Abstract
Sarcopenia coincides with declines in several systemic processes that signal through the MAP kinase and Akt-mTOR-p70S6k cascades typically associated with muscle growth. Effects of aging on these pathways have primarily been examined in limb muscles, which experience substantial activity and neural changes in addition to systemic hormonal and metabolic changes. Head and neck muscles are reported to undergo reduced sarcopenia and disuse with age relative to limb muscles, suggesting muscle activity may contribute to maintaining mass with age. However many head and neck muscles derive from embryonic branchial arches, rather than the somites from which limb muscles originate, suggesting that developmental origin may be important. This study compares the expression and phosphorylation of MAP kinase and mTOR networks in head, neck, tongue, and limb muscles from 8- and 26-month old F344 rats to test the hypothesis that physical activity and developmental origin contribute to preservation of muscle mass with age. Phosphorylation of p38 was exaggerated in aged branchial arch muscles. Phosphorylation of ERK and p70S6k T421/S424 declined with age only in the biceps brachii. Expression of p70S6k declined in all head and neck, tongue and limb muscles although no change in phosphorylation of p70S6k on T389 could be resolved. A systemic change that results in a loss of p70S6k protein expression may reduce the capacity to respond to acute hypertrophic stimuli, while the exaggerated p38 signaling in branchial arch muscles may reflect more active muscle remodeling.
Keywords: aging, muscle, tongue, p70s6 kinase, MAP kinase
INTRODUCTION
Normal aging is associated with a decline in muscle mass, which is correlated with a decline in circulating growth hormones, reduced activity and denervation (Giovannini et al., 2008; Lynch et al., 2007; Nair, 2005). Sarcopenia, the slow, progressive loss of muscle mass and function, reduces independence and quality of life in the elderly (Baumgartner et al., 2004; Janssen et al., 2004). Beginning in the fourth decade, muscle mass declines about 0.5–2% per year (Baumgartner et al., 1998) thus, the extent of muscle loss is often only evident when compared over long periods of time.
The primary cause of sarcopenia is unclear, but systemic changes and reduced muscle activity have been identified as potential sources. Production of growth factors, such as IGF-I, GH and testosterone, and receptor sensitivity or number decline with age (Proctor et al., 1998; Szulc et al., 2004), suggesting that sarcopenia may reflect a systemic loss of anabolic drive. It seems unlikely that any single hormone acts as a master regulator of muscle size (Flueck and Goldspink, 2010), but a general decline in anabolic factors might lead to a general decline in muscle size. Physical activity is also a potent anabolic stimulus and declines with age in humans and animals (Caspersen et al., 2000; Holloszy et al., 1985). Resistance exercise can attenuate the effects of age on muscle as training studies demonstrate elderly can increase muscle strength, size and protein synthesis (Frontera et al., 1988; Trappe et al., 2002; Welle et al., 1995), however, strength training appears to be compensatory and not antagonistic to sarcopenia (Pearson et al., 2002). The decline in activity may reflect compromised neuromuscular function, and aged muscles show evidence of extensive denervation (Larsson and Ansved, 1995; Wang et al., 2005).
Growth signals and physical activity converge on similar biochemical signaling pathways, but the interaction among the stimuli and pathways is poorly understood. Two signaling networks have drawn special interest in integrating diverse stimuli to regulate muscle growth. The Akt-mTOR-p70s6k cascade is a critical pathway regulating protein synthesis (Bodine et al., 2001), and activation of Akt is adequate to increase muscle mass (Blaauw et al., 2009; Bodine et al., 2001). The MAP kinase cascades, including p42/44ERK, p38 and JNK cascades, are important in protein translation, gene transcription and satellite cell activation (Anjum and Blenis, 2008; Long et al., 2004; Widegren et al., 2001). IGF-I activates both Akt and MAP kinase cascades through receptor-mediated mechanisms (Shah et al., 2000; Sherwood et al., 1999). Mechanical stimuli also activate Akt and MAP kinase signaling, although the mechanism of this activation is uncertain (Goodyear et al., 1996; Hornberger et al., 2005; Nader and Esser, 2001; Sakamoto and Goodyear, 2002). AMP-activated protein kinase (AMPK) is activated in response to ATP depletion and inhibits mTOR signaling (Kimball, 2006). Recent studies show AMPK phosphorylation is increased in aged muscles, particularly fast twitch muscles (Thomson and Gordon, 2006).
Comparisons between muscles with differing changes in activity with age may show the influence of muscle activity on growth signaling. Voluntary locomotor activity, measured by wheel running distance or exploratory and home cage behaviors, declines continuously in rats after 10 months of age (Holloszy et al., 1985; Skalicky et al., 1996) and hindlimb mass declines up to 30% in Fischer 344 (F344) rats (Daw et al., 1988). Muscles of the head, neck and tongue are involved in functions such as mastication, respiration and vision that decline less with age in rodents than locomotion. Peng and Kang (1984) reported that running wheel activity declined from 75 minutes/day to under 5 minutes per day in aged Long Evans rats, while feeding behavior changed by less than 30%, with some measures increasing and some decreasing, and similar results are seen in F344 and F344/BN (Zhang et al., 2008). Muscles of the jaw and tongue lose less than 15% of mass and fiber area (Connor et al., 2008; McLoon et al., 2004). Activity, strength and size of head, neck and tongue muscles may decline with age, but those changes are much less pronounced than limb muscles.
Muscles of the head and neck also differ from limb musculature in their innervation and developmental origin, which may contribute to differences in growth signaling. These muscles are innervated by cranial nerves, rather than by spinal motorneurons, and may not be subject to denervation observed in hindlimb muscles (Larsson and Ansved, 1995; Sturrock, 1987). Further, most head and neck, but not tongue, muscles derive from branchial arches of somitomeres, rather than the somites (Noden and Francis-West, 2006; Yamane, 2005), and undergo a distinct myogenic program that may make them differently sensitive to growth stimuli, suggesting that their preservation with age may reflect their unique developmental origin. By contrast, tongue muscles, like limb muscles, derive from somites and represent a unique intersection of features from sarcopenia sensitive and sarcopenia spared muscle.
This study analyzes the phosphorylation state of kinases associated with growth-signaling networks in three classes of muscle to compare the influence of activity and developmental origins. This comparative design will test the hypothesis that physical activity and developmental origin contribute to growth signaling with age. The relative importance of cell lineage and general physical activity in the activation of these signaling networks and in the preservation of muscle mass may be revealed by comparison of head, tongue, and limb muscles from old and young rats. The key findings of the current study show exaggerated ERK and p70S6kT421/S424 phosphorylation in young biceps brachii, exaggerated p38 phosphorylation in aged head and neck muscles, and a system wide decline in p70S6k expression. The systemic loss of p70S6k protein expression may reduce the capacity for muscle hypertrophy in response to periodic vigorous activity.
MATERIALS AND METHODS
Animals and Tissue Preparation
Male Fischer 344 rats aged 8 months or 26 months (n = 8 per group) were obtained from the National Institute on Aging (NIA) colony. Average weight of animals was 403g ± 37 (young: 414g ± 19; old: 397g ± 45). The Fischer 344 strain is supplied by the NIA for use in aging studies. Mean survival age is 24 months with 25% survival to 26 months. Animals were sacrificed by CO2 asphyxiation, and experimental muscles dissected and immediately frozen in liquid nitrogen. Procedures were reviewed and approved by Institutional Animal Care and Use Committee at Georgia Institute of Technology and performed in compliance with the Guide for Care and Use of Laboratory Animals.
