Abstract
Purpose
The purpose of this study was to assess dietary intake habits of Mexican American Hispanic adults participating in the En Balance diabetes education program.
Methods
En Balance is a 3-month culturally sensitive diabetes education intervention for Spanish-speaking Hispanics. Of the 46 participants enrolled, 39 mainly Mexican American Hispanic adults with type 2 diabetes completed the En Balance program. Participants lived in the Riverside and San Bernardino counties of California, and all participants completed the program by June 2008. Dietary intake was assessed at baseline and at 3 months using the validated Southwest Food Frequency Questionnaire.
Results
Clinically important decreases in glycemic control and serum lipid levels were observed at the end of the 3-month program. The baseline diet was characterized by a high intake of energy (2478 ± 1140 kcal), total fat (87 ± 44 g/day), saturated fat (28 ± 15 g/day), dietary cholesterol (338 ± 217 mg/day), and sodium (4236 ± 2055 mg/day). At 3 months, the En Balance group mean intake of dietary fat (P = .045) and dietary cholesterol (P = .033) decreased significantly. Low dietary intakes of docosahexaenoic acid, eicosapentaenoic acid, and vitamin E were also observed in these adults with type 2 diabetes.
Conclusions
The En Balance program improved glycemic control and lipid profiles in a group of Hispanic diabetic participants. En Balance also promoted decreases in dietary fat and dietary cholesterol intake.
According to 2007 prevalence statistics, diabetes affected about 24 million people nationwide, or about 7.4% of the US population.1 Diabetes disproportionately affects minority groups such as Native Americans and Alaska Natives, Blacks, and Hispanics. In 2007, the nationwide prevalence rate for physician-diagnosed diabetes in Hispanics was 10.4% overall and 11.9% for Mexican Americans.1 Hispanics suffer more from diabetic complications when compared to national rates and when compared to non-Hispanic whites.1
Hispanic diabetes health disparities are prevalent at the national, state, and county level in California.2 California is home to the largest population of Hispanics in the United States (ie, 31% of the Hispanics in the US).3 The Hispanic population of California is overwhelmingly Mexican (84%).3 In countywide comparisons, Hispanics still have higher rates of diagnosed diabetes when compared to non-Hispanic whites.2 Along with having higher rates of diagnosed diabetes, Hispanics in California are largely uninsured.2
The US Census Bureau projects that Hispanics will comprise 24% of the population by 2050.4 When compared to non-Hispanic whites, Hispanics continue to bear a disproportionate burden of disease, disability, and death from certain health conditions.5 Limited access to health care, underdiagnosis, low rates of blood glucose self-monitoring, and low income and education may contribute to the diabetes health disparities seen in the Hispanic population. Low literacy and, specifically, low health literacy may be the underlying obstacle to surmount when working with disadvantaged populations.6–9 Culturally appropriate diabetes education interventions are well received by Hispanic groups,10,11 and several have been designed and implemented with the aim of improving glycemic control,11–14 increasing diabetes knowledge and self-efficacy,15–17 or both.18–20 Nonetheless, few diabetes education studies have adequately characterized and addressed the dietary intake patterns of disadvantaged Hispanics. Assessing dietary patterns in Hispanics with diabetes is increasingly relevant given the documented protective effects that nutrients such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) have against cardiovascular disease.21–23 Mexican American adult estimates for DHA and EPA intake vary: according to 2002 dietary intake estimates,24 0.04 to 0.08 g per day for DHA and 0.02 to 0.04 g per day for EPA; according to 2008 dietary intake estimates,25 0.05 g per day for EPA and 0.09 g per day for DHA. As with other dietary intake trends, higher education and acculturation have been associated with higher intakes of DHA and EPA.26 Recent comparisons report lower omega-3 fatty acid consumption in Hispanics when compared to non-Hispanic whites and African Americans.27,28
In light of the projected Hispanic population growth and because alarming diabetes health disparities continue to exist, it is imperative to design effective, culturally competent diabetes intervention programs that address the lifestyle habits that are at the core of the diabetes and obesity epidemics in Hispanics. The purpose of this study was to assess the dietary intake habits of Mexican American Hispanic adults participating in the En Balance diabetes education program. The program objectives are to improve glycemic control, change dietary habits, and increase physical activity in underserved San Bernardino County Hispanics with type 2 diabetes.
Methods
Sample and Setting
A total of 39 Hispanic adults between 25 and 75 years of age with self-reported type 2 diabetes completed this 3-month intervention study. The Southern California En Balance participants were all San Bernardino and Riverside county residents. Program recruitment efforts specifically targeted Hispanic disadvantaged adults with type 2 diabetes by posting recruitment flyers in local grocery stores and by publishing a program hotline in newspaper articles, as well as through announcements in a Spanish radio station and through physician referrals from local medical clinics. The participants were initially screened through telephone interviews and excluded if they had a previous clinical history of drug or alcohol abuse, steroid use, and psychological or other major systemic disease that could affect program compliance, such as end-stage renal disease. The participants were also interviewed in person by a research staff member to determine diabetes history, medication use, and diet and physical activity habits. The En Balance program was conducted in 2 phases: 26 participants finished the program in the first phase and 13 finished in the second. Of the original 46 participants enrolled, 39 completed the En Balance diabetes education program. The participants that dropped out of the program did so for different reasons: 2 traveled out of the country during the most active part of the program; another 2 experienced changes in their work schedules, which could not accommodate the program meetings; another was diagnosed with a serious liver disease; and the last 2 stopped coming to the program meetings. All participants agreed to and signed a Health Insurance Portability and Accountability Act–compliant informed consent form. The study was approved by the Loma Linda University Institutional Review Board.
Data Collection
Baseline and 3-month data collection included fasting blood serum samples; bioelectrical impedance analysis; anthropometric measurements for weight, height, and waist and hip circumferences; and dual-energy X-ray absorptiometry (Hologic Fan Beam, Discovery A Software Version, Hologic Inc, Bedford, MA) values for all 39 participants. Blood serum samples were tested at the Loma Linda University Medical Center laboratory to determine fasting blood glucose, A1C, insulin, and lipid profiles (high-density lipoprotein [HDL], low-density lipoprotein [LDL], total cholesterol, and triglycerides). All anthropometric measurements were taken twice for reliability, using Lohman’s standardized techniques.29 Weight and height were assessed using a balance scale (Detecto, Web City, Missouri) and a wall-mounted stadiometer (Holtain Ltd Crymych, Dyfed, England), respectively. The validated University of Arizona Southwest Food Frequency Questionnaire30 was administered to the En Balance participants during organized questionnaire clinics. All dietary intake records were analyzed using the Metabolize Nutrient Analysis System 2.5.31
The En Balance Diabetes Education Program Approach
The En Balance approach has been described elsewhere.32,33 Briefly, the En Balance Diabetes Education Program is a hands-on, culturally competent diabetes education program for Hispanics. After baseline data collection, the study participants attended a comprehensive diabetes education program hosted at the Loma Linda University School of Public Health. Hispanic professionals (registered dieticians, dentists, physical therapists, and nurses) conducted all classes in Spanish. Clinics were scheduled on Sundays, and classes on weekday evenings, to accommodate participants’ work schedules. Research staff also arranged transportation for participants who were otherwise unable to make the clinic or class appointments. The diabetes education program consisted of four 2-hour presentations. The En Balance participants were taught how to self-monitor their glucose levels and record their blood glucose levels in a log. All participants received free glucose monitors, strips, and lancets. Nutrition topics were taught using a hands-on, culturally relevant approach using food models and comparable hand measurements. Recommendations for changes in diet focused on smaller portion sizes and choosing healthier alternatives within culturally specific food groups, rather than forgoing traditional dishes.
