Abstract
The proton-coupled transporter (PCFT) mediates intestinal folate absorption and folate transport from blood across the choroid plexus. The membrane topology of PCFT has been defined using the substituted cysteine accessibility method; an intramolecular disulfide bond between the Cys 66 and 298 residues, in the first and fourth extracellular loops, respectively, is present but not essential for function. The current report describes Lys 422 mutations (K422C, K422E) that have no effect on transport activity when introduced into wild-type PCFT but result in a marked loss of activity when introduced into a Cys-less PCFT which is otherwise near-fully functional. The loss of activity of both mutant PCFTs was shown to be due to impaired protein stability and expression. Additional studies were conducted with the K422C mutation in Cys-less PCFT. The impact of re-introduction of one, two, three or five, Cys residues was assessed. While there were some differences in the impact of the different Cys residues re-introduced, restoration was more due to a cumulative effect rather than the specific role of individual Cys residues. Preservation of the Cys66-Cys298 intramolecular disulfide bond was not required for stability of the K422C protein. These observations are relevant to studies with Cys-less transporters utilized for the characterization of proteins with the substituted cysteine accessibility method and indicate that functional defects detected in a Cys-less protein when the tertiary structure of the molecule is stressed, are not necessarily relevant to the wild-type protein.
Keywords: proton-coupled folate transporter, hereditary folate malabsorption, substituted cysteine accessibility method, folates
Introduction
The proton-coupled folate transporter (PCFT 1), recently cloned, is the mechanism by which folates are absorbed across the apical brush-border membrane of the proximal small intestine and is required for transport of folates across the blood-choroid plexus – cerebrospinal fluid barrier [1]. Both processes are defective in the rare autosomal inherited disorder, hereditary folate malabsorption (HFM) [2,3], which was shown by this laboratory to be due to mutations in the pcft gene [1,4–7] and subsequently reported by other groups [8–10]. While proton-coupled, this transporter is also operational in the physiological pH range where it has a particularly high affinity for the new-generation antifolate, pemetrexed [11], currently approved for the treatment of mesothelioma and non-squamous, non-small cell lung cancer [12,13]. Most recently, antifolate compounds specifically designed for transport by PCFT have been synthesized [14].
This laboratory recently reported on the PCFT topological structure using the substituted cysteine-accessibility method (SCAM) [15]. This procedure requires that the Cys-less protein retain nearly full activity and is based on the reintroduction of Cys residues, individually, in intracellular and extracellular loops followed by assessment of their accessibility by a membrane impermeant reagent in the presence and absence of membrane permeabilization [15]. This approach, along with localization of its C- and N-termini [16,17], established PCFT to have twelve transmembrane domains with its N- and C-termini directed to the cytosol. An intramolecular disulfide bond between native Cys residues 66 and 298 was also identified, a post-translational modification that does not appear to influence function [15].
Within the context of these studies, along with an assessment of the role of Lys residues in PCFT function, the observation was made that replacement of the fully conserved Lys at position 422 with Cys in the wild-type protein had no functional consequence. However, the same substitution in the Cys-less PCFT resulted in an almost complete loss of activity. The current study explores the basis for these findings and demonstrates the impact that Cys residues play, cumulatively, in stabilizing the PCFT protein independent of their role in the formation of disulfide bonds.
Materials and methods
Key chemicals, antibodies and cell lines
[3’,5’,7-3H(N)]-MTX was obtained from Moravek Biochemicals (Brea, CA). EZ-Link Sulfo-NHS-LC-biotin and streptavidin-agarose were obtained from Pierce Biotechnology (Rockford, IL) and Fisher Scientific (Pittsburg, PA), respectively. Anti-HA antibody (H6908) was purchased from Sigma (St. Lois, MO); anti-actin antibody (49671L) and anti-rabbit IgG-HRP conjugate were obtained from Signaling Technology (Danvers, MA). HeLa R1–11 cells that lack expression of both the reduced folate carrier and PCFT, due to deletion of the former gene and methylation of the latter pcft promoter [18,19] were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin and 100 µg/ml streptomycin. Cry-preserved HeLa R1–11 cells were thawed regularly to assure that PCFT expression was absent or minimal [20].
Site-directed mutagenesis to generate PCFT mutants
All mutations were introduced into a C-terminus HA- (haemagglutinin)-tagged human wild-type- or Cys-less PCFT expression vector [15]. A single mutation was introduced using the QuikChange® II XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA), whereas a combination of mutations was generated with the QuikChange® Multi Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). For both methodologies, a primer contained the designated mutation flanked by twelve nucleotides. For some mutations a temperature (50 or 55 °C) lower than the standard annealing temperature of 60 °C was required. The coding region of the final expression vector was verified by automated sequencing in the Albert Einstein Cancer Center Genomics Shared Resource.
Transient transfection
For transport studies, HeLa R1–11 cells were routinely seeded into 20mL Low Background glass scintillation vials (Research Products International Corporation, Prospect IL) at 0.35 million cells per vial. For surface labeling experiments, HeLa R1–11 cells were grown in 6-well plates at a density of 0.6 million cells per well. In both cases transfections were performed two days later with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Transport and labeling assays were conducted 2 days after transfection.