Muscles
Seven muscles (Table 2) were chosen to represent different developmental origins and different general functions. Furthermore, muscles with opposing functions and different fiber type composition were included to mask phenotypic differences. The biceps brachii (BB) is an elbow flexor, innervated by the musculocutaneous nerve, with motor fibers derived from the embryonic somites. The pectoralis (P) is a flexor and adductor of the shoulder, innervated by the pectoral nerve, and of somitic origin. The styloglossus (SG) is an extrinsic retractor of the tongue, innervated by the hypoglossal or 12th cranial nerve, also of somitic origin. The geniohyoid (GH) is an elevator of the hyoid bone active in deglutition, also innervated by the hypoglossal nerve and of somitic origin. The masseter (M) is a jaw closer, innervated by the trigeminal or 5th cranial nerve, with fibers derived from the first branchial arch of the embryonic somitomere. The posterior digastric (PD) is a jaw opener, innervated by the facial or 7th cranial nerve, with fibers derived from the 2nd branchial arch. These muscles were grouped according to functional role (locomotion or mastication) and by developmental origin (somitic or branchial arch), so muscles will belong to one of three classes or functional Origins: somitic+locomotion (BB), somitic+mastication (SG, GH), or branchial arch+mastication (M, PD). The superior rectus extraocular muscle (EOM) is an eye elevator, innervated by the occulomotor or 3rd cranial nerve, originating from the non-segmented paraxial mesoderm.
Table 2.
Protein and mRNA expression of conventional MHC isoforms in young (8 month) and old (26 month) muscles with different origins and functions. Extraocular (EOM); Masseter (M); Posterior digastric (PD); Geniohyoid (GH); Styloglossus (SG) and Biceps brachii (BB).
| Function | BRANCHIAL ARCH | SOMITIC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eye |
Mastication |
Mastication |
Locomotion |
|||||||||||
| EOM | M | PD | GH | SG | BB | P | ||||||||
| Vision | Jaw Closer | Jaw Opener | Hyoid Elevation | Tongue Retrusion | Stability/Locomotion | Stability/Locomotion | ||||||||
| MHC composition by protein analysis | ||||||||||||||
| EOM | M | PD | GH | SG | BB | P | ||||||||
| Y | O | Y | O | Y | O | Y | O | Y | O | Y | O | Y | O | |
| IIb | 47%±5% | 56%±7% | 51%±14% | 48%±9% | 35%±10% | 33%±5% | 49%±5% | 50%±7% | 29%±5% | 25%±6% | 61%±7% | 53%±12% | 60%±13% | 62%±11% |
| IIx | 27%±3% | 27%±6% | 35%±8% | 37%±5% | 39%±5% | 41%±3% | 34%±4% | 34%±5% | 43%±6% | 43%±4% | 26%±4% | 31%±7% | 29%±8% | 24%±6% |
| IIa | 17%±4% | 12%±3% | 13%±7% | 14%±5% | 20%±6% | 23%±3% | 15%±2% | 16%±3% | 27%±4% | 32%±2% | 10%±4% | 13%±5% | 9%±5% | 10%±5% |
| I | 9%±5% | 6%±4% | 1%±1% | 1%±1% | 6%±3% | 3%±2% | 2%±2% | 0%±1% | 1%±2% | 1%±1% | 3%±1% | 3%±1% | 2%±2% | 3%±2% |
| “Fast” | 0.63 | 0.26 | −0.26 | −0.65 | 0.67 | 0.23 | −1.27 | −1.36 | 1.22 | 0.62 | ||||
| Factor Score | 0.457 | −0.466 | 0.448 | −1.309 | 0.922 | |||||||||
| MHC composition by mRNA analysis | ||||||||||||||
| EOM | M | PD | GH | SG | BB | |||||||||
| Y(n=1) | O(n=1) | Y | O | Y | O | Y | O | Y | O | Y | O | |||
| IIb | 46% | 59% | 59%±16% | 62%±6% | 36%±6% | 26%±9% | 69%±12% | 63%±6% | 33%±10% | 44%±12% | 71%±9% | 68%24% | ||
| IIx | 30% | 21% | 39%±14% | 37%±7% | 46%±6% | 42%±9% | 24%±10% | 27%±14% | 52%±10% | 42%±9% | 21%±5% | 28%±19% | ||
| IIa | 12% | 5% | 2%±2% | 1%±1% | 11%±5% | 13%±4% | 8%±2% | 8%±2% | 14%±2% | 13%±2% | 5%±3% | 3%±3% | ||
| I | 12% | 15% | 0%±0% | 0%±1% | 7%±3% | 20%±13% | 0%±0% | 2%±1% | 2%±2% | 2%±2% | 3%±2% | 2%±2% | ||
Western Blotting
Muscles were by homogenized in a low salt detergent buffer (50mM Tris, pH 7.5; 30mM NaCl; 5mM EDTA, 1% Triton X-100 plus NaF, NaVO3 and protease inhibitors) to minimize myofilament extraction and pelleted at 15,000 × g. The protein concentration of the supernatant was measured by BCA (Pierce) according to manufacturer’s protocol. The pellet, enriched in myofilaments, was resuspended in 0.1M PBS, pH 7.3 with 5% protease inhibitor for myosin heavy chain (MHC) separation. Soluble protein (15ug) was separated by SDS-PAGE, transferred to nitrocellulose membranes, and detected by Western blot. Primary antibody dilutions were: p-ERKT202/T204 1:3000; p-JNK 1:500; p-P38T180/Y182 1:2000; p-Akt S473 1:2000; p-P70S6k T421/S424 1:2000; p-p70S6k T389 1:2500; p-ACC 1:1000; total ERK2 1:2500; total p38 1:2000; total Akt 1:2000; pan p70 1:2000. All antibodies are purchased from Cell Signaling except JNK, from Santa Cruz. Bands were visualized by enhanced chemiluminescence and quantified by scanning densitometry.
Five muscles, the BB, SG, GH, M, and PD, were selected for multivariate analysis. Each young animal was arbitrarily paired with an old animal, and all ten of the paired muscles were loaded on a single gel. Data from each gel was normalized to the average of all young muscles to allow comparisons across muscles and across age groups. EOM and P were analyzed separately, which prevents direct comparison with the five core muscles, but does allow resolution of age-dependent signaling.
Myosin Separation Gels
The MHC isoform content was determined by SDS-PAGE separation on 8% polyacrylamide gels using a procedure derived from Talmadge and Roy (1993). Pellet containing myofilaments was resuspended in 0.1M PBS, pH 7.3 with 5% protease inhibitor, quantified (BCA), diluted in Laemmli buffer (62.5 mmol/L Tris, pH 6.8, 10% glycerol, 2.3% SDS, 5% β-mercaptoethanol, with antiproteolytic factors) and loaded 1ug protein/per lane onto gel. Electrophoresis was carried out at 4°C for 22 hours at 140 V. Bands were visualized by Coomassie staining and MHC I, MHC IIa, MHC IIx and MHC IIb were quantified in Image J software (NIH) by reference to rat MG, soleus and tongue body samples run synchronously.
Real-time PCR
RNA was extracted from muscle by homogenization in 1ml of Trizol reagent (Invitrogen) according to manufacturer’s protocol. Total RNA yield was determined by UV spectroscopy, and reverse transcription was performed with 1ug of total RNA using Multiscribe RT kit (Invitrogen) according to manufacturer’s protocol using random primers. For amplification of myosin heavy chain isoforms, reactions contained 250nM forward and reverse primers in Platinum SYBR Green qPCR Supermix (Invitrogen). MHC standards and primer sets were used as previously described (Rahnert et al., 2010), and all primers and amplicon lengths are given in Table 1. The thermal protocol for all targets was 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 62°C for 1 min. Reaction products were validated at least once by size determination on agarose gel, and by terminal dissociation melting curve for every sample.
Table 1.
Primers used for real time PCR and product size for myosin heavy-chains and calcium channels
| Target | Forward primer 5′ → 3′ | Reverse primer 5′ → 3′ | amplicon size (bp) |
|---|---|---|---|
| B-actin | TTCAACACCCCAGCCATGT | GTAGATGGGCACAGTGTGGGT | 120 |
| MHC-2x | GTACCACAGGGAAACTGGCTT | CTTGGTCATCAATGCTGGG | 222 |
| MHC-2a | GTCTGCCAACTTCCAGAAGC | CAGCTTGTTCAAATTCTCTCTGAA | 307 |
| MHC-2b | AAAGGTGGCCATTTACAAGCT | CAGCAGAGTTCAGACTTGTCAG | 146 |
| MHC-I | AAGTCCTCCCTCAAGCTCATGGC | ATTTTCCCGGTGGAGAGC | 135 |
| CaV1.1 | AGCTACCACCATGCTGATCC | CCCTCGCTTTCTGACTTTTG | 333 |
| RyR-1 | CAAGACCTGAGCTGAGACCC | CCCAATCTCAATTTCTCGGA | 242 |
Statistics
Data are expressed as means ± SD and are log transformed for presentation in figures to equalize visual elevations and reductions of phosphorylation or expression. Statistical analysis was by 2-way ANOVA (functional Origin × Age) with a significance threshold of p < 0.05, followed by post hoc t-tests using the Bonferroni/Dunn correction for multiple comparisons. Factor analysis was used to extract principal components of the data set either as a whole data set or split by age. Factor scores were Varimax transformed and the orthogonal solutions of the whole set were analyzed by 2-way ANOVA (Muscle × Age) with a significance threshold of p < 0.05.