Data Analysis
Statistical analyses were performed using SPSS 17.0. Power calculations suggested that 44 participants were necessary to have at least 80% power to detect a 13% decrease in fasting blood glucose. However, the effective power was reduced to 75% based on the 39 participants who completed the program. Type 1 error was set at α = .05 for statistical significance. In this experimental design, the participants are their own controls. Paired-samples t tests, independent-samples t test, and Wilcoxon signed-rank tests were used to compare baseline and 3-month means; χ2 was used to compare categorical data. The data are shown as mean ± SD. Spearman’s correlation coefficient was used to determine the linear relationship between blood serum changes and dietary changes.
Results
Overall, the mean age of the participant group was 53.95 ± 11.21 years; the mean weight was 81.62 ± 17.66 kg; and the mean body mass index was 31.67 kg/m2. Men made up about 41% of the study sample, and the primary language for most of the group (89.7%) was Spanish. Height and body mass index were significantly different between men and women (see Table 1); the difference in their height largely accounts for the difference in their body mass index. About 48.7% of the participants had less than a high school education, and the majority completed their education outside of the United States.
Table 1.
En Balance Participant Characteristics at Baselinea
| Men (n, 16) | Women (n, 23) | |
|---|---|---|
| Age, years | ||
| 25–34 | 0.0 | 8.7 |
| 35–44 | 18.8 | 0.0 |
| 45–54 | 43.7 | 43.5 |
| 55–64 | 12.5 | 30.4 |
| 65+ | 25.0 | 17.4 |
| Mean ± SD | 52.63 ± 9.91 | 54.87 ± 12.16 |
| P | .122 | |
| Weight,b kg | ||
| 50–64 | 12.5 | 18.2 |
| 65–79 | 56.3 | 27.3 |
| 80–94 | 25.0 | 31.8 |
| 95–109 | 0.0 | 4.5 |
| 110+ | 6.2 | 18.2 |
| Mean ± SD | 78.26 ± 12.51 | 84.07 ± 20.56 |
| P | .401 | |
| Height, cm | ||
| 145–154 | 0.0 | 50.0 |
| 155–164 | 31.3 | 40.9 |
| 165–174 | 50.0 | 9.1 |
| 175+ | 18.7 | 0.0 |
| Mean ± SD | 168.0 ± 6.53 | 155.32 ± 6.23 |
| P | < .001** | |
| Body mass index,b kg/m2 | ||
| 18.5–24.9 | 25.0 | 4.5 |
| 25.0–29.9 | 56.3 | 31.9 |
| 30.0–39.9 | 18.7 | 36.3 |
| 40.0+ | 0.0 | 27.3 |
| Mean ± SD | 27.56 ± 3.30 | 34.66 ± 7.06 |
| P | .022* | |
| Primary language | ||
| Spanish | 93.8 | 87.0 |
| English | 6.2 | 13.0 |
| P | .492 | |
| Birthplace | ||
| United States | 0.0 | 13.0 |
| Mexico | 93.8 | 78.3 |
| Puerto Rico | 6.2 | 4.4 |
| Dominican Republic | 0.0 | 4.3 |
| P | .374 | |
| Highest level of education | ||
| No formal education | 6.3 | 4.3 |
| Some/finished primary | 25.0 | 34.8 |
| Some/finished junior high | 18.7 | 8.7 |
| Some/finished high school | 18.8 | 26.1 |
| Some/finished collegec | 25.0 | 17.4 |
| Some/finished master’sc | 6.2 | 0.0 |
| Missing data | 0.0 | 8.7 |
| P | .620 | |
n, 39. In percentages unless noted otherwise. P values are based on χ2 test.
For weight and body mass index: n, 38.
Six finished college in Mexico; 2 attended a US community college; 1 obtained a US graduate degree.
P < .05.
P < .001.
The group of 39 participants had an average baseline fasting blood glucose of 167.9 mg/dL, a baseline A1C mean of 8.5%, and an insulin mean of 13.7 uU/mL (Table 2). The participants made positive clinical improvements in all three blood glucose management markers. At 3 months, the A1C mean decreased (−0.894%, P = .008) and the insulin mean increased 3.01 uU/mL (P = .05) for the group. Total, LDL, and HDL cholesterol means decreased 13.43 mg/dL (P = .005), 10.28 mg/dL (P = .030), and 2.84 mg/dL (P = .012), respectively. Body weight and body mass index means did not change appreciably from baseline to 3 months.
Table 2.
Changes in Serum Blood Glucose Profiles, Serum Blood Lipid Profiles, Weight, and Body Mass Index After 3 months of the En Balance Diabetes Education Programa
| Variables | Baseline | Three Months | Mean Difference | P |
|---|---|---|---|---|
| FBG (mg/dl) | 167.90 ± 82.46 | 154.26 ± 70.16 | −13.64 | 0.134 |
| A1C, % | 8.53 ± 2.58 | 7.63 ± 1.71 | −0.894 | .008* |
| Insulin, uU/mL | 13.72 ± 11.31 | 16.73 ± 13.33 | 3.01 | .050* |
| Cholesterol, mg/dL | ||||
| Total | 191.38 ± 34.30 | 177.94 ± 40.98 | −13.43 | .005* |
| LDL | 120.67 ± 32.27 | 110.38 ± 34.80 | −10.28 | .030* |
| HDL | 49.74 ± 10.48 | 46.90 ± 9.97 | −2.84 | .012* |
| Cholesterol:HDL | 3.99 ± 1.05 | 3.94 ± 1.12 | −0.048 | .679 |
| Triglycerides, mg/dL | 166.21 ± 83.67 | 170.79 ± 102.77 | 4.59 | .641 |
| Body weight, kg | 81.62 ± 17.66 | 81.62 ± 17.11 | −0.003 | .993 |
| Body mass index, kg/m2 | 31.67 ± 6.73 | 31.45 ± 6.47 | −0.218 | .246 |
Both sexes: n, 39. The data are shown as mean ± SD. P value is based on Wilcoxon signed–rank test for A1C and LDL; otherwise, P value is based on paired-samples t test. FBG, fasting blood glucose; LDL, low-density lipoprotein; HDL, high-density lipoprotein. For body weight and body mass index: n, 38.
P < .05.
Table 3 summarizes changes in food frequency questionnaire dietary intake values at the end of the En Balance Diabetes Education Program. Eight food frequency questionnaire records were excluded from the nutrient intake statistical analyses because they underestimated total nutrient intake (ie, energy estimates fell under 1000 kcal) or because they overestimated dietary intake (ie, energy estimates exceeded 5000 kcal) and were inconsistent with age, occupation, and body mass index. Subsequent dietary intake analyses were performed with the data from the remaining 31 participants. The group consistently decreased overall dietary intake by the end of the 3-month program. The group decreased its mean intake of protein (P = .058) and dietary cholesterol (P = .033) in line with the total decrease in energy intake (see Table 3). Although not statistically significant, the group means for carbohydrates, energy, and saturated fat decreased (see Table 3).
Table 3.