Transport measurements
Cells were washed twice with HBS (20mM HEPES, 5mM dextrose, 140mM NaCl, 5mM KCl, 2mM MgCl2, pH 7.4) and incubated in the same buffer at 37°C for 20 min. The incubation buffer was then aspirated and transport was initiated by the addition of 0.5mL of pre-warmed (37°C) MBS (20mM MES, 140mM NaCl, 5mM KCl, 2mM MgCl2, pH 5.5) containing the 0.5 µM [3H] MTX. Uptake was carried out at 37°C over 1 min and stopped by the addition of 5mL ice-cold HBS. Cells were washed three times with ice-cold HBS and dissolved in 0.5 mL of 0.2M NaOH at 65°C for 40 min. Radioactivity in 0.4mL of lysate was measured on a liquid scintillation spectrometer and normalized to protein levels obtained with the BCA Protein Assay (Pierce, Rockford, IL).
Cell surface biotinylation assay
This assay was described previously [21]. Briefly, cells were washed twice with PBS (pH 8.0) and labeled at room temperature with EZ-Link Sulfo-NHS-LC-Biotin, which reacts specifically with primary amine groups, at a concentration 1 mg/ml in PBS (pH 8.0) for 30 min. Cells were washed twice in PBS and treated with 0.7 ml hypotonic buffer (0.5 mM Na2HPO4, 0.1 mM EDTA, pH 7.0) containing protease inhibitor cocktail (Roche, Indianapolis, IN) on ice for 30 min. The cells were then detached from the plates with disposable cell lifters and centrifuged at 16,000 × g and 4 °C for 5 min. The pellet was then resuspended in 0.4 ml lysis buffer (50 mM TRIS-base, 150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate, pH 7.4) and mixed on a rotisserie shaker for 1 h at 4°C. A 25 µL portion (identified as “crude membrane”) was collected and stored in a freezer. The remaining crude membrane fraction was centrifuged at 16,000 × g and 4°C for 15 min and the supernatant was mixed on a rotisserie shaker overnight at 4°C with 50 µL of streptavidin agarose that was pre-washed three times with the lysis buffer. The agarose beads were washed four times with 0.5 ml lysis buffer, each with a 20 min mix on a rotisserie shaker at 4°C. The precipitated proteins were released from the beads by heating at 95 °C for 5 min in 2X SDS-PAGE sample loading buffer containing dithiothreitol.
Western blot analysis
Samples were resolved on standard 12.5% SDS-PAGE. The precipitated proteins (released from beads as described in the previous section) were loaded directly on gels while the crude membrane fraction was mixed (1:1) with dithiothreitol-containing 2X SDS-PAGE sample loading buffer at room temperature before loading on the gels. After SDS-PAGE, proteins were transferred to Amersham Hybond membranes (GE Healthcare, Piscataway, NJ) and were blocked with 10% dry milk in TBST (20 mM Tris, 135 mM NaCl, 1% Tween 20, pH 7.6) overnight. For crude membrane samples, the blots were probed with anti-actin antibody (1:2000 in TBST, 0.1% milk) then stripped in 100 mM β-mecaptoethanol, 2% SDS, 62.5 mM Tris-Cl at pH 6.7 and re-probed with anti-HA antibody (1:4000 in TBST, 0.1% milk). For precipitated samples, blots were probed directly with anti-HA antibody. After application of the first antibody, the blots were incubated with anti-rabbit IgG-HRP conjugate (1:5000 in TBST). The blots were developed with Amersham ECL Plus reagent (GE Healthcare, Piscataway, NJ) and exposed to autoradiography film (Denville Scientific, Metuchen, NJ).
Results
Effects of substitutions of lysine 422 on PCFT function
Human PCFT contains eleven Lys residues as indicated in Fig. 1. However, eight (Lys 10, 235, 238, 262, 265, 302, 323, 445) are not conserved among 10 species (human, monkey, cow, horse, rat, mouse, dog, opossum, Xenopus and zebra fish) excluding critical roles in PCFT function. Only Lys 378 and 422 are fully conserved (identical) while Lys 381 is semi-conserved (Arg is at this position in cow and opossum). Previous studies indicated that neither Arg 378 nor Arg 381 is required for function [21]. As indicated in Fig. 2A, replacement of Lys 422 in wild-type PCFT with polar Cys (K422C-WT), like-charged Arg (K422R-WT), non-polar Ala (K422A-WT) or opposite-charged Glu (K422E-WT) did not affect PCFT activity. Hence, Lys 422 is fully replaceable. The suffix “WT” in the mutant names indicates that the mutation was introduced into WT PCFT.