RESULTS
Independent factors associated with expression and signaling
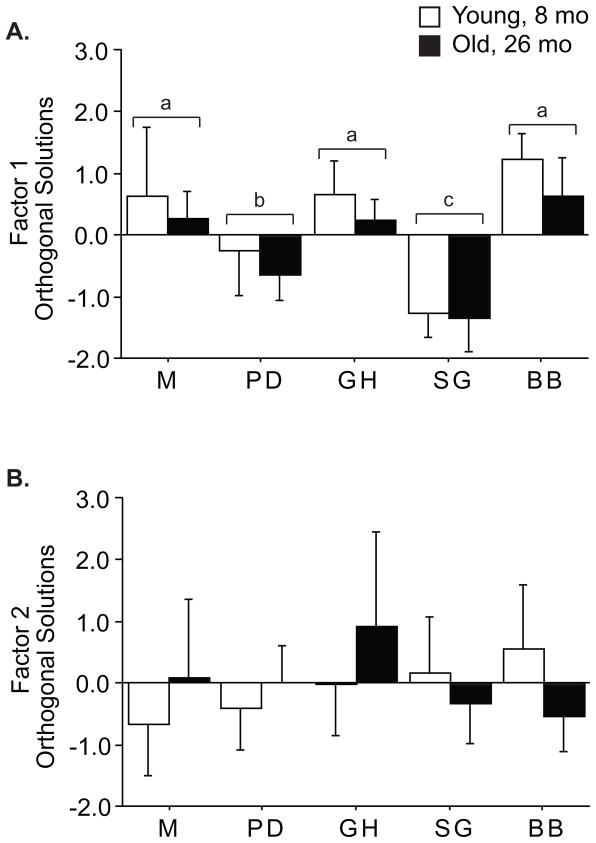
Results of western blots and myosin separation are shown in Tables 2 and 3. To facilitate interpretation of this large data set, which included all muscles except EOM and P, we performed factor analysis, to extract statistically independent sets of correlated variables. Factor analysis revealed two factors that account for 43% of the population variance (Table 5), leaving the remaining 57% of variance distributed across 13 residual factors. The primary factor accounted for 27% of the population variance and combines expression of MHC IIb (factor weight 0.959), MHC IIx (-0.885), and MHC IIa (-0.913) such that faster muscles had greater Factor 1 values. Thus, this factor may be best interpreted as “Fast”. Two-way ANOVA revealed PD and SG were slower than the rest (p<0.0001), that young muscles were faster than old (p=0.01, Figure 1A), and that slowing with age was uniform across all muscles (age × muscle interaction, p=0.86). Expression of p70S6k (factor weight 0.502) and its phosphorylation on T389 (−0.456) were weaker components of Factor 1, which suggests fast muscles express more p70S6k and may have a greater capacity to respond to signals although, at rest p70S6k is less active. Factor 2 contained 16% of the population variance and included most of the kinase phosphorylation levels, particularly p70S6kT421/S424 (0.828) and ERKT202/T204 (0.745), as well as p38T180/Y182, JNK T54 and AktS473. This factor is best interpreted as “Growth Signaling” and suggests the activity of all of these kinases is coordinated, but independent of fiber type. Two-way ANOVA revealed a muscle specific effect of age (muscle × age interaction, p=0.02) in which Factor 2 decreases in BB and increases in M and GH with age, while PD and SG are unchanged, and suggests age-related signaling is different in BB (Figure 1B). The residual factors individually represent 10% or less of the population variance.
Table 3.
Summary of protein expression and phosphorylation in muscles of young (8 month) and old (26 month) F344 rats.
| Branchial Arch- Mastication | Somitic- Mastication | Somitic- Locomotion | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | PD | GH | SG | BB | ||||||
| Y | O | Y | O | Y | O | Y | O | Y | O | |
| p-ACC | 1.12±0.3 | 0.99±0.3 | 0.84±0.3 | 0.89±0.5 | 1.23±0.5 | 1.04±0.4 | 0.71±0.2 | 0.84±0.4 | 0.99±0.2 | 0.97±0.4 |
| Akt | 1.66±0.2 | 1.81±0.5 | 0.89±0.2 | 1.06±0.4 | 0.69±0.3 | 0.85±0.2 | 0.93±0.2 | 1.02±0.2 | 0.86±0.2 | 0.65±0.3 |
| p-Akt | 1.10±0.6 | 1.56±1.3 | 1.05±0.4 | 0.90±0.5 | 0.83±0.2 | 0.88±0.6 | 1.02±0.5 | 0.65±0.4 | 1.05±0.3 | 0.49±0.2 |
| Rel Akt | 0.67±0.4 | 0.93±0.8 | 1.23±0.5 | 0.86±0.4 | 1.66±1.6 | 1.03±0.6 | 1.20±0.7 | 0.62±0.3 | 1.23±0.4 | 0.73±0.5 |
| p70 | 1.02±0.2 | 0.84±0.2 | 1.08±0.4 | 0.83±0.3 | 1.21±0.4 | 0.73±0.2 | 0.54±0.1 | 0.35±0.1 | 1.18±0.4 | 0.51±0.2 |
| p70T421/S424 | 0.72±0.5 | 1.10±1.0 | 0.81±0.5 | 1.10±0.8 | 1.00±0.6 | 2.01±1.6 | 0.98±0.6 | 0.49±0.2 | 1.57±0.9 | 0.52±0.3 |
| Rel T421 | 0.66±0.3 | 1.35±1.1 | 0.85±0.6 | 1.50±1.1 | 1.09±1.2 | 3.26±3.2 | 1.72±0.9 | 1.73±1.1 | 1.32±0.7 | 1.16±0.7 |
| p70T389 | 1.08±0.5 | 0.71±0.3 | 1.13±0.2 | 0.99±0.4 | 0.78±0.3 | 0.76±0.2 | 1.23±0.3 | 1.14±0.4 | 0.89±0.3 | 0.73±0.3 |
| Rel T389 | 1.00±0.6 | 0.72±0.2 | 1.01±0.4 | 1.33±0.6 | 0.73±0.4 | 1.86±1.4 | 2.20±0.2 | 5.44±1.6 | 1.23±0.8 | 2.55±0.8 |
| ERK2 | 1.04±0.3 | 0.88±0.3 | 1.07±0.2 | 1.17±0.4 | 0.82±0.2 | 1.08±0.5 | 1.10±0.3 | 1.01±0.3 | 1.03±0.4 | 0.76±0.2 |
| p-ERK | 1.02±0.7 | 0.84±0.6 | 0.68±0.5 | 0.78±0.4 | 1.03±0.5 | 1.20±0.9 | 0.68±0.3 | 0.69±0.6 | 1.68±0.6 | 0.78±0.3 |
| Rel ERK | 0.95±0.5 | 1.00±0.7 | 0.67±0.5 | 0.52±0.2 | 1.50±1.1 | 1.09±0.7 | 0.72±0.5 | 0.66±0.5 | 1.84±0.8 | 1.06±0.4 |
| p-p38 | 0.79±0.5 | 2.05±1.1 | 0.94±0.3 | 1.51±0.5 | 1.05±0.3 | 1.46±0.7 | 0.96±0.5 | 0.69±0.3 | 1.32±0.6 | 0.95±0.4 |
| p-JNK 46 | 1.11±0.5 | 1.13±0.7 | 0.92±0.3 | 0.98±0.6 | 1.00±0.2 | 0.81±0.2 | 0.87±0.4 | 0.74±0.4 | 1.07±0.2 | 1.03±0.3 |
| p-JNK 54 | 0.83±0.2 | 1.33±0.5 | 0.82±0.4 | 0.90±0.3 | 1.16±0.4 | 1.24±0.4 | 1.06±0.3 | 1.09±0.7 | 1.03±0.3 | 0.84±0.3 |
Table 5.
