Abstract
Primary dystonia has traditionally been viewed as a basal ganglia disorder, but recent studies suggest that the cerebellum plays a crucial role in the disease. Primary dystonia is associated with several genotypes. Among those, DYT1 and DYT6 are inherited in autosomal dominant fashion with reduced penetrance. Extensive structural and functional imaging studies have been performed on manifesting and non-manifesting carriers of these mutations. The results suggest that primary dystonia can be viewed as a neurodevelopmental circuit disorder, involving the cortico-striato-pallido-thalamo-cortical and cerebello-thalamo-cortical pathways. Anatomical disruption of the cerebellar outflow is found in non-manifesting and manifesting mutation carriers, and a second downstream disruption in thalamo-cortical projections appears clinically protective in non-manifesting carriers. The microstructural deficits in cerebellar outflow are linked to an abnormally elevated sensorimotor network (NMRP) in dystonia patients. Abnormal expression of this network is reduced by successful treatment with deep brain stimulation.
Keywords: dystonia, DYT1, DYT6, brain networks, motor activation, imaging marker, neurodevelopmental, positron emission tomography
Introduction
The dystonias comprise a heterogeneous group of movement disorders that are characterized by sustained muscle contractions, resulting in abnormal postures or twisting movements (Oppenheim, 1991). Clinically, dystonia can be described by its distribution, but classifications by etiology are of greater relevance for diagnosis and treatment. Primary dystonias, i.e. those without an acquired cause and not occurring in the context of a broader neurodegenerative process, can be familial or sporadic (Bressman, 2004). Clinically, dystonia can be restricted to isolated muscle groups (i.e. focal dystonias such as blepharospasm or torticollis) or, as in generalized dystonia, may involve several muscle groups.
Primary dystonia is associated with several genotypes. The most common of these, termed DYT1, is a GAG deletion within the coding region for torsinA on chromosome 9q34 (Ozelius et al., 1997; Breakefield et al., 2008). TorsinA is a chaperone protein of the superfamily of AAA+ ATPases. Multiple cellular functions have been associated with torsinA, including vesicle fusion, membrane trafficking, protein folding and cytoskeletal dynamics, but its precise function is unknown (Breakefield et al., 2008; Tanabe et al., 2009; Fuchs et al., 2009). The less frequent DYT6 mutation is linked to the THAP1 gene on chromosome 8q21-22 (Fuchs et al., 2009). THAP1 is a member of a family of DNA-binding factors that regulate cell proliferation(Fuchs et al., 2009).
Both DYT1 and DYT6 mutations are inherited in autosomal dominant fashion with incomplete penetrance. Clinical symptoms are present in about 30% of DYT1 carriers and 60% of DYT6 carriers (Risch et al., 2007; Saunders-Pullman et al., 2007). Unlike the more common adult-onset primary dystonias (Defazio et al., 2007), both DYT1 and DYT6 genotypes are associated with symptom onset in late childhood and adolescence. Thus, studies of non-manifesting gene-positive individuals past the age of clinical onset offers the possibility of identifying genotype-related trait characteristics without the confound of clinical symptoms (Eidelberg, 2003). In both these dystonia genotypes, the pathophysiological link between gene carrier status and clinical penetrance is unknown.
Similarly, the histopathological basis for primary dystonias remains largely unknown. In fact, the challenge of primary dystonia is reflected in its clinical definition: the presence of involuntary, sustained muscle contractions in the absence of identifiable brain lesions. Generally, primary dystonia is regarded as a disorder of the basal ganglia and its efferent connections. Nonetheless, recent evidence in animal and human studies have also implicated the cerebellum and related motor pathways (Jinnah and Hess, 2006; Neychev et al., 2008; Carbon and Eidelberg, 2009).
In this review we will summarize the results of our neuroimaging studies in manifesting and non-manifesting carriers of these dystonia mutations. We will focus on abnormalities in resting brain function, pathway microstructure and anatomical connectivity. We will then discuss these dystonia-related changes at the circuit level and suggest how abnormal network activity can be modulated by treatment.
Microstructural pathway abnormalities
The typical onset of DYT1- and DYT6-associated DYSTONIA suggests a neurodevelopmental basis for this disorder. Clinical signs and symptoms of dystonia generally develop in affected carriers in late childhood and adolescence, when maturation of thalamic and sensorimotor cortical pathways is known to occur (Gogtay et al., 2004; Barnea-Goraly et al., 2005; Paus, 2005). Indeed, magnetic resonance diffusion tensor imaging (DTI) has provided valuable evidence to support this contention as an in vivo probe of pathway microstructure. DTI studies have revealed reduced fractional anisotropy (FA), a measure of axonal coherence and integrity, in the subgyral white matter adjacent to sensorimotor cortex and in the dorsal pons of manifesting and non-manifesting DYT1 and DYT6 carriers (Carbon et al., 2004; 2008; Delmaire et al., 2009). Moreover, the dorsal pontine microstructural abnormalities (in the vicinity of the superior cerebellar peduncle) were found to be greater in manifesting than in non-manifesting carriers (Carbon et al., 2008).
