Abstract
BACKGROUND
The association of elevated serum uric acid with the development of hypertension is established outside of pregnancy. We investigated whether first trimester uric acid was associated with the development of the following: gestational hypertension or preeclampsia, these outcomes stratified by presence of hyperuricemia at delivery since this denotes more severe disease, preterm birth or small for gestational age (SGA).
METHODS
Uric acid was measured in 1541 banked maternal plasma samples from a prior prospective cohort study that were collected at a mean gestational age of 9.0 (± 2.5) weeks. Polytomous regressions were performed and adjusted for parity and pre-pregnancy body mass index.
RESULTS
First trimester uric acid in the highest quartile (>3.56 mg/dL) compared to lowest three quartiles was associated with an increased risk of developing preeclampsia (adjusted OR = 1.82; 95% CI, 1.03–3.21) but not gestational hypertension. In women with hypertensive disease complicated by hyperuricemia at delivery, high first trimester uric acid was associated with a 3.22-fold increased risk of hyperuricemic gestational hypertension and a 3.65-fold increased risk of hyperuricemic preeclampsia. High first trimester uric acid was not associated with gestational hypertension or preeclampsia without hyperuricemia at delivery, preterm birth, or SGA. In women who developed hypertensive disease, elevated uric acid at delivery was only partly explained by elevated uric acid in the first trimester (r2 = .23).
CONCLUSIONS
First trimester elevated uric acid was associated with later preeclampsia and more strongly with preeclampsia and gestational hypertension with hyperuricemia.
Keywords: gestational hypertension, hyperuricemia, preeclampsia, uric acid
INTRODUCTION
Elevated uric acid is associated with both cardiovascular disease events and risk factors, such as hypertension, metabolic syndrome, chronic kidney disease, obesity, and diabetes in non-pregnant adults.1 All of these are also known to increase the risk of developing the pregnancy syndrome preeclampsia.2 While uric acid has been mainly described as a biomarker for these diseases, evidence is emerging that uric acid not only predicts the development of hypertension, but may be part of the causal pathway.3 In pregnancy, hyperuricemia frequently occurs before the development of hypertension and proteinuria, and has been proposed to have a pathogenic role in the development of preeclampsia.4, 5
While the association of elevated serum uric acid with development of hypertension is established outside of pregnancy, less is known about whether women who begin pregnancy with elevated serum uric acid have an increased risk of developing hypertensive disease during pregnancy. In healthy pregnancies, uric acid decreases from an average of 4.2 mg/dl pre-pregnancy to 3.1 ± 1.1 mg/dl in the first trimester, and slowly increases during gestation to an average of 5.1 ± 1.2 mg/dl from 35 weeks gestation to term.4, 6 Serum uric acid concentration was higher in the first trimester in women with pre-gestational diabetes who developed preeclampsia in the third trimester compared to women with pre-gestational diabetes who did not develop preeclampsia.7 In non-diabetic women, the average serum uric acid concentration at 13 weeks gestation was higher in women who developed both gestational hypertension and preeclampsia, and hypertensive disease complicated by SGA, although the numbers with these outcomes were small.8
We have previously reported on the association between elevated first trimester uric acid and increased risk for development of gestational diabetes.9 In this study, we initially explored whether first trimester hyperuricemia would be associated with the development of gestational hypertension and preeclampsia. In prior studies we have demonstrated that gestational hypertension with hyperuricemia (HU) or preeclampsia with hyperuricemia (HPU) defines a more severe form of hypertensive disease, with increased risk of adverse fetal outcomes including preterm birth and small for gestational age (SGA) compared to women with gestational hypertension or preeclampsia without hyperuricemia (H or HP).10 Therefore we further explored whether first trimester uric acid concentration was associated with increased risk for developing hypertensive disease during pregnancy with (HU, HPU) and without hyperuricemia at delivery (H, HP). Finally, since hyperuricemia at delivery in women with gestational hypertensive disease is associated with increased risk of preterm birth and having an SGA infant, we also explored whether first trimester uric acid concentration is associated with an increased risk for these outcomes.
METHODS
Study design and population
We used banked maternal plasma samples from specimens drawn at < 15 weeks gestation from subjects enrolled in the Pregnancy Exposures and Preeclampsia Prevention Study (PEPP). PEPP is an ongoing study of preeclampsia approved by the University of Pittsburgh Institutional Review Board. Women with a singleton gestation were recruited from the outpatient clinics and private practices. Of the 4045 women who were approached, 2891 (71.5%) agreed to participate. We investigated 2211 pregnancies in the longitudinal component of this study from 1997–2002, of which 1604 had samples available. Baseline demographic information and medical history were collected via a structured interview. Pregnancy outcomes were also recorded from the medical record as part of the study and were available for analysis. Eight women had two or more samples available in the first trimester, and in these cases the earliest gestational age sample was used. We excluded women with chronic hypertension (n=23) and women with incomplete data or unknown uric acid concentration at delivery. Patients with previous preeclampsia were not excluded since these women may evidence elevated uric acid, and therefore represent an interesting study group (n=4). A total of 1541 samples were available < 15 weeks for analysis. This subset of women did not differ from the larger longitudinal cohort as far as maternal entry age (25.2 versus 25.2 years, p > 0.9), pre-pregnancy BMI (25.4 versus 25.5 kg/m2, p > 0.9), or at least high school education (81.6% versus 80.2%, p = 0.3).