Changes in Selected Food Frequency Questionnaire Dietary Intake Variables After 3 Months of the En Balance Diabetes Education Programa
| Variables | Baseline | Three Months | Mean Difference | P |
|---|---|---|---|---|
| Energy, kcal | 2478.89 ± 1140.39 | 2084.96 ± 741.48 | −393.92 | .065 |
| Carbohydrate, g | 331.10 ± 160.42 | 286.28 ± 119.66 | −44.82 | .161 |
| Protein, g | 105.38 ± 45.75 | 89.32 ± 30.60 | −16.06 | .058 |
| Total fat, g | 87.27 ± 44.42 | 71.06 ± 26.46 | −16.20 | .045* |
| Total fiber, g | 39.46 ± 18.92 | 37.97 ± 23.01 | −1.49 | .327b |
| Cholesterol, mg | 338.63 ± 217.50 | 259.41+ ± 163.21 | −79.22 | .033b* |
| Saturated fat, g | 28.00 ± 15.45 | 23.09 ± 9.12 | −4.91 | .073 |
| Monounsaturated fatty acid, g | 33.79 ± 17.89 | 27.38 ± 10.26 | −6.41 | .136b |
| Polyunsaturated fatty acid, g | 17.27 ± 8.25 | 13.95 ± 5.88 | −3.31 | .112b |
| Linoleic acid, g | 15.37 ± 7.36 | 12.31 ± 5.22 | −3.06 | .112b |
| α-Linolenic acid, g | 1.50 ± 0.83 | 1.30 ± 0.58 | −0.20 | .232b |
| Palmitic acid, g | 15.60 ± 8.24 | 12.91 ± 4.92 | −2.69 | .158b |
| Arachidonic acid, g | 0.17 ± 0.09 | 0.13 ± 0.10 | −0.04 | .158b |
| DHA, g | 0.108 ± 0.139 | 0.090 ± 0.095 | −0.017 | .922b |
| EPA, g | 0.038 ± 0.046 | 0.033 ± 0.033 | −0.005 | .891b |
| Vitamin E, mg | 13.45 ± 9.39 | 10.07 ± 4.66 | −3.38 | .100b |
| Vitamin C, mg | 226.6 ± 156.4 | 197.72 ± 106.11 | −28.88 | .327b |
| Vitamin A, μg | 2125.0 ± 1985.8 | 2402.7 ± 2773.1 | 277.7 | .668 |
| Beta carotene, μg | 6741.5 ± 5855.5 | 7282.1 ± 6046.2 | 540.56 | .493b |
| Selenium, μg | 130.01 ± 60.25 | 107.16 ± 40.14 | −22.85 | .112b |
| Sodium, mg | 4236.6 ± 2055.7 | 3650.7 ± 1304.1 | −585.8 | .272b |
| Calories, % | ||||
| Total fat | 31.48 ± 5.71 | 30.89 ± 4.46 | −0.59 | .628 |
| Saturated fat | 10.05 ± 2.35 | 10.10 ± 2.08 | 0.099 | .845 |
| Monounsaturated fatty acid | 12.13 ± 2.81 | 11.90 ± 1.81 | −0.231 | .678 |
| Polyunsaturated fatty acid | 6.33 ± 1.27 | 5.93 ± 1.06 | −0.398 | .129 |
| Protein | 17.31 ± 2.89 | 17.60 ± 3.26 | 0.284 | .654 |
| Carbohydrates | 53.39 ± 7.93 | 54.09 ± 8.13 | 0.700 | .666b |
| Alcohol | 0.166 ± .370 | 0.198 ± 0.613 | 0.032 | .775b |
Both sexes: n, 31. All values are based on per day. The data are shown as mean ± SD. Eight food frequency questionnaire records were excluded from these analyses owing to total calorie underestimation (< 1000 kcal) or overestimation (> 5000 kcal) at baseline or 3 months. DHA, 22:6 docosahexaenoic acid; EPA, 20:5 eicosapentaenoic acid.
P value based on Wilcoxon signed–rank test; otherwise, P value based on paired-samples t test.
P < .05.
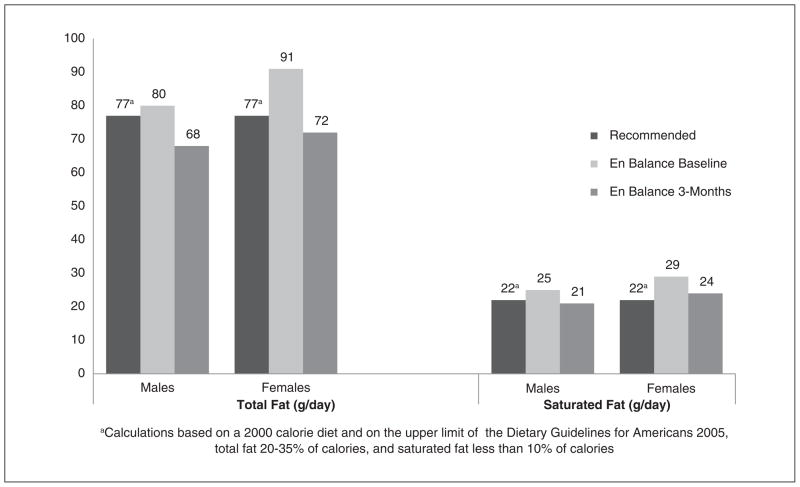
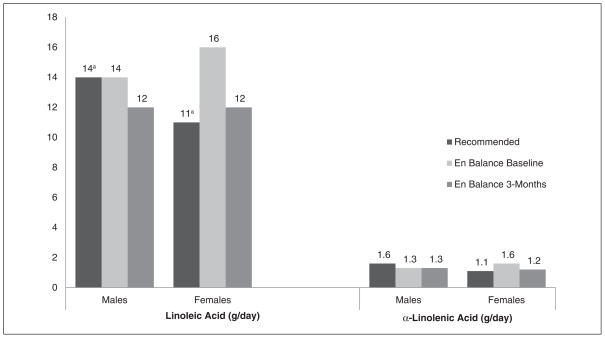
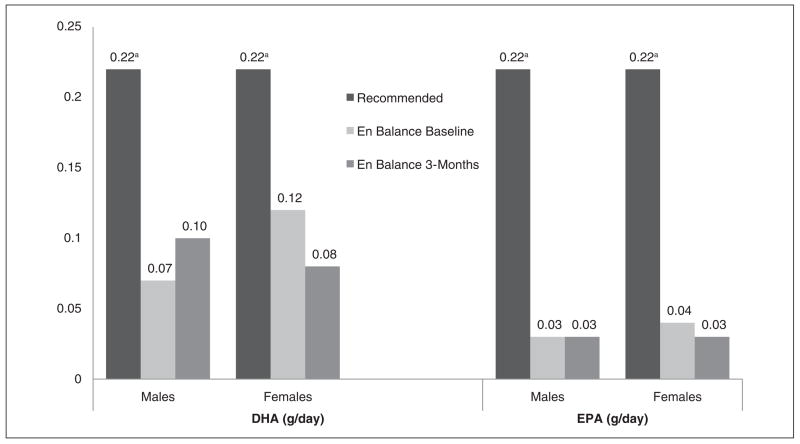
Table 4 displays the dietary fat intake profile for the En Balance participants at baseline and 3 months, by sex and as compared to recommended national guidelines.34 Total fat intake was high for both men and women at baseline when compared to the acceptable macronutrient distribution range (see Figure 1).35 At 3 months, men’s and women’s mean total fat intake fell within recommended guidelines (see Table 4 and Figure 1). Saturated fat intake was high for men and women at baseline, but men decreased their mean intake as a group and achieved normal mean intake levels at 3 months. Linoleic and α-linolenic acid intake varied between men and women (see Figure 2). DHA and EPA mean intake was low for the men and women of the program when compared to the adequate intake recommendations set forth by the Workshop on the Essentiality of and Recommended Dietary Intakes for Omega-6 and Omega-3 Fatty Acids (see Figure 3).36 Only the En Balance men were well within the recommended guideline to consume less than 10% of calories from saturated fat.