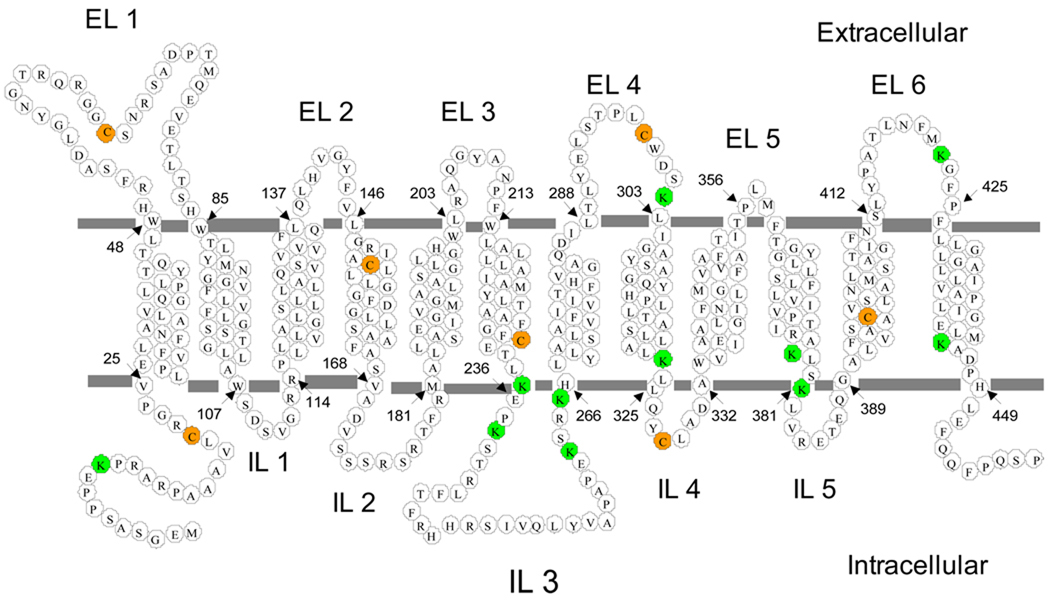
Figure 1. Membrane topology of PCFT and localization of Cys and Lys residues.
Membrane topology of human PCFT was established in a previous study [15]. The start and end of each transmembrane domain is marked by arrows and numbers. All seven native cysteine residues (orange) were replaced with Ser in a CL-PCFT. All eleven lysine residues are indicated by green circles; most are located either in loops or boundaries of loops and transmembrane domains. EL, extracellular loop; IL: intracellular loop.
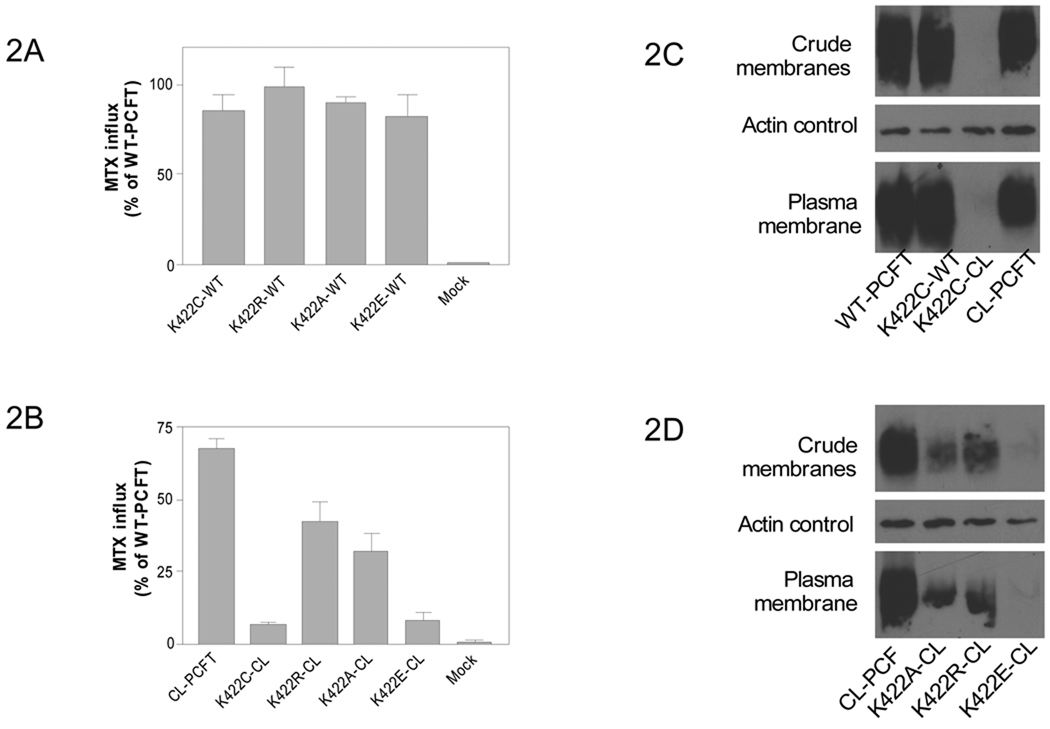
Figure 2. Effects of mutations at Lys 422 in wild-type and CL-PCFT.
[3H]MTX influx was assessed in Hela R1–11 cells, that lack endogenous folate transporters, transfected with PCFT constructs in which the K422 mutations were introduced into WT-PCFT (Panel A) or into CL-PCFT (Panel B). The MTX concentration was 0.5 µM; the pH 5.5. Uptake was assessed over 1 min; the data is normalized to the wild-type PCFT level indicated as 100%. Data are the mean ± SEM from three independent experiments. PCFT expression in the crude membrane fraction and biotinylated at the plasma membrane is indicated for the K422C-CL mutant (Panel C) and K422A-CL, K422R-CL as well as K422E-CL mutants (Panel D). Actin is the loading control for the crude membrane fraction. The Western blots are representative of at least two separate experiments.
