Abstract
Current cocaine-dependent users show reductions in white matter (WM) integrity, especially in cortical regions associated with cognitive control that have been associated with inhibitory dysfunction. A key question is whether these white matter differences are present following abstinence from drug use. To address this, WM integrity was examined using diffusion tensor imaging (DTI) obtained on 43 cocaine abstinent patients (abstinence duration ranged between five days and 102 weeks) and 43 non-using controls. Additionally, a cross-sectional comparison separated the patients into three groups (short-term, mid-term and long-term) based upon duration of cocaine abstinence. The 43 cocaine abstinent patients showed lower fractional anisotropy (FA) in the left anterior callosal fibers, left genu of the corpus callosum, right superior longitudinal fasciculus, right callosal fibers and the superior corona radiata bilaterally when compared against non-using controls. Higher FA in the cocaine abstinent patients was observed in the splenium of the corpus callosum and right superior longitudinal fasciculus. Differences between the cocaine abstinent groups were observed bilaterally in the inferior longitudinal fasciculus, right anterior thalamic radiation, right ventral posterolateral nucleus of the thalamus, left superior corona radiata, superior longitudinal fasciculus bilaterally, right cingulum and the WM of the right precentral gyrus. The results identified WM differences between cocaine abstinent patients and controls as well as distinct differences between abstinent subgroups. The findings suggest that specific white matter differences persist throughout abstinence while other, spatially distinct, differences discriminate as a function of abstinence duration. These differences may, therefore, represent brain changes that mark recovery from addiction.
Keywords: Diffusion Tensor Imaging, Relapse, Cingulum, Corpus Callosum, Fractional Anisotropy
1. INTRODUCTION
A persistent problem with cocaine dependence is that most users will often return to drug use after periods of abstinence (Bossert et al., 2005). Understanding the neurobiology of relapse or, more particularly, the neurobiology of those who successfully avoid relapse is thus an important issue with clear therapeutic implications. However, there is relatively little empirical research on the neurobiological characteristics of successful abstainers. Exceptions include a positron emission tomography study (Bolla et al., 2004) which showed less activation in the left anterior cingulate cortex and right lateral prefrontal cortex and greater activation in the right anterior cingulate cortex during performance on a modified Stroop task in recently abstinent cocaine dependent (CD) patients when compared to non-using controls. Another positron emission tomography study showed that recently abstinent CD patients showed greater activation while performing the Iowa Gambling Task in the right orbitofrontal cortex and less activation in the right dorsolateral prefrontal cortex and left medial prefrontal cortex when compared against non-using controls (Bolla et al., 2003). An fMRI study showed decreased activation in the putamen, anterior cingulate, parahippocampal gyrus amygdala, mesencephalon and thalamus while increased activation was observed in prefrontal and parietal cortical regions involved in attentional processes in abstinent CD patients when compared against non-using controls during performance of a verbal working memory task (Tomasi et al., 2007). Additionally, another fMRI study found positive correlations between self-reported duration of abstinence and activation during a cognitive control task in the left posterior cingulate cortex, left ventral medial prefrontal cortex and right putamen (Brewer et al., 2008). Abstinent CD patients have shown reduced gray matter volume in the prefrontal cortex (Fein et al., 2002; Matochik et al., 2003), lateral and medial aspects of the orbitofrontal cortex and right cingulate gyrus (Matochik et al., 2003) when compared against non-using controls. Finally, utilizing diffusion tensor imaging (DTI), Xu et al. (2010) found that self-reported days of cocaine abstinence were positively correlated with increased fractional anisotropy (FA) in the right superior longitudinal fasciculus, right body of the CC, right posterior limb of the internal capsule and the left cerebellum that was measured before entering a treatment program for CD. Additionally, Xu et al. (2010) split their sample into a short term abstinent group (mean duration of abstinence = 2.5 weeks) and a long-term abstinent group (mean duration of abstinence = 7.3 weeks) and found that the long-duration group had significantly higher FA than the short-duration group. All of these studies examined CD patients at relatively early stages of abstinence (mean = 7.3 weeks was the longest period of abstinence) and thus little is known about the effects of long-term abstinence.
Neuroimaging studies of currently-using CD individuals have shown differences in brain function (Hanlon et al., 2009; Kaufman et al., 2003; Garavan et al., 2008; Li et al., 2007), gray matter volume (Sim et al., 2007; Franklin et al., 2002), and white matter (WM) between CD individuals and healthy controls. With regard to WM, structural magnetic resonance imaging (MRI) has found an increased number of WM hyperintensities in CD individuals compared to healthy controls (Bartzokis et al., 1999) and to opiate dependent patients (Lyoo et al., 2004). A single period of intense cocaine use may even cause WM hyperintensities. After a single “binge” use of cocaine, a patient with a past history of drug dependence but no history of CD was diagnosed with bilateral WM hyperintensities of the palladi and splenium of the corpus callosum (CC) attributed to the cocaine overdose (De Roock et al., 2007).
DTI has been used to identify WM differences in current CD users. DTI provides information about WM integrity based upon the flow of water molecules through white matter tissue using FA as the dependent variable (Beaulieu, 2002). The FA value varies from 0 (completely isotropic) to 1 (free diffusion in one direction only). Studies utilizing DTI have found lower FA in the WM of the inferior prefrontal cortex (Lim et al., 2002; Romero et al., 2010), internal capsule (Lim et al., 2008), genu of the CC (Moeller et al., 2005) and the isthmus, body and splenium of the CC (Ma et al., 2009; Lim et al., 2008) in current cocaine users when compared to non-using controls. Increased FA has been observed in current users compared to controls in the WM of the anterior cingulate (Romero et al., 2010). A DTI study also demonstrated that better performance on executive functioning measures was associated with higher FA in the left frontal callosal fibers and that faster performance on a set-shifting task was associated with higher FA in the right frontal projection fibers in children between the ages of 12 and 14 who were exposed in utero to cocaine (Warner et al., 2006). Finally, a recent DTI study using an animal model of cocaine exposure, thereby permitting causal relationships to be concluded, has shown that rats infused with cocaine for a four-week period exhibit reduced FA in the splenium of the CC (Narayana et al., 2009).
Gene expression studies have also provided evidence that cocaine use may be responsible for deficits in WM integrity. An investigation of myelin-related gene expression in postmortem striatal tissue of CD users revealed decreased levels of proteolipid protein (PLP1), claudin 11 (CLDN11) and transferrin (TFN) in every region examined except the nucleus accumbens (Kristiansen et al., 2009). PLP1, CLDN11 and TFN are involved in creating and maintaining the structural integrity of myelin (Kursula, 2008; Campagnoni, 1988). However, another postmortem gene expression study that was conducted specifically on the nucleus accumbens found decreased levels of myelin basic protein (MBP), PLP1 and myelin-associated oligodendrocyte basic protein (MOBP) in CD users (Albertson et al., 2004). MBP, along with PLP1, is responsible for about 80% of CNS myelin protein (Albertson et al., 2004). Reduced gene expression of MBP has also been shown in the splenium of rodents infused with cocaine (Narayana et al., 2009).