Table 4.
Sex-Specific Fat Intake Profile for the En Balance Participants at Baseline and 3 Monthsa
| Lipid Intake | Recommended | Baseline | Three Months | P |
|---|---|---|---|---|
| Men: n, 13 | ||||
| Total fat | 44–78b | 80.91 ± 29.32 | 68.82 ± 22.80 | .279 |
| Saturated fat | ≤ 22b | 25.29 ± 9.09 | 21.22 ± 7.65 | .311 |
| Linoleic acid | 14–17c | 14.46 ± 5.29 | 12.16 ± 5.05 | .382 |
| α-Linolenic acid | 1.6c | 1.36 ± 0.68 | 1.34 ± 0.55 | .753 |
| DHA | 0.22d | 0.07 ± 0.05 | 0.10 ± 0.09 | .650 |
| EPA | 0.22d | 0.03 ± 0.02 | 0.03 ± 0.03 | .600 |
| Calories, % | ||||
| Total fat | 20–35e | 31.84 ± 6.58 | 29.75 ± 4.93 | .552 |
| Saturated fat | ≤ 10f | 9.97 ± 2.08 | 9.25 ± 2.25 | .507 |
| Monounsaturated fatty acid | NR | 12.53 ± 3.27 | 11.71 ± 1.76 | .807 |
| Polyunsaturated fatty acid | NR | 6.35 ± 1.42 | 5.85 ± 1.13 | .507 |
| Women: n, 18 | ||||
| Total fat | 44–78b | 91.86 ± 53.12 | 72.68 ± 29.37 | .248 |
| Saturated fat | ≤ 22b | 29.96 ± 18.79 | 24.44 ± 10.04 | .327 |
| Linoleic acid | 11–12c | 16.02 ± 8.65 | 12.42 ± 5.48 | .231 |
| α-Linolenic acid | 1.1c | 1.60 ± 0.93 | 1.27 ± 0.62 | .094 |
| DHA | 0.22d | 0.12 ± 0.17 | 0.08 ± 0.08 | .557 |
| EPA | 0.22d | 0.04 ± 0.05 | 0.03 ± 0.03 | .616 |
| Calories, % | ||||
| Total fat | 20–35e | 31.23 ± 5.18 | 31.72 ± 4.02 | .557 |
| Saturated fat | ≤ 10f | 10.02 ± 2.58 | 10.71 ± 1.77 | .231 |
| Monounsaturated fatty acid | NR | 11.85 ± 2.48 | 12.04 ± 1.88 | .983 |
| Polyunsaturated fatty acid | NR | 6.32 ± 1.20 | 5.99 ± 1.03 | .112 |
With the exception of calories, values based on grams per day. The data are shown as mean ± SD. P value is based on Wilcoxon signed–rank test comparing En Balance baseline and 3-month values. DHA, 22:6 docosahexaenoic acid; EPA, 20:5 eicosapentaenoic acid; NR, no recommendation established.
Calculations based on a 2000-kcal diet and on the Dietary Guidelines for Americans34: total fat, 20% to 35% of calories; saturated fat, < 10% of calories.
Adequate intakes for age: ≥ 19 years.
Adequate intake recommendations for adults from the Workshop on the Essentiality of and Recommended Dietary Intakes for Omega-6 and Omega-3 Fatty Acids.36
Acceptable macronutrient distribution range based on the Dietary Reference Intake recommendations.
Based on the Dietary Guidelines for Americans34: saturated fat < 10% of calories.
Figure 1.
En Balance participants’ dietary intake of total fat and saturated fat (in grams per day) compared with recommendations from the Dietary Guidelines for Americans.34
aCalculations based on a 2000-kcal diet and on the upper limit of the Dietary Guidelines for Americans: total fat, 20% to 30% of calories; saturated fat, < 10% of calories.
Figure 2.
En Balance participants’ dietary intake of linoleic acid and α-linolenic acid (in grams per day) compared with the adequate intake guidelines.
aBased on the lower limit of the adequate intake recommendation for the En Balance age group (25–75 years).
Figure 3.
En Balance participants’ dietary intake of DHA and EPA (grams per day) compared with adequate intake guidelines for adults.
DHA, 22:6 docosahexaenoic acid; EPA, 20:5 eicosapentaenoic acid.
aAdequate intakes recommendations for adults from the Workshop on the Essentiality of and Recommended Dietary Intakes for Omega-6 and Omega-3 Fatty Acids.36
Table 5 summarizes the En Balance participant anti-oxidant intake profile. In general, the antioxidant intake for this group was well above the dietary reference intakes for both sexes, with the exception of vitamin E. Intake of vitamin E was lower than the Dietary Reference Intake recommendations for both sexes at baseline, and it was particularly low at 3 months (see Table 5).35 Both sexes decreased their overall antioxidant mean intake, except that men increased vitamin A and beta carotene intake at 3 months. The 3-month mean increase in vitamin A and beta carotene observed in the En Balance male group was due to 2 influential outliers. Only the vitamin C decrease in women was statistically significant from baseline to 3 months (see Table 5).
Table 5.
Sex-Specific Antioxidant Intake Profile for the En Balance Participants at Baseline and 3 Monthsa
| Antioxidant Intake | Recommended | Baseline | Three Months | P |
|---|---|---|---|---|
| Men: n, 13 | ||||
| Vitamin E, mg | 15b,c | 13.19 ± 9.77 | 10.32 ± 4.81 | .552 |
| Vitamin C, mg | 90b | 208.73 ± 172.12 | 229.60 ± 123.78 | .552 |
| Vitamin A, μg | 900b,d | 1815.12 ± 1645.48 | 3264.11 ± 3868.18 | .196 |
| Beta carotene, μg | NR | 7648.05 ± 7440.54 | 9512.80 ± 7829.29 | .196 |
| Selenium, μg | 55b | 119.78 ± 40.76 | 108.93 ± 36.89 | .600 |
| Women: n, 18 | ||||
| Vitamin E, mg | 15b,c | 13.64 ± 9.39 | 9.89 ± 4.68 | .078 |
| Vitamin C, mg | 75b | 239.51 ± 147.75 | 174.70 ± 87.84 | .018* |
| Vitamin A, μg | 700b,d | 2348.88 ± 2218.51 | 1780.65 ± 1425.40 | .286 |
| Beta carotene, μg | NR | 6086.84 ± 4510.71 | 5671.04 ± 3832.85 | .500 |
| Selenium, μg | 55b | 137.40 ± 71.39 | 105.88 ± 43.35 | .112 |
NR, no recommendation established. Values based on per day. Data are shown as mean ± SD. P value is based on Wilcoxon signed–rank test comparing En Balance baseline and 3-month values.
Recommended dietary allowances.
Vitamin E recommendations expressed as α-tocopherol
Vitamin A recommendations expressed as retinol activity equivalents.
P < .05.