During the course of another study using SCAM to define the membrane topology of PCFT, Cys residues were individually substituted for residues in loops of the transporter in which all seven native Cys residues were replaced with Ser (Cys-less or CL-PCFT). When the K422C mutation was introduced into the CL-PCFT (K422C-CL), activity was markedly reduced to 10% that of WT-PCFT (Fig. 2B). The suffix “CL” in the mutant names indicates that the mutation was introduced into CL-PCFT. Substitution of Lys 422 with Ala or Arg in CL-PCFT led to only a modest reduction in activity (54 or 38 % respectively) as compared to WT-PCFT. However, replacement of K422 with Glu resulted in a loss of ~90% of activity.
The levels of expression of the K422C-CL PCFT mutant protein in the crude membrane fraction and accessible at the plasma membrane were markedly decreased as compared to WT-PCFT, K422C-WT, or CL-PCFT (Fig 2C). Expression of the K422E-CL mutant was also barely detectable in the crude membrane fraction or at the plasma membrane (Fig. 2D). Expression of K422A-CL and K422R-CL mutant proteins were detectable; however, the reduction in protein was greater than the reduction in activity as compared to CL-PCFT (Fig 2D). Hence, the adverse effects of substitutions at Lys 422 of CL-PCFT were due to impaired protein stability and expression.
Impact of the disulfide bond between Cys 66 and Cys 298 on the stability of the K422C PCFT mutant
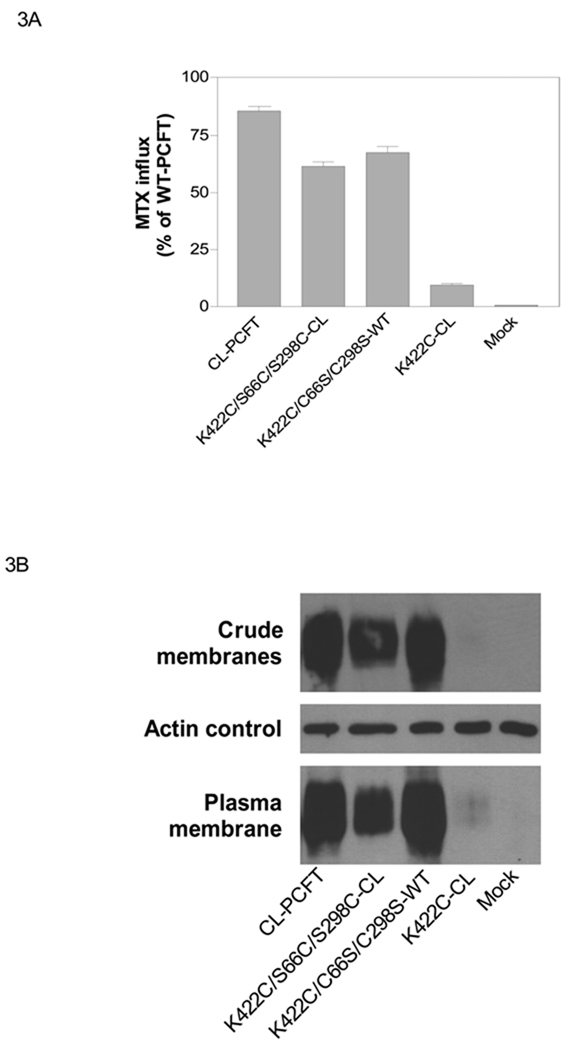
There is a disulfide bond located between Cys66 and Cys298 in the first and fourth extracellular loops, respectively, that is not required for protein stability nor function. To determine if this bond is required for stability of the K422 mutants, Cys66 and Cys298 were re-introduced into the K422C-CL PCFT mutant, the least active of the CL-PCFT mutants. As indicated in Fig. 3A, MTX influx mediated by this mutant (K422C/S66C/S298C-CL) was 72% that of CL-PCFT and six-fold higher than K422C-CL. This partial restoration of activity correlated with PCFT expression in the crude membrane fraction and accessible at the plasma membrane (Fig. 3B). Hence, the presence of Cys 66 and 298 neutralized the negative impact of the K422C mutation.
Figure 3. Effects of additions of Cys66 and Cys298 to CL-PCFT, and substitutions of these residues with Ser in WT-PCFT, on protein expression and function.
Panel A: MTX influx was assessed in transfectants as described in Figure 2 and indicated as percentage of WT-PCFT. Data are the mean ± SEM from three independent experiments. Panel B: PCFT expression in the crude membrane fraction and biotinylated at the plasma membrane. Actin is the loading control for the crude membrane fraction. The Western blots are representative of two separate experiments.