To date, there has been one published investigation of WM differences in CD patients at different stages of abstinence, see Xu et al., 2010 mentioned above (also see preliminary results published in abstract form in Nierenberg, et al., 2005). Given the evidence for WM deficits in current cocaine users, and evidence of early recovery of WM in abstinent cocaine users, the present study assessed WM over longer periods of abstinence. Using a cross-sectional design, we examined a cohort of abstinent cocaine patients who varied in the duration of their abstinence. Comparisons between a non-using control population and abstinent patients and within the abstinent patient group (split into three groups based on the duration of abstinence) allowed us to test for WM integrity differences related to cocaine use and to assess if these differences changed with abstinence duration. Although the present study used a between-subject cross-sectional design rather than a within-subject longitudinal design, the observation of WM differences as a function of abstinence duration would nonetheless identify neurobiological characteristics associated with successful abstinence: these may be of therapeutic importance (i.e., they may facilitate ongoing abstinence) and may provide useful biomarkers for other longitudinal investigations.
2. METHODS
2.1. Subjects
Forty-three abstinent cocaine patients were recruited from in-patient and out-patient addiction treatment centers located in New York State. The 43 controls were recruited through the Volunteer Recruitment Pool at the Nathan S. Kline Institute for Psychiatric Research. All 43 patients received a primary Axis I diagnosis of Cocaine Dependence and from the onset of treatment were closely monitored for continued abstinence with random urine toxicology testing for multiple substances at least two times a week. Patients would also meet at least once a week with a personal counselor who was accredited through the state of New York as an alcoholism and substance abuse counselor. Duration of abstinence, as assessed through negative biweekly random urine screens for the durations noted, was confirmed by the counselors at the addiction treatment centers. Exclusion criteria were as follows: 1) Any DSM IV, Axis 1 diagnosis excluding dependence or a past diagnosis of depression caused by CD based on the Structured Clinical Interview for the DSM IV (SCID); 2) Head trauma resulting in loss of consciousness for longer than 30 minutes; 3) Presence of any past or current brain pathology; 4) A diagnosis of HIV: 5) The presence of any contradictions to an MRI; 6) Over the age of 55 years; 7) Under the age of 19 years; 8) Presence of WM hyperintensities (only one patient was excluded from the analysis because of clinically significant WM hyperintensities). Because of the high rates of comorbidity of alcohol and drug abuse among this population patients were not excluded if they had abused other drugs or alcohol prior to the onset of their cocaine abstinence (3 individuals had comorbid alcohol dependence and 7 individuals had comorbid heroin dependence). None of the patients were currently using any amount of alcohol or drugs. Years of drug use were recorded during the initial SCID interviews. Controls were excluded if they had any major Axis 1 disorder or alcohol/drug dependence diagnosis based upon a SCID for the DSM IV. The study received Institutional Review Board approval at the Nathan S. Kline Institute for Psychiatric Research. All participants were screened for any contradictions to an MRI and signed an informed consent document administered by HIPAA-certified staff.
The sample consisted of 43 patients (2 women) and 43 controls (7 women) (see table 1). The patients and controls did not differ in age (37.5 ± 8.0, 38.8 ± 10.8, respectively; p =.55) but did differ in years of education (12.3 ± 2.0, 14.6 ± 2.2, respectively; p =.001). Consequently, education was included as a covariate in the patient-control group comparisons. The patients were split by abstinence duration into three categories consisting of 13 short term (ST) abstainers (0.7–5.1 weeks), 14 mid-term (MT) abstainers (10–40.3 weeks) and 16 long-term (LT) abstainers (44–102 weeks). The three patient groups did not differ on age at time of testing (F (2, 40) =.83, p=.44) or years of use prior to the most recent period of abstinence (F (2, 40) =2.03, p =.15) but did differ on years of education (F (2, 40) =3.44, p=.04; pairwise contrasts revealed a significant difference between the MT and LT group) and therefore years of education was included as a covariate in the between-patient group analyses. Finally, the ST group was compared to an age and education-matched subset of the control group. The ST group consisted of 13 men and the controls consisted of 11 men and 2 women. Neither the mean age of short-term patients (36.7 ± 8.2) and controls (41.1 ± 11.2) nor the mean years of education of short-term patients (12.5 ± 1.6) and controls (12.7 ± .95) were significantly different (p ≤.26 for age, p ≤.66 for education).
Table 1.
Participant Demographics
| All patients | Group | F | p | ||
|---|---|---|---|---|---|
| Short-term (N=13) | Mid-term (N=14) | Long-term (N=16) | |||
| Length of abstinence (weeks) | 0.7–5.1 | 10–40.3 | 44–102 | - | - |
| Age (years) | 36.7 (8.2) | 39.8 (9.4) | 36.2 (6.5) | 0.83 | 0.42 |
| Years of education | 12.5 (1.6) | 13.2 (1.4) | 11.4 (2.3) | 3.44 | 0.01a |
| Years of use | 11.9 (6.0) | 8.8 (5.5) | 7.14 (7.1) | 2.03 | 0.15 |
| Comorbid (Alcohol/Heroin) | 0/1 | 2/4 | 1/2 | - | - |
| Sex (Male/Female) | 13/0 | 14/0 | 14/2 | - | 0.17b |
| All patients vs. All controls | Group | p | |||
| CD (N=43) | Controls (N=43) | ||||
| Age (Years) | 37.4 (8.0) | 38.8 (10.8) | 0.55 | ||
| Years of education | 12.3 (2.0) | 14.6 (2.2) | 0.01 | ||
| Sex (Male/Female) | 41/2 | 36/7 | 0.08b | ||
| Short-term patients vs. controls | Group | p | |||
| ST (N=13) | Controls (N=13) | ||||
| Age (years) | 36.7 (8.2) | 41.1 (11.2) | 0.26 | ||
| Years of education | 12.5 (1.6) | 12.7 (.95) | 0.66 | ||
| Sex (Male/Female) | 13/0 | 11/2 | 0.14b | ||
Note:
Significant between MT and LT groups
Chi-Square significance level
2.2. Image Acquisition
MRI scans were performed on a 1.5T Siemens Vision system (Erlangen, Germany) at the Center for Advanced Brain Imaging (CABI) at the Nathan S. Kline Institute. Image sequences acquired included: magnetization-prepared rapid gradient echo (MPRAGE) (TR/TE=11.6/4.9 ms, flip angle = 8%, 172 slices, 1.20mm slice thickness, 307 mm FOV, 256 × 256 matrix, pixel size = 1.20 × 1.20mm2, no gap). For 27 of the controls the sequence had 190 slices, 1mm slice thickness, 300mm FOV, 256 × 256 matrix, pixel size 1 × 1mm2, no gap. T2 (TR=5000 ms, TE = 22/90 ms, 26 slices, 5 mm slice thickness, no gap, 192 × 256 matrix, flip angle = 90%, 224 mm FOV, pixel size = .88 × .88 mm2) and diffusion-tensor images (TR=6000 ms, TE=100 ms, 128 × 128 matrix, 320 mm FOV, b-value=1000 s/mm2, 8 non-collinear gradient orientations, NEX=7, 19 slices, 5 mm slice thickness, no gap, pixel size = 2.5 × 2.5 mm2. A double spin echo, pulsed-gradient echo planar acquisition was employed to minimize distortion due to eddy currents (Reese et al., 2003).
