments
This work was supported by grants to CGC from AT Still University (Strategic Research Grant), the Association Française contre les Myopathies (AFM; 11832, 13980), the NIH (1R15AR055360-01A2) and Charley’s Fund, and by the MS program in Biology at Truman State University (MTM). The authors also are thankful for the help provided by Bonnie King and the KCOM mouse facility staff (Alan Coonfield and RaElla Wiggins). The authors wish to thank Drs. Dianne Janick-Buckner, Brent Buckner, and Dean DeCock for their insight and recommendations regarding this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ludin HP. Microelectrode study of dystrophic human skeletal muscle. Europ Neurol. 1970;3:116–121. doi: 10.1159/000113963. [DOI] [PubMed] [Google Scholar]
- 2.Sakakibara H, Engel AG, Lambert EH. Duchenne dystrophy: Ultrastructural localization of the acetylcholine receptor and intracellular microelectrode studies of neuromuscular transmission. Neurology. 1977;27:741–745. doi: 10.1212/wnl.27.8.741. [DOI] [PubMed] [Google Scholar]
- 3.Nagel A, Lehmann-Horn F, Engel AG. Neuromuscular transmission in the mdx mouse. Muscle Nerve. 1990;13:742–749. doi: 10.1002/mus.880130813. [DOI] [PubMed] [Google Scholar]
- 4.Kurihara T, Kishi M, Komoto N, Hidaka T, Kinoshita M. Electrical myotonia and cataract in X-linked muscular dystrophy (mdx) mouse. J Neurol Sci. 1990;99(1):83–92. doi: 10.1016/0022-510x(90)90202-x. [DOI] [PubMed] [Google Scholar]
- 5.Carlson CG, Roshek D. Adult dystrophic mdx endplates exhibit reduced quantal sensitivity and enhanced quantal variation. Pflugers Arch Europ J Physiol. 2001;442:369–375. doi: 10.1007/s004240100561. [DOI] [PubMed] [Google Scholar]
- 6.Carlson CG, Samadi A, Siegel A. Chronic treatment with agents that stabilize cytosolic IκB-α enhances survival and improves RP in mdx muscle fibers subjected to chronic passive stretch. Neurobiol Dis. 2005;20:719–730. doi: 10.1016/j.nbd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Ludin HP. Action potentials of normal and dystrophic human muscle fibers. In: Desmedt JE, editor. New developments in electromyography and clinical neurophysiology. Vol. 1. Basel: Karger; 1973. pp. 400–406. [Google Scholar]
- 8.Woods CE, Novo D, DiFranco M, Vergara JL. The action potential-evoked sarcoplasmic reticulum calcium release is impaired in mdx mouse muscle fibers. J Physiol. 2004;557.1:59–74. doi: 10.1113/jphysiol.2004.061291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillis JM. Membrane abnormalities and Ca homeostasis in muscles of the mdx mouse, an animal model of Duchenne muscular dystrophy. Acta Physiol Scand. 1996;156:397–406. doi: 10.1046/j.1365-201X.1996.201000.x. [DOI] [PubMed] [Google Scholar]
- 10.Carlson CG. The dystrophinopathies: An alternative to the structural hypothesis. Neurobiol Dis. 1998;5:3–15. doi: 10.1006/nbdi.1998.0188. [DOI] [PubMed] [Google Scholar]
- 11.DeBacker F, Vandenbrouk C, Gailly P, Gillis JM. Long-term study of Ca2+ homeostasis and of survival in collagenase-isolated muscle fibers from normal and mdx mice. J Physiol. 2002;542.3:855–865. doi: 10.1113/jphysiol.2002.020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson CG, Gueorguiev A, Roshek DM, Ashmore R, Chu JS, Anderson JE. Extra-junctional resting Ca2+ influx is not increased in a severely dystrophic expiratory muscle (triangularis sterni)of the mdx mouse. Neurobiol Dis. 2003;14(2):229–239. doi: 10.1016/s0969-9961(03)00128-1. [DOI] [PubMed] [Google Scholar]