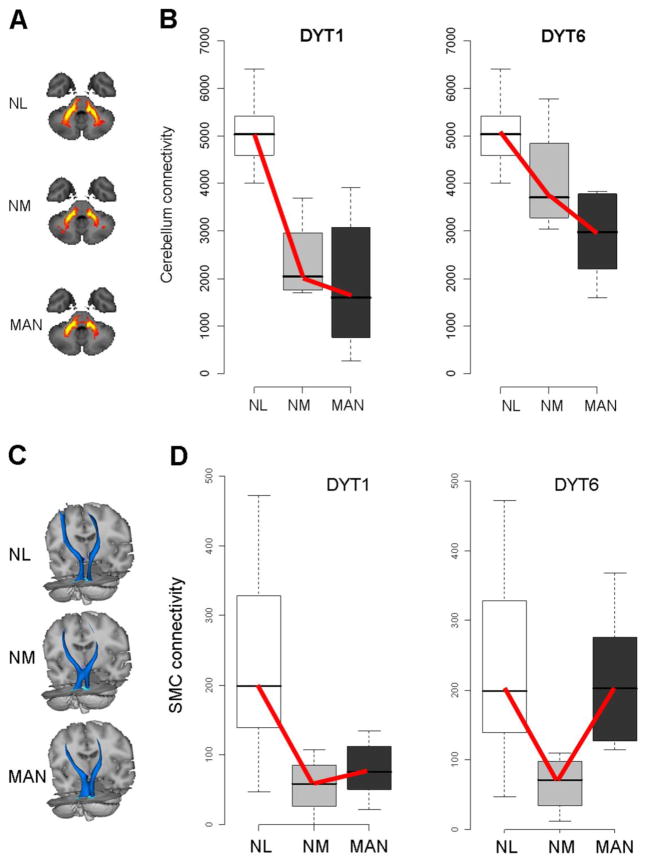
These observations implicate abnormalities in the cerebello-thalamo-cortical pathways in the pathogenesis of dystonia. Nonetheless, this regional measure of altered microstructure failed to accurately classify individual gene-positive subjects according to clinical penetrance. Moreover, these studies were limited by their inability to localize the microstructural changes to specific neuroanatomical fiber tracts. Recently, we used higher field (3T) magnetic resonance DTI in conjunction with probabilistic tractography to identify the specific circuit abnormalities that underlie clinical penetrance in dystonia mutation carriers (Argyelan et al., 2009). The resulting connectivity data were then compared voxel-wise across groups. This approach revealed reduced integrity of cerebello-thalamo-cortical fiber tracts, in both clinically manifesting and non-manifesting DYT1 and DYT6mutation carriers (Figure 1A, 1B). Post-hoc analysis of the connectivity data from each genotype (Figure 1B) revealed reduced connectivity in this region in both manifesting and non-manifesting carriers, with the latter occupying an intermediate position between manifesting carriers and controls.
Figure 1. Reduced cerebellar outflow (A, B) and thalamocortical (C, D) connectivity in dystonia gene carriers.
A. Two-dimensional projections of the group mean probabilistic connectivity tracts generated from seed masks in normal control subjects (NL, n=8), and in non-manifesting (NM, n=8) and manifesting (MAN, n=12) dystonia mutation carriers. Reduced fiber tract integrity is evident in the proximal portion of the cerebellar outflow pathway in the NM and MAN groups. B. Connectivity values measured in a significant white matter cluster identified by comparing cerebello-thalamo-cortical tracts in dystonia mutation carriers with controls (Argyelan et al., 2009). Connectivity in this region was abnormally reduced in both NM and MAN gene carriers (p<0.01 and p<0.001, Mann-Whitney U tests). A significant decreasing group-wise trend (NL>NM>MAN) was present in both the DYT1 (left) and DYT6 (right) carriers across the three groups (p<0.001 and p=0.002 for the two genotypes; tests of trends). C. Three-dimensional displays of the group mean probabilistic connectivity tracts generated from seed masks in the same subjects. The size of this fiber tract is reduced distally, most pronounced in the non-manifesting carriers. D. Connectivity values measured in a significant cluster identified by comparing cerebello-thalamo-cortical tracts in NM mutation carriers with controls (Argyelan et al., 2009). This cluster was localized to the distal segment of the pathway in the subgyral white matter of the precentral region. A different group-wise trend (NL>MAN>NM) was evident for this cluster in the DYT1 (left) and DYT6 (right) carriers (p<0.002 and p=0.0025 for the two genotypes; tests of trends).
[A-D: Adapted from J Neurosci, Cerebellothalamocortical connectivity regulates penetrance in dystonia, 9740-9747, Copyright 2009, with permission from the Society for Neuroscience.]
Interestingly, reductions in cerebellothalamic connectivity in DYT1 carriers correlated with motor activation responses determined by measuring regional cerebral blood flow (rCBF) in the same subjects at rest and during movement (Argyelan et al., 2009). Lower cerebellar connectivity was associated with greater activation in the sensorimotor cortex (SMC) and supplementary motor area (SMA). These results are consistent with the notion that abnormalities of cerebellar pathway development in dystonia are associated with loss of inhibition at the cortical level.
Thalamocortical pathways and clinical penetrance
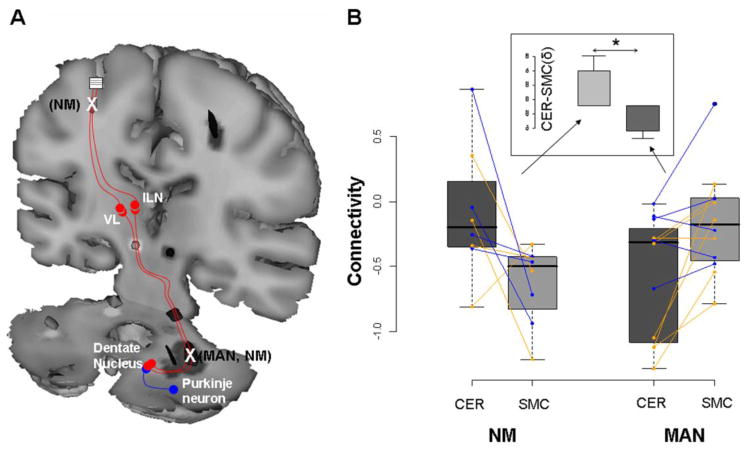
Comparison of non-manifesting carriers and controls revealed that non-manifesting mutation carriers were distinguished by an additional area of reduced fiber tract integrity situated distally along the thalamocortical segment of the cerebello-thalamo-cortical pathway, in tandem with the proximal cerebellar outflow abnormality. Pathway microstructure in this region was more perturbed in the non-manifesting carriers than in the manifesting subjects (Figure 1C, 1D). These data support a compelling model for clinical penetrance in primary dystonia: symptoms develop when the distal segment of the cerebello-thalamo-cortical motor pathway is less disrupted, which allows aberrant cerebellar outflow signals to gain access to cortical motor regions and cause abnormal involuntary movements. Greater disruption in this segment of the pathway blocks transmission to motor cortex and thus exerts a clinically protective non-manifesting state (Figure 2A). Indeed, we found that clinical status (i.e., penetrant or non-penetrant) could be predicted accurately by the difference in tract integrity (measured as “connectivity” in the probabilistic tractography maps) at the two sites (Figure 2B). This observation was further supported by Monte Carlo simulation in which stochastic estimates of this difference resulted in a DYT1 penetrance rate of approximately 30%, analogous to that observed in population studies.