2.3. Image Processing
FA was calculated using a C++ program created by Babak Ardekani at the Center for Advanced Brain Imaging (CABI) at the Nathan S. Kline Institute and has been used in previous DTI studies (Hoptman et al., 2008). The MPRAGE images were skull striped using Freesurfer Version 1.3 (Segonne et al., 2004) and registered to the original MPRAGE volume and saved as a binary mask using the Automated Registration Toolbox (ART) (Ardekani et al., 1995; Klein et al., 2009). The binary mask was then applied to the original MPRAGE images. The resulting volumes were then spatially transformed into MNI space using ART. The skull stripped MPRAGE volume and b=0 volumes were also registered to the raw T2 images using a rigid body linear transformation. The b=0 image was then corrected for susceptibility-induced distortion by matching it to the raw T2 image using a 2-D nonlinear registration in ART. Finally, the transformations performed above were combined in one step and were applied to the native FA image to obtain a distortion- corrected and spatially normalized FA volume.
A WM mask was created using the normalized FA volumes from all of the participants employed in each analysis. The threshold for this image was obtained using Otsu’s (1979) nonparametric method. The images also were masked so that only voxels with data present for all participants were included in the analyses. An exploratory whole-brain voxelwise analysis of covariance (VANCOVA) was performed using the FA maps and WM masks for each analysis. The VANCOVA is in-house software that uses a general linear model to evaluate variance. We conducted three voxelwise analyses: 1) a comparison of 43 abstinent patients vs. 43 non-using controls with years of education as a covariate 2) a comparison of 13 short-term abstinent patients against a control cohort of 13 age and education-matched individuals 3) a comparison in which abstinent group (ST, MT, and LT) was the between-subject variable and years of education was included as a covariate. In the first two of these, we identified clusters of at least 200 contiguous voxels (200mm3) each significant at p < .005 with the additional constraint that at least one voxel was significant at p < .001. In the last analysis, we used a lower voxelwise threshold of at least 200 contiguous voxels (200 mm3) each significant at p < .05 with the constraint of at least one voxel being significant at p < .001. This staged thresholding procedure was based on Baudewig (2003) and has been used in other publications by our group (e.g. Hoptman et al., 2008, 2009). A lower threshold was adopted for the three abstinent groups analysis because of the lower number of participants in this analysis and because any WM differences between patient groups was expected to be smaller than would be expected between patients and controls. For significant clusters from the abstinent group comparison, each participant’s mean FA value for that cluster was calculated and then entered into SPSS version 12 (SPSS Inc, Chicago, Illinois). Cluster-level ANCOVA’s were then performed which provided cluster-level, education-adjusted summary statistics and pairwise comparisons between groups. The WM areas were identified using the MRI Atlas of Human White Matter (Mori et al., 2005). Talairach coordinates were automatically derived from the MNI coordinates using the “whereami” tool available as part of AFNI (Cox, 1996).
3. RESULTS
3.1. Abstinent patients versus controls
The voxelwise comparison between all abstinent patients and all controls identified lower FA in the WM of CD individuals in seven regions and higher FA in the WM of CD individuals in two regions (see table 2). Abstinent patients exhibited lower FA in the left anterior callosal fibers, left genu of the CC, right superior longitudinal fasciculus, right callosal fibers and the superior corona radiata bilaterally. Abstinent patients expressed higher FA in the splenium of the CC and right superior longitudinal fasciculus (see figure 1).
Table 2.
Talairach coordinates of FA differences between all patients and all controls
| Anatomical region | Talairach coordinate | Cluster size | t- value | ||
|---|---|---|---|---|---|
| Decreased FA in patients | X | Y | Z | ||
| 1. L. anterior callosal fibers | −19 | 50 | 5 | 440 | −3.54 |
| 2. L. genu of the corpus callosum | −12 | 31 | 10 | 251 | −3.18 |
| 3. R. superior longitudinal fasciculus | 34 | −3 | 26 | 205 | −3.30 |
| 4. L. superior corona radiata | −26 | −20 | 32 | 282 | −3.14 |
| 5. R. superior corona radiata | 26 | −22 | 30 | 501 | −3.45 |
| 6. L. superior corona radiata | −25 | −9 | 34 | 310 | −3.24 |
| 7. R. callosal fibers | 11 | 1 | 49 | 205 | −3.33 |
| Increased FA in patients | |||||
| 1. Splenium of the corpus callosum | 2 | −36 | 15 | 286 | 3.10 |
| 2. R. superior longitudinal fasciculus | 31 | −14 | 19 | 217 | 3.42 |
Figure 1. Brain areas that show differences between all patients and all controls.
Blue signifies lower FA and red signifies higher FA in the patient group. Number refers to region (see Table 2). Images are in radiological orientation with left hemisphere shown on the right side of the image.
3.2. Short-term abstinent patients vs. controls
The previous analysis comparing all abstinent patients and all controls is likely to be most sensitive to differences in FA that hold for the duration of cocaine abstinence. In contrast, the comparison between ST abstinent patients and controls should be most sensitive to differences associated with recent use and should, therefore, be most similar to previous comparisons of current users and controls. The voxelwise t-test between short-term abstinent patients and an age and education matched control cohort showed lower FA in the CD group in seven WM regions (see table 3). Regions included the right anterior limb of the internal capsule, right genu of the CC, left anterior portion of the cingulum, superior corona radiata bilaterally, right cingulum superior to the isthmus of the CC and the right precentral gyrus WM. There were no regions with higher FA in the ST patients.
Table 3.
