Abstract
Objective
Cell cycle checkpoints guarantee movement through the cell cycle. Mitotic arrest deficiency 2 (Mad2), a mitotic checkpoint protein, appears crucial for generating the wait anaphase signal to prevent onset of anaphase. We evaluated effects of Mad2 haploinsufficiency on hematopoietic stem (HSC) and progenitor (HPC) function in response to stress.
Materials and Methods
We studied effects of Mad2+/− on in vivo recovery of bone marrow HPC from cytotoxic effects and also effects of cytostatic agents on HPC growth in vitro using Mad2+/− mice.
Results
Mad2+/− HPCs were protected from cytotoxic effects in vivo of a cell cycle specific agent, Ara-C, events consistent with Mad2+/− HPCs being in a slow or noncycling state, but not from recovery of functional HPC after treatment with non-cycle specific cyclophosphamide or sub-lethal irradiation. There were no differences in phenotyped HSCs in Mad2+/− & Mad2+/+ mice, information confirmed by no changes in short or long term repopulating HSC assay. To better understand Mad2+/− HPC function, E3330, a cytostatic agent, was used to assess redox function of Ape1/Ref-1; colony growth was examined under 5% and 20% O2 tension. Mad2+/− HPCs were less responsive to E3330 than Mad2+/+ HPCs, and E3330 was more effective under lowered O2 tension. Mad2+/− HPCs were not enhanced at lowered oxygen, as were Mad2+/+ HPCs.
Conclusions
Our studies have unexpectedly found that Mad2 haploinsufficiency is protective in the presence of a cycle specific DNA synthesis agent in vivo, and Ape1/Ref-1 inhibitor in vitro.
Introduction
During eukaryotic cell division, accurate transmission of the genome is essential for survival of daughter cells and is ensured by intrinsic properties of cell cycle machinery and checkpoints [1]. Checkpoint mechanisms monitor cell cycle progression and guarantee faithful duplication and precise segregation of the genome [2]. The spindle assembly checkpoint (SAC), also known as the mitotic or spindle checkpoint, is a highly conserved signal transduction pathway that links initiation of anaphase to spindle assembly and completion of chromosome microtubule attachment [3–7]. The presence of even a single unattached or misaligned chromosome is sufficient to activate the SAC, which inhibits the anaphase promoting complex/cyclosome (APC/C) and arrests cells at the transition from metaphase to anaphase [2, 8–11]. Arrest caused by an unattached chromosome is overcome by laser ablation of the kinetochore, the structure that mediates chromosome-microtubule attachment [12], indicating that the signal for checkpoint-dependent arrest arises from, or is transduced through, kinetochores [2].
Genes involved in the spindle assembly checkpoint were first identified in the budding yeast S. cerevisiae and include mitotic arrest defective genes MAD1-3 [6] and the budding uninhibited by benzimidazole genes BUB1-3 [5]. All six of these genes are dispensable for normal growth, apparently because mitosis in S. cerevisiae lasts long enough for all chromosomes to attach to the spindle before anaphase begins, even in absence of a checkpoint. Addition of anti-microtubule drugs causes cells to proceed through mitosis without having established chromosome-microtubule attachment, resulting in extensive chromosome loss and cell death [5, 6]. Mitotic checkpoint proteins are likely to be highly conserved in mammals [13, 14], and homologues of these checkpoint proteins were later found in other organisms, including mammals: Mad1, Mad2, Mad3/BubR1, Bub1-3, and Mps1[15].
The principal system for regulating APC/C-Cdc20 interaction in response to kinetochore microtubule attachment status is the SAC [13, 16, 17]. One model regarding SAC function hypothesizes that in response to kinetochores which lack microtubule attachment and/or tension, the primary downstream effect of SAC activation is generation of a conformation of Mad2 capable of sequestering cellular Cdc20, thereby preventing APC/C activation and hence cell cycle progression [18, 19]. Although the complete mechanism of SAC signaling in response to improperly attached kinetochores remains incompletely resolved, the net effect is APC/C-Cdc20 inhibition for which Mad2 is of central importance [17].
The mammalian hematopoietic system is a complex tissue made up of various cell types capable of performing a wide range of functions, such as protection against infection and oxygen and nutrient delivery. All the cells that compose the blood and immune system originate from hematopoietic stem (HSC) and progenitor (HPC) cells. Our group established that Mad2+/− BM and spleen manifested decreased absolute numbers and cycling status of immature, but not mature hematopoietic progenitor cells. Also, Mad2+/− BM granulocyte-macrophage colony-forming units (CFU-GM) did not proliferate synergistically in response to stem cell factor (SCF) plus granulocyte/macrophage-colony stimulating factor (GM-CSF), and Mad2 associated with the c-Kit receptor in the human growth factor-dependent cell line MO7e [20]. In the present study, we examined various stresses in vivo on recovery of Mad2+/− HPC from cell cycle (Ara-C) and non-cell cycle (cyclophosphamide and irradiation) cytotoxic agents, and effects in vitro on Mad2+/− HPCs of a cytostatic agent (E3330) that blocks the redox function of Ape1/Ref-1, a multifunctional protein involved in both the DNA base excision repair pathway and redox regulation of various transcription factors, and also effects of lowered oxygen tension. The redox function, but not the DNA repair activity, of Ape1/Ref-1 is required for normal embryonic hematopoiesis [21], but the function of this protein has not been thoroughly evaluated in normal adult hematopoiesis
Materials and methods
Cytokines, antibodies, and other agents
Purified recombinant preparations of murine GM-CSF and SCF were purchased from R&D Systems (Minneapolis, MN). Purified recombinant human Epo was purchased from Amgen (Thousand Oaks, CA). Pokeweed mitogen mouse spleen cell-conditioned medium (PWMSCM) was prepared as described [22].