Figure 2. Penetrance is related to the integrity of tandem pathway segments.
A. Schematic representation of the proposed circuit model for gene penetrance in primary dystonia (Argyelan et al., 2009). In this model, serial connectivity reductions in the proximal and distal segments of the cerebello-thalamo-cortical pathway inhibit the development of clinical manifestations (see text). B. Connectivity values for the cerebellar (CER) and sensorimotor cortical (SMC) clusters measured in the individual gene carriers. For each subject, connectivity values (z-transformed) for each region were right-left averaged and connected by lines (DYT1, orange; DYT6, blue). Subject differences in connectivity between regions (δ=CER–SMC; insert) were compared for the MAN and NM carriers. Differences in δ were found to be significant (p=0.001, Mann-Whitney U test), with positive values for the NM group and negative values for the MAN group.
[B: Reprinted from J Neurosci, Cerebellothalamocortical connectivity regulates penetrance in dystonia, 9740-9747, Copyright 2009, with permission from the Society for Neuroscience.]
Abnormal functional connectivity at rest
Changes in pathway microstructure can lead to widespread alterations in regional brain function measured in the rest state. [18F]-fluorodeoxyglucose (FDG) positron emission tomography (PET) has been used to map spatially distributed metabolic changes in neurological disease. In particular, spatial covariance approaches based on principal components analysis (PCA) have been employed to characterize disease-specific patterns of regional metabolic activity in patients with a variety of movement disorders (see e.g., Eidelberg, 2009). A specific covariance topography was originally identified in sporadic primary dystonia patients (Eidelberg et al., 1995); this pattern was subsequently validated in several independent cohorts of individuals with sporadic and genetic forms of the illness (Eidelberg et al., 1998; Hutchinson et al., 2000). This pattern, termed torsion dystonia-related pattern (TDRP), is characterized by relative increases of metabolic activity in the posterior putamen/globus pallidus, cerebellum and SMA.
Interestingly, abnormal TDRP expression appears to be unrelated to the presence of symptoms in carriers of dystonia mutations (i.e., gene penetrance), and is also unrelated to the somatotopic distribution of clinical manifestations in these individuals (i.e., phenotypic variability). Indeed, significant elevations in the expression of this metabolic pattern have been observed in forms of dystonia that would not typically be classified as “torsion dystonia”, such as essential blepharospasm (Hutchinson et al., 2000). Indeed, the TDRP was originally identified in non-manifesting DYT1 carriers and its expression proved to be similar in affected and non-penetrant gene positive subjects (Eidelberg et al., 1998; Trost et al., 2002). Moreover, TDRP expression in manifesting individuals remained abnormally elevated even after the dystonic movements were suppressed by sleep induction (Eidelberg et al., 1998; Hutchinson et al., 2000). The distinctive TDRP metabolic topography has since been confirmed in a larger cohort of non-manifesting DYT1 carrier and control scans analyzed using routine voxel-based univariate comparisons (Carbon and Eidelberg, 2009). That said, TDRP expression is not a universal feature of all dytonias. In contrast to DYT1, in non-manifesting carriers of the DYT6 mutation, the regional abnormalities involved metabolic reductions in the putamen and cerebellum, and in the upper brainstem extending into the thalamus (Carbon et al., 2004).
Although trait-related metabolic abnormalities appear to differ across genotypes, affected individuals share a number of distinct metabolic characteristics in the rest state, notably increased activity in the pre-SMA and parietal association regions (Carbon et al., 2004). These cortical abnormalities were not related to specific clinical characteristics of dystonia, but instead represent a common feature in individuals with clinically heterogeneous manifestations. Multifocal or generalized signs of dystonia are evident in the majority of manifesting subjects in the DYT1 genotype, but in only a third of DYT6 haplotype carriers. Conversely, in DYT1, the cranial musculature is involved in less than 15% of manifesting carriers, while in DYT6 cervico-cranial dystonia is present in 60% (Bressman, 2004; Saunders-Pullman et al., 2007).
Spatial covariance analysis has also been used to identify a distinct metabolic pattern related to clinical penetrance in mutation carriers (Carbon and Eidelberg, 2009; cf. Eidelberg et al., 1998; Hutchinson et al., 2000). This pattern is characterized by relative metabolic increases in the pre-SMA and parietal association cortices, and covarying decreases in the inferior cerebellum, brainstem, and ventral thalamus (Carbon and Eidelberg, 2009). Expression of this dystonia manifestation-related pattern (DYT-RP) distinguished manifesting from non-manifesting mutation carriers in both DYT1 and DYT6 subjects. Manifesting carriers of either genotype showed increased expression of DYT-RP compared to non-manifesting carriers and controls. This is consistent with the notion that in dystonia, clinical penetrance is associated with abnormal sensorimotor integration. Interestingly, expression of this pattern was actually reduced in non-manifesting carriers compared to controls (Carbon et al., 2004), raising the possibility of specific adaptive responses in these unaffected carriers. These findings again indicate that penetrance-related cortical metabolic increases are distinct from genotype-related trait features.
These effects are less easily separated in brain areas with penetrance-related reductions in metabolic activity. In particular, metabolic abnormalities in the cerebellum may reflect an interaction of trait and phenotype effects. Network-related metabolic reductions are seen in the rostral pons and midbrain, and ventral thalamus (Carbon and Eidelberg, 2009), areas that include the targets of internal pallidal inhibitory output. Regional metabolism is related to afferent synaptic activity (e.g., Eidelberg et al., 1997; Lin et al., 2008). Thus, in manifesting gene carriers, the relative metabolic reductions observed in these regions can be attributed to the functional loss of inhibitory output from the internal globus pallidus (GPi) (e.g., Starr et al., 2005).