- 13.Hayakawa M, Miyashita H, Sakamoto I, Kitagawa M, Tanaka H, Yasuda H, Karin M, Kikugawa K. Evidence that reactive oxygen species do not mediate NF-κB activation. EMBO J. 2003;22 (13):3356–3366. doi: 10.1093/emboj/cdg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messina S, Bitto A, Aguennouz M, Minutoli L, Monici MC, Altavilla D, Sqauadrito F, Vita G. Nuclear factor kappa-B blockade reduces skeletal muscle degeneration and enhances muscle function in Mdx mice. Exp Neurol. 2006;198:234–241. doi: 10.1016/j.expneurol.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Acharyya S, Villalta A, Bakkar N, Bupha-Intr T, Janssen PML, Carathers M, Li Z-W, Beg AA, Ghosh S, Sahenk Z, Weinstein M, Gardner KL, Rafael-Fortney JA, Karin M, Tidball JG, Baldwin AS, Guttridge DC. Interplay of IKK/NF-κB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hnia V, Hugon G, Rivier V, Masmoudi V, Mercier V, Mornet V. Modulation of p38 mitogen-activated protein kinase cascade and metalloproteinase activity in diaphragm muscle in response to free radical scavenger administration in dystrophin-deficient mdx mice. Am J Pathol. 2007;170(2):633–643. doi: 10.2353/ajpath.2007.060344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan Y, Chen C, Shen Y, Zhu C-H, Wang G, Wang X-C, Chen HQ, Zhu MS. Curcumin alleviates dystrophic muscle pathology in mdx mice. Mol Cells. 2008;25 (4):531–537. [PubMed] [Google Scholar]
- 18.Whitehead NP, Pham C, Gervasio OL, Allen DG. N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J Physiol. 2008;586(7):2003–2014. doi: 10.1113/jphysiol.2007.148338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegel AL, Bledsoe C, Lavin J, Gatti F, Berge J, Millman G, Turin E, Winders WT, Rutter J, Palmeiri B, Carlson CG. Treatment with inhibitors of the NF-kappaB pathway improves whole body tension development in the mdx mouse. Neuromuscul Disord. 2009;19:131–139. doi: 10.1016/j.nmd.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Siegel AL, Henley S, Zimmerman A, Miles M, Plummer R, Kurz J, Balch F, Rhodes JA, Shinn GL, Carlson CG. The influence of passive stretch and NF-κB inhibitors on the morphology of dystrophic (mdx) muscle fibers. Anat Rec. 201X doi: 10.1002/ar.21294. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miles MT, Carlson CG. Acute inhibition of NF-κB with pyrrolidine dithiocarbamate (PDTC) improves the resting membrane potential of the triangularis sterni (TS) muscle in the dystrophic (mdx) mouse. Neuroscience Abstracts. 2007;167.5 [Google Scholar]
- 22.Dunn JF, Bannister N, Kemp GJ, Publicover SJ. Sodium is elevated in mdx muscles: ionic interactions in dystrophic cells. J Neurol Sci. 1993;114:76–80. doi: 10.1016/0022-510x(93)90052-z. [DOI] [PubMed] [Google Scholar]
- 23.Yeung EW, Head SI, Allen DG. Gadolinium reduces short-term stretch-induced muscle damage in isolated mdx mouse muscle fibers. J Physiol. 2003;552.2:449–458. doi: 10.1113/jphysiol.2003.047373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miura T, Ouchida R, Yoshikawa N, Okamoto K, Makino Y, Nakamura T, Morimoto C, Makino I, Tanaka H. Functional modulation of the glucocorticoid receptor and suppression of NF-κB-dependent transcription by ursodeoxycholic acid. J Biol Chem. 2001;276:47371–47378. doi: 10.1074/jbc.M107098200. [DOI] [PubMed] [Google Scholar]
- 25.Diarra A, Sheldon C, Church J. In situ calibration and [H+] sensitivity of the fluorescent Na+indicator SBFI. Am J Physiol Cell Physiol. 2001;280:C1623–C1633. doi: 10.1152/ajpcell.2001.280.6.C1623. [DOI] [PubMed] [Google Scholar]
- 26.Gao J, Wang W, Cohen IS, Mathias RT. Transmural gradients in Na+/K+ pump activity and [Na+]i in canine ventricle. Biophys J. 2005;89:1700–1709. doi: 10.1529/biophysj.105.062406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh R, Millman G, Turin E, Polisiakeiwicz L, Lee B, Gatti F, Berge J, Smith E, Rutter J, Sumski C, Winders WT, Samadi A, Carlson CG. Increases in nuclear p65 activation in dystrophic skeletal muscle are secondary to increases in the cellular expression of p65 and are not solely produced by increases in IκB-α kinase activity. J Neurol Sci. 2009;285:159–171. doi: 10.1016/j.jns.