To determine whether the Cys66 and Cys298 residues as well as the bond between them are absolutely required to negate the effect the K422C mutation, Cys66 and Cys298 were simultaneously replaced with Ser in the K422C-WT background to obtain the K422C/C66S/C298S-WT in which the other five native cysteine residues were intact. As indicate in Fig. 3A, transport activity mediated by K422C/C66S/C298S-WT was essentially the same as that of K422C/S66C/S298C-CL PCFT. As indicated in Fig. 3B, expression of 422C/C66S/C298S WT-PCFT protein was comparable to, or slightly greater, than that of K422C/S66C/S298C-CL PCFT. Hence, it is not the presence of the Cys 66 and Cys 298 residues, per se, or the disulfide bond between them, that restored activity for the K422C mutant in CL-PCFT, rather it was the presence of added Cys residues that was the important factor as indicated by the high level of activity and expression associated with the mutant construct in which these residues were absent, but the other Cys residues in WT PCFT remained.
Finally, confirming the lack of importance of the Cys66-Cys298 disulfide bond in the stability of the K422C/S66C/S298C-CL, transient transfectants expressing this mutant along with WT-PCFT were treated with β-mercaptoethanol, a process known to disrupt the disulfide bond in WT-PCFT [15]. Based up three independent experiments, there was no significant change in MTX influx (0.5 µM) conducted at pH 5,5 over 1 min after β-mercaptoethanol incubation, either for K422C/S66C/S298C-CL mutant (24.3 ± 0.3 vs 26.1 ± 2.4 nmol/mg protein of control, P=0.49 from paired t-test) or for WT-PCFT (41.6 ± 3.2 vs 48.3 ± 3.7 nmol/mg protein of control, P=0.11 from paired t-test). Therefore, if a disulfide bond indeed forms between Cys 66 and C298 in K422C/S66C/S298C-CL mutant as in WT-PCFT, it was apparently not important for function of the mutant.
The cumulative effects of Cys residues in the stabilization of the K422C-CL PCFT
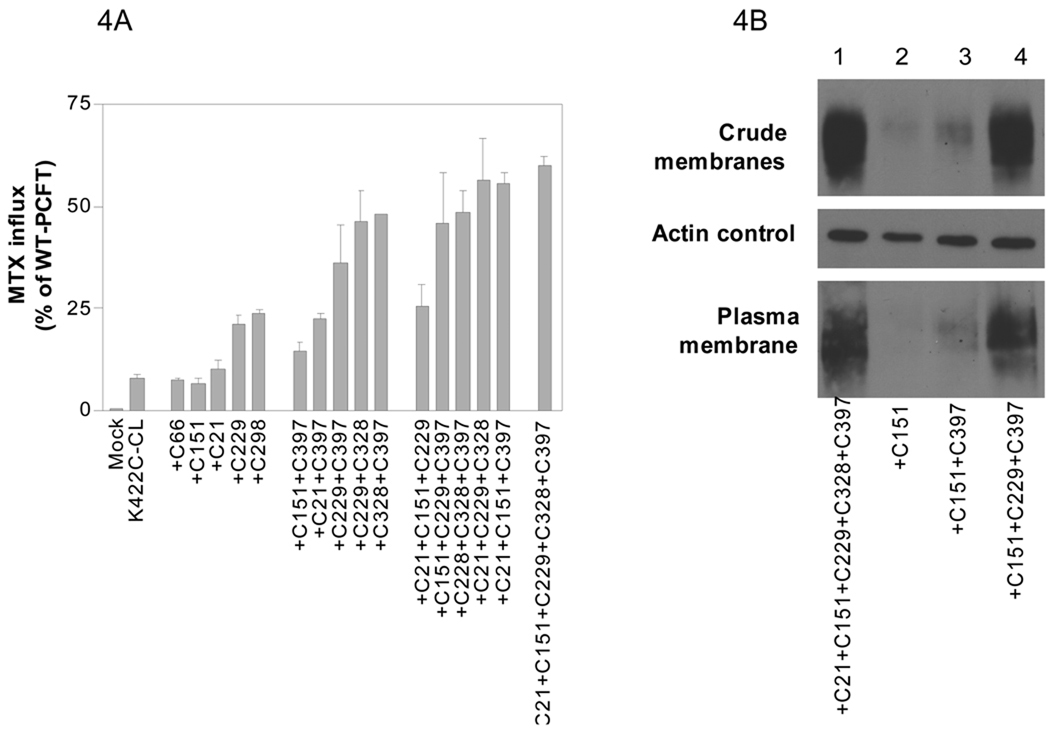
As indicated above, the K422C/C66S/C298S-WT mutant, in which five native cysteine residues (Cys 21, 151, 229, 328 and 397) were intact, sustained 60% of WT-PCFT activity and was expressed at a level similar to CL-PCFT. In the next series of experiments, the role of each of the remaining five Cys residues, or a combination of these residues, in sustaining stability of the K422C-CL PCFT was assessed. To accomplish this, a spectrum of mutants, in which K422C was always present along with a variable number of Cys residues, was generated by a single multi site-directed mutagenesis. Two additional mutants in which Cys 66 or Cys 298 were reintroduced into K422C-CL PCFT were generated separately. As indicated in Fig. 4A, the activities of 15 mutants, all of which had the K422C substitution, were assessed and compared to K422C-CL and WT-PCFT. Five mutants contained only one Cys residue, five contained two Cys residues, five contained three Cys residues, and one contained five Cys residues. There was a general correlation between the number of Cys residues and function. The greater the number of Cys residues, the greater the transport function. This relationship becomes clear if mutants which represent consecutive additions of Cys residue are compared (+C151 vs +C151+C397 vs +C151+C229+C397) and (+C21 vs +C21+C397 vs +C21+C151+C397). There was a large increase in function when K422C mutants containing one Cys residue were compared to mutants containing two Cys and when mutants containing two, are compared to mutants containing three, Cys residues. There was no further increase in activity between constructs containing the three added residues and the one containing five Cys residues. However, not all Cys residues contributed equally to maintaining activity of these mutants. For example, Cys 229 and 298 were more efficient than Cys 21, 66, 151 when a single Cys residue was compared. Cys 21 and 151 were also not as efficient as Cys 229, C328 and 397 when a combination of two Cys residues were compared.