Orthogonal solutions of 2 factors revealed by factor analysis of whole data set.
| Factor 1 (Fast) | Factor 2 (Signaling) | |
|---|---|---|
| p-ACC | 0.402 | 0.113 |
| p-Akt | 0.113 | 0.513 |
| p70T421/S424 | 0.136 | 0.828 |
| p70389 | −0.456 | −0.060 |
| p-ERK | 0.405 | 0.745 |
| p-p38 | 0.067 | 0.560 |
| p-JNK 46 | 0.065 | 0.107 |
| p-JNK 54 | −0.078 | 0.650 |
| Akt | 0.021 | −0.129 |
| p70S6k | 0.502 | −0.084 |
| ERK | −0.202 | 0.116 |
| IIb | 0.959 | 0.144 |
| IIx | −0.885 | −0.213 |
| IIa | −0.913 | −0.049 |
| I | 0.149 | −0.018 |
Figure 1.
The major factors identified by factor analysis were analyzed by 2-way ANOVA. For Factor 1 (A), significant effects of age (p=0.01), and muscle (p<0.0001), but not age × muscle (p=0.86), were found. Muscles designated with the same letter were indistinguishable in post hoc T-tests. Factor 2 (B) had a significant interaction of age × origin (p=0.02), but no main effects (age p=0.63; origin p=0.23). The interaction effect indicates that the decrease with age in BB is different than the increase seen in M, GH. Mean ± S.D. of linear factor scores.
Factor analysis was also performed separately on muscles from old and young animals to examine age-related changes in signaling patterns. The primary factor in both age groups was a combination of MHC isoforms (data not shown); however, p70S6k expression and phosphorylation (T389) were components of Factor 1 only in young animals (0.631 and −0.488, respectively), and not in old animals (0.268 and −0.335, respectively). Interestingly, there was no core “Growth Signaling” factor in old animals. The weighting of all phosphorylations was greater than 0.480 in Factor 2 of young muscles, but in old animals these components were spread across Factors 2, 3 and 4, suggesting that several signaling modules become more independent or discoordinated with age.
MHC and calcium channel expression does not differ with age
Although factor analysis was able to distinguish an age-related change in MHC profile, no individual MHC isoform had a significant change in expression by mRNA or protein with age in these animals (Table 2). The significant decrease in the “Fast” factor with age (p=0.01) was resolvable because the correlations between MHC IIb, IIx, and IIa were able to overcome the inter-sample variability. Expression of the L-type calcium channel (CaV1.1) and the calcium release channel, ryanodine receptor (RyR-1) may shift or down-regulate prior to changes in MHC isoforms, however, neither the calcium channel expression nor the ratio of CaV1.1 to RyR-1 differ with age in any muscle (Table 6). This is consistent with similar transcript data from mice (Zheng et al., 2001), although there may be a decrease in CaV1.1 protein (Renganathan et al., 1998).
Table 6.
mRNA expression of L-type calcium channel (CaV) and ryanodine receptor (RyR) isoforms in muscles of young (8 month) and old (26 month) F344 rats.
| Calcium channel mRNA expression (fold β-actin) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Branchial Arch - Mastication | Somitic - Mastication | Somitic - Locomotion | ||||||||
| M | PD | GH | SG | BB | ||||||
| Y | O | Y | O | Y | O | Y | O | Y | O | |
| CaV1.1 | 0.7±0.2 | 1.3±1.1 | 1.8±1.4 | 1.5±0.6 | 2.3±1.4 | 2.3±1.3 | 1.5±1.0 | 1.4±0.4 | 2.2±1.0 | 1.2±0.6 |
| RyR-1 | 1.6±1.3 | 1.1±1.3 | 1.1±0.7 | 1.0±0.7 | 1.3±1.0 | 1.3±1.2 | 0.9±0.9 | 1.2±1.3 | 1.0±0.6 | 0.9±1.1 |
Aging does not increase ACC phosphorylation
Acetyl coenzyme A carboxylase (ACC) is a primary physiological target of AMPK and its phosphorylation was used as an index of in vivo AMPK activity. ACC phosphorylation was extremely uniform (Table 3), and no effect of age (p=0.74) or muscle origin (p=0.99) could be resolved. This suggests that the resting metabolic requirements of these muscles are similarly satisfied and that persistent metabolic stress is not a likely contributor to sarcopenia.
Akt-p70 signaling cascade
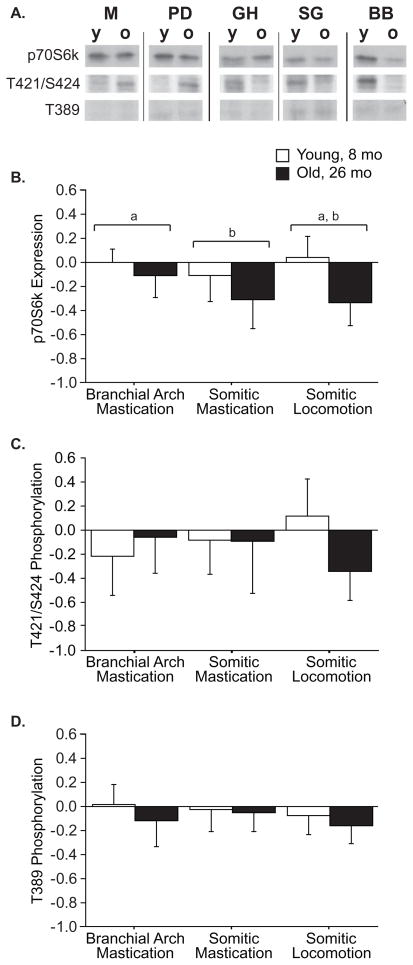
The effect of age was more apparent on p70S6k than on Akt. There was no effect of age on Akt expression (p=0.84) or phosphorylation (p=0.16) (Table 3), but expression was exaggerated in the branchial arch (BA) muscles (p<0.0001), and there was a trend (p=0.060) for increased phosphorylation in BA muscles. By contrast, significant effects of age (p<0.0001) and origin (p=0.03) were found for expression of p70S6k, with lower expression in aged muscle (Figure 2B) and lower expression in the tongue than in branchial arch muscles (Figure 2B). Phosphorylation of p70S6k on T389 is closely correlated with activity, and this tended (p=0.07) to decrease with age, independent of muscle origin (origin effect, p=0.27, interaction effect p=0.66, Figure 2D). Neither age (p=0.54) nor origin (p=0.62) had an effect on p70S6kT421/S424 phosphorylation. There was a significant interaction (Origin × Age p=0.03, Figure 2C) within the five core muscles, indicating reduced phosphorylation specifically in aged BB, however, this effect could not confirmed in P (Table 4), so this reduction seems unique to BB.
Figure 2.
Expression and phosphorylation of p70S6k in young and old muscles by origin. Representative western blots (A) in which each antigen row is from a single blot, with the muscles rearranged for clarity. Expression of p70S6k (B) differs by muscle origin (p=0.03) and age (p<0.0001), but no significant interaction effect (p=0.10) was found. Origins designated by the same letter were statistically indistinguishable in post hoc T-tests. Phosphorylation of p70S6k on T421/S424 (C) had a significant interaction of age × origin (p=0.03), in which phosphorylation declines with age in somitic locomotion muscles, but is unchanged with age in branchial arch and somitic mastication muscles. No significant effects on phosphorylation of p70S6k on T389 were found (D). Data are presented as difference of logs from young averages, expressed as mean ± S.D..
Table 4.