The notion of a stepwise breakdown of cortical sensorimotor disintegration gains further traction from study of an allelic polymorphism in the torsinA gene. Residue 216 has been associated with penetrance in DYT1 (Kock et al., 2006; Risch et al., 2007). In one American DYT1 population, the 216H allele is found in increased frequency in non-manifesting DYT1 carriers. This haplotype is believed to be clinically “protective” as compared to the more common D216 allele (Risch et al., 2007). Regional metabolism in the parietal association cortex and pre-SMA of non-manifesting DYT1 carriers with the D216 (“unprotected”) allele fell intermediately between manifesting and control values (Carbon et al., 2008). Metabolism in the dorsal premotor cortex (PMC) of the D216 allele carriers was in the range of manifesting subjects. By contrast, in 216H non-manifesting carriers, regional metabolism in pre-SMA was in the normal range, and was actually below normal in the parietal association and PMC regions. It is not known whether carriers of the D216H polymorphism also differ in cerebello-thalamo-cortical microstructure, particularly with respect to the relative integrity of the distal thalamo-cortical segment of this pathway.
Increased sensorimotor network activity in dystonia mutation carriers
The studies outlined above demonstrate altered resting metabolic activity in dystonia gene carriers. However, abnormalities of the sensorimotor network have been described in dystonia involving activated as well as resting conditions. At least in the case of the focal dystonias, studies have related the disorder to multiple cortical phenomena, including increased excitability, decreased inhibition, impaired plasticity, and impaired sensorimotor integration (Quartarone et al., 2006). Neurophysiological studies have demonstrated decreased intracortical inhibition in the hand area of patients with focal hand dystonia or blepharospasm (Sommer et al., 2002), and in penetrant and non-penetrant DYT1 gene carriers (Edwards et al., 2003). The sensory discrimination threshold, a measure of sensory cortical organization, has been found to be abnormal in relatives of patients with sporadic dystonia (Walsh et al., 2007).
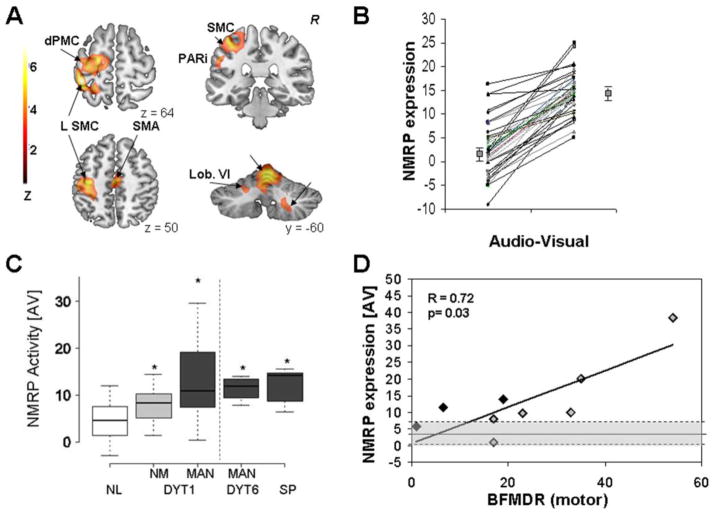
Functional imaging has extended these electrophysiological observations. Beyond the alterations in resting state metabolism, we have found increased activation of auxiliary cortical motor regions and the inferior parietal cortex, accompanied by reduced activation in the right cerebellum, in non-manifesting DYT1 carriers performing a kinematically controlled motor sequence learning task (Ghilardi et al., 2003). We recently used a new multivariate network-modeling approach to identify a reproducible motor activation spatial covariance pattern in healthy subjects, and quantify the expression of this pattern in dystonia mutation carriers (Carbon et al., 2010). The analytical technique that was employed was ordinal trends canonical variates analysis (OrT/CVA) (Habeck et al., 2005). This is a form of supervised principal components analysis (Bair et al., 2006) that can be used to characterize specific spatial covariance patterns associated with consistent within-subject change in expression across behavioral conditions (e.g., Stern et al., 2008). OrT/CVA was applied to O15-labeled water (H215O) PET activation data from 18 normal control subjects (37.2±13.4 years) who were scanned while performing repetitive counterclockwise reaching movements (Ghilardi et al., 2000; 2003). Each subject was also scanned in a non-motor and audiovisual condition while attending to the cues without moving. The resulting activation topography, termed the normal motor-related activation pattern (NMRP), was characterized by contributions from the major nodes of the cortico-striato pallidal-thalamo-cortical and cerebello-thalamo-cortical motor circuits (left sensorimotor cortex, premotor cortex, and inferior parietal cortex, with additional contributions bilaterally in the cerebellar vermis and hemispheres, Figure 3A). This activation pattern exhibited an ordinal trend in that, without exception, subject expression in the counterclockwise reaching task was greater than in the non-motor audiovisual condition for all pairs of runs obtained in the derivation cohort (Figure 3B).
Figure 3. Normal motor-related activation pattern (NMRP).
A. NMRP as identified by ordinal trends canonical variates analysis (OrT/CVA) of 78 H215O PET scans from 18 healthy volunteers (Carbon et al., 2010). [Color scale: Positive voxel weights thresholded at Z=3.09, corresponding to regions that contributed significantly (p<0.001) to network activity. The displayed voxel weights were found to be reliable (p<0.0001) on bootstrap estimation]. B. NMRP expression in the subjects comprising the original derivation sample. For all subjects and runs, pattern expression increased during the performance of the motor task (p<0.0001, (Carbon et al., 2010)). C. NMRP scores for manifesting (MAN) and non-manifesting (NM) DYT1 carriers and controls (left panel), and for MAN DYT6 carriers and subjects with sporadic (SP) primary dystonia (right panel). For all subjects, the network values were measured in H215O PET scans acquired in the audio-visual (AV) condition (Carbon et al., 2010). In this non-motor (“sensory”) condition pattern expression was elevated in both groups of mutation carriers and in the SP subjects. [Significant post-hoc comparisons with controls (p<0.05) are denoted by asterisks]. D. NMRP expression in MAN DYT1 carriers measured in the audio-visual (AV) condition correlated with clinical severity ratings according to the Burke-Fahn-Marsden Dystonia Rating Scale (BFMDRS). [Black diamonds: subjects without contractions at rest; gray diamonds: subjects with constant or with occasional contractions at rest. The normal mean and range are indicated by shading].