Gestational hypertension (H) was defined as new-onset elevated blood pressure 140 mmHg systolic or ≥ 90 mm/Hg diastolic after 20 weeks of gestation. Preeclampsia was characterized as gestational hypertension with proteinuria (HP) as defined by ≥ 300 mg in a timed 24 hour urine collection, > 2+ on a voided or >1+ on a catheterized random urine specimen, or a spot urine protein to creatinine ratio ≥ 0.3. Uric acid concentration was assessed at delivery and hyperuricemia (U) was defined as ≥ 1 standard deviation above the mean value of uric acid at the gestational age the sample was obtained.6 Normotensive women were those without gestational blood pressure elevations. Neonatal outcomes assessed included birth weight and birth weight centile, adjusted for race, sex and gestational age at delivery from a reference population of over 10,000 births at Magee-Womens Hospital.10 Small for gestational age (SGA) was defined as birth weight < 10th centile. Maternal race was classified as either non-black or black because of the low frequencies of non-white, non-black participants. The baseline characteristics of the non-white, non-black participants were comparable to white participants, and therefore we elected to combine these groups. Education was categorized as less than high school (12 years), or at least a high school level (≥ 12 years). Gestational age was determined by best obstetrical assessment, using early ultrasound data where available.
Plasma samples
Banked maternal plasma samples were stored at −70° C until assayed. We have previously performed analyses demonstrating that uric acid concentrations are stable with prolonged storage over 10 years and multiple freeze thaws in our laboratory. Uric acid was measured using an uricase based colorimetric assay from Pointe Scientific, Inc. (Canton, MI) Kit U7581-120 with a lower detection limit of 1 mg/dL. The coefficient of variance was 9.0%.
Statistical Analysis
Demographic data were summarized by uric acid quartiles, as means and standard deviations or numbers of subjects and percents, and test for trend was performed using linear or logistic regression. Mean first trimester uric acid concentrations were compared among groups with analysis of variance (ANOVA) and Scheffe test. Polytomous logistic regression was performed to calculate the adjusted odds ratio (OR) for developing hypertensive disease (preeclampsia or gestational hypertension; and H, HU, HP, or HPU) compared to remaining normotensive given the first trimester uric acid concentrations. Uric acid concentration was evaluated as both a continuous and a categorical variable. Our final analysis evaluated uric acid concentration by quartile for the entire study population, comparing the highest quartile to the lower three quartiles. Covariates that were considered included maternal age at entry to the study, parity, pre-pregnancy body mass index (BMI, kg/m2 calculated from maternal pre-pregnancy weight by self report and measured height), maternal race, smoking, education, gestational age at sampling and gestational age at delivery. Only parity and pre-pregnancy body mass index significantly contributed to the final model with a p-value < .05. Additional adjustment for gestational age at sampling did not change the results, so we did not adjust for this covariate. An interaction between uric acid and BMI was tested in our final analysis and was not significant (P = .3). There were ten women who were potential influential or leverage points. They all had a BMI > 30 kg/m2, and nine had a BMI > 43 kg/m2. Three of these women with morbid obesity had first trimester uric acid concentrations > 7.5 mg/dL. When evaluating the association between hypertensive disease and first trimester uric acid as a continuous variable, the significant relative risk ratios remained significant after dropping these ten women from the model, although the association was mildly decreased. When evaluating the association between hypertensive disease and uric acid in the highest quartile, the results did not change significantly when these influential and leverage points were dropped from the model, so they were retained for all analyses. For the overall model an α level of < 0.05 was considered statistically significant.
We examined the relation between each standard deviation change in first trimester uric acid concentration (z-score) and the odds of developing hypertensive disease compared to remaining normotensive using polytomous regression and predicted probabilities for the specific hypertensive outcome (i.e. HU or HPU). Nonparametric smoothing with locally weighted regression (lowess) was used. Differences were considered significant for P < 0.05.
Linear or logistic regression was also performed to investigate the association between first trimester uric acid concentrations and birth weight, birth weight percentile, having an SGA infant and delivering an infant preterm. The proportions of women with first trimester uric acid in the fourth quartile who developed hypertensive disease (H, HU, HP, or HPU) and had a preterm birth or SGA infant were compared to the proportion of women with first trimester uric acid in the fourth quartile who remained normotensive (N) and had a preterm birth or SGA infant using a two sample test of proportion. Analyses were performed with Stata (StataCorp LP, College Station, TX), version 10.0 for Windows.