Talairach coordinates of FA differences between short-term abstinent patients and matched controls
| Anatomical region | Talairach coordinate | Cluster size | t -value | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| R. anterior limb of the internal capsule | 16 | 6 | 10 | 393 | −3.42 |
| R. genu of the corpus callosum | 8 | 25 | 13 | 301 | −3.37 |
| L. anterior portion of the cingulum | −8 | 26 | 15 | 228 | −3.56 |
| L. superior corona radiata | −26 | −20 | 30 | 817 | −3.89 |
| R. superior corona radiata | 26 | −23 | 29 | 567 | −3.91 |
| R. cingulum superior to isthmus of CCa | 14 | 5 | 32 | 529 | −3.95 |
| R. precentral gyrus WM | 39 | −2 | 35 | 303 | −4.14 |
Note:
CC stands for corpus callosum
a negative t -value denotes a decrease in patients
3.3. ANCOVA results for abstinent patients
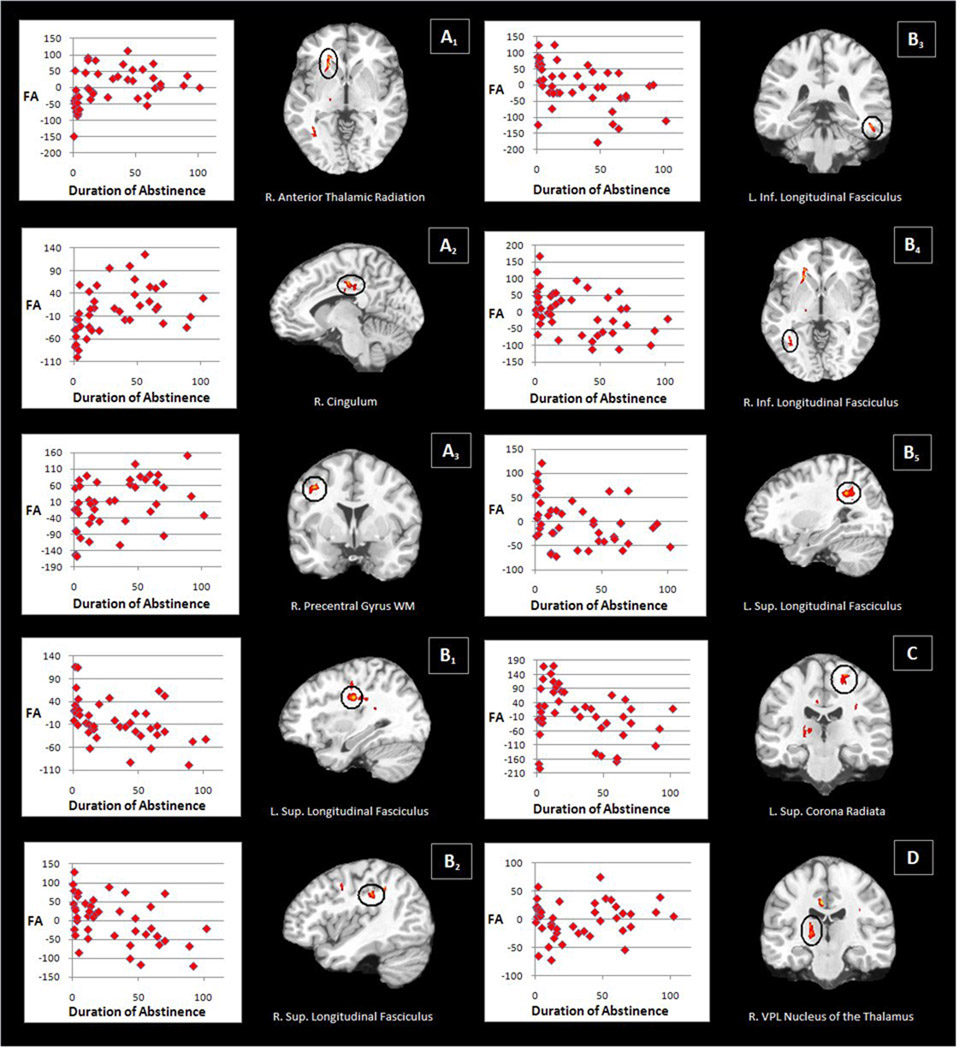
Significant differences between the ST, MT, and LT abstinent groups were observed in a number of WM areas (see table 4). Groups differed bilaterally in the inferior longitudinal fasciculus, right anterior thalamic radiation, right ventral posterolateral nucleus (VPL) of the thalamus, left superior corona radiata, superior longitudinal fasciculus bilaterally, right cingulum and the WM of the right precentral gyrus. A number of different patterns of pairwise effects were observed across these ten areas (see figure 2).
Table 4.
Talairach coordinates of FA differences between abstinent patient groups
| Anatomical region | Talairach coordinate | Cluster size | F | Pairwise p- valuesa | ||||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ST vs. MT | MT vs. LT | ST vs. LT | |||
| A. Increase in FA with abstinence | ||||||||
| 1. R. anterior thalamic radiation | 20 | 23 | 0 | 754 | 5.01 | 0.0001 | - | 0.0001 |
| 2. R. cingulum | 7 | −20 | 32 | 433 | 5.11 | 0.028 | - | 0.0001 |
| 3. R. precentral gyrus WM | 36 | −3 | 38 | 215 | 4.88 | - | 0.004 | 0.0003 |
| B. Decrease in FA with abstinence | ||||||||
| 1. L. superior longitudinal fasciculus | −32 | −15 | 33 | 595 | 4.64 | 0.005 | - | 0.0001 |
| 2. R. superior longitudinal fasciculus | 39 | −28 | 30 | 321 | 5.18 | - | 0.0001 | 0.0001 |
| 3. L. inferior longitudinal fasciculus | −47 | −36 | −12 | 214 | 4.61 | - | 0.011 | .0001 |
| 4. R. inferior longitudinal fasciculus | 34 | −58 | 0 | 349 | 4.34 | - | 0.003 | 0.0001 |
| 5. L. superior longitudinal fasciculus | −28 | −47 | 27 | 500 | 4.49 | 0.002 | - | 0.003 |
| C. Increase and then decrease in FA with abstinence | ||||||||
| L. superior corona radiata | −19 | −23 | 54 | 266 | 4.80 | 0.008 | 0.0001 | - |
| D. Decrease and then increase in FA with abstinence | ||||||||
| R. VPLb nucleus of the thalamus | 17 | −20 | 5 | 561 | 4.54 | 0.003 | 0.0001 | - |
Notes:
p-values for groups that showed significant differences in FA
ventral posterolateral
Figure 2. Brain areas that show differences between abstinence groups.