[A-D: Adapted from Brain, Increased sensorimotor network activity in DYT1 dystonia: a functional imaging study, 690-700, Copyright 2010, with permission from Oxford University Press]
We postulated that NMRP is elevated in dystonia mutation carriers and that network activity is relatively greater in manifesting carriers. For this, NMRP expression was computed prospectively on an individual basis in each counterclockwise and audiovisual run obtained in the gene carrier groups and in a subsequent healthy control group (whose scans had not been used in NMRP derivation). Significant group differences in NMRP expression were evident in both behavioral conditions (p<0.001, ANOVA). In the motor task, manifesting but not non-manifesting DYT1 carriers showed greater NMRP values than controls. Although network activity did not differ between non-manifesting DYT1 carriers and controls during movement tasks, these values were abnormally elevated (p<0.01) in the non-motor condition (Figure 3C, left). Moreover, NMRP elevations were not limited to DYT1 carriers. Both manifesting DYT6 carriers with task-specific dystonia and individuals with sporadic cervical dystonia showed significantly greater network activity than controls during both counterclockwise movements and the audiovisual condition (Figure 3C, right). Moreover, NMRP expression measured in the audiovisual condition correlated closely with Burke-Fahn-Marsden dystonia rating scores (Figure 3D). Notably, the increases in NMRP expression were unlikely to be an artifact of intercurrent involuntary movements, as (i) no movements were evident during the scanning, and (ii) significant elevations of NMRP were evident in non-manifesting carriers. Thus, we attribute the observed increases in NMRP activity in dystonia to decreased inhibition of motor circuitry (Defazio et al., 2007; Carbon et al., 2010).
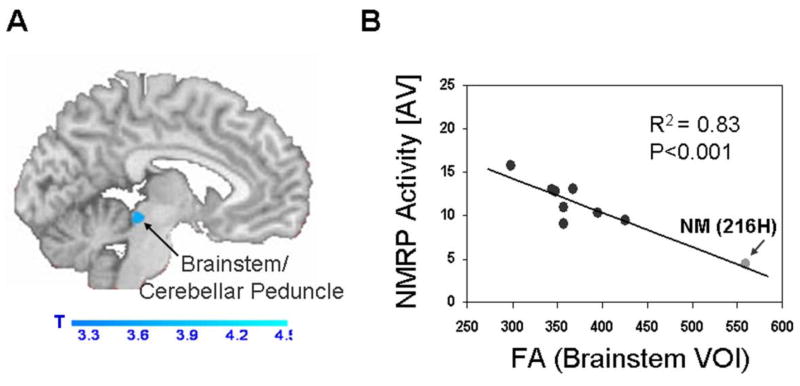
Abnormal NMRP expression is likely associated with the microstructural abnormalities described above (Argyelan et al., 2009), specifically the abnormal transmission of cerebellar outflow to the thalamus and sensorimotor cortex (Molinari et al., 2002; Daskalakis et al., 2004). We explored this potential structure-function relationship by conducting a voxel-wise search for significant correlations between subject NMRP expression and the corresponding fractional anisotropy maps generated by 3T magnetic resonance DTI in nine DYT1 mutation carriers This analysis revealed only a single cluster in which fractional anisotropy values correlated negatively with NMRP expression, such that reduced local microstructural integrity was associated with increased NMRP activity in the non-movement audiovisual condition. This significant region was situated in the dorsal aspect of the upper brainstem in the vicinity of the superior cerebellar peduncle (Figure 4). Indeed, fractional anisotropy measurements in this brain area were found to differentiate between manifesting and non-manifesting mutation carriers in an earlier lower field MR DTI study (Carbon et al., 2008). Of note, the only subject with normal fractional anisotropy in this region (as well as normal NMRP expression) was a non-manifesting individual carrying the 216H allele (arrow). This suggests that this polymorphism is indeed “protective” in that the proximal segment of the cerebello-thalamo-cortical pathway may be spared in this cohort of non-manifesting DYT1 carriers. Needless to say, the correlation described above remained significant (p<0.02) even after this one influential subject was excluded from the analysis.
Figure 4. Microstructural correlates of NMRP activity.
A. Statistical parametric map (SPM) of significant correlations between individual subject expression of the normal motor-related activation pattern (NMRP) and the corresponding maps of fractional anisotropy (FA) generated by 3T magnetic resonance (MR) diffusion tensor imaging (DTI) acquired in nine DYT1 mutation carriers. Significant regions were thresholded at p<0.001, uncorrected and superimposed on a single-subject MRI T1 template. A significant correlation between NMRP expression and FA was identified in the dorsal aspect of the upper brainstem in the vicinity of the superior cerebellar peduncle. B. Post-hoc analysis of the significant cluster identified in (A). An inverse correlation was present between NMRP expression in the non-movement audiovisual (AV) condition and local FA values such that reduced microstructural integrity in brainstem pathways was associated with relatively greater network activity. We note that the non-manifesting (NM) carrier with the “protective” 216H allele (arrow) was normal with respect to both variables. Nonetheless, the correlation remained significant following the exclusion of this subject (R2=0.62, p<0.02).
Modulation of network activity by treatment
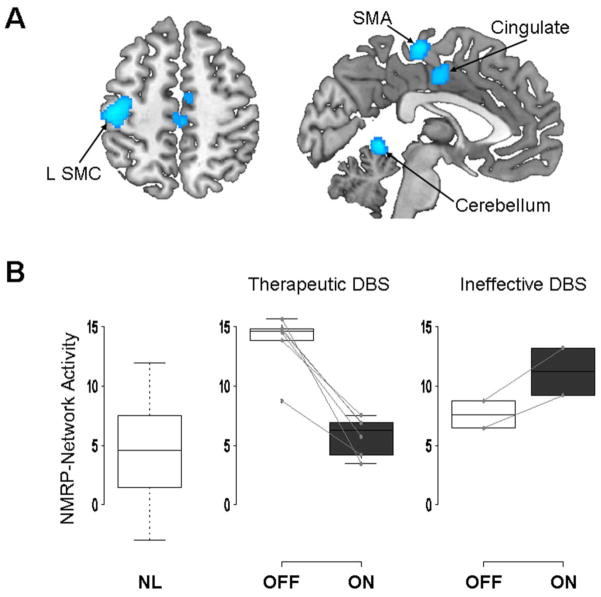
Deep brain stimulation (DBS) of the internal globus pallidus (GPi) has been shown to be an effective treatment option in patients with generalized or focal dystonia (Vidailhet et al., 2007; Hung et al., 2007; Ostrem and Starr, 2008). The mechanism by which DBS exerts its therapeutic benefit remains unknown, but imaging studies have shown that DBS can decrease abnormal motor activation in the primary and secondary motor cortices, as well as prefrontal regions, putamen and thalamus (Kumar et al., 1999; Detante et al., 2004). In a pilot study, we determined whether GPi DBS can lower the NMRP elevations that typify primary dystonia. Five subjects underwent H215O PET while performing the counterclockwise motor task and in the non-motor audiovisual condition before and during GPi stimulation. Voxel-wise analysis revealed a significant treatment effect on rCBF (p<0.001, uncorrected), with stimulation-mediated reductions in the SMC, pre-SMA, inferior prefrontal cortex, dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC), and anterior-superior cerebellar cortex (Figure 5A). These regional findings accord with published studies on the effect of pallidal stimulation in dystonia (Detante et al., 2004; Thobois et al., 2008).