For flow cytometric analysis, anti-mouse antibodies c-Kit, Sca-1, FcγR III/II, IL-7Rα, Lineage Cocktail, and isotype controls were purchased from BD Biosciences (San Diego, CA). Anti-mouse CD34 antibody was purchased from eBioscience (San Diego, CA) & sca-1 was also purchased from BioLegend (San Diego, CA).
The following drugs purchased from Sigma-Aldrich (St. Louis, MO) were used to stress mice and examine BM progenitor recovery: cyclophosphamide monohydrate (cytoxan), and Ara-C. To condition mice before BM transplantation or examine BM recovery, γ radiation (137Cs source, single dose) was used. E3330 and its synthesis have been previously described [23].
Mice
Mad2+/+ wild type and Mad2+/− mutant mice were originally generated by interbreeding the wild type and mutant mice (kindly provided by Dr Robert Benezra, Memorial Sloan-Kettering Cancer Center, New York, NY). For the present studies, mice were backcrossed with C57Bl/6J (Bl/6; CD45.2) mice (Jackson Laboratories, Bar Harbor, ME, USA) for at least 8 generations to a Bl/6 (CD45.2) background before experimental use.
All of the mice were used at 6 to 10 weeks of age. For competitive repopulation assay, C57/BoyJ F1 (F1; CD45.1/CD45.2) and B6.SJL –PtrcaPep3b/BoyJ (Boy J; CD45.1) mice were obtained from an on-site core breeding colony. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine.
Phenotypic Analysis
The following phenotypically identified populations were assessed in Mad2+/+ and Mad2+/− bone marrow (BM): lineage− sca-1+ c-kit+ IL-7Rα− cells (enriched for HSC), common lymphoid progenitor (CLP; Lin− Sca1+ c-kit+ IL-7Rα+), megakaryocyte/erythroid progenitor (MEP; Lin− Sca1− c-kit+ CD34− FcγR−/lo), common myeloid progenitor (CMP; Lin− Sca1− c-kit+ CD34+ FcγR−/lo), and granulocyte/macrophage progenitor (GMP; Lin − Sca1− c-kit+ CD34+ FcγR+/hi). BM was collected and stained with antibodies to surface markers previously described. Data was collected from the samples using a LSR II (BD) instrument and BD FACSDiva software (BD, San Diego, CA), and was analyzed using WinList software (Verity Software House, Topsham, ME).
Clonogenic progenitor cell assay
Bone marrow from mice were assessed for granulocyte-macrophage colony-forming unit (CFU-GM), erythroid burst-forming unit (BFU-E), and multipotential granulocyte, erythroid, monocyte, megakaryocyte colony-forming units (CFU-GEMM) progenitor cells, as described elsewhere [24]. In short, 5 × 104 BM cells or 5 × 105 spleen cells were plated and cultured in 0.9% methylcellulose culture medium per ml with the combination of 30% fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT), 5% vol/vol pokeweed mitogen mouse spleen cell-conditioned medium (PWMSCM), 50 ng/mL rmuSCF, 1 U/mL rhuEpo, 2 mM glutamine (Cambrex Bio Science, Walkersville, MD), 10−4 M 2-mercaptoethanol, and 0.1 mM hemin (Sigma-Aldrich, St. Louis, MO). Also, 5 × 104 cells were plated and cultured in 0.3% methylcellulose culture medium in the presence of 50 ng/mL rmuSCF, 10 ng/mL rmuGM-CSF, or a combination of the two. Absolute numbers of progenitors were calculated from the nucleated cellularity per femur and the number of colonies formed per number of cells plated. The percentage of progenitors in the S phase of the cell cycle was estimated by use of the tritiated thymidine kill technique, as described previously [25]. For assays examining the effect of E3330 on colony formation, 5 × 104 BM cells were incubated with varying concentrations of the inhibitor for 4 hours at 37°C prior to plating in methylcellulose as described above. Colonies were scored after 7 days of incubation at 37°C, in lowered (5%) O2, unless otherwise noted, and 5% CO2.
Recovery of BM progenitors from cytotoxic effects
Mad2+/+ and Mad2+/− mice were treated with cyclophosphamide/cytoxan (200 mg/kg, i.p.), high sub-lethal levels of γ irradiation (650 cGy whole body irradiation), or Ara-C (500 mg/kg, s.c.) on day zero of the experiment. For the mice treated with Ara-C, we analyzed mice at days 1, 3, and 5. Mice treated with Cytoxan were analyzed on days 1, 3, 5, and 7 after injection. Mice treated with γ irradiation were analyzed on days 7, 10, and 14 after irradiation. For each treatment, both control Mad2+/+ and Mad2+/− mice were assessed at day zero without any treatment. For each treatment used, the days that we assessed have been shown by our laboratory and others to be optimal for assessing rebound hematopoiesis [26–29]. For analysis, BM and spleen were harvested from the treated mice at the various time points stated above, and colony assays were set up as previously described.
Competitive repopulation assay
Hematopoietic stem cell engraftment in lethally irradiated hosts was performed using a competitive repopulation assay (CRA) as adapted from Harrison and colleagues [30, 31]. In CRAs performed in ablated hosts, varying ratios of freshly isolated Mad2+/+ or Mad2+/− Bl/6 donor cells were mixed with freshly isolated Boy J competitor cells, and transplanted into 950 cGy-conditioned (137Cs source, single dose) F1 recipients as described previously [32]. Donor, competitor, and recipient peripheral blood chimerism was determined at 1, 2, 4, and 6 months posttransplantation. Peripheral blood was collected by tail-vein bleeding into heparinized microcapillary tubes (Fisher Scientific, Pittsburg, PA). Red blood cells (RBCs) in the PB were lysed by incubating with RBC lysis buffer (0.155 M NH4Cl, 0.01 M KHCO3, 0.1 mM EDTA in H2O and filter sterilized) and samples were washed in PBS + 2% BSA. Cells were stain with antibodies to anti-CD45.1 PE and anti-CD45.2 APC (BD-Pharmingen) and analyzed by FACS [32]. Peripheral blood from BoyJ, F1, and C57/Bl6 mice was used as controls. Data analysis was done using FCS Express V3 software (De Novo Software, Ontario, Canada).