Figure 4. Comparison of function and expression of PCFT mutants in which a variety, and variable number, of Cys residues have been re-introduced into the K422C-CL.
Panel A: MTX influx was assessed in transfectants as described in Figure 2 and is indicated as percentage of WT-PCFT. Data are the mean ± SEM from three independent experiments. The mutants are grouped according to the number of Cys residues and arranged from low to high activity within each group. All mutants contain the K422C mutation. “+C66” indicates a mutant in which native Cys66 was reintroduced into K422C-CL; +C151+C397” indicates a mutant with both native Cys 151 and 397 were simultaneously introduced into K422C-CL. Other mutants are designated in the same fashion. Panel B: PCFT in the crude membrane fraction and accessible at the plasma membrane. Actin is the loading control for the crude membrane fraction. The Western blot analysis is representative of two separate experiments.
Preservation of PCFT protein expression correlated with preservation of PCFT function. As indicated in Fig. 4B, the mutants containing the C151 or C151+C397 additions, with only a low level of function, expressed only low levels of protein. While the mutant containing three added Cys residues (C151+C397+C229) expressed higher levels of protein comparable to the mutant containing five added Cys residues (+C21+C151+C229+C328+C397) both of which had high and comparable levels of function.
Discussion
SCAM is widely used in defining membrane topology and the role of specific residues or domains in transport function [22]. The key requirement for this methodology is retention of function when all Cys residues are replaced, in most cases, with Ser. Indeed, the majority of Cys-less transporters are functional as is the case for cysteine-less PCFT [15]. However, in some transporters Cys residues are irreplaceable, such as the proton coupled amino acid transporter hPAT1, due to the formation of a functionally important disulfide bond between Cys 180 and 329 residues [23] and the human vesicle monoamine transporter (VMAT2), due to a critical disulfide bond between the Cys 126 and 333 residues [24]. In SCAM, Cys resides are introduced into the Cys-less transporter at specific sites following which function of these mutants is assessed, and accessibility of these Cys residues to sulfhydryl reagents is probed. However, whenever a resulting mutation results in functional impairment, it is usually assumed that the residue has a comparable role in the wild-type transporter. The results in this report clearly indicate that functional defects detected in a Cys-less protein may not be extrapolated to the wild-type protein.
The molecular basis for the functional impairment of the various K422 mutants in CL-PCFT was the lack of protein expression most likely due to impaired protein folding and stability. An attempt was made to determine whether specific Cys residues were responsible for the preservation of protein stability. Two obvious candidates were the Cys66 and Cys298 residues that form an intramolecular disulfide bond in the WT-PCFT and, while this bond is not required for function in that setting, it was possible that the bond was required in a carrier structurally stressed by a drastic mutation. Indeed, simultaneous reintroduction of these two Cys residues in the K422C-CL PCFT preserved a majority of protein expression and function. However, abrogation of this bond with β-mercaptoethanol did not alter the impact of these added residues and substitution of both residues with Ser in the K422C-WT PCFT did not result in a loss of protein and function. Hence, the data suggested that it was the presence of these Cys residues, rather than their interaction, that improved stability of the mutated PCFT.
Although the number of mutants in which Cys residues were reintroduced in K422C-CL PCFT was limited, a pattern of restoration of protein expression and function emerged: (i) No single Cys residue restored more than 25% of WT activity, although Cys229 and Cys298 were more potent than Cys21, 66 and 151. (ii) When the second Cys residue was introduced the activity in the resulting mutant was always increased as compared to a mutant with only one Cys residue. (ii) Maximal activity could be reached with two Cys residues as in the case of “+C66+C298”, “C229+C328” or “C328+C397” or, in most cases, with the addition of three Cys residues. These data suggest that Cys residues in WT-PCFT cumulatively, though not equally, contribute to the stability of K422C-WT PCFT. This pattern of restoration of function by Cys residues may also be relevant to the K422E mutant since this substitution, similar to K422C, had no impact on WT-PCFT but decreased function and protein expression of the CL-PCFT. The vulnerability of CL-PCFT may not only be limited to mutations at Lys 422. For instance, the R114C mutation had no functional impact on WT-PCFT but reduced activity by ~ 86 % in CL-PCFT (unpublished results). Further, the vulnerability of CL-PCFT associated with substitution of seven native cysteine residues with serine may not be cysteine-specific; it is possible that this might also be produced by mutations at other residues.