Figure 5. Treatment-mediated network modulation: Effect of GPi DBS in primary dystonia.
NMRP expression was measured in five patients with intractable cervical dystonia (Hung Neurology 2007) who underwent H215O PET imaging on and off pallidal stimulation. A. Statistical parametric map of voxel-wise analysis, showing a significant treatment effect on regional cerebral blood flow (rCBF), with stimulation-mediated reductions in the sensorimotor cortex (SMC), supplementary motor area (SMA), inferior prefrontal cortex, dorsolateral prefrontal cortex, cingulate cortex (p<0.001, uncorrected). B. Baseline NMRP values (left) measured in the non-motor audiovisual (AV) condition were found to be significantly elevated relative to normal control subjects (p<0.01, t-test). Effective stimulation (middle) was associated with a highly significant reduction in network activity (p<0.001, paired t-test). However, NMRP expression did not change significantly in the one subject (two runs), who did not improve with treatment (right).
On a network level, NMRP expression values were abnormally elevated in the off-stimulation condition (Figure 5B, middle). Clinically effective GPi stimulation resulted in a significant reduction in NMRP expression (p<0.001; paired Student’s t-test), recorded during the audiovisual condition. By contrast, stimulation-mediated declines in network activity were not evident in scans without an associated clinical response to treatment (Figure 5B, right).
Overall, these findings are consistent with the notion that GPi stimulation for dystonia acts to normalize baseline overactivity of sensorimotor networks. By directly modulating the activity of pallido-thalamic projections, and/or by indirectly altering the activity of distal thalamocortical pathways, this intervention can be construed to relieve symptoms by making affected gene carriers functionally resemble their counterparts without clinical manifestations.
Conclusion
Primary dystonia, which often begins in late childhood or adolescence, has traditionally been attributed to basal ganglia dysfunction, but no specific pathological lesions of these structures have been consistently described on postmortem examination. The recent studies summarized in this review expand this picture and create a paradigm for understanding dystonia as a potential neurodevelopmental circuit disorder. Anatomically, both manifesting and non-manifesting mutation carriers exhibit disruptions of the cerebello-thalamo-cortical pathways. Moreover, non-manifesting individuals show additional, distal disruptions in the thalamo-cortical projection pathways. This distal defect is clinically “protective” in that it blocks transmission of the aberrant cerebellar output to the motor cortex.
These microstructural deficits in the cerebellar outflow pathways are associated with localized functional changes at rest and during motor activation. To understand these alterations at the circuit level, we identified and validated a specific activation pattern in normal subjects performing motor tasks. We found the activity of this network to be elevated in dystonia patients (even in non-movement conditions) and in non-manifesting mutations carriers, potentially reflecting the impairment of integrative motor processing (Carbon and Eidelberg, 2009). Moreover, this network abnormality proved to be potentially correctable by treatment with GPi DBS. By analogy to Parkinson’s disease (Asanuma et al., 2006; Feigin et al., 2007), spatial covariance patterns like NMRP may ultimately find use as adjunct outcome measures in clinical trials, and potentially also as endophenotypic biomarkers in gene-finding population studies.
Future studies will need to address how the microstructural lesions and changes in functional network activity account for the varied anatomical distribution of dystonia signs and symptoms in individuals with identical genetic mutations. It will also be interesting to see if patients with sporadic dystonia, including task-specific dystonias, have underling structural and network changes that predispose them to the development of dystonia later in life.
Research Highlights.
Structural lesions in cerebellar outflow pathways are present in DYT1/DYT6 carriers
Penetrance in DYT1 and DYT6 carriers is determined by a second structural lesion
Dystonia mutation carriers show increased activity of sensorimotor networks
Effective DBS reduces abnormal network activity in primary dystonia
Acknowledgments
This work was supported by the National Institutes of Health (NIH NINDS R01 047668), the Bachmann-Strauss Dystonia & Parkinson Foundation and The Feinstein Institute for Medical Research General Clinical Research Center (National Centre for Research Resources, a component of the NIH, M01 RR 018535).