Secondary Non-competitive Transplantation
Six months after the primary competitive repopulation assay was started, BM cells were aseptically harvested from primary F1 recipient mice, resuspended in IMDM, and 2 × 106 cells injected into secondary F1 recipient mice irradiated with 950 cGy total body irradiation. Peripheral blood was collected from secondary animals at 1, 2, 4, and 6 months after transplantation and analyzed for CD45.1 and CD45.2 chimerism as described above.
ID50 Analysis of Mad2+/+ and Mad2+/− HPC in Response to E3330
To further examine if the response to E3330 was different between Mad2+/+ and Mad2+/− HPC, we performed an ID50 (inhibitory dose50) analysis. A linear regression model was used to fit a line to the dose-response curve generated from the data of the percentage of colonies inhibited by each dose of E3330 used. Each equation was then solve for y=50 to give the dose needed to inhibit 50% of the colony growth.
Statistical analysis
Data were analyzed statistically using the Student t test. A P value less than 0.05 was considered statistically significant.
Results
Mad2 haploinsufficiency protects HPC from cytotoxic effects of cell-cycle specific chemotherapeutic agents
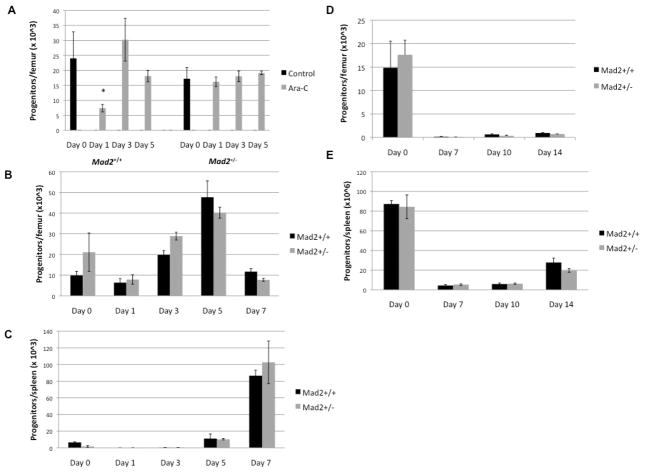
We evaluated the in vivo cytotoxic effects of irradiation and chemotherapeutic agents on HPC from Mad2+/− and Mad2+/+ mice. Mice were treated with either cyclophosphamide (200 mg/kg, i.p.), a high sub-lethal dose (650 cGy) of γ radiation, or cytarabine (Ara-C) (500 mg/kg, s.c.) and then assessed for recovery of HPC, expressed as absolute numbers per femur. Mad2 haploinsufficiency protected BM HPCs from the cytotoxic affects of Ara-C (Figure 1A). Numbers of Mad2+/− HPCs, in contrast to that of Mad2+/+ littermate controls, did not decrease during the nadir period of recovery from Ara-C treatment, demonstrating that Mad2+/− protected mice from the cytotoxic effects of Ara-C. No significant differences were observed in recovery of functional Mad2+/− and Mad2+/+ BM or spleen HPC after treatment with cyclophosphamide (Figure 1B, C) or γ radiation (Figure 1D, E). Thus, our studies unexpectedly found that Mad2 haploinsufficiency does not render the hematopoietic system more susceptible than control mice to suppressive effects of cytotoxic agent treatment, but is protective for a cycle specific agent, Ara-C.
Figure 1. Mad2 haploinsufficiency protects HPC in vivo from the cytotoxic effects of a cell-cycle specific chemotherapeutic agent.
(A) BM cells collected from Mad2+/+ and Mad2+/− mice treated with Ara-C (500 mg/kg, s.c.) were assessed for total progenitor cells (stimulated in vitro with Epo, PWMSCM, and SCF) at days 1, 3, and 5 after treatment. BM (B) and spleen cells (C) collected from Mad2+/+ and Mad2+/− mice treated with cyclophosphamide (200 mg/kg, i.p.) were assessed for total progenitor cells at days 0, 1, 3, 5, and 7 after treatment. BM (D) and spleen cells (E) collected from Mad2+/+ and Mad2+/− mice treated with sub-lethal dose of irradiation (650 cGy) were assessed for total progenitor cells at days 0, 7, 10, and 14 after treatment. In each experiment, 5 mice of each genotype were used for every data point. Data were analyzed using the Student t test. A P value less than 0.05 was considered statistically significant. *p<0.01
Decreases in progenitor numbers and cycling status are observed in BM HPC from Mad2+/− mice
We had previously reported that Mad2+/− BM HPC were in a slow or non-cycling state and their absolute numbers were decreased compared to Mad2+/+ BM. This might possibly be a reason for the protected state of Mad2+/− HPC after in vivo treatment of mice with Ara-C. The mice used our published studies [20] were on a mixed background, but the mice used in present studies were on a C57Bl/6 background. Hence, we assessed absolute numbers and cycling status of Mad2+/− and Mad2+/+ HPC on a C57Bl/6 background (N=9 mice/group). We found significantly decreased absolute numbers by 35% (p<0.014), 32% (p<0.019), and 47% (p<0.016), respectively, of immature progenitors, CFU-GM, CFU-GEMM, and BFU-E (responsive to stimulation by multiple growth factors), in Mad2+/− BM compared with Mad2+/+ BM. Moreover, the percentage of immature progenitor cells in the S phase of the cell cycle was significantly decreased in Mad2+/− BM (respectively for Mad2+/+ vs Mad2+/−: CFU-GM, 42 ± 3 vs 2 ± 5; CFU-GEMM, 60 ± 8 vs 17 ± 8; and BFU-E, 58 ± 4 vs −15 ± 13 ). These results are in agreement with our previous findings using mice on a mixed strain background [20], demonstrating that Mad2 also plays an important role in HPC proliferation in mice on a Bl/6 background.