RESULTS
Plasma samples analyzed for the 1,541 women in the study were collected, on average, at 9.0 ± 2.5 weeks gestation. With higher first trimester uric acid, women were slightly younger, the gestational age at sampling decreased, and pre-pregnancy BMI increased (Table 1).
Table 1.
Characteristics of the women and their newborns by uric acid quartile.
| First Trimester Uric Acid Quartile (range, mg/dL) | |||||
|---|---|---|---|---|---|
| Characteristics | 1st (0.91–2.47) | 2nd (>2.47–2.96) | 3rd (>2.96–3.56) | 4th (>3.56–8.30) | P-value for trend |
| n = 385 | n = 385 | n = 385 | n = 386 | ||
| Maternal age (years) – mean (S.D.) | 25.7 (6.3) | 25.3 (5.9) | 24.6 (5.6) | 25.0 (5.7) | 0.02 |
| BMI (kg/m2) – mean (S.D.) | 23.0 (4.2) | 24.0 (5.1) | 25.8 (6.2) | 28.9 (7.5) | <0.001 |
| ≥ 25 kg/m2 – % | 26.5 | 31.4 | 44.2 | 64.2 | |
| Gestational age at sampling (weeks) – mean (S.D.) | 9.3 (2.5) | 9.0 (2.3) | 8.8 (2.5) | 8.7 (2.6) | 0.008 |
| Maternal Race – % | 0.6 | ||||
| Non-black | 61.8 | 70.6 | 62.1 | 66.3 | |
| Black | 38.2 | 29.4 | 37.9 | 33.7 | |
| Smokers – % | 24.5 | 36.1 | 32.7 | 26.9 | 0.7 |
| Education – % < 12 years | 18.2 | 17.7 | 20.3 | 17.6 | 0.9 |
| Parity – % | 0.5 | ||||
| 0 | 56.9 | 62.6 | 62.4 | 59.4 | |
| 1 | 24.4 | 23.1 | 23.1 | 23.8 | |
| 2+ | 18.7 | 14.3 | 14.5 | 16.8 | |
| GA at delivery (weeks) – mean(S.D.) | 39.1 (2.1) | 39.4 (1.5) | 39.0 (2.6) | 39.2 (2.2) | 0.8 |
| Preterm birth – % | 9.1 | 6.0 | 10.4 | 10.4 | 0.2 |
| Birth weight (grams) – mean(S.D.) | 3241.3 (597.8) | 3325.5 (524.1) | 3212.0 (633.5) | 3317.5 (697.1) | 0.4 |
| Percentile – mean(S.D.) | 51.8 (30.4) | 52.9 (29.2) | 51.7 (29.1) | 54.2 (29.5) | 0.4 |
| SGA – % | |||||
| ≤ 10th percentile | 10.1 | 8.3 | 9.6 | 7.0 | 0.2 |
BMI, body mass index; GA, gestational age; Preterm birth, delivery < 37 weeks gestation; SGA, small for gestational age and is adjusted for race, sex and gestational age.
Uric Acid and Gestational Hypertensive Disease
Overall, 111 (7.2%) women developed gestational hypertension, 60 (3.9%) women developed preeclampsia, and 1370 (88.9%) women remained normotensive. The mean first trimester uric acid concentration for women who remained normotensive was 3.1 ± 0.8 mg/dL, which was similar to concentrations in women who developed gestational hypertension (mean 3.1 ± 0.8 mg/dL, P = .9) but significantly less than the mean first trimester uric acid concentration of women who developed preeclampsia (mean 3.4 ± 1.0 mg/dL, P = .01).
First trimester uric acid in the highest quartile compared to the lowest three quartiles was not associated with developing gestational hypertension (adjusted OR 1.12; 95% CI, 0.71 –1.78) compared to remaining normotensive, but was associated with an increased risk of developing preeclampsia (adjusted OR 1.82; 95% CI, 1.03–3.21, Table 2).
Table 2.
Development of hypertensive disease in relationship to first trimester uric acid.
| Hypertensive Status N (%) | First Trimester Uric Acid Quartile (range, mg/dL) |
P-value for trend | OR (95% CI) | |||
|---|---|---|---|---|---|---|
| 1 (0.91–2.47 ) | 2 (>2.47–2.96 ) | 3 (>2.96–3.56 ) | 4 (>3.56–8.30) | |||
| Normotensive | 348 (90.4) | 354 (91.9) | 337 (87.5) | 331 (85.8) | .1 | Referent |
| Gestational hypertension | 28 (7.3) | 19 (5.0) | 33 (8.6) | 31 (8.0) | .5 | 1.12 (0.71, 1.78) |
| H | 26 | 18 | 25 | 19 | .4 | 0.79 (0.45, 1.37) |
| HU | 2 | 1 | 8 | 12 | .002 | 3.22 (1.33, 7.76) |
| Preeclampsia | 9 (2.3) | 12 (3.1) | 15 (3.9) | 24 (6.2) | .03 | 1.82 (1.03, 3.21) |
| HP | 9 | 7 | 5 | 7 | .1 | 0.74 (0.29, 1.85) |
| HPU | 0 | 5 | 10 | 17 | < .001 | 3.65 (1.73, 7.73) |
H, hypertension; HP, hypertension + proteinuria; HU, hypertension + hyperuricemia defined as 1 SD above the mean for gestational age at diagnosis of hypertensive disease; HPU, hypertension, proteinuria + hyperuricemia.