Summary of protein expression and phosphorylation in extraocular (EOM) and pectoralis (P) muscles of young (8 month) and old (26 month) F344 rats.
| EOM | P | |||
|---|---|---|---|---|
| Y | O | Y | O | |
| p-ACC | 1.00±0.4 | 1.45±1.5 | 1.00±0.3 | 1.34±0.7 |
| Akt | 1.00±0.3 | 0.78±0.3 | 1.00±0.3 | 1.59±0.5 |
| p-Akt | 1.00±0.5 | 1.32±1.1 | 1.00±0.3 | 0.92±0.5 |
| Rel Akt | 1.00±0.5 | 1.94±1.5 | 1.00±0.3 | 0.64±0.4 |
| p70 | 1.00±0.4 | 0.99±0.7 | 1.00±0.3 | 0.92±0.5 |
| p70T421/S424 | N.D. | N.D. | 1.00±0.5 | 0.98±0.5 |
| Rel T421 | N.D. | N.D. | 1.00±0.8 | 0.97±0.5 |
| p70T389 | N.D. | N.D. | 1.00±0.4 | 0.99±0.3 |
| Rel T389 | N.D. | N.D. | 1.00±0.8 | 1.07±0.5 |
| ERK2 | 1.00±0.5 | 1.17±0.7 | 1.00±0.2 | 1.11±0.6 |
| p-ERK | 1.00±0.3 | 0.83±0.5 | 1.00±0.7 | 1.09±0.4 |
| Rel ERK | 1.00±0.4 | 0.72±0.3 | 1.00±0.6 | 1.05±0.6 |
| p-p38 | 1.00±0.3 | 0.79±0.4 | 1.00±0.4 | 1.45±0.5 |
| p-JNK 46 | 1.00±0.7 | 0.63±0.3 | 1.00±0.2 | 1.09±0.4 |
| p-JNK 54 | 1.00±0.3 | 1.84±0.6 | 1.00±0.3 | 1.69±0.6 |
MAP kinases
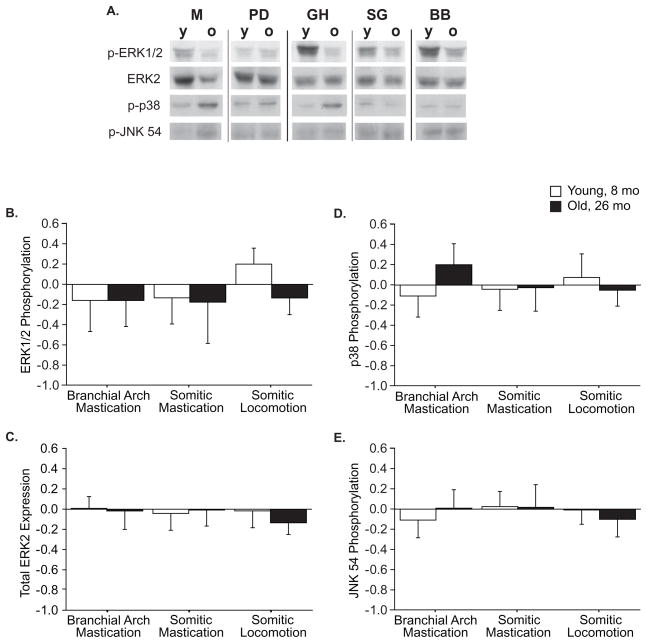
No main effects of muscle origin or age were found among MAP kinases (Figure 3), although interaction effects revealed origin-specific responses to aging for ERK and p38 phosphorylation. The significant origin X age interaction (p=0.03) shows that ERK phosphorylation declines with age in BB muscles while remaining unchanged in jaw and tongue muscles (Figure 3B), primarily because phosphorylation is exaggerated in the young muscle relative to all other groups. This effect was not seen in P (Table 4), and also appears to be unique to BB rather than to muscles of locomotion. The interaction effect (p=0.002) on p38 phosphorylation resulted from an increase with age in branchial arch muscles, with other groups remaining unchanged (Figure 3D), which suggest a greater sensitivity to stress in branchial arch muscles. Generally, phosphorylation of MAP kinases was slightly greater in the faster muscle from each origin, suggesting that the specific function or phenotype of the muscle is more important to MAPK signaling than is the generalized task.
Figure 3.
MAPK expression and phosphorylation in young and old muscles by origin. Representative western blots (A) in which each antigen row is from a single blot, with the muscles rearranged for clarity. A significant interaction effect (p=0.03) on ERK phosphorylation (B) indicates that the decline with age in somitic locomotion muscles is different than the conservation in mastication muscles. No effects on ERK2 expression (C) were found. The significant interaction effect (p=0.002) on phosphorylation of p38 (D) indicates that the increase with age in branchial arch muscles is different than the conservation seen in somitic muscles. No effects on JNK phosphorylation on T54 (E) were found. Data are presented as difference of logs from young averages, expressed as mean ± S.D..
Signaling in EOM and P muscles
EOM and P were separately analyzed to validate observations from the five core muscles. BA muscles showed exaggerated expression of Akt, which was not observed in EOM, but the BA-specific increase in JNK T54 phosphorylation was (p=0.03, Table 4). The BB-specific phosphorylation of p70S6kT421/S424 and ERK2 with age was not observed in P, and aged P showed significantly greater phosphorylation of JNK54 and expression of Akt (p=0.02 and p=0.016, respectively, Table 4).
DISCUSSION
The key findings of the current study show p70S6k expression declines with age in all muscle groups; phosphorylation of ERK and p70S6kT421/S424 is elevated in young BB muscles; Akt expression is elevated in branchial arch muscles; and p38 phosphorylation is much greater in aged branchial arch muscles. Factor analysis revealed coordinated phosphorylation of most signaling molecules, independent of fiber-type or developmental origin, and that this coordination breaks down with aging.
Statistical resolution of individual kinase differences may have been hampered by at least two constraints. First, these comparisons are among muscles in their basal or resting state, at which phosphorylation levels are expected to be low. Second, due to the nature of the dissections, there was a substantial lag between euthanasia and freezing of each muscle. During this time, endogenous phosphatases and proteases may have reduced differences among muscles. Specimens were chilled after euthanasia to minimize this activity as much as possible and pilot experiments indicated that basal phosphorylation was stable for at least an hour. However, one interpretation of factor 2 containing all of the phosphorylations is that samples varied in the preservation of phosphorylation.
Factor analysis identifies sets of correlations between all measures within each muscle and across all groups of muscles. The strongest correlations were between the MHC isoforms and distinguished between fast and slow muscles. The quantification of MHC content by Coomassie-stained gels was associated with variance of less than 20% for fast isoforms. In contrast, variance of kinase phosphorylations detected by Western blot ranged from a low of 20% to a high of 95% within a muscle. This difference in variance encourages the inclusion of MHC isoforms, and facilitates the resolution of systematic biological patterns, such as the shift from IIb to IIx/IIa with age. Coordination among kinases from the Akt-p70S6k cascade and the different MAP kinase cascades is consistent with the involvement of multiple pathways in growth signaling, and it is particularly interesting that this factor is increased in aged M and GH, muscles associated with jaw closing and tongue protrusion, but decreased in aged BB. This suggests that there may be activity dependent signaling within the muscles of mastication at a level too subtle to resolve by Western blot of individual kinases.
The goal of this study was to evaluate the influence of developmental and functional origin on growth signaling during aging. Aging is associated with a decline in systemic growth signals, muscle activity and with motor neuron degeneration that may differentially affect somitic and branchial arch muscles (Giovannini et al., 2008; Larsson and Ansved, 1995; Lynch et al., 2007; Nair, 2005). Such differences may contribute to declines up to 30% in hindlimb muscle mass in F344 rats with age (Daw et al., 1988; Yu et al., 1982), while head, neck and tongue muscles of F344/BN or F344 show reductions of 15% or less in mass and force at equivalent age (Connor et al., 2008; Norton et al., 2001). Although we did not collect head, neck and tongue muscles intact enough to assess mass, EDL and soleus mass from these animals decreased by 19 and 26% respectively (data not shown), which is appropriate for this strain and age (Daw et al., 1988; Yu et al., 1982). BB from old animals were visibly smaller than from young animals and expected to be a forelimb correlate to the commonly studied fast hindlimb muscles. Size differences of the tongue and jaw muscles were not as visibly distinct.