Table 6 summarizes selected correlations between changes in blood serum values and dietary intake variables at 3 months. At the end of the 3-month En Balance diabetes education program, changes (ie, 3 months minus baseline) in serum A1C were positively correlated to changes in percentage calories from saturated fat (P = .036). Changes in serum total cholesterol values were negatively correlated to changes in calcium, phosphorus, zinc, vitamin A, and vitamin C intake. Likewise, changes in serum LDL cholesterol were negatively correlated to changes in calcium, phosphorus, zinc, vitamin A, vitamin C and arachidonic acid intake, as well as energy, linolenic acid, and arachidonic acid intake (see Table 6).
Table 6.
En Balance Spearman Correlation Coefficients Using Laboratory and Dietary Intake Change (Δ) Variablesa
| Δ Variables | Δ A1C, % | Δ Cholesterol, mg/dL | Δ HDL, mg/dL | Δ LDL, mg/dL | Δ Triglycerides, mg/dL |
|---|---|---|---|---|---|
| Energy, kcal | .078 (.675) | −.318 (.081) | −.115 (.537) | −.372* (.039) | .160 (.391) |
| Alcohol, g | −.026 (.888) | .106 (.570) | −.316 (.083) | .017 (.929) | .461* (.009) |
| Calcium, mg | .143 (.443) | −.410* (.022) | −.080 (.669) | −.463* (.009) | .233 (.207) |
| Phosphorus, mg | .093 (.619) | −.377* (.037) | −.082 (.661) | −.410* (.022) | .122 (.514) |
| Zinc, mg | .067 (.719) | −.411* (.022) | −.176 (.344) | −.456* (.010) | .093 (.620) |
| Vitamin A, mcg (RE) | −.101 (.587) | −.488* (.005) | −.349 (.054) | −.502* (.004) | .156 (.402) |
| Vitamin E, mg (ATE) | −.007 (.969) | −.315 (.085) | −.311 (.089) | −.371* (.040) | .190 (.306) |
| Vitamin C, mg | −.239 (.196) | −.368* (.042) | −.362* (.045) | −.436* (.014) | .226 (.222) |
| Linolenic acid, g | −.102 (.585) | −.290 (.113) | −.243 (.188) | −.363* (.044) | .199 (.283) |
| Arachidonic acid, g | .040 (.832) | −.338* (.005) | −.177 (.340) | −.363* (.045) | −.116 (.534) |
| Calories, % | |||||
| Saturated fat | .378* (.036) | −.011 (.953) | .101 (.588) | −.014 (.942) | −.136 (.467) |
| Protein | −.169 (.362) | −.099 (.597) | .146 (.434) | .013 (.943) | −.489* (.005) |
| Carbohydrate | −.178 (.337) | .105 (.573) | −.057 (.759) | .067 (.720) | .369* (.041) |
| Alcohol | −.037 (.843) | .154 (.408) | −.330 (.070) | .084 (.653) | .467* (.008) |
Change variables equal 3-month minus baseline for selected variables. P values in parentheses. Both sexes: n, 31. Eight food frequency questionnaire records were excluded from this analysis owing to total calorie underestimation (< 1000 kcal) or overestimation (> 5000 kcal) at baseline or 3 months. All other laboratory and dietary intake change variables were not statistically significant. HDL, high-density lipoprotein; LDL, low-density lipoprotein; RE, retinol equivalents; ATE, α-tocopherol equivalents.
P < .05.
Discussion
The En Balance Effect on Glycemic Control and Body Composition
The participants of the En Balance Diabetes Education Program were able to improve glycemic control and serum lipid management levels. Although the decrease in fasting blood glucose from baseline to 3 months was not statistically significant (mainly due to power considerations), the decrease was clinically important. As a group, the participants were able to lower total cholesterol and LDL cholesterol. HDL cholesterol also decreased, due to the overall total cholesterol decrease, but this HDL decrease was relatively small. The participants did not improve in mean triglyceride levels. This finding is consistent with what the American Diabetes Association calls “the most common pattern of dyslipidemia in type 2 diabetes patients”: elevated triglyceride levels and decreased HDL cholesterol levels.37 According to the American Diabetes Association, the initial therapy for elevated triglyceride levels is better glycemic control. The En Balance Diabetes Education Program approach was effective in accomplishing similar clinical improvements in a previous Hispanic participant group (n, 34).32 Note, however, that the En Balance participants (n, 38) did not lose weight at the end of the 3-month program. In the CoDE program (ie, the Community Diabetes Education program) for Mexican Americans, body mass index did not significantly change from baseline to 12 months in spite of significant improvements in A1C at 12 months.14 In another diabetes intervention tailored for Mexican Americans, significant weight loss was not necessarily associated with significant A1C decreases.20
Cultural competency is a term that has been used to define diabetes education programs that provide interventions that are accessible to the community and reflect the cultural characteristics and preferences of that community.18 Brown et al documented similar glycemic management success using a culturally competent diabetes education program for Mexican Americans along the Texas-Mexico border.13,15,16,18 Although the En Balance participant group (n, 39 for clinical data) was smaller than the group sample in the Starr County study (n, 256), En Balance participants experienced similar significant improvements in A1C levels.18 They successfully improved glycemic control in a 3-month interval with only 8 hours of diabetes education, whereas the Starr County intervention required 52 contact hours.18 Culica et al found that A1C was significantly reduced (P < .01) in patients who participated in a low-cost, culturally appropriate 12-month CoDE intervention that targets Mexican Americans.14 Another diabetes management program, called Project Dulce, reported improved clinical outcomes similar to the En Balance findings on A1C and total cholesterol in a group of 210 high-risk Hispanics with type 2 diabetes.19
Food Frequency Questionnaire Use in Hispanics
Although other culturally competent diabetes education programs have reported positive clinical changes, En Balance is one of the first, to our knowledge, to report detailed changes in dietary intake and lifestyle habits. The survey instrument used, the University of Arizona Southwest Food Frequency Questionnaire, was validated for a Hispanic population in Arizona; it is an adaptation of the Arizona Food Frequency Questionnaire,30,38 which is a version of the Health Habits and History Questionnaire, developed at the National Cancer Institute.38 The questionnaire contains foods common in the Southwest region of the United States, and it is printed in Spanish with English translation. The complete adult questionnaire includes a list of about 159 foods, and it asks for frequency and portion size (in small, medium, and large).30 The Arizona Food Frequency Questionnaire and the Southwest Food Frequency Questionnaire have been used in other studies.39,40
The use of food frequency questionnaires in minority populations warrants special attention.41–43 The Southwest Food Frequency Questionnaire has been validated in a mainly Mexican American Hispanic group; it lists commonly consumed Mexican foods; and it reports dis-attenuated correlations that range from r = .55 for energy means to r = .68 for protein.30 Due to the overall low literacy of the participants and the length of the questionnaire, special clinics were organized to administer the questionnaire by interview. Validation of food frequency questionnaires and low literacy among disadvantaged Hispanics are well-documented concerns that can be addressed by administering questionnaires through interview.43–45 Eight of 39 En Balance participants still did not accurately estimate food intake, and their records were excluded from the nutritional analyses.