The percentage was calculated by the number of women in the uric acid quartile for the hypertensive group divided by the total number of women in that first trimester uric acid quartile.
P-value for trend was adjusted for parity and pregestational body mass index.
OR, odds ratios, are for the risk for developing the hypertensive disorder compared to remaining normotensive if first trimester uric acid was in the highest quartile compared to the lower 3 quartiles, adjusted for parity and pregestational body mass index.
Uric Acid and Gestational Hypertensive Disease by Hyperuricemia Status
Of the 111 women who developed gestational hypertension, 23 (20.7%) had hyperuricemia at delivery (HU). For the 60 women who developed preeclampsia, 32 (53.3%) had hyperuricemia at delivery (HPU).
When comparing women who remained normotensive to those women who developed hypertensive disease by the presence or absence of hyperuricemia, the mean first trimester uric acid concentration was significantly lower in normotensive women (3.1 ± 0.8 mg/dL) compared to women who developed the hyperuricemic forms of gestational hypertension (HU, mean 3.7 ± 0.8 mg/dL, P = .03) and preeclampsia (HPU, mean 3.7 ± 0.8 mg/dL, P < .01), but not compared to women who developed gestational hypertension or preeclampsia without hyperuricemia (data not shown).
There was a significantly increasing risk for women to develop either of the hyperuricemic forms of hypertensive disease (HU or HPU) with increasing first trimester uric acid quartile (Table 2). This was not the case for women who developed the non-hyperuricemic forms of hypertensive disease (H or HP) or for women who remained normotensive. The risk of developing HU or HPU compared to women who remained normotensive was more than 3-fold higher if women had first trimester uric acid in the highest quartile (> 3.56 mg/dL) compared to the lower three quartiles (Table 2).
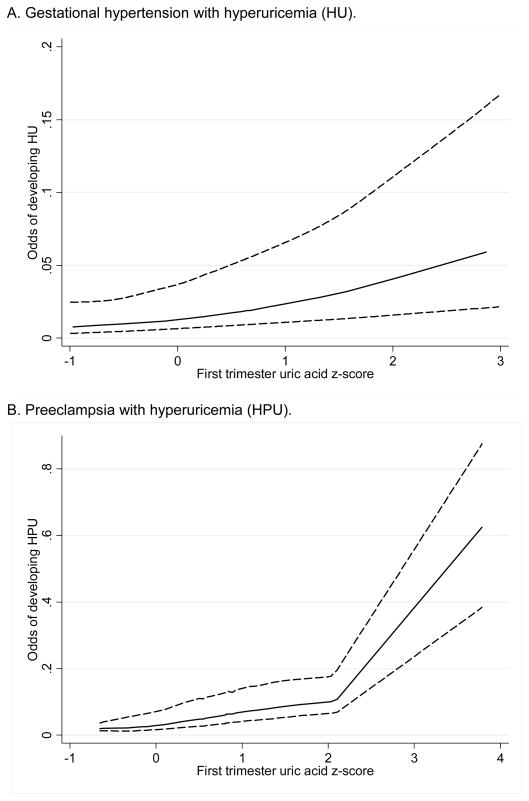
Among women with hypertension and hyperuricemia (HU or HPU), there was a positive relationship between increasing first trimester uric acid concentration (expressed as z-score) and the odds of developing hyperuricemic hypertensive disease compared to remaining normotensive (Figure 1). For each standard deviation increase in first trimester uric acid, the adjusted odds of developing HU compared to remaining normotensive increased by 1.74 (95% CI, 1.20–2.52) and the adjusted odds of developing HPU compared to remaining normotensive increased by 1.96 (95% CI, 1.42–2.71).
Figure 1. First trimester uric acid z score and the odds of developing A. hyperuricemic gestational hypertension, or B. hyperuricemic preeclampsia.
Uric acid and neonatal outcomes.
Association between first trimester uric acid z score and the odds of developing A. hyperuricemic gestational hypertension (HU); or B. hyperuricemic preeclampsia (HPU) compared to remaining normotensive, adjusted for parity and pregestational body mass index. Of note the estimates were imprecise beyond a z score of 2 in figure 2b. because there was only one data point. The solid lines indicate point estimates; the upper and lower dashed lines indicate 95% confidence bands by nonparametric smoothing with locally weighted regression (lowess).