General effects of age
The effect of systemic changes on growth signaling was expected to be revealed by age-associated alterations in signaling molecule activation independent of origin. Expression of p70S6k declines in every muscle group of our 26 month-old Fischer 344 animals. Similar declines are seen in 30 and 36 month-old F344/Brown Norway (FBN) rats (Kinnard et al., 2005) and 27 month-old Sprague-Dawley rats (Kimball et al., 2004). Although the regulation of p70S6k phosphorylation and activation has been reported in detail, control of p70S6k expression has been less extensively reported. The phosphorylation of p70S6k shortly after exercise is correlated with eventual hypertrophy (Baar and Esser, 1999), and acute performance of high force contractions results in an extended period of p70S6k phosphorylation (Glover et al., 2008; Nader and Esser, 2001). In this study, factor analysis revealed p70S6k expression was greater in fast muscles suggesting p70S6k signaling may be particularly important in muscles that stereotypically produce greater forces but are subject to infrequent activity. The general decline in p70S6k expression suggests that aged muscle may have reduced capacity to respond to maintenance signals provided by infrequent high force contractions.
Influence of lineage
The effect of lineage is revealed by differences between the branchial arch muscles and the somitic muscles. Branchial arch muscles displayed two unique features: hyperexpression of Akt and hyperphosphorylation of p38 with age. Head and neck muscles in general have several characteristics that differ from somitic limb muscles, including a unique myogenic program and increased myonuclear turnover (Evans et al., 2008; Noden and Francis-West, 2006). Masseter and extraocular muscles have a larger population of activated satellite cells than limb muscle, but also appear to undergo accelerated apoptosis, suggesting that these branchial arch muscles are subject to continual myonuclear turnover (Evans et al., 2008; McLoon et al., 2004).
P38 is activated in response to cellular stress and important in cellular processes including myogenesis and apoptosis (Keren et al., 2006; Lluis et al., 2006). Phosphorylation of p38 overall was unchanged with age in somitic muscles, which is similar to other studies (Mylabathula et al., 2006), but in branchial arch muscles, p38 phosphorylation greatly increased with age. Hyperactivation of p38 is consistent with accelerated myonuclear turnover, and a more active population of satellite cells in the branchial arch muscles might contribute to preservation of function during aging. However, the unique developmental origin of the head and neck muscles may make them hypersensitive to cellular stresses. Further studies are needed to elucidate the role of p38 with age in these muscles.
Influence of physical activity
Activity was not measured in this study but qualitative observations from other studies are extremely consistent, and a reduction in locomotor activity is observed using any measure of home-cage or voluntary activity (Holloszy et al., 1985; Skalicky et al., 1996). In contrast, tasks of head, neck and tongue muscles - mastication, respiration, and swallowing - are required throughout life and show either minimal change (Connor et al., 2008) or even increased activity (Peng and Kang, 1984; Zhang et al., 2008). We hypothesized these relative changes in muscle activity with age based on muscle function (mastication versus locomotion) would reveal the effect of muscle activity on growth signaling. Although analysis of the five core muscles indicated that phosphorylation of ERK and p70S6kT421/S424 declines with age only in somitic BB muscles, failure to confirm this with P suggests that home cage activity plays little role in supporting basal activation of growth signaling of young locomotor muscles. This is consistent with highly variable literature reports of basal ERK phosphorylation, including either no change (pectoralis, present study; tibialis anterior and plantaris, (Parkington et al., 2004)), increase (extensor digitorum longus, (Mylabathula et al., 2006)), or decrease (biceps brachii, present study) in limb muscles with age. Acutely, ERK phosphorylation is increased in response to electrical stimulation and is closely correlated with mechanical force (Martineau and Gardiner, 2001; Widegren et al., 2001; Wretman et al., 2001). Such acute, force-dependent responses may allow infrequent but intense activity to provide a transient stimulus and maintain muscle mass.
Separate Mechanisms Regulating Expression and Phosphorylation
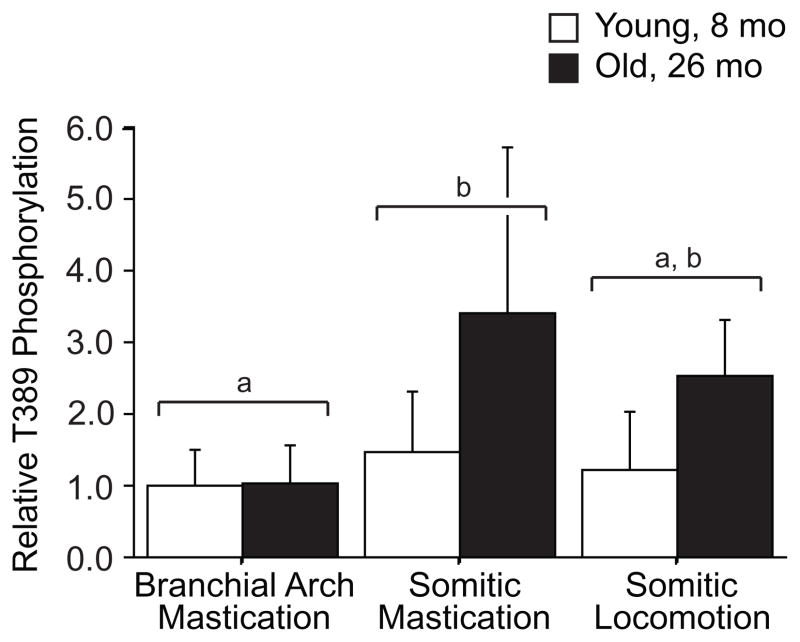
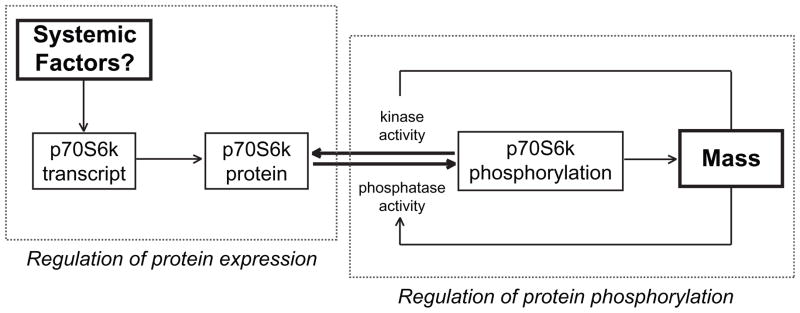
Similar p70S6kT389 phosphorylation in spite of down-regulation of p70S6k expression with age indicates a greater fraction of p70S6k molecules are active as reflected in relative phosphorylation status (Figure 4), and implies a decoupling of the control of expression and activity. Discoordination of p70S6k expression and T389 phosphorylation has been reported in other aging models (Kimball et al., 2004; Kinnard et al., 2005; Thomson and Gordon, 2006) suggesting the regulation of expression and post-translational control of phosphorylation may be regulated by independent processes. Phosphorylation of p70S6k at T389 is the residue most closely representative of activity (Pullen and Thomas, 1997; Weng et al., 1998) and is highly correlated with protein synthesis rate and muscle growth (Baar and Esser, 1999; Thomson and Gordon, 2006). Protein synthesis is higher in old animals despite losing muscle mass (Kimball et al., 2004), which is consistent with neutral or elevated p70S6k phosphorylation. We propose a feedback model for T389 phosphorylation, dependent on muscle force and a separate feedforward model for p70S6k expression dependent on systemic factors (Figure 5). This suggests that functional overload stimulates phosphorylation and activation of p70S6k, whereas systemic factors promote cell growth possibly through acetyl-group modifications leading to stabilization of p70S6k protein (Fenton et al., 2010). Withdrawal of systemic factors, such as growth factors, associated with aging would then reduce the pool of p70S6k and limit the ability of muscle to increase protein synthesis in response to acute stimuli like exercise.
Figure 4.
Relative phosphorylation of p70S6k on T389 to total p70S6k expression in young and old muscles by origin. Relative p70S6k phosphorylation differs by muscle origin (p=0.01) and age (p=0.01), but no significant interaction effect (p=0.10) was found. Origins designated by the same letter were indistinguishable in post hoc T-tests. Data are presented as difference of logs from young averages, expressed as mean ± S.D..
Figure 5. Suggested mechanisms for regulation of p70S6k expression and phosphorylation.