Letter A refers to higher FA with abstinence. Letter B refers to lower FA with abstinence. Letter C refers to higher and then lower FA with abstinence. Letter D refers to lower and then higher FA with abstinence. Number refers to region (see Table 4). Scatterplots show education-adjusted FA values (y-axis) plotted against abstinence duration (x-axis). FA values are scaled from 0–1000. Images are in radiological orientation.
Higher FA with longer abstinence was observed in the right anterior thalamic radiation (higher FA from ST to MT and stable FA between MT and LT) and the right cingulum (higher FA from ST to MT and a trend [p≤.055] towards higher FA from MT to LT). The WM of the right precentral gyrus showed stable FA between ST and MT and lower FA between MT and LT.
Lower FA with longer abstinence was also observed. The left superior longitudinal fasciculus showed lower FA at two different locations from ST to MT and stable FA between MT and LT. The left inferior longitudinal fasciculus, the right inferior longitudinal fasciculus and the right superior longitudinal fasciculus all exhibited stable FA from ST to MT and lower FA from MT to LT.
Finally, two areas showed a nonmonotonic pattern. The left superior corona radiata showed higher FA from ST to MT and then lower FA from MT to LT. The right VPL nucleus of the thalamus showed an opposite pattern with lower FA from ST to MT and then higher FA from MT to LT.
4. DISCUSSION
Our results provide evidence of significant brain structural differences between abstinent patients and non-using controls. These differences were present in a group of recently-abstinent patients (up to five weeks since last cocaine use) and also in a larger group of 43 patients who were abstinent from 0.7 weeks to almost two years. While the locations of the differences were not identical in the two contrasts, the results provide evidence of WM differences that persist for former users through all three periods of abstinence assessed. That said, a more specific test of WM changes with abstinence did show that there are differences between users who vary in abstinence duration and that these are regionally distinct from the ones showing persistent impairment. Thus, the results suggest that abstinence of up to two years duration is characterized by one set of structural brain differences relative to the recently abstinent and a second set of differences relative to the healthy controls. While the former unique features may give insight into brain changes that arise from or perhaps enable long-term abstinence, the latter may reflect deficits that either arose from cocaine use and persisted or preceded cocaine use and may even have predisposed towards such use.
The comparison between ST patients and age and education - matched controls showed lower FA in seven WM tracts. Because of the short period of abstinence in the ST group (0.7–5.1 weeks), one might expect their WM structures to be the most similar to current users. Indeed, previous studies have identified lower FA in current users, some of which (the internal capsule and the genu of the CC) are consistent with the present results (Lim et al., 2008; Moeller et al., 2005). The administration of cocaine is known to have adverse vascular effects. In an MRI study, Bartzokis et al., (1999) found that 28% of CD patients compared to 7% of non-using controls displayed severe WM hyperintensities that are thought to be the result of cerebrovascular toxicity. Individuals who abuse cocaine can suffer from vascular toxicity leading to stroke that is not accounted for by other risk factors (Fessler et al., 1997; Nolte et al., 1996; Levine and Welch, 1996). In a study of depressed geriatric patients, Hoptman et al., (2009) showed that increased blood pressure, a cerebrovascular risk factor, was associated with lower FA in frontostriatal regions. Thus, the net effect of such cerebrovascular toxicity associated with cocaine use could be responsible for the lower FA values that were observed.
The contrast between all patients and all controls could reflect WM differences that persist despite varying lengths of abstinence. A possible reason for the overall lower FA values in the patient group could be cerebrovascular toxicity resulting from cocaine use as described above. Former CD users have exhibited deficits in attention and motor skills even after one year of abstinence (Toomey et al., 2003). Indeed, we saw that our patient group had lower FA bilaterally in the superior corona radiata through which corticospinal and corticobulbar motor tracts as well as sensory tracts descend (Blumenfield, 2002). It has been shown that damage to the corona radiata can lead to sensory deficits (Shinoura et al., 2009). Sensorimotor deficits have been observed in CD users and include an increase in choreoathetoid movements (Bartzokis et al., 1999), dystonia and tremors (Cardoso and Jankovic, 1993). Additionally, an fMRI study found that current CD users exhibited less efficient activation of the dorsal striatum during a motor task than a control cohort (Hanlon et al., 2009). The dorsal striatum is believed to be involved in sensorimotor integration (Balleine et al., 2007). It seems plausible that these sensorimotor deficits persist into abstinence. Additionally, in the patient group, multiple callosal regions displayed lower FA. Existing literature on current CD individuals shows lower FA in the CC (Moeller et al., 2005; Ma et al., 2009; Lim et al., 2008) and higher impulsivity scores have been correlated with lower FA in the anterior CC (Moeller et al., 2005; Lim et al., 2008). Therefore, the lower FA values of the CC in our patient group could explain why deficits in executive functioning are still evident even after six months of abstinence (Sclafani et al., 2002). It may be that the CC is disrupted before the onset of CD and thus leads to CD, or that prolonged cocaine use causes either irreparable damage to the CC or damage that takes more than two years to fully repair. Whichever situation pertains, we see that there are clear differences that cannot be attributed to current cocaine use between individuals who were addicted to cocaine and individuals who have no history of CD.
The analysis of the abstinent patient groups revealed FA differences, almost all of which were regionally distinct from the FA differences stated above. The differences were varied, including both higher and lower FA as a function of the length of abstinence. As stated above, current cocaine use is associated with lowered FA that is believed to account for decreased cognitive functioning. Therefore, higher FA values with longer abstinence would be consistent with a restoration of WM integrity. Higher FA was associated with longer duration of abstinence in the right superior longitudinal fasciculus, right body of the CC, right posterior limb of the internal capsule and in the left cerebellum (Xu et al., 2010). Increased FA has been shown in a longitudinal study assessing patients suffering from severe traumatic brain injury; notably, the increase over time followed an initial decrease in FA that was observed immediately after the injury suggesting that the pattern of FA changes accompanying brain recovery from insult may be complex (Sidaros et al., 2008).
Moreover, lower values of FA with longer abstinence may also be consistent with a normalization of white matter. Since extended cocaine use is associated with various changes in neural functioning, it may be that certain WM tracts of current CD individuals are utilized more, either to compensate for the loss of function in other affected areas or because they are associated strongly with specific addictive behavior. For example, higher FA has been observed in current CD users when compared to non-using controls in the WM of the anterior cingulate (Romero et al., 2010), an area shown to be hyperactive in current CD individuals when viewing cocaine stimuli (Garavan et al., 2000). A possible reason for lower values of FA with continued abstinence could be that once an individual is recovering from his or her addiction, these tracts are no longer over-utilized resulting in lower FA. However, this explanation would predict higher FA in these areas in the ST group relative to the controls which was not observed, albeit possibly due to the smaller sample sizes of this contrast or WM changes that may have already occurred within the five weeks of abstinence in the ST group.