Lys residues often form a charge pair with negatively charged residues that are important to the structural integrity of facilitative transporters. For examples, charge pairs between Lys358 and Asp237, as well as between Lys319 and Asp 240, have been identified in the Lac transporter [25]. Data in the current report, along with our previously results from this laboratory [21], indicate that none of the eleven Lys residue in PCFT forms a charge pair. Eight of these residues are not conserved; replacement of Lys378 (conserved) and Lys381 (semi-conserved) with Ala had no functional consequence [21]. Lys 422 is fully conserved but function was fully preserved even after substitution with the negatively charged Glu in the wild-type PCFT. The functional roles of all His and Asp residues, along with several Arg and Glu residues, in human PCFT have been defined among the charged residues [6,7,21,26,27].
Research Highlights.
-
>
The highly conserved Lys 422 is fully replaceable in wild-type PCFT.
-
>
K422C and K422E mutants are inactive in the functional Cys-less PCFT.
-
>
The K422C in Cys-less PCFT loses activity due to decreased protein expression.
-
>
Additions of Cys residues cumulatively restore K422C PCFT expression and activity.
Acknowledgements
This work was supported by a grant from the National Institutes of Health, CA-82621
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: CL, Cysteine-less; HA, haemagglutinin ; MTX, methotrexate; PCFT, the proton-coupled folate transporter; HFM, hereditary folate malabsorption; SCAM, substituted cysteine accessibility method
References
- 1.Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127:917–928. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 2.Geller J, Kronn D, Jayabose S, Sandoval C. Hereditary folate malabsorption: family report and review of the literature. Medicine (Baltimore) 2002;81:51–68. doi: 10.1097/00005792-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Mahadeo KM, Min SH, Diop-Bove N, Kronn D, Goldman ID. Hereditary Folate Malabsorption. In: Pagon RA, Bird TC, Dolan CR, Stephens K, editors. GeneReviews [Internet] Seattle, Seattle, WA: University of Washington; 2010. [Google Scholar]
- 4.Zhao R, Min SH, Qiu A, Sakaris A, Goldberg GL, Sandoval C, Malatack JJ, Rosenblatt DS, Goldman ID. The spectrum of mutations in the PCFT gene, coding for an intestinal folate transporter, that are the basis for hereditary folate malabsorption. Blood. 2007;110:1147–1152. doi: 10.1182/blood-2007-02-077099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Min SH, OH SY, Karp GI, Poncz M, Zhao R, Goldman ID. The clinical course and genetic defect in the PCFT in a 27-year-old woman with Hereditary folate malabsorption. J. Pediatr. 2008;153:435–437. doi: 10.1016/j.jpeds.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahadeo K, Diop-Bove N, Shin D, Unal E, Teo J, Zhao R, Chang MH, Fulterer A, Romero MF, Goldman ID. Properties of the Arg376 residue of the proton-coupled folate transporter (PCFT-SLC46A1) and a glutamine mutant causing hereditary folate malabsorption. Am. J Physiol Cell Physiol. 2010;299:C1153–C1161. doi: 10.1152/ajpcell.00113.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin DS, Min SH, Russell L, Zhao R, Fiser A, Goldman ID. Functional roles of aspartate residues of the proton-coupled folate transporter (PCFT; SLC46A1); a D156Y mutation causing hereditary folate malabsorption. Blood. 2010;116:5162–5169. doi: 10.1182/blood-2010-06-291237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lasry I, Berman B, Straussberg R, Sofer Y, Bessler H, Sharkia M, Glaser F, Jansen G, Drori S, Assaraf YG. A novel loss of function mutation in the proton-coupled folate transporter from a patient with hereditary folate malabsorption reveals that Arg 113 is crucial for function. Blood. 2008;112:2055–2061. doi: 10.1182/blood-2008-04-150276. [DOI] [PubMed] [Google Scholar]
- 9.Borzutzky A, Crompton B, Bergmann AK, Giliani S, Baxi S, Martin M, Neufeld EJ, Notarangelo LD. Reversible severe combined immunodeficiency phenotype secondary to a mutation of the proton-coupled folate transporter. Clin. Immunol. 2009;133:287–294. doi: 10.1016/j.clim.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer E, Kurian MA, Pasha S, Trembath RC, Cole T, Maher ER. A novel PCFT gene mutation (p.Cys66LeufsX99) causing hereditary folate malabsorption. Mol Genet. Metab. 2010;99:325–328. doi: 10.1016/j.ymgme.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao R, Qiu A, Tsai E, Jansen M, Akabas MH, Goldman ID. The proton-coupled folate transporter (PCFT): impact on pemetrexed transport and on antifolate activities as compared to the reduced folate carrier. Mol. Pharmacol. 2008;74:854–862. doi: 10.1124/mol.108.045443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther. 2007;6:404–417. doi: 10.1158/1535-7163.MCT-06-0343. [DOI] [PubMed] [Google Scholar]