Abbreviations
- DTI
magnetic resonance diffusion tensor imaging
- FA
fractional anisotropy
- rCBF
regional cerebral blood flow
- SMC
sensorimotor cortex
- SMA
supplementary motor area
- FDG
[18F]-fluorodeoxyglucose
- PET
positron emission tomography
- PCA
principal components analysis
- TDRP
torsion dystonia-related pattern
- DYT-RP
dystonia manifestation-related pattern
- Gpi
internal globus pallidus
- PMC
premotor cortex
- OrT
ordinal trends
- CVA
canonical variates analysis
- CCW
counterclockwise
- AV
audiovisual
- NMRP
normal motor-related activation pattern
- CSPTC
cortico-striato pallidal-thalamo-cortical
- BFMDR
Burke-Fahn-Marsden dystonia rating scores
- DBS
deep brain stimulation
- ACC
anterior cingulate cortex
- DLPFC
dorsolateral prefrontal cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argyelan M, Carbon M, Niethammer M, Ulug AM, Voss H, Bressman SB, Dhawan V, Eidelberg D. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J Neurosci. 2009;29:9740–9747. doi: 10.1523/JNEUROSCI.2300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma K, Tang C, Ma Y, Dhawan V, Mattis P, Edwards C, Kaplitt MG, Feigin A, Eidelberg D. Network modulation in the treatment of Parkinson’s disease. Brain. 2006;129:2667–2678. doi: 10.1093/brain/awl162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair E, Hastie T, Paul D, Tibshirani R. Prediction by supervised principal components. J Am Stat Assoc. 2006;101:119–137. [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat RevNeurosci. 2008;9:222 –234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- Bressman SB. Dystonia genotypes, phenotypes, and classification. Adv Neurol. 2004;94:101–107. [PubMed] [Google Scholar]
- Carbon M, Argyelan M, Habeck C, Ghilardi MF, Fitzpatrick T, Dhawan V, Pourfar M, Bressman SB, Eidelberg D. Increased sensorimotor network activity in DYT1 dystonia: a functional imaging study. Brain. 2010;133:690–700. doi: 10.1093/brain/awq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Eidelberg D. Abnormal structure-function relationships in hereditary dystonia. Neuroscience. 2009;164:220–229. doi: 10.1016/j.neuroscience.2008.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Kingsley P, Su S, Smith GS, Spetsieris P, Bressman SB, Eidelberg D. Microstructural white matter changes in carriers of the DYT1 gene mutation. Ann Neurol. 2004;56:283–286. doi: 10.1002/ana.20177. [DOI] [PubMed] [Google Scholar]
- Carbon M, Kingsley PB, Tang C, Bressman S, Eidelberg D. Microstructural white matter changes in primary torsion dystonia. Mov Disord. 2008;23:234–239. doi: 10.1002/mds.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Ozelius L, Raymond D, Bressman S, Eidelberg D. Metabolic correlates of genetic susceptibility in DYT1 torsion dystonia. Neurology. 2008;11 (Suppl 1):A432. [Google Scholar]
- Carbon M, Su S, Dhawan V, Raymond D, Bressman SB, Eidelberg D. Regional metabolism in primary torsion dystonia: effects of penetrance and genotype. Neurology. 2004;62:1384–1390. doi: 10.1212/01.wnl.0000120541.97467.fe. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Paradiso GO, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol. 2004;557:689–700. doi: 10.1113/jphysiol.2003.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defazio G, Berardelli A, Hallett M. Do primary adult-onset focal dystonias share aetiological factors? Brain. 2007;130:1183–1193. doi: 10.1093/brain/awl355. [DOI] [PubMed] [Google Scholar]
- Delmaire C, Vidailhet M, Wassermann D, Descoteaux M, Valabregue R, Bourdain F, Lenglet C, Sangla S, Terrier A, Deriche R, Lehericy S. Diffusion abnormalities in the primary sensorimotor pathways in writer’s cramp. Arch Neurol. 2009;66:502–508. doi: 10.1001/archneurol.2009.8. [DOI] [PubMed] [Google Scholar]
- Detante O, Vercueil L, Thobois S, Broussolle E, Costes N, Lavenne F, Chabardes S, Lebars D, Vidailhet M, Benabid AL, Pollak P. Globus pallidus internus stimulation in primary generalized dystonia: a H215O PET study. Brain. 2004;127:1899–1908. doi: 10.1093/brain/awh213. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Huang YZ, Wood NW, Rothwell JC, Bhatia KP. Different patterns of electrophysiological deficits in manifesting and non-manifesting carriers of the DYT1 gene mutation. Brain. 2003;126:2074–2080. doi: 10.1093/brain/awg209. [DOI] [PubMed] [Google Scholar]
- Eidelberg D. Brain networks and clinical penetrance: lessons from hyperkinetic movement disorders. Curr Opin Neurol. 2003;16:471–474. doi: 10.1097/01.wco.0000084224.82329.75. [DOI] [PubMed] [Google Scholar]
- Eidelberg D. Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci. 2009;32:548–557. doi: 10.1016/j.tins.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Antonini A, Kazumata K, Nakamura T, Dhawan V, Spetsieris P, deLeon D, Bressman SB, Fahn S. Functional brain networks in DYT1 dystonia. Ann Neurol. 1998;44:303–312. doi: 10.1002/ana.410440304. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Przedborski S, Fahn S. The metabolic topography of idiopathic torsion dystonia. Brain. 1995;118:1473–1484. doi: 10.1093/brain/118.6.1473. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Kazumata K, Antonini A, Sterio D, Dhawan V, Spetsieris P, Alterman R, Kelly PJ, Dogali M, Fazzini E, Beric A. Metabolic correlates of pallidal neuronal activity in Parkinson’s disease. Brain. 1997;120 ( Pt 8):1315–1324. doi: 10.1093/brain/120.8.1315. [DOI] [PubMed] [Google Scholar]
- Feigin A, Kaplitt MG, Tang C, Lin T, Mattis P, Dhawan V, During MJ, Eidelberg D. Modulation of metabolic brain networks after subthalamic gene therapy for Parkinson’s disease. Proc Natl Acad Sci U S A. 2007;104:19559–19564. doi: 10.1073/pnas.0706006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs T, Gavarini S, Saunders-Pullman R, Raymond D, Ehrlich ME, Bressman SB, Ozelius LJ. Mutations in the THAP1 gene are responsible for DYT6 primary torsion dystonia. Nat Genet. 2009;41:286–288. doi: 10.1038/ng.304. [DOI] [PubMed] [Google Scholar]