Alternatively, lower FA with ongoing abstinence may signify a reparative process in which damaged areas exhibit an increase in astrocytes during early abstinence that would result in higher FA values. Glial fibrillary acidic protein (GFAP) is a protein that is upregulated by astrocytes after chemical or physical insult (Aronica et al., 2000) and changes in GFAP levels can be used to assess neuronal insult or injury (Eng et al., 2000). A study assessing the levels of GFAP three weeks after mice were given cocaine intravenously once a day for one week found significantly increased GFAP levels in the nucleus accumbens shell, nucleus accumbens core and prefrontal cortex (Bowers and Kalivas, 2003). In addition, vimentin, a marker for immature or reactive astrocytes showed an increase in the PFC after a three week withdrawal period (Bowers and Kalivas, 2003). A study measuring regional cerebral blood flow (rCBF) in abstinent patients (mean duration of abstinence = 49.02 ± 64.07 weeks) found increased rCBF in the globus pallidus and frontal white matter in abstinent users (Ernst et al., 2000) which these authors postulated could be due to the high energy requirements of astrocytes. Therefore, higher FA in WM tracts between patients could be explained by the increased preponderance of astrocytes that are evident after neuronal injury caused by repeated cocaine administration. The timing of this increase in FA could be a result of the specific tract that is affected, the amount of neuronal injury that was incurred and how long it takes to recover. Higher FA earlier in abstinence that then reduces with continued abstinence may thus reflect an early proliferation of astrocytes in the WM tracts that are responding to the neuronal injury caused by cocaine administration.
The right cingulum showed higher FA in those with the longest abstinence. The cingulum is one of the primary WM tracts involved with limbic system functioning. This tract has been shown to connect the anterior thalamus with the hippocampus (Burgel et al., 2006) and carries afferent connections from the cingulate gyrus to the entorhinal cortex (Mori et al., 2005). fMRI studies in current CD individuals using response inhibition paradigms consistently show hypoactivation in the cingulate gyrus when compared to controls (Li et al., 2007; Kaufman et al., 2003) and co-activation of both the anterior cingulate and the hippocampus has been shown for tasks that require learning from one’s errors (Hester et al., 2008). Chronic cocaine use is frequently associated with deficits in cognitive functioning including decision making, judgment, attention, planning and mental flexibility (e.g. Bolla et al., 1998; Kubler et al., 2005), and abstinent cocaine users still show deficits in executive functioning six months after abstinence (Sclafani et al., 2002). It is tempting to speculate that lower FA in the cingulum may underlie some of these cognitive and executive deficits including compromised learning of the negative consequences of one’s behavior. Given that the ST group exhibited lower FA in two areas of the cingulum when compared to non-using controls, it is notable that higher FA in the cingulum was observed for the LT group relative to the ST group indicating that heightened integrity in this pathway may be related to longer abstinence.
Another tract exhibiting higher FA over time between the abstinent groups was the right anterior thalamic radiation. The anterior thalamic radiation connects the dorsomedial and anterior thalamic nuclei with the prefrontal cortex (Sprooten et al., 2009) and lower FA values within it has previously been linked to the abnormal prefrontal function of those with schizophrenia and bipolar disorder (McIntosh et al., 2008; Sprotten et al., 2009). Increased prefrontal functioning with abstinence may be related to the higher FA of the anterior thalamic radiation and may also be related to the observed higher FA in the vicinity of the right precentral gyrus.
It is notable that all areas showing lower FA with increasing abstinence fell on either the inferior or superior longitudinal fasciculus. The superior longitudinal fasciculus II showed higher FA bilaterally in the ST group relative to the MT or LT groups. In a longitudinal study, Xu et al., (2010) found that higher FA in the superior longitudinal fasciculus at the onset of treatment predicted a longer duration of abstinence. The superior longitudinal fasciculus II has been characterized as a WM tract that links the prefrontal and parietal cortices (Makris et al., 2005). It may play an important role in coordinating prefrontal-parietal processes involved in working memory, visuospatial perception and attention (Makris et al., 2005). A meta-analysis of studies examining subcortical lesions that resulted in visuospatial neglect found the lesions to be located at or near the superior longitudinal fasciculus (Bartolomeo et al., 2007). Utilizing fMRI and a verbal working memory task, recently abstinent users showed hyperactivation in the prefrontal and parietal cortices and prefrontal hyperactivation on a visuospatial attention task when compared to control subjects (Tomasi et al., 2007). The authors postulated that this hyperactivation could be due to increased attention and control processes to compensate for a decrease in executive functioning that is associated with cocaine use. Though speculative, the lower FA in the MT and LT groups might reflect diminishing reliance on a prefrontal-parietal compensatory mechanism through recovery of executive functions.
The inferior longitudinal fasciculus is an association tract that connects the parietal and temporal cortices and the occipital and temporal cortices with very strong connections to temporal polar cortex (Hua et al., 2009). The temporal polar cortex is located at the junction of higher-order visual, auditory, and olfactory/insular association cortices (Ding et al., 2009) and is hypothesized to be responsible for binding processed perceptual inputs to visceral emotional responses (Olson et al., 2007). The higher FA observed bilaterally in this tract in the ST group relative to the MT and LT groups may reflect increased utilization of this tract during addiction, perhaps related to a role in drug craving (Garavan et al., 2000; Bonson et al., 2002). Conceivably, with continued abstinence this tract exhibits lower FA mirroring a reduction in strong cue-induced visceral emotional responses that are associated with CD.
There are specific limitations in the present study that need to be acknowledged. First, when between-groups analyses were conducted on the three sub-groupings of abstinent patients, the sample sizes were relatively small with the attendant reductions in power that this imposes. Also, images in the present study were collected using a 1.5T scanner with 5 mm slice thickness and just 8 gradient orientations. It is now possible to collect thinner slices and more orientations at higher field strength. Nonetheless, because of the slice thickness and the fact that seven excitations were collected for each direction, excellent signal-to-noise ratios were achieved here. We also did not collect any health-related information on study participants and it is known that factors such as cardiovascular disease or diabetes can influence the development of white matter and therefore cannot be ruled out as factors in the observed differences in white matter between abstinent groups. Another potential issue was raised by a reviewer of this manuscript who pointed out that although not statistically significant, there is a numerical difference between the short- and long-term abstinence groups in terms of overall years of cocaine use. Long-term patients, on average, had shorter durations of use than short-term patients and it is possible that the relative success these long-term patients have had in resisting relapse might arise because of shorter original exposure periods to the drug of abuse. In turn, this may have been reflected in differences in WM integrity. However, the long-term group in this study used cocaine for an average of 7 or more years, and to our minds, it seems highly unlikely that such a protracted period of use could result in significantly less impairment in this group. Lastly, it also needs to be pointed out that this study was conducted predominantly in male participants and that in view of this; appropriate care should be taken in generalizing these results to abstinent female CD patients.