Phenotypic characterization of HSC and HPC populations in Mad2+/− and Mad2+/+ mice
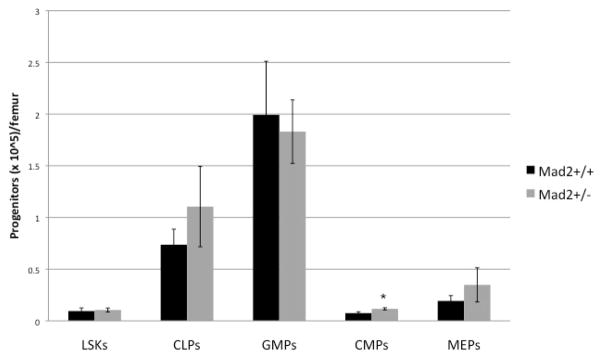
To further understand BM HSC/HPC characteristics, we performed a phenotypic analysis of these cell populations (Figure 2). We observed no significant difference in the lineage− sca-1+ c-kit+ IL-7Ra− cells (LSK; a population enriched for HSC) in the Mad2+/− and Mad2+/+ mice. Moreover, there were no significant differences in CLP, MEP, and GMP populations in both types of mice. We did note that absolute numbers of CMP were slightly (45%, p<0.01), but significantly increased in the Mad2+/− mice. The fact that the numbers of phenotyped populations of CMP and GMP were respectively slightly increased or not different in Mad2+/− and Mad2+/+ mice, yet there were significant decreases in the numbers and cycling status of Mad2+/− CFU-GM, BFU-E, and CFU-GEMM suggests that phenotypes of cells in this case do not provide telling information about the functional proliferative capacity of these cells.
Figure 2. Phenotypic analysis of HSC and HPC populations reveals no changes in LSK, CLP, GMP, and MEP, but a slight increase in CMPs in Mad2+/− mice.
BM from Mad2+/+ and Mad2+/− mice was assessed for the number of phenotypically defined progenitors per femur. Whole BM was collected and stained with fluorescent antibodies to various surface markers; cells were then analyzed by flow cytometry. Results shown are the average of 3 independent experiments. *p<0.05.
Mad2 haploinsufficiency does not affect long-term engrafting ability of HSC
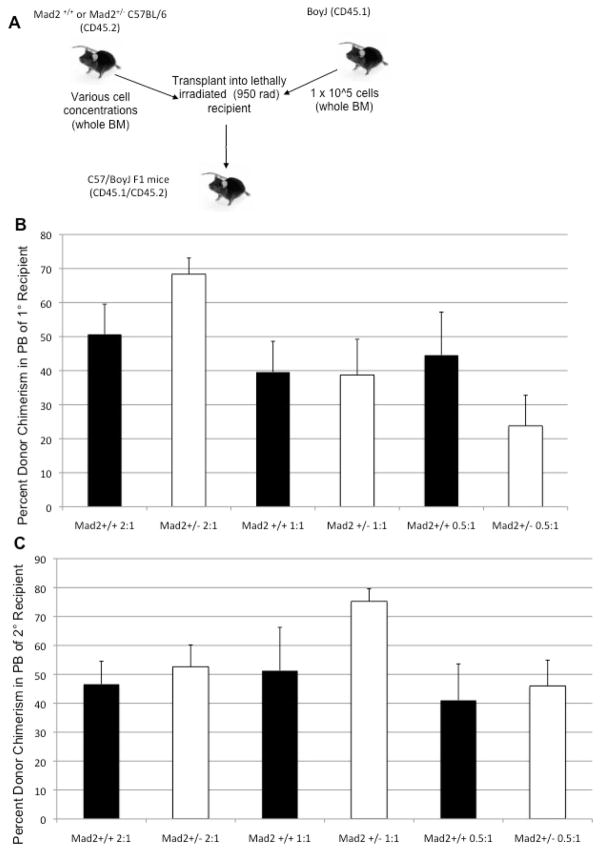
The discrepancy between phenotype and function of Mad2+/− HPCs made it clear that a functional assessment of Mad2+/− HSC was warranted. In order to determine if Mad2 haploinsufficiency affects the function of HSC, we performed CRA to examine the short and long term repopulating ability of Mad2+/− compared to Mad2+/+ HSC. Various concentrations of Mad2+/+ or Mad2+/− Bl/6 freshly isolated BM (CD45.2 donor) cells were mixed with 1 x 105 freshly isolated Boy J BM (CD45.1 competitor) cells, and these mixtures were transplanted into 950 cGy-conditioned F1 (CD45.1/CD45.2) recipients (Figure 3A). Peripheral blood donor cell chimerism was determined at 1, 2, 4, and 6 months posttransplant. The percent donor chimerism for 6 months posttransplant is shown in Figure 3B. For the various different donor to competitor ratios used, no significant difference in the percent peripheral blood chimerism was observed between Mad2+/+ or Mad2+/− cells. This indicates that Mad2 haploinsufficiency does not affect the ability of HSC to engraft and compete in a transplant setting. After 6 months, the recipient F1 mice were sacrificed and BM was collected to perform a secondary non-competitive transplant into new 950 cGy-conditioned F1 recipients. Peripheral blood donor cell chimerism was determined at 1, 2, 4, and 6 months posttransplant, and the 6 month time point is shown in Figure 3C. Data from the secondary transplant showed no consistent significant difference in percent peripheral blood chimerism between Mad2+/+ and Mad2+/− cells. This suggests that Mad2 haploinsufficiency does not affect either engrafting capability, numbers of functional HSC, or self-renewal of HSCs.