Nutritional Intake Patterns in Mexican Americans
The baseline nutritional profile of the En Balance participants characterizes an overall group diet that is high calorie, high fat, high cholesterol, and high sodium (see Table 3). However, this group of En Balance participants consumed higher-than-recommended levels of fiber and certain antioxidants, such as vitamin C, vitamin A, and selenium (see Tables 3 and 5), and the mean intakes for these antioxidants remained within the recommended values at 3 months.35 The group made clinically important nutritional intake decreases in the major macronutrients: energy in total calories, carbohydrates, protein, total fat, dietary cholesterol, and saturated fat (see Table 3). Of these mean decreases, only total fat (P = .045) and dietary cholesterol (P = .033) were statistically significant, but more important, the decreases measured at 3 months bring the group mean intake of total fat and cholesterol within recommended ranges.35 Other researchers who have attempted to characterize the Mexican American diet have found that, traditionally, it is characterized by a high intake of fiber as well as cholesterol and a greater proportion of energy from fat.46,47 If primary language and country of birth (see Table 1) are used as a proxy for acculturation in the En Balance group, then the fiber and vitamin A baseline and 3-month intakes support previous findings that Mexican Americans born in Mexico and those that are less acculturated consume more fiber and higher levels of vitamin A.48–50 However, choosing a healthier traditional Mexican diet may be confounded by the overall low literacy and low English literacy of the En Balance participants.8,9,26,51 The baseline and 3-month male and female mean intakes of vitamin E were lower than dietary reference intake recommendations but comparable to the low national estimates of intake across ethnic groups, according to data from the National Health and Nutrition Examination Survey, 2005–2006.25,35 Likewise, the baseline En Balance participant mean intake of sodium was much higher than the recommended adequate intake values, and it exceeded tolerable upper intake levels but was similar to the general high-sodium American diet.25,35
The total fat, saturated fat, and dietary cholesterol decreases at 3 months may account for the significant decreases in serum levels of LDL, HDL, and total cholesterol, despite the lack of significant weight loss in the participant group. Although the decrease in carbohydrate intake at 3 months was not statistically significant, the mean decrease of 44 g per day is roughly equivalent to a reduction of 3 starch servings or 3 corn or flour tortillas a day, according to the American Diabetes Association exchange system.52 These results imply that the En Balance participants responded to program recommendations to decrease tortilla intake to 1 per meal. The group decrease in serum A1C probably resulted from this decrease in carbohydrate intake. Also, the positive correlation between change in A1C and change in percentage calories from saturated fat, from baseline to 3 months (see Table 6), suggests that those who decreased their A1C levels also decreased their intake from saturated fat. The largest percentage decreases in dietary intake, from baseline to 3 months, came from total fat (−18%), saturated fat (−17%), and dietary cholesterol (−23%) (see mean differences, Table 3). In comparison, carbohydrate and protein intake decreased by 13% and 15%, respectively. Notably, the group decrease in serum LDL levels at 3 months was negatively correlated with several dietary intake variables, including energy, vitamin A, vitamin E, vitamin C, linolenic, and arachidonic acid, suggesting that for those who decreased in LDL levels, intake of those nutrients increased. An increase in vitamin C intake, from baseline to 3 months, was significantly correlated to a decrease in LDL, HDL, and total cholesterol serum levels.
Fatty Acid Intake Profile
The baseline dietary intake profile of the En Balance group of type 2 diabetes participants shows a diet that exceeds the recommended intake of total fat and saturated fat. At baseline, the En Balance group mean and the means for men and women (when analyzed separately) were well above the recommended intakes according to the Dietary Guidelines for Americans that advise that total fat intake should be within 44 g to 78 g of fat and saturated fat should be less than 22 g of total intake, based on a 2000-kcal diet (see Table 3 and Figure 1).34 These findings are consistent with the Action for Health in Diabetes (Look AHEAD) trial, which found that overweight adults with type 2 diabetes were consuming too much fat, saturated fat, and sodium.53 The En Balance male mean intake of linoleic acid, already at the minimum adequate intake guideline recommendation at baseline, fell below the adequate intake guideline at 3 months, and although the male mean intake for α-linolenic acid did not change from baseline to 3 months, the mean intake reflects a lower-than-recommended intake of α-linolenic acid.34 These findings suggest that dietary interventions should be tailored for male Hispanics who might be consuming low intakes of polyunsaturated fatty acids. The overall En Balance group mean intake of DHA and EPA is alarmingly low (about 108 mg and 38 mg per day, respectively). Furthermore, the male mean intake of DHA and EPA was lower than that of females at baseline (about 70 mg and 30 mg per day vs 120 mg and 40 mg per day). En Balance men increased DHA intake, and women decreased DHA intake at 3 months. Low intakes of omega-3 fatty acids in Hispanics were also reported by the Action for Health in Diabetes (Look AHEAD) study, where Hispanics had a combined DHA + EPA intake of 152 mg per day.28
Dietary recommendations for DHA and EPA are not well established. In 1999, the Workshop on the Essentiality of and Recommended Dietary Intakes for Omega-6 and Omega-3 Fatty Acids made recommendations for adequate intake for adults—namely, a DHA + EPA intake of 0.65 g per day; DHA, at least 0.22 g per day; and EPA, at least 0.22 g per day.36 The En Balance mean intakes are compared to those recommendations. According to the Dietary Guidelines for Americans, the range of DHA + EPA intake that results in the lowest risk of the coronary events is 246 mg per day to 919 mg per day, which roughly translates to a recommendation of 2 servings of fish high in omega-3 fatty acids per week.34 Others who have reviewed the scientific evidence suggest that a therapeutically protective intake should be between 250 mg and 500 mg per day of DHA + EPA.54,55 In a 2007 position statement, the American Dietetic Association and the Dieticians of Canada recommended a weekly intake of 8 oz (227 g) of fatty fish, or about 500 mg of DHA + EPA per day.56 To date, no dietary reference intakes have been nationally established.57
Even by the most conservative recommendations for cardiovascular disease protection, the En Balance group of Hispanic participants with type 2 diabetes was consuming very low intakes of DHA and EPA at baseline. The men of this group were at high risk for nutritional deficiencies due to their already low α-linolenic acid intake and the demonstrated low conversion of α-linolenic acid to DHA or EPA. A high-calorie, high-fat, high-cholesterol and high-sodium diet, and low DHA and EPA intakes-coupled with uncontrolled diabetes, obesity, and uninsured status—makes this group of disadvantaged adults with type 2 diabetes particularly vulnerable to diabetic complications and cardiovascular disease.
Yet the En Balance results demonstrate that Hispanic adults with type 2 diabetes can make significant clinical improvements in glycemic control, serum lipid profiles, and dietary intake as part of a culturally competent diabetes education program that uses few health care resources.
Study Limitations
The En Balance Diabetes Education Program study had several limitations. First, the results may not apply to the population at large, due to the small sample size and convenience sampling method and because only Hispanics were chosen for the program. Second, the program followed the participants for only 3 months, and it is not clear if the positive clinical and dietary changes were sustained beyond then. Finally, dietary intake was assessed via a food frequency questionnaire that was validated for use in this population but is not free from response bias and errors in estimating dietary intake.
Conclusion
The culturally competent and language-sensitive En Balance Diabetes Education Program was able to improve glycemic control and lipid profiles in a group of Hispanic participants with type 2 diabetes. En Balance also promoted decreases in dietary fat and dietary cholesterol intake, which could prevent future diabetic complications or comorbid conditions in this group of disadvantaged diabetic adults. More studies and a longer follow-up are needed to see if Hispanic adults with type 2 diabetes can make lasting lifestyle changes in their dietary intake habits.
Implications for Diabetes Educators
A culturally relevant approach to diabetes education may lead to meaningful decreases in dietary fat and cholesterol intake when Hispanic adults with type 2 diabetes are taught to focus on smaller portion sizes and healthier choices within culturally specific food groups. Dietary interventions should address the low DHA and EPA intakes prevalent in this population group, by stressing consumption of a variety of culturally sensitive food choices that include walnuts, almonds, and fatty fish, such as salmon, tuna, and sardines.