Because of this study’s cross-sectional design, it is impossible to determine if the observed FA differences between patients at different stages of abstinence were due to dynamic intra-individual changes that perhaps reflect recovery of function, or if they reflect pre-existing differences between patients. Whichever situation pertains, these cross-sectional results suggest that although WM deficits exist in the abstinent users as a whole, there may also be additional differences in brain structure in those who have attained a relatively long period of abstinence compared to those who are more recently abstinent. The regions showing these differences include white matter tracts thought to be involved in motor, cognitive and emotional processes but without precise assessments of psychological changes occurring with abstinence, the interpretations offered for the functional changes that accompany WM differences between the groups must remain speculative. The caveats notwithstanding, the patterns of FA differences across abstinence durations may reflect dynamic patterns of changing reliance on different psychological processes as drug users escape their drug dependence.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, Bannon MJ. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. Journal of Neurochemistry. 2004;88:1211–1219. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardekani BA, Braun M, Hutton BF, Kanno I, Iida H. A fully automatic multimodality image registration algorithm. J. Comput. Assist. Tomogr. 1995;19:615–623. doi: 10.1097/00004728-199507000-00022. [DOI] [PubMed] [Google Scholar]
- Aronica E, van Vliet EA, Mayboroda OA, Troost D, da Silva FH, Gorter JA. Upregulation of metabotropic gluatamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur. J. Neurosci. 2000;12:2333–2344. doi: 10.1046/j.1460-9568.2000.00131.x. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neuroscience. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomeo P, Thiebaut de Schotten M, Doricchi F. Left unilateral neglect as a disconnection syndrome. Cerebral Cortex. 2007;17:2479–2490. doi: 10.1093/cercor/bhl181. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Hance DB, Lu PH, Foster JA, Mintz J, Ling W, Bridge P. Magnetic resonance imaging evidence of “silent” cerebrovascular toxicity in cocaine dependence. Biol. Psychiatry. 1999;45:1203–1211. doi: 10.1016/s0006-3223(98)00228-5. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Wirshing DA, Lu PH, Foster JA, Mintz J. Choreoathetoid movements in cocaine dependence. Biol. Psychiatry. 1999;45:1630–1635. doi: 10.1016/s0006-3223(98)00238-8. [DOI] [PubMed] [Google Scholar]
- Baudewig J, Dechent P, Merboldt KD, Frahm J. Thresholding in correlational analyses of magnetic resonance functional neuroimaging. Magn. Reson. Imaging. 2003;21:1121–1130. doi: 10.1016/j.mri.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NWR in Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Blumenfield H. Neuroanatomy through clinical cases. Sunderland, MA: Sinauer Associates; 2002. [Google Scholar]
- Bolla KI, Cadet JL, London ED. The neuropsychiatry of chronic cocaine abuse. J. Neuropsychiatry Clin. Neurosci. 1998;10:280–289. doi: 10.1176/jnp.10.3.280. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. NeuroImage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J. Neuropsychiatry Clin. Neurosci. 2004;16(4):456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl L, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacology. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Bowers SM, Kalivas PW. Forebrain astroglial plasticity is induced following withdrawal from repeated cocaine administration. Eur J Neuroscience. 2003;17:1273–1278. doi: 10.1046/j.1460-9568.2003.02537.x. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pre-treatment brain activation during stroop task is associated with outcomes in cocaine dependent patients. Biol. Psychiatry. 2008;64(11):998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgel U, Amunts K, Hoemke L, Mohlberg H, Gilsbach JM, Zilles K. White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography, and intersubject variability. Neuroimage. 2006;29:1092–1105. doi: 10.1016/j.neuroimage.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Campagnoni AT. Molecular biology of myelin proteins from the central nervous system. J Neurochem. 1988;51:1–14. doi: 10.1111/j.1471-4159.1988.tb04827.x. [DOI] [PubMed] [Google Scholar]
- Cardoso FE, Jankovic J. Cocaine-related movement disorders. Mov. Disord. 1993;8:175–178. doi: 10.1002/mds.870080210. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- De Roock S, Hantson P, Laterre PF, Duprez T. Extensive pallidal and white matter injury following cocaine overdose. Intensive Care Med. 2007;33:2030–2031. doi: 10.1007/s00134-007-0773-1. [DOI] [PubMed] [Google Scholar]
- Ding SL, Van Hoesen GW, Cassell MD, Poremba A. Parcellation of human temporal polar cortex: a combined analysis of multiple cytoarchitectonic, chemoarchitectonic, and pathological markers. J Comparative Neurology. 2009;514:595–623. doi: 10.1002/cne.22053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem. Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Oropilla G, Gustavson A, Speck O. Cerebral perfusion abnormalities in abstinent cocaine abusers: a perfusion MRI and SPECT study. Psychiatry Research: Neuroimaging. 2000;99:63–74. doi: 10.1016/s0925-4927(00)00056-1. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68(1):87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler RD, Esshaki CM, Stankewitz RC, Johnson RR, Diaz FG. The neurovascular complications of cocaine. Surg. Neurol. 1997;47:339–345. doi: 10.1016/s0090-3019(96)00431-4. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol. Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Garavan H, Kaufman JN, Hester R. Acute effects of cocaine on the neurobiology of cognitive control. Philos Trans R Soc Lond B Biol Sci. 2008;363:3267–3276. doi: 10.1098/rstb.2008.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho J, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Porrino LJ. Loss of functional specificity in the dorsal striatum of chronic cocaine users. Drug and Alcohol Depend. 2009;102:88–94. doi: 10.1016/j.drugalcdep.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Barre N, Murphy K, Silk TJ, Mattingly JB. Human medial frontal cortex activity predicts learning from errors. Cerebral Cortex. 2008;18:1933–1940. doi: 10.1093/cercor/bhm219. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Gunning-Dixon FM, Murphy CF, Ardekani BA, Hrabe J, Lim KO, Etwaroo GR, Kanellopoulos D, Alexopoulos GS. Blood pressure and white matter integrity in geriatric depression. J Affect. Disord. 2009;115:171–176. doi: 10.1016/j.jad.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, Nierenberg J, Bertisch HC, Catalano D, Ardekani BA, Branch CA, Delisi LE. A DTI study of white matter microstructure in individuals at high genetic risk for schizophrenia. Schizophrenia Research. 