Figure 3. Mad2 haploinsufficiency does not affect the engrafting/repopulating ability of HSC compared to wild type HSC.
(A) Donor (CD45.2) and competitor (CD45.1) BM cells were transplanted into a lethally irradiated recipient (CD45.1/45.2) mice at the ratios indicated. (B) Peripheral blood (PB) was collected at months 1,2,4, and 6 after primary transplant; the 6 month time point is shown to examine long-term engraftment [N=3]. (C) 6 months after the primary transplants, secondary transplants were performed & PB was collected at 1,2,4, and 6 months; the 6 month time point is shown to examine long-term engraftment [N=2]. PB was assessed for percent donor and competitor chimerism, which is indicative of the levels of BM chimerism. To analyze PB samples for chimerism, the blood was stained with fluorescent antibodies to CD45.1 and CD45.2 and analyzed by flow cytometry. Five mice were used as recipients each transplant group. *p<0.05.
Mad2+/− BM HPCs are less sensitive to cytostatic effects caused by blocking the redox function of Ape1/Ref-1, and do not respond well to effects of lowered O2 tension
After finding that functional differences exist between Mad2+/− and Mad2+/+ HPC, but not HSC, we decided to further examine HPC function in these mice. To examine how Mad2+/− BM HPCs respond to cytostatic effects of drugs in comparison to Mad2+/+ HPC, we treated these cells with E3330. E3330 is a highly specific inhibitor of Ape1/Ref-1 redox activity. E3330 does not increase the percentage of cells undergoing apoptosis, but rather stops them from proliferating [23, 33]. Ape1/Ref-1 is a multifunctional protein involved in both the DNA base excision repair pathway and redox regulation of various transcription factors, including AP-1, HIF-1α, and p53 [34–36]. It was reported that the redox function, but not the DNA repair activity, of Ape1/Ref-1 is required for normal embryonic hematopoiesis [21]; however, the function of this protein has not been thoroughly evaluated in normal adult hematopoiesis. Using E3330, we examined colony-forming ability of both Mad2+/+ and Mad2+/− HPC in vitro at normoxic (~20%) and at lowered (5%) oxygen tension. Lowered O2 tension much more closely mimics the O2 tension in bone marrow, and is more physiological. BM HPC colonies grow better at 5% O2 tension compared to colonies grown at 20% O2 tension [37]. Hence the reason all of our above studies assessing HPC were done at 5% O2.
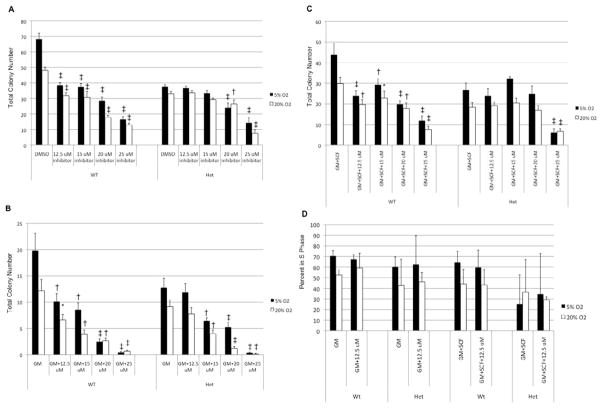
For Mad2+/+ (wild type; WT) BM HPC, E3330 causes decreases in colony formation responsive to stimulation by multiple growth factors (PWMSCM, Epo, SCF; Figure 4A), GM-CSF alone (Figure 4B), and the combination of SCF and GM-CSF (Figure 4C), regardless of the concentration of E3330 used. Upon examination of colony formation from Mad2+/− HPC with the lowest concentration of E3330 used, we found no significant differences in colony formation between E3330 and vehicle control treated cells, while wild type HPC colony formation was significantly inhibited at both oxygen tensions. However, by increasing the concentration of E3330 used, we were able to inhibit Mad2+/− HPC colony formation, but still not the level of Mad2+/+ HPCs. ID50 analysis revealed the dose of E3330 required for inhibition of 50% of Mad2+/− HPC is greater than that required for inhibition of 50% of Mad2+/+ HPC at lowered oxygen tension (ID50 of 23 vs 18, 17 vs 13, and 23 vs 17 for Mad2+/− vs Mad2+/+, respectively for cells stimulated by multiple growth factors, GM-CSF alone, and GM-CSF+SCF). Of interest, Mad2+/− HPCs do not grow better at lower O2 tension as do wild type HPC.
Figure 4. Mad2+/− BM cells are less sensitive to the cytostatic effects caused by blocking the redox function of Ape1/Ref-1 compared to Mad2+/+ BM cells.
BM cells collected from Mad2+/+ and Mad2+/− mice were treated with various concentrations of E3330 or DMSO control for 4 hours before placing cells into colony assays. (A) Cells plated in the presence of Epo, PWMSCM, and SCF; (B) Cells plated in the presence of GM-CSF. (C) Cells plated in the presence of SCF and GM-CSF. (D) The percentage of BM progenitors in S phase. Data were analyzed using the Student t test. A P value less than 0.05 was considered statistically significant. N ≥ 3. *p<0.05, †p<0.01, ‡p<0.001 compared to DMSO control.