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 28.Logvinenko NS, Dulobova I, Fedosova N, Larsson SH, Nairn AC, Esmann M, Greengard P, Aperia A. Phosphorylation by protein kinase C of serine-23 of the α-1 subunit of rat Na+, K+ - ATPase affects its conformational equilibrium. Proc Natl Acad Sci U S A. 1996;93:9132–9137. doi: 10.1073/pnas.93.17.9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chibalin AV, Ogimoto G, Pedemonts CH, Pressley TA, Katz AI, Feraille E, Berggren PO, Bertorello AM. Dopamine-induced endocytosis of Na+, K+, -ATPase is initiated by phosphorylation of Ser-18 in the rat α subunit and is responsible for the decreased activity in epithelial cells. J Biol Chem. 1999;274(4):1920–1927. doi: 10.1074/jbc.274.4.1920. [DOI] [PubMed] [Google Scholar]
- 30.Feraille E, Carranza ML, Gonin S, Beguin P, Pedemonte C, Rousselot M, Caverzasio J, Geering K, Martin PY, Favre H. Insulin-induced stimulation of Na+, K+ -ATPase activity in kidney proximal tubule cells depends on phosphorylation of the α-subunit at Tyr-10. Mol Biol Cell. 1999;10:2847–2859. doi: 10.1091/mbc.10.9.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chibalin AV, Kovalenko MV, Ryder JW, Feraille E, Wallberg-Henriksson H, Zierath JR. Insulin- and glucose-induced phosphorylation of the Na+, K+ -Adenosine Triphosphatase α–subunits in rat skeletal muscle. Endocrinology. 2001;142(8):3474–3482. doi: 10.1210/endo.142.8.8294. [DOI] [PubMed] [Google Scholar]
- 32.Hatou S, Yamada M, Mochizuki H, Shiraishi A, Joko T, Nishida T. The effects of dexamethasone on the Na, K-ATPase activity and pump function of corneal endothelial cells. Curr Eye Res. 2009;34:347–354. doi: 10.1080/02713680902829624. [DOI] [PubMed] [Google Scholar]
- 33.Anderson JA. Myotube phospholipid synthesis and sarcolemmal ATPase activity in dystrophic mdx mouse muscle. Biochem Cell Biol. 1991;69:835–841. doi: 10.1139/o91-124. [DOI] [PubMed] [Google Scholar]
- 34.Dunn JF, Burton KA, Dauncey MJ. Ouabain sensitive Na+/K+ ATPase content is elevated in mdx mice: Implications for the regulation of ions in dystrophic muscle. J Neurol Sci. 1995;133:11–15. doi: 10.1016/0022-510x(95)00167-z. [DOI] [PubMed] [Google Scholar]
- 35.Chibalin AV. Regulation of the Na, K-ATPase: Special implications for cardiovascular complications of metabolic syndrome. Pathophysiology. 2007;14:153–158. doi: 10.1016/j.pathophys.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Koksoy AA. Na+, K+-ATPase: A review. J of Ankara Med Sch. 2002;24(2):73–81. [Google Scholar]
- 37.Aizman O, Uhlen P, Lal M, Brismar H, Aperia A. Ouabain, a steroid hormone that signals with slow calcium oscillations. PNAS. 2001;98(23):13420–13424. doi: 10.1073/pnas.221315298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Araki S, Mawatari S. Ouabain and erythrocyte-ghost adenosine triphosphatase. Arch Neurol. 1971;24:187–190. doi: 10.1001/archneur.1971.00480320115012. [DOI] [PubMed] [Google Scholar]
- 39.Peter JB, Worsfold M, Pearson CM. Erythrocyte ghost adenosine triphosphatase ATPase in Duchenne dystrophy. J Lab and Clin Med. 1969;74(1):103–108. [PubMed] [Google Scholar]
- 40.Lloyd SJ, Emery AEH. A possible circulating plasma factor in Duchenne muscular dystrophy. Clin Chim Acta. 1981;112:85–90. doi: 10.1016/0009-8981(81)90271-0. [DOI] [PubMed] [Google Scholar]
- 41.Ljubuncic P, Abu-Salach O, Bomzon A. Ursodeoxycholic acid and superoxide anion. World J Gastroenterol. 2005;11(31):4875–4878. doi: 10.3748/wjg.v11.i31.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreydiyyeh SI, Al-Sadi R. The signal transduction pathway that mediates the effect of interleukin-1 beta on the Na+ - K+ATPase in LLC-PK1 cells. Pflugers Arch Europ J Physiol. 2004;448:231–238. doi: 10.1007/s00424-004-1242-0. [DOI] [PubMed] [Google Scholar]
- 43.Kreydiyyeh SI, Abou-Chahine C, Hilal-Dandan R. Interleukin-1 beta inhibits Na+-K+ ATPase activity and protein expression in cardiac myocytes. Cytokine. 2004;26(1):1–8. doi: 10.1016/j.cyto.2003.11.014. [DOI] [PubMed] [Google Scholar]