First Trimester Uric Acid and Preterm Birth
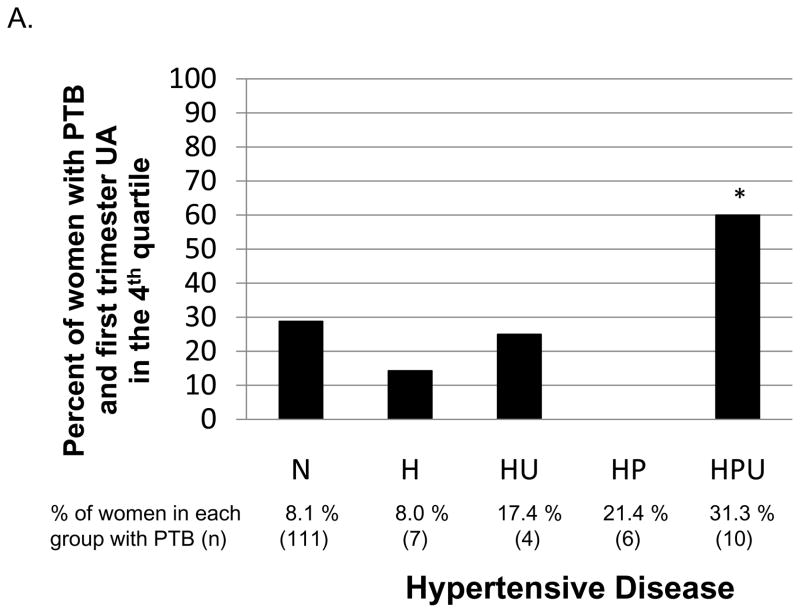
There were 138 (9.0%) births that were preterm < 37 weeks gestation. First trimester uric acid in the fourth quartile (> 3.56 mg/dL) compared to the lowest three quartiles was not associated with an increased risk of preterm birth in all women (OR, 1.25; 95% CI, 0.85–1.84), nor in women who developed gestational hypertension (H and HU) or preeclampsia (HP and HPU, OR, 0.53; 95% CI, 0.23–1.22 and OR, 0.87; 95% CI, 0.27–2.81, respectively). There were too few outcomes to investigate the association of having first trimester UA in the 4th quartile and a preterm birth for women who developed gestational hypertension or preeclampsia by hyperuricemia at delivery status (H, HU, HP or HPU). However, women who developed HPU and delivered preterm (n=10) were more likely to have a first trimester uric acid in the fourth quartile compared to women who remained normotensive and delivered preterm (n=111, Figure 2A).
Figure 2.
Figure 2A. For women who had a preterm birth, percent with first trimester uric acid concentration in the fourth quartile. The reference category is for women who remained normotensive (N). In women who developed hypertensive disease (H=gestational hypertension, HU= hyperuricemic gestational hypertension, HP= preeclampsia, or HPU= hyperuricemic preeclampsia), only women who developed HPU had a significantly higher proportion of first trimester uric acid concentration in the fourth quartile (P = .04)*.
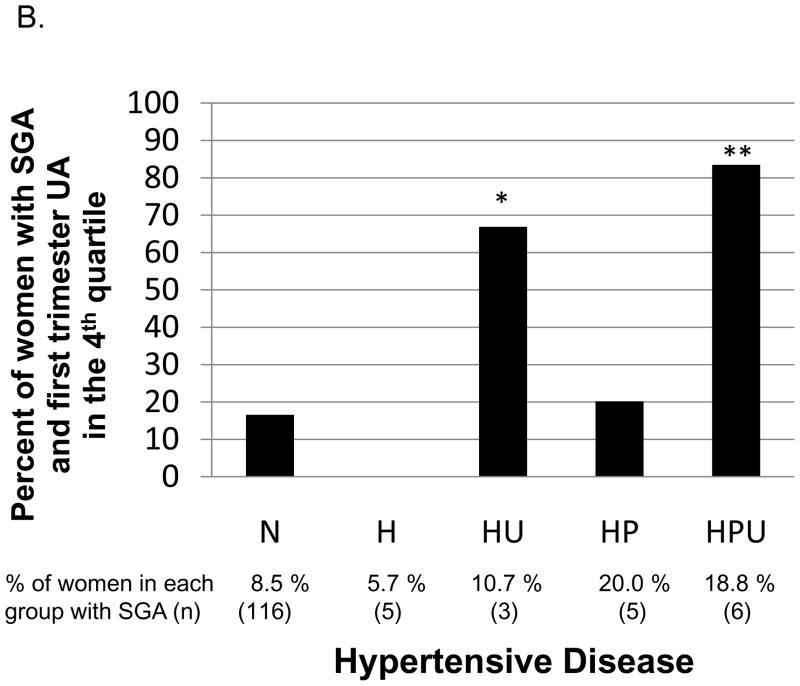
B. For women who had a small for gestational age infant, percent with first trimester uric acid concentration in the fourth quartile. The reference category is for women who remained normotensive (N). In women who developed hypertensive disease (H, HU, HP or HPU), only women who developed HU or HPU had a significantly higher proportion of first trimester uric acid concentration in the fourth quartile (P = .02*and P = .001**, respectively).