- Ghilardi M, Ghez C, Moeller J, Dhawan V, Eidelberg D. Patterns of regional brain activation associated with different aspects of motor learning. Brain Research. 2000;871:127–145. doi: 10.1016/s0006-8993(00)02365-9. [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Carbon M, Silvestri G, Dhawan V, Tagliati M, Bressman SB, Ghez C, Eidelberg D. Impaired sequence learning in carriers of the DYT1 dystonia mutation. Ann Neurol. 2003;54:102–109. doi: 10.1002/ana.10610. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck C, Krakauer JW, Ghez C, Sackeim HA, Eidelberg D, Stern Y, Moeller JR. A new approach to spatial covariance modeling of functional brain imaging data: ordinal trend analysis. Neural Comput. 2005;17:1602–1645. doi: 10.1162/0899766053723023. [DOI] [PubMed] [Google Scholar]
- Hung SW, Hamani C, Lozano AM, Poon YY, Piboolnurak P, Miyasaki JM, Lang AE, Dostrovsky JO, Hutchison WD, Moro E. Long-term outcome of bilateral pallidal deep brain stimulation for primary cervical dystonia. Neurology. 2007;68:457–459. doi: 10.1212/01.wnl.0000252932.71306.89. [DOI] [PubMed] [Google Scholar]
- Hutchinson M, Nakamura T, Moeller JR, Antonini A, Belakhlef A, Dhawan V, Eidelberg D. The metabolic topography of essential blepharospasm: a focal dystonia with general implications. Neurology. 2000;55:673–677. doi: 10.1212/wnl.55.5.673. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Hess EJ. A new twist on the anatomy of dystonia: the basal ganglia and the cerebellum? Neurology. 2006;67:1740–1741. doi: 10.1212/01.wnl.0000246112.19504.61. [DOI] [PubMed] [Google Scholar]
- Kock N, Naismith TV, Boston HE, Ozelius LJ, Corey DP, Breakfield XO, Hanson PI. Effects of genetic variations in the dystonia protein torsin A: identification of polymorphism at residue 216 as protein modifier. Hum Mol Genet. 2006;15:1355–1364. doi: 10.1093/hmg/ddl055. [DOI] [PubMed] [Google Scholar]
- Kumar R, Dagher A, Hutchison WD, Lang AE, Lozano AM. Globus pallidus deep brain stimulation for generalized dystonia: clinical and PET investigation. Neurology. 1999;53:871–874. doi: 10.1212/wnl.53.4.871. [DOI] [PubMed] [Google Scholar]
- Lin TP, Carbon M, Tang C, Mogilner AY, Sterio D, Beric A, Dhawan V, Eidelberg D. Metabolic correlates of subthalamic nucleus activity in Parkinson’s disease. Brain. 2008;131:1373–1380. doi: 10.1093/brain/awn031. [DOI] [PubMed] [Google Scholar]
- Molinari M, Filippini V, Leggio MG. Neuronal plasticity of interrelated cerebellar and cortical networks. Neuroscience. 2002;111:863–870. doi: 10.1016/s0306-4522(02)00024-6. [DOI] [PubMed] [Google Scholar]
- Neychev VK, Fan X, Mitev VI, Hess EJ, Jinnah HA. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131:2499–2509. doi: 10.1093/brain/awn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim H. Uber Eine eigenartige Kramotkrankheit des Kindlichen und jugendlichen Alters (Dysbasia lordotica progressiva, Dystonia musculorum deformans. Neurologie Centralblatt. 1991;30:1090–1107. [Google Scholar]
- Ostrem JL, Starr PA. Treatment of dystonia with deep brain stimulation. Neurotherapeutics. 2008;5:320–330. doi: 10.1016/j.nurt.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, deLeon D, Brin MF, Raymond D, Corey DP, Fahn S, Risch NJ, Buckler AJ, Gusella JF, Breakefield XO. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17:40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Siebner HR, Rothwell JC. Task-specific hand dystonia: can too much plasticity be bad for you? Trends Neurosci. 2006;29:192–199. doi: 10.1016/j.tins.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Risch NJ, Bressman SB, Senthil G, Ozelius LJ. Intragenic Cis and Trans modification of genetic susceptibility in DYT1 torsion dystonia. Am J Hum Genet. 2007;80:1188–1193. doi: 10.1086/518427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders-Pullman R, Raymond D, Senthil G, Kramer P, Ohmann E, Deligtisch A, Shanker V, Greene P, Tabamo R, Huang N, Tagliati M, Kavanagh P, Soto-Valencia J, Aguiar Pde C, Risch N, Ozelius L, Bressman S. Narrowing the DYT6 dystonia region and evidence for locus heterogeneity in the Amish-Mennonites. Am J Med Genet A. 2007;143A:2098–2105. doi: 10.1002/ajmg.a.31887. [DOI] [PubMed] [Google Scholar]
- Sommer M, Ruge D, Tergau F, Beuche W, Altenmuller E, Paulus W. Intracortical excitability in the hand motor representation in hand dystonia and blepharospasm. Mov Disord. 2002;17:1017–1025. doi: 10.1002/mds.10205. [DOI] [PubMed] [Google Scholar]
- Starr PA, Rau GM, Davis V, Marks WJ, Jr, Ostrem JL, Simmons D, Lindsey N, Turner RS. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson’s disease and normal macaque. J Neurophysiol. 2005;93:3165–3176. doi: 10.1152/jn.00971.2004. [DOI] [PubMed] [Google Scholar]
- Stern Y, Zarahn E, Habeck C, Holtzer R, Rakitin BC, Kumar A, Flynn J, Steffener J, Brown T. A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cereb Cortex. 2008;18:959–967. doi: 10.1093/cercor/bhm134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe LM, Kim CE, Alagem N, Dauer WT. Primary dystonia: molecules and mechanisms. Nat Rev Neurol. 2009;5:598–609. doi: 10.1038/nrneurol.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thobois S, Ballanger B, Xie-Brustolin J, Damier P, Durif F, Azulay JP, Derost P, Witjas T, Raoul S, Le Bars D, Broussolle E. Globus pallidus stimulation reduces frontal hyperactivity in tardive dystonia. J Cereb Blood Flow Metab. 2008;28:1127–1138. doi: 10.1038/sj.jcbfm.9600610. [DOI] [PubMed] [Google Scholar]
- Trost M, Carbon M, Edwards C, Raymond D, Mentis M, Moeller JR, Bressman SB, Eidelberg D. Primary dystonia: is abnormal functional brain architecture linked to genotype? Ann Neurol. 2002;52:853–856. doi: 10.1002/ana.10418. [DOI] [PubMed] [Google Scholar]
- Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Lagrange C, Yelnik J, Bardinet E, Benabid AL, Navarro S, Dormont D, Grand S, Blond S, Ardouin C, Pillon B, Dujardin K, Hahn-Barma V, Agid Y, Destee A, Pollak P. Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol. 2007;6:223–229. doi: 10.1016/S1474-4422(07)70035-2. [DOI] [PubMed] [Google Scholar]
- Walsh R, O’Dwyer JP, Sheikh IH, O’Riordan S, Lynch T, Hutchinson M. Sporadic adult onset dystonia: sensory abnormalities as an endophenotype in unaffected relatives. J Neurol Neurosurg Psychiatry. 2007;78:980–983. doi: 10.1136/jnnp.2006.105585. [DOI] [PMC free article] [PubMed] [Google Scholar]