Acknowledgments
This study was funded by National Institutes of Health award No. 5P20MD001632 through the Loma Linda University Center for Health Disparities and Molecular Medicine.
We would like to thank Gina Wheeler for her valuable input in discussing the concept of this article.
Footnotes
For reprints and permission queries, please visit SAGE’s Web site at http://www.sagepub.com/journalsPermissions.nav.
References
- 1.Centers for Disease Control and Prevention. Estimates of Diagnosed Diabetes Now Available for all US Counties. Atlanta, GA: Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 2.University of California San Francisco, Institute for Health and Aging. California Diabetes Program: Diabetes in California Counties. Sacramento, CA: California Department of Public Health; 2009. [Google Scholar]
- 3.Pew Hispanic Center. [Accessed December 8, 2010];Latinos in California, Texas, New York, Florida and New Jersey [fact sheet] http://pewhispanic.org/files/factsheets/10.pdf. Published March 19, 2004.
- 4.US Census Bureau. [Accessed December 8, 2010];Facts for features: Hispanic Heritage Month 2007: Sept 15–Oct 15 2007. http://www.census.gov/newsroom/releases/pdf/cb07-ff14.pdf. Published July 16, 2007.
- 5.Centers for Disease Control and Prevention, Office of Minority Health, Office of the Director. Health Disparities Experienced by Hispanics: United States. Atlanta, GA: Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 6.Kanjilal S, Gregg EW, Cheng YJ, et al. Socioeconomic status and trends in disparities in 4 major risk factors for cardiovascular disease among US adults, 1971–2002. Arch Intern Med. 2006;166(21):2348–2355. doi: 10.1001/archinte.166.21.2348. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Quintero C, Berry EM, Neumark Y. Limited English proficiency is a barrier to receipt of advice about physical activity and diet among Hispanics with chronic diseases in the United States. J Am Diet Assoc. 2009;109(10):1769–1774. doi: 10.1016/j.jada.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Thompson FE, McNeel TS, Dowling EC, Midthune D, Morrissette M, Zeruto CA. Interrelationships of added sugars intake, socioeconomic status, and race/ethnicity in adults in the United States: National Health Interview Survey, 2005. J Am Diet Assoc. 2009;109(8):1376–1383. doi: 10.1016/j.jada.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klohe-Lehman DM, Freeland-Graves J, Anderson ER, et al. Nutrition knowledge is associated with greater weight loss in obese and overweight low-income mothers. J Am Diet Assoc. 2006;106(1):65–75. doi: 10.1016/j.jada.2005.09.047. [DOI] [PubMed] [Google Scholar]
- 10.Mauldon M, Melkus GD, Cagganello M. Tomando Control: a culturally appropriate diabetes education program for Spanish-speaking individuals with type 2 diabetes mellitus. Evaluation of a pilot project. Diabetes Educ. 2006;32(5):751–760. doi: 10.1177/0145721706291999. [DOI] [PubMed] [Google Scholar]
- 11.Gagliardino JJ, Etchegoyen G. A model educational program for people with type 2 diabetes: a cooperative Latin American implementation study (PEDNID-LA) Diabetes Care. 2001;24(6):1001–1007. doi: 10.2337/diacare.24.6.1001. [DOI] [PubMed] [Google Scholar]
- 12.California Medi-Cal Type 2 Diabetes Study Group. Closing the gap: effect of diabetes case management on glycemic control among low-income ethnic minority populations: the California Medi-Cal Type 2 Diabetes Study. Diabetes Care. 2004;27(1):95–103. doi: 10.2337/diacare.27.1.95. [DOI] [PubMed] [Google Scholar]
- 13.Brown SA, Blozis SA, Kouzekanani K, Garcia AA, Winchell M, Hanis CL. Dosage effects of diabetes self-management education for Mexican Americans: the Starr County Border Health Initiative. Diabetes Care. 2005;28(3):527–532. doi: 10.2337/diacare.28.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Culica D, Walton JW, Prezio EA. CoDE: Community Diabetes Education for uninsured Mexican Americans. Proc (Bayl Univ Med Cent) 2007;20(2):111–117. doi: 10.1080/08998280.2007.11928263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown SA, Hanis CL. Culturally competent diabetes education for Mexican Americans: the Starr County Study. Diabetes Educ. 1999;25(2):226–236. doi: 10.1177/014572179902500208. [DOI] [PubMed] [Google Scholar]
- 16.Brown SA, Blozis SA, Kouzekanani K, Garcia AA, Winchell M, Hanis CL. Health beliefs of Mexican Americans with type 2 diabetes: The Starr County border health initiative. Diabetes Educ. 2007;33(2):300–308. doi: 10.1177/0145721707299728. [DOI] [PubMed] [Google Scholar]
- 17.Lorig KR, Ritter PL, Jacquez A. Outcomes of border health Spanish/English chronic disease self-management programs. Diabetes Educ. 2005;31(3):401–409. doi: 10.1177/0145721705276574. [DOI] [PubMed] [Google Scholar]
- 18.Brown SA, Garcia AA, Kouzekanani K, Hanis CL. Culturally competent diabetes self-management education for Mexican Americans: the Starr County border health initiative. Diabetes Care. 2002;25(2):259–268. doi: 10.2337/diacare.25.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Philis-Tsimikas A, Walker C. Improved care for diabetes in under-served populations. J Ambul Care Manage. 2001;24(1):39–43. doi: 10.1097/00004479-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Vincent D, Pasvogel A, Barrera L. A feasibility study of a culturally tailored diabetes intervention for Mexican Americans. Biol Res Nurs. 2007;9(2):130–141. doi: 10.1177/1099800407304980. [DOI] [PubMed] [Google Scholar]
- 21.Stirban A, Nandrean S, Gotting C, et al. Effects of n-3 fatty acids on macro- and microvascular function in subjects with type 2 diabetes mellitus. Am J Clin Nutr. 2010;91(3):808–813. doi: 10.3945/ajcn.2009.28374. [DOI] [PubMed] [Google Scholar]
- 22.Adkins Y, Kelley DS. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem. 2010;21(9):781–92. doi: 10.1016/j.jnutbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Jung UJ, Torrejon C, Tighe AP, Deckelbaum RJ. n-3 Fatty acids and cardiovascular disease: mechanisms underlying beneficial effects. Am J Clin Nutr. 2008;87(6):2003S–2009S. doi: 10.1093/ajcn/87.6.2003S. [DOI] [PubMed] [Google Scholar]
- 24.Bialostosky K, Wright JD, Kennedy-Stephenson J, McDowell M, Johnson CL. Dietary intake of macronutrients, micronutrients, and other dietary constituents: United States 1988–94. Vital Health Stat 11. 2002;245:1–158. [PubMed] [Google Scholar]
- 25.US Department of Agriculture, Agricultural Research Service. Nutrient Intakes From Food: Mean Amounts Consumed per Individual, by Race/Ethnicity and Age, One Day, 2005–2006. Washington, DC: US Department of Agriculture; 2008. [Google Scholar]
- 26.Lora KR, Lewis NM, Eskridge KM, Stanek-Krogstrand K, Travnicek DA. Correlation of omega-3 fatty acids intakes with acculturation and socioeconomic status in Midwestern Latinas [published online ahead of print January 23, 2010] J Immigr Minor Health. doi: 10.1007/s10903-009-9314-z. [DOI] [PubMed] [Google Scholar]