First Trimester Uric Acid and Small for Gestational Age
There were 135 (8.8%) neonates that were small for gestational age (SGA). There was no linear association between first trimester uric acid and birth weight or birth weight percentile in the entire cohort (P = .3 and P = .1, respectively). First trimester uric acid in the fourth quartile (> 3.56 mg/dL) compared to the lowest three quartiles was not associated with an increased risk of having an SGA infant in the entire cohort (OR, 0.73; 95% CI, 0.47–1.13), nor in women who developed gestational hypertension or preeclampsia (OR, 0.88; 95% CI, 0.17–4.63 and OR, 2.07; 95% CI, 0.55–7.45, respectively). There were too few outcomes to investigate the association of having first trimester UA in the 4th quartile and an SGA infant for women who developed gestational hypertension or preeclampsia by hyperuricemia at delivery status (H, HU, HP or HPU). However, women who developed HU or HPU and had an SGA infant (n=3 and n=6, respectively) were more likely to have a high first trimester uric acid compared to women who remained normotensive and had an SGA infant (n=116, Figure 2B).
First Trimester Uric Acid and Uric Acid at Delivery
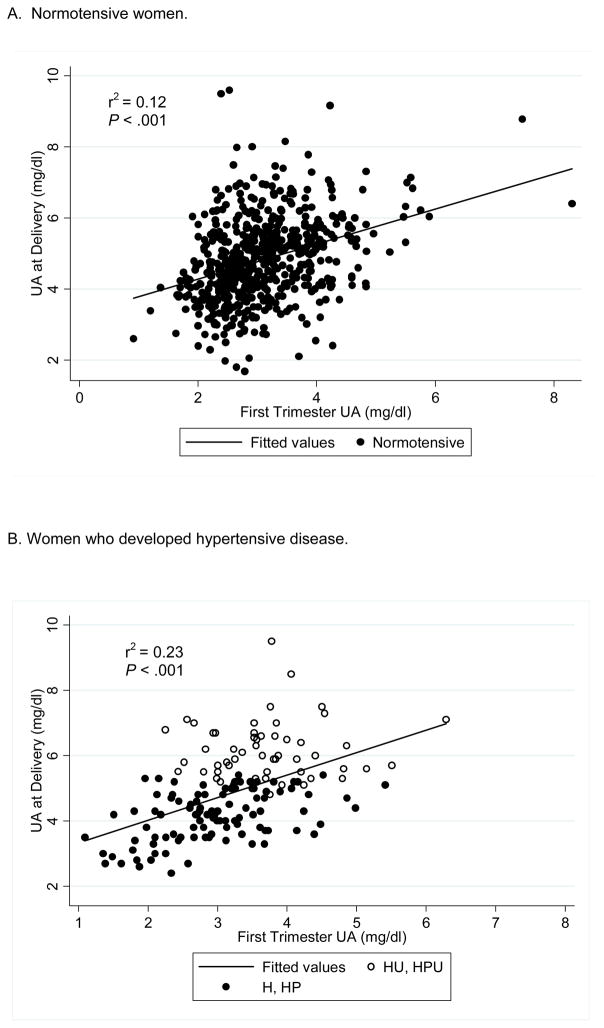
The extent to which elevated uric acid at delivery was the result of having elevated uric acid in the first trimester was determined (Figure 3). In women who remained normotensive, 12% of the variance in uric acid concentrations at delivery was explained by uric acid concentrations in the first trimester (P < .001, Figure 3A). In women who developed hypertensive disease (H, HP, HU or HPU), 23% of the variance in uric acid concentrations at delivery was explained by uric acid concentrations in the first trimester (P < .001, Figure 3B).
Figure 3. Uric acid in the first trimester compared to uric acid at delivery in women who A. remained normotensive, or B. developed hypertensive disease.
Figure 3A. In women who remained normotensive, 12% of the variance of uric acid at delivery was explained by uric acid in the first trimester; B. In women who developed hypertensive disease (H=gestational hypertension, HP= preeclampsia, HU= hyperuricemic gestational hypertension, and HPU= hyperuricemic preeclampsia), 23% of the variance of uric acid at delivery was explained by uric acid in the first trimester. The closed circles represent women who developed H or HP and the open circles represent women who developed HU or HPU.
DISCUSSION
Higher uric acid concentrations in the first trimester (> 3.56 mg/dL) were associated with an increased risk of developing preeclampsia but not gestational hypertension. Women with elevated uric acid in the first trimester had a higher risk of developing gestational hypertension and preeclampsia with hyperuricemia (HU or HPU), but not without hyperuricemia (H or HP), an important association because prior studies have found hyperuricemic forms of gestational hypertension and preeclampsia to be associated with increased risk for adverse neonatal outcomes of preterm birth and SGA.10. Despite the obvious possibility that higher uric acid concentrations in later pregnancy were merely an extension of higher uric acid in early pregnancy, most of the higher uric acid at delivery was not explained by elevated uric acid in the first trimester.