Schematic representation of a feed-forward mechanism in which systemic factors regulate p70S6k expression and a feed-back mechanism in which p70S6k phosphorylation and activation is regulated by muscle mass and modulated by muscle load. In aged muscle, maintaining p70S6k phosphorylation despite a decline in amount of p70S6k may require increased (mTOR) kinase activity. The age-related decline in p70S6k may limit the muscles’ ability to respond to stimuli with increased mass.
Conclusions
In summary, this study provides a comparative analysis of the phosphorylation state of proteins associated with muscle growth in muscles with different functional roles, and different developmental origins. Phosphorylation within the mTOR and MAP kinase cascades was strikingly independent of age, developmental origin, or functional task, but an underlying coordination among phosphorylation of ERK, JNKT54, and p70S6kT421/S424 and Akt was found. One unique signaling structure observed in branchial arch muscles, characterized by hyperexpression of Akt and exaggerated phosphorylation of p38, may contribute to their exaggerated myonuclear turnover and relative sparing from sarcopenia. The discoordination of p70S6k expression and phosphorylation of T389 suggests these aspects are independently regulated, potentially by systemic factors and muscle load.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9:747–758. doi: 10.1038/nrm2509. [DOI] [PubMed] [Google Scholar]
- Baar K, Esser K. Phosphorylation of p70S6k correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol. 1999;45:C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Baumgartner R, Wayne S, Waters D, Janssen I, Gallagher D, Morley J. Sarcopenic Obesity Predicts Instrumental Activities of Daily Living Disability in the Elderly. Obes Res. 2004;12:1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- Blaauw B, Canato M, Agatea L, Toniolo L, Mammucari C, Masiero E, Abraham R, Sandri M, Schiaffino S, Reggiani C. Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J. 2009;23:000–000. doi: 10.1096/fj.09-131870. [DOI] [PubMed] [Google Scholar]
- Bodine S, Stitt T, Gonzalez M, Kline W, Stover G, Bauerlein R, Zlotchenko E, Scrimgeourt A, Lawrence J, Glass D, Yancopoulos G. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Caspersen CJ, Pereira MA, Curran KM. Changes in physical activity patterns in the United States, by sex and cross-sectional age. Medicine and Science in Sports and Exercise. 2000;32:1601–9. doi: 10.1097/00005768-200009000-00013. [DOI] [PubMed] [Google Scholar]
- Connor N, Ota F, Nagai H, Russell J, Leverson G. Differences in age-related alterations in muscle contraction properties in rat tongue and hindlimb. J Speech Lang Hear Res. 2008;51:818–827. doi: 10.1044/1092-4388(2008/059). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw CK, Starnes JW, White TP. Muscle atrophy and hypoplasia with aging: impact of training and food restriction. Journal of Applied Physiology. 1988;64:2428–32. doi: 10.1152/jappl.1988.64.6.2428. [DOI] [PubMed] [Google Scholar]
- Evans M, Morine K, Kulkarni C, Barton ER. Expression profiling reveals heightened apoptosis and supports fiber size economy in the murine muscles of mastication. Physiol Genomics. 2008;35:86–95. doi: 10.1152/physiolgenomics.00232.2007. [DOI] [PubMed] [Google Scholar]
- Fenton TR, Gwalter J, Ericsson J, Gout IT. Histone acetyltransferases interact with and acetylate p70 ribosomal S6 kinases in vitro and in vivo. International Journal of Biochemistry and Cell Biology. 2010;42:359–66. doi: 10.1016/j.biocel.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Flueck M, Goldspink G. Point:Counterpoint: IGF is/is not the major physiological regulator of muscle mass. Counterpoint: IGF is not the major physiological regulator of muscle mass. Journal of Applied Physiology. 2010;108:1821–3. doi: 10.1152/japplphysiol.01246.2009a. discussion 1823–4; author reply 1833. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Meredith CN, O’Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64:1038–1044. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- Giovannini S, Marzetti E, Borst SE, Leeuwenburgh C. Modulation of GH/IGF-1 axis: potential strategies to counteract sarcopenia in older adults. Mech Age Develop. 2008;129:593–601. doi: 10.1016/j.mad.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EI, Oates BR, Tang JE, Moore DR, Tarnopolsky MA, Phillips SM. Resistance exercise decreases eIF2Bepsilon phosphorylation and potentiates the feeding-induced stimulation of p70S6K1 and rpS6 in young men. Am J Physiol Reg Integr Comp Physiol. 2008;295:R604–R610. doi: 10.1152/ajpregu.00097.2008. [DOI] [PubMed] [Google Scholar]
- Goodyear L, Chang PY, Sherwood D, Dufresne S, Moller D. Effects of exercise and insulin on mitogen-activated protein kinase signaling pathways in rat skeletal muscle. Am J Physiol Endocrinol Metab. 1996;271:E403–E408. doi: 10.1152/ajpendo.1996.271.2.E403. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Smith EK, Vining M, Adams S. Effect of voluntary exercise on longevity of rats. Journal of Applied Physiology. 1985;59:826–31. doi: 10.1152/jappl.1985.59.3.826. [DOI] [PubMed] [Google Scholar]
- Hornberger TA, Armstrong DD, Koh TJ, Burkholder TJ, Esser KA. Intracellular signaling specificity in response to uniaxial vs. multiaxial stretch: implications for mechanotransduction. American Journal of Physiology. 2005;288:C185–94. doi: 10.1152/ajpcell.00207.2004. [DOI] [PubMed] [Google Scholar]
- Janssen I, Baumgartner R, Ross R, Rosenberg I, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159:413–421. doi: 10.1093/aje/kwh058. [DOI] [PubMed] [Google Scholar]
- Keren A, Tamir Y, Bengal E. The p38 MAPK signaling pathway: a major regulator of skeletal muscle development. Mol Cell Endocrinol. 2006;252:224–230. doi: 10.1016/j.mce.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Kimball SR. Interaction between the AMP-Activated Protein Kinase and mTOR Signaling Pathways. Med Sci Sports Exerc. 2006;38:1958–1964. doi: 10.1249/01.mss.0000233796.16411.13. [DOI] [PubMed] [Google Scholar]
- Kimball SR, O’Malley JP, Anthony JC, Crozier SJ, Jefferson LS. Assessment of biomarkers of protein anabolism in skeletal muscle during the life span of the rat: sarcopenia despite elevated protein synthesis. Am J Physiol Endocrinol Metab. 2004;287:E772–E780. doi: 10.1152/ajpendo.00535.2003. [DOI] [PubMed] [Google Scholar]
- Kinnard RS, Mylabathula DB, Uddemarri S, Rice KM, Wright GL, Blough ER. Regulation of p70S6k, GSK-3beta, and calcineurin in rat striated muscle during aging. Biogerontology. 2005;6:173–184. doi: 10.1007/s10522-005-7953-6. [DOI] [PubMed] [Google Scholar]
- Larsson L, Ansved T. Effects of ageing on the motor unit. Progress in neurobiology. 1995;45:397–458. doi: 10.1016/0301-0082(95)98601-z. [DOI] [PubMed] [Google Scholar]
- Lluis F, Perdiguero E, Nebreda AR, Munoz-Canoves P. Regulation of skeletal muscle gene expression by p38 MAP kinases. Trends Cell Biol. 2006;16:36–44. doi: 10.1016/j.tcb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Long YC, Widegren U, Zierath JR. Exercise-induced mitogen-activated protein kinase signalling in skeletal muscle. Proc Nutr Soc. 2004;63:227–232. doi: 10.1079/PNS2004346. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Schertzer JD, Ryall JG. Therapeutic approaches for muscle wasting disorders. Pharmacol Ther. 2007;113:461–487. doi: 10.1016/j.pharmthera.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Martineau LC, Gardiner PF. Insight into skeletal muscle mechanotransduction: MAPK activation is quantitatively related to tension. Journal of Applied Physiology. 2001;91:693–702. doi: 10.1152/jappl.2001.91.2.693. [DOI] [PubMed] [Google Scholar]