- 27.He K, Liu K, Daviglus ML, et al. Associations of dietary long-chain n-3 polyunsaturated fatty acids and fish with biomarkers of inflammation and endothelial activation (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2009;103(9):1238–1243. doi: 10.1016/j.amjcard.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belalcazar LM, Reboussin DM, Haffner SM, et al. Marine omega-3 fatty acid intake: associations with cardiometabolic risk and response to weight loss intervention in the Look AHEAD (Action for Health in Diabetes) study. Diabetes Care. 2010;33(1):197–199. doi: 10.2337/dc09-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- 30.Taren D, de Tobar M, Ritenbaugh C, Graver E, Whitacre R, Aickin M. Evaluation of the Southwest Food Frequency Questionnaire. Ecol Food Nutr. 2000;38:515–547. [Google Scholar]
- 31.Arizona Cancer Center. Metabolize Nutrient Analysis System [version 2.5] Tucson, AZ: University of Arizona; 2004. [Google Scholar]
- 32.Metghalchi S, Rivera M, Beeson L, et al. Improved clinical outcomes using a culturally sensitive diabetes education program in a Hispanic population. Diabetes Educ. 2008;34(4):698–706. doi: 10.1177/0145721708320913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ojo E, Beeson L, Schulz E, et al. Effect of the En Balance, a culturally and language-sensitive diabetes education program on dietary changes and plasma lipid profile in Hispanic diabetics. Int J Body Compos Res. 2010;7:S69–S76. [PMC free article] [PubMed] [Google Scholar]
- 34.US Department of Health and Human Services. Dietary Guidelines for Americans, 2005. 6. Washington, DC: US Government Printing Office; 2005. [Google Scholar]
- 35.Otten JJ, Pitzi Hellwig J, Meyers LD. The Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 36.Simopoulos AP, Leaf A, Salem N., Jr Workshop statement on the essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2000;63(3):119–121. doi: 10.1054/plef.2000.0176. [DOI] [PubMed] [Google Scholar]
- 37.Haffner SM. Management of dyslipidemia in adults with diabetes. Diabetes Care. 2003;26(suppl 1):S83–S86. doi: 10.2337/diacare.26.2007.s83. [DOI] [PubMed] [Google Scholar]
- 38.Thompson FE, Byers T. Dietary assessment resource manual. J Nutr. 1994;124(11 suppl):2245S–2317S. doi: 10.1093/jn/124.suppl_11.2245s. [DOI] [PubMed] [Google Scholar]
- 39.Martinez ME, Marshall JR, Graver E, et al. Reliability and validity of a self-administered food frequency questionnaire in a chemoprevention trial of adenoma recurrence. Cancer Epidemiol Biomarkers Prev. 1999;8(10):941–946. [PubMed] [Google Scholar]
- 40.Jones LA, Gonzalez R, Pillow PC, et al. Dietary fiber, Hispanics, and breast cancer risk? Ann N Y Acad Sci. 1997;837:524–536. doi: 10.1111/j.1749-6632.1997.tb56897.x. [DOI] [PubMed] [Google Scholar]
- 41.Coates RJ, Monteilh CP. Assessments of food-frequency questionnaires in minority populations. Am J Clin Nutr. 1997;65(4 suppl):1108S–1115S. doi: 10.1093/ajcn/65.4.1108S. [DOI] [PubMed] [Google Scholar]
- 42.Teufel NI. Development of culturally competent food-frequency questionnaires. Am J Clin Nutr. 1997;65(4 suppl):1173S–1178S. doi: 10.1093/ajcn/65.4.1173S. [DOI] [PubMed] [Google Scholar]
- 43.Block G, Wakimoto P, Jensen C, Mandel S, Green RR. Validation of a food frequency questionnaire for Hispanics. Prev Chronic Dis. 2006;3(3):A77. [PMC free article] [PubMed] [Google Scholar]
- 44.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, et al. Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Ann Epidemiol. 1999;9(5):314–324. doi: 10.1016/s1047-2797(98)00070-2. [DOI] [PubMed] [Google Scholar]
- 45.Kristal AR, Feng Z, Coates RJ, Oberman A, George V. Associations of race/ethnicity, education, and dietary intervention with the validity and reliability of a food frequency questionnaire: the Women’s Health Trial Feasibility Study in Minority Populations. Am J Epidemiol. 1997;146(10):856–869. doi: 10.1093/oxfordjournals.aje.a009203. [DOI] [PubMed] [Google Scholar]
- 46.Carrera PM, Gao X, Tucker KL. A study of dietary patterns in the Mexican-American population and their association with obesity. J Am Diet Assoc. 2007;107(10):1735–1742. doi: 10.1016/j.jada.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 47.Murtaugh MA, Herrick JS, Sweeney C, et al. Diet composition and risk of overweight and obesity in women living in the southwestern United States. J Am Diet Assoc. 2007;107(8):1311–1321. doi: 10.1016/j.jada.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Guendelman S, Abrams B. Dietary intake among Mexican-American women: generational differences and a comparison with white non-Hispanic women. Am J Public Health. 1995;85(1):20–25. doi: 10.2105/ajph.85.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dixon LB, Sundquist J, Winkleby M. Differences in energy, nutrient, and food intakes in a US sample of Mexican-American women and men: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2000;152(6):548–557. doi: 10.1093/aje/152.6.548. [DOI] [PubMed] [Google Scholar]
- 50.Neuhouser ML, Thompson B, Coronado GD, Solomon CC. Higher fat intake and lower fruit and vegetables intakes are associated with greater acculturation among Mexicans living in Washington State. J Am Diet Assoc. 2004;104(1):51–57. doi: 10.1016/j.jada.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 51.Fitzgerald N, Damio G, Segura-Perez S, Perez-Escamilla R. Nutrition knowledge, food label use, and food intake patterns among Latinas with and without type 2 diabetes. J Am Diet Assoc. 2008;108(6):960–967. doi: 10.1016/j.jada.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 52.American Diabetes Association. Choose Your Foods: Exchange Lists for Diabetes. Chicago, IL: American Dietetic Association; 2008. [Google Scholar]
- 53.Vitolins MZ, Anderson AM, Delahanty L, et al. Action for Health in Diabetes (Look AHEAD) trial: baseline evaluation of selected nutrients and food group intake. J Am Diet Assoc. 2009;109(8):1367–1375. doi: 10.1016/j.jada.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gebauer SK, Psota TL, Harris WS, Kris-Etherton PM. n-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am J Clin Nutr. 2006;83(6 suppl):1526S–1535S. doi: 10.1093/ajcn/83.6.1526S. [DOI] [PubMed] [Google Scholar]
- 55.Harris WS, Mozaffarian D, Lefevre M, et al. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J Nutr. 2009;139(4):804S–819S. doi: 10.3945/jn.108.101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kris-Etherton PM, Innis S American Dietetic Association, Dietitians of Canada. Position of the American Dietetic Association and Dietitians of Canada: dietary fatty acids. J Am Diet Assoc. 2007;107(9):1599–1611. [PubMed] [Google Scholar]
- 57.Kris-Etherton PM, Grieger JA, Etherton TD. Dietary reference intakes for DHA and EPA. Prostaglandins Leukot Essent Fatty Acids. 2009;81(2–3):99–104. doi: 10.1016/j.plefa.2009.05.011. [DOI] [PubMed] [Google Scholar]