2008;106:115–124. doi: 10.1016/j.schres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Hua K, Oishi K, Zhang J, Wakana S, Yoshioka T, Zhang W, Akhter KD, Li X, Huang H, Jiang H, van Zijl P, Mori S. Mapping of functional areas in the human cortex based on connectivity through association fibers. Cerebral Cortex. 2009;19:1889–1895. doi: 10.1093/cercor/bhn215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Garavan H. Cingulate hypoactivity in cocaine users during a go-nogo task as revealed by event-related functional magnetic resonance imaging. J Neuroscience. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen LV, Meador-Woodruff JH, Bannon MJ. Expression of transcripts for myelin related genes in postmortem brain from cocaine abusers. Neurochem Res. 2009;34:46–54. doi: 10.1007/s11064-008-9655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubler A, Murphy K, Garavan H. Cocaine dependence and attention switching within and between verbal and visuospatial working memory. Eur J Neuroscience. 2005;21:1984–1992. doi: 10.1111/j.1460-9568.2005.04027.x. [DOI] [PubMed] [Google Scholar]
- Kursula P. Structural properties of proteins specific to the myelin sheath. Amino Acids. 2008;34:175–185. doi: 10.1007/s00726-006-0479-7. [DOI] [PubMed] [Google Scholar]
- Levine SR, Welch KMA. Cocaine and stroke. Stroke. 1996;19:779–783. doi: 10.1161/01.str.19.6.779. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Yan P, Bhagwagar Z, Millivojevic V, Sinha R. Neural correlates of impulse control during stop signal inhibition in cocaine-dependent men. Neuropsychopharmacology. 2007;33(8):1–9. doi: 10.1038/sj.npp.1301568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wokin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol. Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, Silverman AB, Rotrosen JP. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug and Alcohol Depend. 2008;92:164–172. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo IK, Streeter CC, Ahn KH, Lee HK, Pollack MH, Silveri MM, Nassar L, Levin JM, Sarid-Segal O, Ciraulo DA, Renshaw PF, Kaufman MJ. White matter hyperintensities in subjects with cocaine and opiate dependence and healthy comparison subjects. Psychiatry Research: Neuroimaging. 2004;131:135–145. doi: 10.1016/j.pscychresns.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Ma L, Hasan KM, Steinberg JL, Narayana PA, Lane SD, Zuniga EA, Kramer LA, Moeller FG. Diffusion tensor imaging in cocaine dependence: regional effects of cocaine on corpus callosum and effect of cocaine administration route. Drug and Alcohol Depend. 2009;104:262–267. doi: 10.1016/j.drugalcdep.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorenson GA, Wang R, Caviness VS, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Munoz-Maniega S, Lymer KS, McKirdy J, Hall J, Sussmann JED, Bastin ME, Clayden JD, Johnstone EC, Lawrie SM. White matter tractography in bipolar disorder and schizophrenia. Biol. Psychiatry. 2008;64:1088–1092. doi: 10.1016/j.biopsych.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KH, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PCM. MRI atlas of human white matter. Amsterdam: Elsevier; 2005. [Google Scholar]
- Narayana PA, Ahobila-Vaijula P, Ramu J, Herrera J, Steinberg JL, Moeller FG. Diffusion tensor imaging of cocaine-treated rodents. Psychiatry Research: Neuroimaging. 2009;171:242–251. doi: 10.1016/j.pscychresns.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierenberg J, Nash L, Hoptman MJ, Hrabe J, Ardekani BA, Bunt G, Lim KO, Rotrosen J. White matter changes in cocaine dependence. Neuropsychopharmacology. 2005;30:S210–S270. [Google Scholar]
- Nolte KB, Brass LM, Fletterick CF. Intracranial hemorrhage associated with cocaine abuse: A prospective autopsy study. Neurology. 1996;46:1291–1296. doi: 10.1212/wnl.46.5.1291. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Otsu N. A thresholding selection method from gray-level histogram. IEEE Trans. System Man Cybernet. 1979;9(1):62–66. [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn. Reson. Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Romero MJ, Asensio S, Palau C, Sanchez A, Romero FJ. Cocaine addiction: Diffusion tensor imaging study of the inferior frontal and anterior cingulate white matter. Psychiatry Research: Neuroimaging. 2010;181:57–63. doi: 10.1016/j.pscychresns.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Sclafani VD, Tolou-Shams M, Price LJ, Fein G. Neuropsychological performance of individuals dependent on crack-cocaine, or crack-cocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug and Alcohol Depend. 2002;66:161–171. doi: 10.1016/s0376-8716(01)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salvolini U, Hahn HK, Fischl B. A hybrid approach to the skull-stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Shinoura N, Suzuki Y, Yoshida M, Yamada R, Tabei Y, Saito K, Yagi K. Assessment of the corona radiata sensory tract using awake surgery and tractography. J Clin Neuroscience. 2009;16:764–770. doi: 10.1016/j.jocn.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Sidaros A, Engberg AW, Sidaros K, Liptrot MG, Herning M, Petersen P, Paulson OB, Jernigan TL, Rostrup E. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain. 2008;131:559–572. doi: 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, Kim MJ, Kaufman MJ, Yurgelun-Todd DA, Renshaw PF. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;10:2229–2237. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- Sprooten E, Lymer GKS, Munoz-Maniega S, McKirdy J, Clayden JD, Bastin ME, Porteous D, Johnstone EC, Lawrie SM, Hall J, McIntosh AM. The relationship of anterior thalamic radiation integrity to psychosis risk associated neuregulin-1 variants. Molecular Psychiatry. 2009;14:237–238. doi: 10.1038/mp.2008.136. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Thompson L, Dalwani M, Owens E, Crowley T, Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance dependent individuals. Biol. Psychiatry. 2009;65(2):160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Research. 2007;1171:83–92. doi: 10.1016/j.brainres.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomey R, Lyons MJ, Eisen SA, Xian H, Chantarujikapong S, Seidman LJ, Faraone SV, Tsuang MT. A twin study of the neuropsychological consequences of stimulant abuse. Arch Gen Psychiatry. 2003;60:303–310. doi: 10.1001/archpsyc.60.3.303. [DOI] [PubMed] [Google Scholar]
- Warner TD, Behnke M, Elyer FD, Padgett K, Leonard C, Hou W, Garavan CW, Schmalfuss IM, Blackband SJ. Diffusion tensor imaging of frontal white matter and executive functioning in cocaine-exposed children. Pediatrics. 2006;118:2014–2024. doi: 10.1542/peds.2006-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. White matter integrity is associated with treatment outcome measures in cocaine dependence. Neuropsychopharmacology. 2010;35:1541–1549. doi: 10.1038/npp.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]