- McLoon LK, Rowe J, Wirtschafter JD, McCormick KM. Continuous myofiber remodeling in uninjured extraocular myofibers: myonuclear turnover and evidence for apoptosis. Muscle and Nerve. 2004;29:707–715. doi: 10.1002/mus.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylabathula DB, Rice KM, Wang Z, Uddemarri S, Kinnard RS, Blough ER. Age-associated changes in MAPK activation in fast- and slow-twitch skeletal muscle of the F344/NNiaHSD X Brown Norway/BiNia rat model. Experimental Gerontology. 2006;41:205–14. doi: 10.1016/j.exger.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Nader GA, Esser KA. Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. Journal of Applied Physiology. 2001;90:1936–42. doi: 10.1152/jappl.2001.90.5.1936. [DOI] [PubMed] [Google Scholar]
- Nair KS. Aging muscle. Am J Clin Nutr. 2005;81:953–963. doi: 10.1093/ajcn/81.5.953. [DOI] [PubMed] [Google Scholar]
- Noden D, Francis-West P. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn. 2006;235:1194–1218. doi: 10.1002/dvdy.20697. [DOI] [PubMed] [Google Scholar]
- Norton M, Verstegeden A, Maxwell LC, McCarter RM. Constancy of masseter muscle structure and function with age in F344 rats. Archives of oral biology. 2001;46:139–46. doi: 10.1016/s0003-9969(00)00107-2. [DOI] [PubMed] [Google Scholar]
- Parkington JD, LeBrasseur NK, Siebert AP, Fielding RA. Contraction-mediated mTOR, p70S6k, and ERK1/2 phosphorylation in aged skeletal muscle. Journal of Applied Physiology. 2004;97:243–8. doi: 10.1152/japplphysiol.01383.2003. [DOI] [PubMed] [Google Scholar]
- Pearson SJ, Young P, Macaluso A, Devito G, Nimmo MA, Cobbold M, Harridge SDR. Muscle function in elite master weightlifters. Med Sci Sports Exerc. 2002;34:119–1206. doi: 10.1097/00005768-200207000-00023. [DOI] [PubMed] [Google Scholar]
- Peng MT, Kang M. Circadian rhythms and patterns of running-wheel activity, feeding and drinking behaviors of old male rats. Physiology and Behavior. 1984;33:615–20. doi: 10.1016/0031-9384(84)90380-9. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Balagopal P, Nair KS. Age-related sarcopenia in humans is associated with reduced synthetic rates of specific muscle proteins. J Nutr. 1998;128:351S–355S. doi: 10.1093/jn/128.2.351S. [DOI] [PubMed] [Google Scholar]
- Pullen N, Thomas G. The modular phosphorylation and activation of p70s6k. FEBS Letters. 1997;410:78–82. doi: 10.1016/s0014-5793(97)00323-2. [DOI] [PubMed] [Google Scholar]
- Rahnert JA, Sokoloff AJ, Burkholder TJ. Sarcomeric myosin expression in the tongue body of humans, macaques and rats. Cell Tissues Organs. 2010;191:431–442. doi: 10.1159/000258678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renganathan M, Messi ML, Delbono O. Overexpression of IGF-1 exclusively in skeletal muscle prevents age-related decline in the number of dihydropyridine receptors. Journal of Biological Chemistry. 1998;273:28845–51. doi: 10.1074/jbc.273.44.28845. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Goodyear LJ. Invited review: intracellular signaling in contracting skeletal muscle. Journal of Applied Physiology. 2002;93:369–83. doi: 10.1152/japplphysiol.00167.2002. [DOI] [PubMed] [Google Scholar]
- Shah J, Anthony J, KimballI S, Jefferson L. 4E-BP1 and S6K1: translational integration sites for nutritional and hormonal information in muscle. Am J Physiol Endocrinol Metab. 2000;279:E715–E729. doi: 10.1152/ajpendo.2000.279.4.E715. [DOI] [PubMed] [Google Scholar]
- Sherwood D, Dufresne S, Markuns J, Cheatham B, Moller D, Aronson D, Goodyear L. Differential regulation of MAP kinase, p70S6K, and Akt by contraction and insulin in rat skeletal muscle. Am J Physiol Endocrinol Metab. 1999;39:E870–E878. doi: 10.1152/ajpendo.1999.276.5.E870. [DOI] [PubMed] [Google Scholar]
- Skalicky M, Bubna-Littitz H, Viidik A. Influence of physical exercise on aging rats: I. Life-long exercise preserves patterns of spontaneous activity. Mechanisms of Ageing and Development. 1996;87:127–39. doi: 10.1016/0047-6374(96)01707-1. [DOI] [PubMed] [Google Scholar]
- Sturrock RR. Changes in the number of neurons in the mesencephalic and motor nuclei of the trigeminal nerve in the ageing mouse brain. J Anat. 1987;151:15–25. [PMC free article] [PubMed] [Google Scholar]
- Szulc P, Duboeuf F, Marchand F, Delmas PD. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: the MINOS study. Am J Clin Nutr. 2004;80:496–503. doi: 10.1093/ajcn/80.2.496. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ, Roy RR. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. Journal of Applied Physiology. 1993;75:2337–40. doi: 10.1152/jappl.1993.75.5.2337. [DOI] [PubMed] [Google Scholar]
- Thomson D, Gordon S. Impaired overload-induced muscle growth is associated with diminished translational signalling in aged rat fast-twitch skeletal muscle. J Physiol. 2006;574:291–305. doi: 10.1113/jphysiol.2006.107490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappe S, Williamson D, Godard M. Maintenance of whole muscle strength and size following resistance training in older men. J Gerontol A Biol Sci Med Sci. 2002;57:B138–B143. doi: 10.1093/gerona/57.4.b138. [DOI] [PubMed] [Google Scholar]
- Wang ZM, Zheng Z, Messi ML, Delbono O. Extension and magnitude of denervation in skeletal muscle from ageing mice. J Physiol. 2005;565:757–764. doi: 10.1113/jphysiol.2005.087601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle S, Thornton C, Statt M. Myofibrillar protein synthesis in young and old human subjects after three months of resistance training. Am J Physiol Endocrinol Metab. 1995;268:E422–E427. doi: 10.1152/ajpendo.1995.268.3.E422. [DOI] [PubMed] [Google Scholar]
- Weng QP, Kozlowski M, Belham C, Zhang A, Comb MJ, Avruch J. Regulation of the p70 S6 kinase by phosphorylation in vivo. Analysis using site-specific anti-phosphopeptide antibodies. J Biol Chem. 1998;273:16621–16629. doi: 10.1074/jbc.273.26.16621. [DOI] [PubMed] [Google Scholar]
- Widegren U, Ryder J, Zierath J. Mitogen-activated protein kinase signal transduction in skeletal muscle: effects of exercise and muscle contraction. Acta Physiol Scand. 2001;172:227–238. doi: 10.1046/j.1365-201x.2001.00855.x. [DOI] [PubMed] [Google Scholar]
- Wretman C, Lionikas A, Widegren U, Lannergren J, Westerblad H, Henriksson J. Effects of concentric and eccentric contractions on phosphorylation of MAPK(erk1/2) and MAPK(p38) in isolated rat skeletal muscle. Journal of Physiology. 2001;535:155–64. doi: 10.1111/j.1469-7793.2001.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane A. Embryonic and postnatal development of masticatory and tongue muscles. Cell Tissue Res. 2005;322:183–189. doi: 10.1007/s00441-005-0019-x. [DOI] [PubMed] [Google Scholar]
- Yu BP, Masoro EJ, Murata I, Bertrand HA, Lynd FT. Life span study of SPF Fischer 344 male rats fed ad libitum or restricted diets: Longevity, growth, lean body mass and disease. J Gerontol. 1982;37:130–141. doi: 10.1093/geronj/37.2.130. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bethel CS, Smittkamp SE, Stanford JA. Age-related changes in orolingual motor function in F344 vs F344/BN rats. Physiology and Behavior. 2008;93:461–6. doi: 10.1016/j.physbeh.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Messi ML, Delbono O. Age-dependent IGF-1 regulation of gene transcription of Ca2+ channels in skeletal muscle. Mechanisms of Ageing and Development. 2001;122:373–84. doi: 10.1016/s0047-6374(00)00236-0. [DOI] [PubMed] [Google Scholar]