To ensure that differences in inhibition were not due to inherent changes in cell cycle status between wild type and Mad2+/− HPC and to ensure that E3330 is not cell cycle specific, a high specific activity tritiated thymidine kill assay was performed in the presence and absence of E3330 to estimate the percentage of cells in S phase. The percentage of wild type cells in S phase is similar in the presence and the absence of E3330 (Figure 4D) and the same is true for Mad2+/− HPC; this indicates that the inhibitor is not cell cycle specific. The inhibition of Mad2+/− HPC colony formation only under higher concentrations of E3330 suggests that Mad2+/− HPC are less sensitive to the cytostatic effects of this inhibitor compared to Mad2+/+ HPC.
Discussion
In this study, we found decreases in progenitor numbers and cycling status in BM HPC from Bl/6 strain Mad2+/− mice, demonstrating that the previously described effects of Mad2 haploinsufficiency of mice on a mixed strain background on hematopoiesis [20] were not strain dependent. However, our present data on functional HPC numbers on the surface seem to be in disagreement with our phenotypic analysis showing that numbers of GMPs and MEPs are not different, and CMPs are slightly, but significantly increased in the BM of Mad2+/− mice compared to Mad2+/+ mice. One explanation for this could be that although there are greater numbers of CMPs in Mad2+/− BM, these cells could in fact just be less functional than their wild type counterparts due to the decreases in Mad2 protein levels. But because of the massive reserve of hematopoietic progenitor cells that normally exist in the BM of mice [24], functional differences may not be reflected in the overall phenotypically defined population. However, when this reserve of progenitors is depleted, as it is in the case of Ara-C treatment, we do see that Mad2+/− HPC are not affected by the cytotoxic effects as Mad2+/+ HPC are. We hypothesized that this might be due to the differences in cycling status of these progenitor, but it might also be due to the increased numbers of phenotypically defined CMP observed in Mad2+/− bone marrow. This might suggest that the differences between functional and phenotypic data are in fact masked by the massive reserve of progenitors found in the bone marrow.
To examine if functional differences exist between Mad2+/− HSC compared to Mad2+/+ HSC, we set up a CRA. CRA defines the competitive nature of the HSC, and secondary repopulation studies allow for measurement of self-renewal capabilities of the HSC. We did not find significant differences in the short or long term engraftment of Mad2+/− HSC and Mad2+/+ HSC in either the primary or secondary transplants. This indicates that Mad2 haploinsufficiency does not have a negative effect on the ability of the HSC to compete, engraft, or self-renew. Only subtle differences in morbidity rates occur between colonies of Mad2+/+ and Mad2+/− mice; overall Mad2+/− animals appear largely normal [4], although Mad2+/− mice exhibit increased germinal center formation in the spleen and a possible increase in tumor incidence when compared to age-matched Mad2+/+ mice [4].
Previous studies in our lab found a higher percentage of cell death in KL (c-kit+ lin−) BM of Mad2+/− mice compared to Mad2+/+ BM when cells were cultured in SCF and GM-CSF [20]. However, there was no significant difference between Mad2+/− and Mad2+/+ mice in the percentage of cell death of BM cells before cell culture, suggesting that greater cell death may occur in stress conditions, but not in steady state conditions [20]. To examine this, we exposed Mad2+/− and Mad2+/+ mice to various cytotoxic and cytostatic agents. No significant differences were observed in the recovery of functional BM or spleen HPC from Mad2+/− and Mad2+/+ mice after treatment with cyclophosphamide or γ radiation. In contrast, we found that Mad2+/− HPC were not affected by the cytotoxic affects of Ara-C even though Mad2+/+ HPC were. In fact, Mad2 haploinsufficiency was myeloprotective to this specific DNA synthesis inhibitor. The decreased percentage of cycling Mad2+/− HPCs compared to Mad2+/+ HPCs might in part account for the differences observed in the recovery of HPCs from treatment with Ara-C, which selectively inhibits DNA synthesis, mainly affecting rapidly dividing cells. The differences might also be due to the increased absolute numbers of CMP observed in Mad2+/− mice.
Examination of the cytostatic effects of E3330 on BM HPC populations of Mad2+/− and Mad2+/+ mice found that the drug had a greater effect in cell cultures growing in 5%, compared to 20%, O2 tension. However, Mad2+/− HPCs are much less sensitive the effects of E3330 than Mad2+/+ HPCs. Also, Mad2+/− HPCs do not grow better at lower O2 tension as do wild type HPC; we observed similar colony numbers formed from Mad2+/− HPCs with mixed growth factors at 5% and 20% O2 tension. This phenomenon has not been shown before with Mad2+/− HPC colony formation; earlier studies in our lab only examined Mad2+/− HPC growth under 5% O2 tension [20]. These data suggest that the redox function of Ape1/Ref-1 is important in normal adult hematopoiesis, particularly for HPC growing under lower oxygen tension. This also suggests that transcription factors that are normally upregulated under hypoxic conditions may be important for colony formation and growth. The growth inhibitory effects of E3330 have been shown to be accentuated by hypoxia in pancreatic cancer cells [38], also suggesting an enhanced requirement for Ape1/Ref-1 redox function in hypoxia.