- 13.Goldman ID, Chattopadhyay S, Zhao R, Moran RG. The Antifolates: Evolution, New Agents in the Clinic, and How targeting delivery via specific membrane transporters is driving the development of a next generation of folate analogs. Curr. Opin. Investig. Drugs. 2010;11:1409–1423. [PubMed] [Google Scholar]
- 14.Kugel DS, Wang Y, Wu J, Stout M, Hou Z, Fulterer A, Chang MH, Romero M, Cherian C, Gangjee A, Matherly L. Targeting the proton-coupled folate transporter for selective delivery of 6-substituted pyrrolo[2,3-d]pyrimidine antifolate inhibitors of de novo purine biosynthesis in the chemotherapy of solid tumors. Mol Pharmacol. 2010;78:577–587. doi: 10.1124/mol.110.065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao R, Unal ES, Shin DS, Goldman ID. Membrane Topological Analysis of the Proton-Coupled Folate Transporter (PCFT-SLC46A1) by the Substituted Cysteine Accessibility Method. Biochemistry. 2010;49:2925–2931. doi: 10.1021/bi9021439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unal ES, Zhao R, Qiu A, Goldman ID. N-linked glycosylation and its impact on the electrophoretic mobility and function of the human proton-coupled folate transporter (HsPCFT) Biochim. Biophys. Acta. 2008;1178:1407–1414. doi: 10.1016/j.bbamem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu A, Min SH, Jansen M, Malhotra U, Tsai E, Cabelof DC, Matherly LH, Zhao R, Akabas MH, Goldman ID. Rodent intestinal folate transporters (SLC46A1): secondary structure, functional properties, and response to dietary folate restriction. Am. J. Physiol Cell Physiol. 2007;293:C1669–C1678. doi: 10.1152/ajpcell.00202.2007. [DOI] [PubMed] [Google Scholar]
- 18.Zhao R, Gao F, Hanscom M, Goldman ID. A prominent low-pH methotrexate transport activity in human solid tumor cells: Contribution to the preservation of methotrexate pharmacological activity in HeLa cells lacking the reduced folate carrier. ClinCancer Res. 2004;10:718–727. doi: 10.1158/1078-0432.ccr-1066-03. [DOI] [PubMed] [Google Scholar]
- 19.Diop-Bove NK, Wu J, Zhao R, Locker J, Goldman ID. Hypermethylation of the human proton-coupled folate transporter (SLC46A1) minimal transcriptional regulatory region in an antifolate-resistant HeLa cell line. Molecular Cancer Therapeutics. 2009;8:2424–2431. doi: 10.1158/1535-7163.MCT-08-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao R, Chattopadhyay S, Hanscom M, Goldman ID. Antifolate resistance in a HeLa cell line associated with impaired transport independent of the reduced folate carrier. Clin. Cancer Res. 2004;10:8735–8742. doi: 10.1158/1078-0432.CCR-04-0932. [DOI] [PubMed] [Google Scholar]
- 21.Unal ES, Zhao R, Goldman ID. Role of the glutamate 185 residue in proton translocation mediated by the proton-coupled folate transporter SLC46A1. Am. J. Physiol Cell Physiol. 2009;297:C66–C74. doi: 10.1152/ajpcell.00096.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karlin A, Akabas MH. Substituted-cysteine accessibility method. Methods Enzymol. 1998;293:123–145. doi: 10.1016/s0076-6879(98)93011-7. 123–145. [DOI] [PubMed] [Google Scholar]
- 23.Dorn M, Weiwad M, Markwardt F, Laug L, Rudolph R, Brandsch M, Bosse-Doenecke E. Identification of a disulfide bridge essential for transport function of the human proton-coupled amino acid transporter hPAT1. J. Biol. Chem. 2009;284:22123–22132. doi: 10.1074/jbc.M109.023713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thiriot DS, Sievert MK, Ruoho AE. Identification of human vesicle monoamine transporter (VMAT2) lumenal cysteines that form an intramolecular disulfide bond. Biochemistry. 2001;14:6346–6353. doi: 10.1021/bi015779j. [DOI] [PubMed] [Google Scholar]
- 25.Guan L, Kaback HR. Lessons from lactose permease. Annu. Rev. Biophys. Biomol. Struct. 2006;35:67–91. doi: 10.1146/annurev.biophys.35.040405.102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unal ES, Zhao R, Chang MH, Fiser A, Romero MF, Goldman ID. The functional roles of the His247 and His281 residues in folate and proton translocation mediated by the human proton-coupled folate transporter SLC46A1. J. Biol. Chem. 2009;284:17846–17857. doi: 10.1074/jbc.M109.008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lasry I, Berman B, Glaser F, Jansen G, Assaraf YG. Hereditary folate malabsorption: a positively charged amino acid at position 113 of the proton-coupled folate transporter (PCFT/SLC46A1) is required for folic acid binding. Biochem. Biophys. Res. Commun. 2009;386:426–431. doi: 10.1016/j.bbrc.2009.06.007. [DOI] [PubMed] [Google Scholar]