The increased glomerular filtration rate early in pregnancy is normally associated with a decrease in serum uric acid concentration. Our group previously demonstrated that uric acid concentrations are elevated across pregnancy in women who develop the hyperuricemic forms of gestational hypertension (HU) and preeclampsia (HPU) compared to controls and these elevations were not related significantly to concomitant serum creatinine concentration.4 A limitation of our study is that we did not measure serum creatinine to assess renal function; however, in women who developed HU and HPU, the uric acid concentration followed a similar curve to controls, but at higher concentrations, and had a steeper increase near the time of diagnosis. The elevated uric acid in the first trimester may represent concentrations before pregnancy, such as from metabolic syndrome or pre-hypertension, or also could be a result of poor adaptation to pregnancy (i.e. abnormal placentation). Furthermore, we have previously reported striking increases in xanthine oxidase, a synthetic enzyme for uric acid, in the cytotrophoblast and skin of preeclamptic women.11, 12
First trimester uric acid was not associated with preterm birth or SGA. The lack of association between elevated first trimester uric acid and neonatal outcomes may be due to our small numbers, but alternatively it may be explained by the different relations of uric acid to other risk factors in early pregnancy. For example, we have previously demonstrated that uric acid in mid-gestation is associated with insulin resistance and that hyperuricemia without insulin resistance is associated with decreased birth weight.13 Thus, the relationship between elevated first trimester uric acid and birth weight may be complex because of the strong association of higher uric acid with maternal BMI and insulin resistance, both of which are known to promote rather than restrict fetal growth. We were not able to assess the association of elevated first trimester uric acid and SGA or preterm birth in women with hypertensive disease by hyperuricemia status because there were too few women with the combination of these outcomes. Uric acid may simply be a biomarker for the development of hyperuricemic gestational hypertension and preeclampsia (HU, HPU), although there is mounting evidence that it may be involved in the pathogenesis of hypertensive disorders.5 In epidemiologic studies of non-pregnant adults, uric acid is an independent risk factor for developing hypertensive disease, and it is elevated prior to the development of hypertension.14–16 A causal role for uric acid in the development of hypertension is also supported by animal data. In rats, increasing uric acid by blocking uricase leads to the development of hypertension, an effect that can be reversed when an agent to either block the production or increase excretion of uric acid is given.17 In vitro, elevated uric acid decreases endothelial cell proliferation and migration, which could lead to poor placental development, and ultimately preeclampsia.5 In our studies, uric acid in vitro inhibits placental amino acid uptake, trophoblast invasion and the incorporation of trophoblast into endothelial monolayers.18, 19 These observations support an association between uric acid and the development of preeclampsia.
In conclusion, we have demonstrated that elevated serum uric acid in early pregnancy is associated with the development of preeclampsia which is consistent with the association of elevated serum uric acid with the development of hypertension outside of pregnancy.1 While first trimester uric acid concentration may not be useful as a clinical predictor, it potentially could be used in combination with other clinical and biochemical factors to improve prediction of preeclampsia, and also as a screening tool to enrich the sample of preeclampsia in research studies. Our findings also support the concept of preeclampsia as a heterogeneous syndrome since first trimester uric acid was associated with hyperuricemic forms of hypertensive disease, HU and HPU, but not the less serious non-hyperuricemic forms of hypertensive disease, H and HP. Perhaps gestational hypertension and preeclampsia with hyperuricemia are separate disease processes with different etiologies than gestational hypertension and preeclampsia without hyperuricemia.20 Long term cohort studies are needed to investigate whether women with hyperuricemia early in pregnancy are at risk for developing both hypertensive disease with more adverse outcomes during pregnancy and diseases in their lifetime including hypertension, metabolic disease, diabetes and cardiovascular disease.1
Acknowledgments
Sources of Funding:
NIH P01 HD030367, Pregnancy Exposures and Preeclampsia Prevention Study, Magee-Womens Hospital
UL1 RR024153, Clinical Translational Research Center funded by the University of Pittsburgh Clinical and Translational Science Award
Footnotes
Conflict of interest statement: We have no conflicts of interest to disclose.