Lack of inhibition of Mad2+/− HPC by low concentrations of E3330, and inhibition of Mad2+/− HPC colony formation only under higher concentrations of E3330 suggests that Mad2+/− HPC are less sensitive to the cytostatic effects of the inhibitor compared to Mad2+/+ HPC. Initially we thought that the differences between Mad2+/− HPC and Mad2+/+ HPC colony formation in the presence of E3330 might be due to inherent differences in the cycling status of the progenitors. However, we found that the E3330 is not cell cycle specific and therefore the cycle status of Mad2+/− HPC cannot account for the differences in sensitivity of E3330 observed in Mad2+/− and Mad2+/+ HPC. A study in mouse embryonic stem (ES) cells and ES cell-derived embryoid body (EB) cells revealed that siRNA knockdown of Ape1/Ref-1 expression induced G1 arrest and decreased the percent of ES cells in S phase, indicating Ape1/Ref-1 regulates cell-cycle status in ES cells and also positively regulates the G1/S transition of the cell cycle in EB cells [21]. It is possible that Mad2+/− HPCs are less sensitive to the effects of E3330 because they already exhibit cell cycle defects. However, knockdown of APE1 removes not only its redox signaling function, but also its DNA repair function and protein-protein interactions with other DNA repair and other proteins independent of the redox or DNA repair functions. Therefore, a specific small molecule inhibitor of APE1 redox is not directly comparable to siRNA knockdown studies. Further studies will be needed to determine how Ape1/Ref-1 is involved in regulating the cell cycle of HPC.
In conclusion, the data presented in this study suggests that Mad2 haploinsufficiency affects the function of HPCs, but not the HSC population. Although Mad2+/− HPCs have decreased colony numbers and cycling status compared to Mad2+/+ HPCs, they appear to be protected from cytotoxic effects in vivo of a cell-cycle specific agent, as well as the cytostatic effects of an Ape1/Ref-1 inhibitor. We also found that the redox function of Ape1/Ref-1 positively regulates adult hematopoiesis, and control of the cell cycle also appears to be important in this regulation process, events associated with HPC growing at lowered O2 tension.
Acknowledgments
Public Health Service Grants NIH R01 HL 56416, NIH R01 HL 67384, and a project in NIH P01 HL 53586 to HEB supported this work. SLR was supported by the Cancer Biology Training Grant T32 CA 111198 for parts of this study. MRK is supported by the National Institutes of Health, National Cancer Institute (CA94025, CA106298, CA114571, and CA 121168 to M.R.K.) and Riley Children’s Foundation.
Footnotes
Conflict of interest disclosure
MRK has patents and IP licensed to ApeX Therapeutics relating to the E3330 molecule. The other authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gillett ES, Sorger PK. Tracing the pathway of spindle assembly checkpoint signaling. Dev Cell. 2001;1:162–164. doi: 10.1016/s1534-5807(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Gorbea C, Mahaffey D, Rechsteiner M, Benezra R. MAD2 associates with the cyclosome/anaphase-promoting complex and inhibits its activity. Proc Natl Acad Sci U S A. 1997;94:12431–12436. doi: 10.1073/pnas.94.23.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Benezra R. Identification of a human mitotic checkpoint gene: hsMAD2. Science. 1996;274:246–248. doi: 10.1126/science.274.5285.246. [DOI] [PubMed] [Google Scholar]
- 4.Dobles M, Liberal V, Scott ML, Benezra R, Sorger PK. Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2. Cell. 2000;101:635–645. doi: 10.1016/s0092-8674(00)80875-2. [DOI] [PubMed] [Google Scholar]
- 5.Hoyt MA, Totis L, Roberts BT. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 1991;66:507–517. doi: 10.1016/0092-8674(81)90014-3. [DOI] [PubMed] [Google Scholar]
- 6.Li R, Murray AW. Feedback control of mitosis in budding yeast. Cell. 1991;66:519–531. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- 7.Taylor SS, McKeon F. Kinetochore localization of murine Bub1 is required for normal mitotic timing and checkpoint response to spindle damage. Cell. 1997;89:727–735. doi: 10.1016/s0092-8674(00)80255-x. [DOI] [PubMed] [Google Scholar]
- 8.Rieder CL, Schultz A, Cole R, Sluder G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J Cell Biol. 1994;127:1301–1310. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Nicklas RB. Mitotic forces control a cell-cycle checkpoint. Nature. 1995;373:630–632. doi: 10.1038/373630a0. [DOI] [PubMed] [Google Scholar]
- 10.Hwang LH, Lau LF, Smith DL, et al. Budding yeast Cdc20: a target of the spindle checkpoint. Science. 1998;279:1041–1044. doi: 10.1126/science.279.5353.1041. [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Lin D, Matsumoto S, Kitazono A, Matsumoto T. Fission yeast Sip1: an effector of the Mad2-dependent spindle checkpoint. Science. 1998;279:1045–1047. doi: 10.1126/science.279.5353.1045. [DOI] [PubMed] [Google Scholar]
- 12.Rieder CL, Cole RW, Khodjakov A, Sluder G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J Cell Biol. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musacchio A, Hardwick KG. The spindle checkpoint: structural insights into dynamic signalling. Nat Rev Mol Cell Biol. 2002;3:731–741. doi: 10.1038/nrm929. [DOI] [PubMed] [Google Scholar]
- 14.Page AM, Hieter P. The anaphase-promoting complex: new subunits and regulators. Annu Rev Biochem. 1999;68:583–609. doi: 10.1146/annurev.biochem.68.1.583. [DOI] [PubMed] [Google Scholar]