References
- 1.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. The New England journal of medicine. 2008;359(17):1811–21. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sibai BM, Gordon T, Thom E, Caritis SN, Klebanoff M, McNellis D, Paul RH. Risk factors for preeclampsia in healthy nulliparous women: a prospective multicenter study. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. American journal of obstetrics and gynecology. 1995;172(2 Pt 1):642–8. doi: 10.1016/0002-9378(95)90586-3. [DOI] [PubMed] [Google Scholar]
- 3.Johnson RJ, Segal MS, Srinivas T, Ejaz A, Mu W, Roncal C, Sanchez-Lozada LG, Gersch M, Rodriguez-Iturbe B, Kang DH, Acosta JH. Essential hypertension, progressive renal disease, and uric acid: a pathogenetic link? J Am Soc Nephrol. 2005;16(7):1909–19. doi: 10.1681/ASN.2005010063. [DOI] [PubMed] [Google Scholar]
- 4.Powers RW, Bodnar LM, Ness RB, Cooper KM, Gallaher MJ, Frank MP, Daftary AR, Roberts JM. Uric acid concentrations in early pregnancy among preeclamptic women with gestational hyperuricemia at delivery. American journal of obstetrics and gynecology. 2006;194(1):160. doi: 10.1016/j.ajog.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 5.Bainbridge SA, Roberts JM. Uric acid as a pathogenic factor in preeclampsia. Placenta. 2008;29 (Suppl A):S67–72. doi: 10.1016/j.placenta.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lind T, Godfrey KA, Otun H, Philips PR. Changes in serum uric acid concentrations during normal pregnancy. British journal of obstetrics and gynaecology. 1984;91(2):128–32. doi: 10.1111/j.1471-0528.1984.tb05895.x. [DOI] [PubMed] [Google Scholar]
- 7.Winocour PH, Taylor RJ. Early alterations of renal function in insulin-dependent diabetic pregnancies and their importance in predicting pre-eclamptic toxaemia. Diabetes Res. 1989;10(4):159–64. [PubMed] [Google Scholar]
- 8.de Jong CL, Paarlberg KM, van Geijn HP, Schipper EJ, Bast A, Kostense PJ, Dekker GA. Decreased first trimester uric acid production in future preeclamptic patients. J Perinat Med. 1997;25(4):347–52. doi: 10.1515/jpme.1997.25.4.347. [DOI] [PubMed] [Google Scholar]
- 9.Laughon SK, Catov J, Provins T, Roberts JM, Gandley RE. Elevated first-trimester uric acid concentrations are associated with the development of gestational diabetes. American journal of obstetrics and gynecology. 2009;201(4):402, e1–5. doi: 10.1016/j.ajog.2009.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts JM, Bodnar LM, Lain KY, Hubel CA, Markovic N, Ness RB, Powers RW. Uric acid is as important as proteinuria in identifying fetal risk in women with gestational hypertension. Hypertension. 2005;46(6):1263–9. doi: 10.1161/01.HYP.0000188703.27002.14. [DOI] [PubMed] [Google Scholar]
- 11.Many A, Hubel CA, Fisher SJ, Roberts JM, Zhou Y. Invasive cytotrophoblasts manifest evidence of oxidative stress in preeclampsia. Am J Pathol. 2000;156(1):321–31. doi: 10.1016/S0002-9440(10)64733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bainbridge SA, Deng JS, Poberts JM. Increased Xanthine Oxidase in the Skin of Preeclamptic Women. Reproductive Sciences. 2009;16(5):468–78. doi: 10.1177/1933719108329817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laughon SK, Catov J, Roberts JM. Uric acid concentrations are associated with insulin resistance and birthweight in normotensive pregnant women. American journal of obstetrics and gynecology. 2009;201(6):582, e1–6. doi: 10.1016/j.ajog.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyer AR, Liu K, Walsh M, Kiefe C, Jacobs DR, Jr, Bild DE. Ten-year incidence of elevated blood pressure and its predictors: the CARDIA study. Coronary Artery Risk Development in (Young) Adults. J Hum Hypertens. 1999;13(1):13–21. doi: 10.1038/sj.jhh.1000740. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan E, Kwoh CK, Schumacher HR, Kuller L. Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension. 2007;49(2):298–303. doi: 10.1161/01.HYP.0000254480.64564.b6. [DOI] [PubMed] [Google Scholar]
- 16.Mellen PB, Bleyer AJ, Erlinger TP, Evans GW, Nieto FJ, Wagenknecht LE, Wofford MR, Herrington DM. Serum uric acid predicts incident hypertension in a biethnic cohort: the atherosclerosis risk in communities study. Hypertension. 2006;48(6):1037–42. doi: 10.1161/01.HYP.0000249768.26560.66. [DOI] [PubMed] [Google Scholar]
- 17.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38(5):1101–6. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 18.Bainbridge SA, von Versen-Hoynck F, Roberts JM. Uric acid inhibits placental system A amino acid uptake. Placenta. 2009;30(2):195–200. doi: 10.1016/j.placenta.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bainbridge SA, Roberts JM, von Versen-Höynck F, Koch J, Hubel CA. Uric Acid Attenuates Trophoblast Invasion and Integration into Endothelial Cell Monolayer. American Journal of Physiology. 2009 doi: 10.1152/ajpcell.00593.2008. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ness RB, Roberts JM. Heterogeneous causes constituting the single syndrome of preeclampsia: a hypothesis and its implications. American journal of obstetrics and gynecology. 1996;175(5):1365–70. doi: 10.1016/s0002-9378(96)70056-x. [DOI] [PubMed] [Google Scholar]