- 15.Yu H. Regulation of APC-Cdc20 by the spindle checkpoint. Curr Opin Cell Biol. 2002;14:706–714. doi: 10.1016/s0955-0674(02)00382-4. [DOI] [PubMed] [Google Scholar]
- 16.Taylor SS, Scott MI, Holland AJ. The spindle checkpoint: a quality control mechanism which ensures accurate chromosome segregation. Chromosome Res. 2004;12:599–616. doi: 10.1023/B:CHRO.0000036610.78380.51. [DOI] [PubMed] [Google Scholar]
- 17.Homer HA. Mad2 and spindle assembly checkpoint function during meiosis I in mammalian oocytes. Histol Histopathol. 2006;21:873–886. doi: 10.14670/HH-21.873. [DOI] [PubMed] [Google Scholar]
- 18.De Antoni A, Pearson CG, Cimini D, et al. The Mad1/Mad2 complex as a template for Mad2 activation in the spindle assembly checkpoint. Curr Biol. 2005;15:214–225. doi: 10.1016/j.cub.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 19.Nasmyth K. How do so few control so many? Cell. 2005;120:739–746. doi: 10.1016/j.cell.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Ito S, Mantel CR, Han MK, et al. Mad2 is required for optimal hematopoiesis: Mad2 associates with c-Kit in MO7e cells. Blood. 2007;109:1923–1930. doi: 10.1182/blood-2006-06-030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou GM, Luo MH, Reed A, Kelley MR, Yoder MC. Ape1 regulates hematopoietic differentiation of embryonic stem cells through its redox functional domain. Blood. 2007;109:1917–1922. doi: 10.1182/blood-2006-08-044172. [DOI] [PubMed] [Google Scholar]
- 22.Cooper S, Broxmeyer H. Clonogenic methods in vitro for the enumeration of granulocyte-macrophage progenitor cells (CFU-GM) in human bone marrow and mouse bone marrow and spleen. J of Tissue Cult Methods. 1991;13:77–82. [Google Scholar]
- 23.Luo M, Delaplane S, Jiang A, et al. Role of the multifunctional DNA repair and redox signaling protein Ape1/Ref-1 in cancer and endothelial cells: small-molecule inhibition of the redox function of Ape1. Antioxid Redox Signal. 2008;10:1853–1867. doi: 10.1089/ars.2008.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broxmeyer HE, Cooper S, Lasky LA, De Sauvage F. Identification of a massive reserve of hematopoietic progenitors in mice. Stem Cells Dev. 2005;14:105–110. doi: 10.1089/scd.2005.14.105. [DOI] [PubMed] [Google Scholar]
- 25.Cooper S, Mantel C, Broxmeyer HE. Myelosuppressive effects in vivo with very low dosages of monomeric recombinant murine macrophage inflammatory protein-1 alpha. Exp Hematol. 1994;22:186–193. [PubMed] [Google Scholar]
- 26.Broxmeyer HE, Pelus LM, Kim CH, Hangoc G, Cooper S, Hromas R. Synergistic inhibition in vivo of bone marrow myeloid progenitors by myelosuppressive chemokines and chemokine-accelerated recovery of progenitors after treatment of mice with Ara-C. Exp Hematol. 2006;34:1069–1077. doi: 10.1016/j.exphem.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Hromas R, Cooper S, Broxmeyer HE. The chemokine CCL21 protects normal marrow progenitors from Ara-C cytotoxicity. Cancer Chemother Pharmacol. 2002;50:163–166. doi: 10.1007/s00280-002-0486-7. [DOI] [PubMed] [Google Scholar]
- 28.Radley JM, Scurfield G. Effects of 5-fluorouracil on mouse bone marrow. Br J Haematol. 1979;43:341–351. doi: 10.1111/j.1365-2141.1979.tb03761.x. [DOI] [PubMed] [Google Scholar]
- 29.DeWys WD, Goldin A, Man, El N. Hematopoietic recovery after large doses of cyclophosphamide: correlation of proliferative state with sensitivity. Cancer Res. 1970;30:1692–1697. [PubMed] [Google Scholar]
- 30.Harrison DE. Competitive repopulation: a new assay for long-term stem cell functional capacity. Blood. 1980;55:77–81. [PubMed] [Google Scholar]
- 31.Harrison DE, Jordan CT, Zhong RK, Astle CM. Primitive hemopoietic stem cells: direct assay of most productive populations by competitive repopulation with simple binomial, correlation and covariance calculations. Exp Hematol. 1993;21:206–219. [PubMed] [Google Scholar]
- 32.Campbell TB, Basu S, Hangoc G, Tao W, Broxmeyer HE. Overexpression of Rheb2 enhances mouse hematopoietic progenitor cell growth while impairing stem cell repopulation. Blood. 2009;114:3392–3401. doi: 10.1182/blood-2008-12-195214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fishel ML, Colvin ES, Luo M, Kelley MR, Robertson KA. Inhibition of the redox function of APE1/Ref-1 in myeloid leukemia cell lines results in a hypersensitive response to retinoic acid-induced differentiation and apoptosis. Exp Hematol. 2010 doi: 10.1016/j.exphem.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xanthoudakis S, Miao G, Wang F, Pan YC, Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992;11:3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lando D, Pongratz I, Poellinger L, Whitelaw ML. A redox mechanism controls differential DNA binding activities of hypoxia-inducible factor (HIF) 1alpha and the HIF-like factor. J Biol Chem. 2000;275:4618–4627. doi: 10.1074/jbc.275.7.4618. [DOI] [PubMed] [Google Scholar]
- 36.Jayaraman L, Murthy KG, Zhu C, Curran T, Xanthoudakis S, Prives C. Identification of redox/repair protein Ref-1 as a potent activator of p53. Genes Dev. 1997;11:558–570. doi: 10.1101/gad.11.5.558. [DOI] [PubMed] [Google Scholar]
- 37.Bradley TR, Hodgson GS, Rosendaal M. The effect of oxygen tension on haemopoietic and fibroblast cell proliferation in vitro. J Cell Physiol. 1978;97:517–522. doi: 10.1002/jcp.1040970327. [DOI] [PubMed] [Google Scholar]
- 38.Zou GM, Maitra A. Small-molecule inhibitor of the AP endonuclease 1/REF-1 E3330 inhibits pancreatic cancer cell growth and migration. Mol Cancer Ther. 2008;7:2012–2021. doi: 10.1158/1535-7163.MCT-08-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]