Abstract
The NIMH's new strategic plan, with its emphasis on the “4P's” (Prediction, Preemption, Personalization, & Populations) and biomarker-based medicine requires a radical shift in animal modeling methodology. In particular 4P's models will be non-determinant (i.e. disease severity will depend on secondary environmental and genetic factors); and validated by reverse-translation of animal homologues to human biomarkers. A powerful consequence of the biomarker approach is that different closely-related disorders have a unique fingerprint of biomarkers. Animals can be validated as a highly-specific model of a single disorder by matching this `fingerprint'; or as a model of a symptom seen in multiple disorders by matching common biomarkers.
Here we illustrate this approach with two Abnormal Repetitive Behaviors (ARBs) in mice: stereotypies; and barbering (hair pulling). We developed animal versions of the neuropsychological biomarkers that distinguish human ARBs, and tested the fingerprint of the different mouse ARBs. As predicted, the two mouse ARBs were associated with different biomarkers. Both barbering and stereotypy could be discounted as models of OCD (even though they are widely used as such), due to the absence of limbic biomarkers which are characteristic of OCD and hence are necessary for a valid model. Conversely barbering matched the fingerprint of trichotillomania (i.e. selective deficits in set-shifting), suggesting it may be a highly specific model of this disorder. In contrast stereotypies were correlated only with a biomarker (deficits in response shifting) correlated with stereotypies in multiple disorders, suggesting that animal stereotypies model stereotypies in multiple disorders.
Keywords: Autism, OCD, Trichotillomania, Stereotypy, Perservation, Executive, Prefrontal Cortex, Orbitofrontal Cortex, Basal ganglia
1. Introduction
The NIMH's new strategic plan, with its emphasis on Predicting at-risk individuals, Preemption of disease development, Personalized medicine, and involvement of diverse Populations (the “4P's”), and consequently on biomarker-based medicine [55], calls for a radical shift in animal modeling methodology. The biomarker-based approach views disease not as a static final symptomatic physiology predetermined by `genes for' a disease, but as a progression of empirically observable developmental bottlenecks and points of divergence (the biomarkers), with increasing environmental influence, decreasing genetic penetrance, and increasing specificity to the final symptomatology [36]. The best biomarkers are both measurable and manipulable – thus they provide the theoretical and empirical basis to Predict, Preempt, and Personalize medicine, and a fundamental translational connection between bench research and bedside treatment.
Traditional animal models usually involve a determinant manipulation of physiology (e.g. genetic, drug or surgical lesions), which may not be directly involved in the typical development of the human disease, and which may override pertinent environmental influences. Therefore, contrary to most traditional models, Biomarker-based 4P's models have three requirements. First, they will be non-determinant and multifactorial – 4P's research requires variation in symptom severity, development, or phenomenology in response to environmental and genetic risk factors. Second, they will be validated by reverse translation – they will show the same specific complex multifactorial epidemiology, and the same specific biomarkers as the human disease. Third, they will employ experimental designs which investigate or control individual variation in response to environmental and biological variability, such as epidemiological (e.g. [34]) and matched-pair (e.g. [31,64,65]) designs.
The need for a change in approach is evident in the lack of predictive validity of traditional models – approximately 8% of CNS drugs succeed in human trials, and approximately 80–90% of these failures are due to lack of efficacy in humans [40]. This 80–90% false positive rate of traditional animal models is arguably the major financial burden in drug discovery, as the number of drugs being approved is insufficient to meet the cost of future development or growth [40]. Thus industry, as well as the NIMH, has argued the need for new methods and models that prioritize specificity (the proportion of negatives correctly identified), not sensitivity (the proportion of positives correctly identified) [16,41]. Reasons for false-positives and the over-sensitivity of models in behavioral neuroscience are discussed elsewhere (e.g. [21,35,65,78]). Here we illustrate the utility of a biomarker-based 4P's approach to validate the specificity of spontaneously occurring Abnormal Repetitive Behaviors (ARB)s in mice as models of distinct and differentially diagnosed symptoms in humans.
ARBs are behaviors that are: (i) heavily repeated; (ii) invariant in motor output, environmental interaction, goal, or topic; and (iii) functionless, maladaptive, self-injurious, or inappropriate [26,44,50,73,74]. ARB covers a diverse symptomatology in humans, and occurs in many disorders. For instance `stereotypies' (inappropriate, identical and unvarying, non-goal directed, repeated motor patterns) and compulsive behaviors (flexible and varying goal-directed behavior directed at an inappropriate and excessively repeated goal) are mutually exclusive categories of ARB both within (e.g. autism [44,73]) and between disorders (e.g. stereotypy precludes a diagnosis of OCD [7])
ARB in animals is equally diverse and heterogeneous. However there is little consensus on categorizing these behaviors, or their relationships to human symptoms (e.g. [26,49,52]). This confusion is well illustrated in the use of mouse stereotypies (e.g. jumping and bar-mouthing [80]) and barbering (fur and whisker plucking [33]) as models in behavioral neuroscience. Barbering has been used as a model of OCD [39] and trichotillomania (TTM) [34] (which are very distinct neurobiologically [13]), and as a sign of normal social interaction [45] (which is inconsistent with other interpretations, inconsistent with wild mouse behavior, and refuted by papers testing this hypothesis [27,75]). Similarly stereotypies have been used as models of stereotypies (e.g. in autism [44]), and of OCD [17]. One or both of these interepretations must be false, because one behavior in animals cannot possibly be a model for two mutually exclusive symptoms in humans. The consequence of using the wrong behavior to model the wrong disorder is that any positive result will automatically be a false positive.
We therefore set out to test whether animal ARB can be subdivided into the same basic categories as in humans using the reverse translation of biomarkers unique to each form of ARB in humans. Our experiments were informed by the dissociations of these categories within autism [73] and between OCD and TTM [12,13] using neuropsychological biomarkers; and by the emerging consensus that the natural history of ARBs stems from the biology of behavioral organization [1,5,8,18,25,26,56,73].
Although the different behavioral disciplines may differ in terminology, each describes a hierarchy of behavioral organization with four basic layers: motivations; goals; motor patterns; and muscle movements (Table 1). Functional behavior involves both selection and inhibition at each level, and co-ordination between levels. For instance, for reproductive motivation to be selected, other motivations (e.g. feeding) must be inhibited. Then each goals must be selected in the correct order (e.g. there is no point in displaying to a mate before one has been found) before particular motor programs within each goal are enacted. Within each motor program every muscle movement must be coordinated in turn. Although control further down the hierarchy is directed by selections higher up, processing continues in higher levels as lower aspects of behavior are executed. Thus, motor patterns can be interrupted by a higher change in motivation (e.g. if a predator appears – stop eating and run away) [38,56]. Successful behavioral transitions require a balance between inhibition and selection. Thus disinhibition, over-activation, or alterations in hysteresis potentially predispose the system to inappropriate, repetitive or chaotic behavioral transitions [2,8,9,26,48,53,56].
Table 1. Elements of behavior, corticostriatal loops, symptoms, and biomarkers.
The rationale and results of the current experiments are summarized. Each of the classic levels of behavioral organization is linked to a discrete corticostriatal loop. Symptoms arising from either pre-emptive or post-emptive disinhibtion are proposed. The biomarkers of post-emptive disinhibition used in this study are detailed, and the fingerprint characteristic of the three disorders discussed (OCD, autism and TTM) are detailed. Two additional levels of organization (individual muscle movements, and eye movements) are included for a complete overview, although they are not investigated in this study. This table summarises hypotheses and results from [1,10,15,22,23,26,47,49,63,70,73]. Affective shifting and Set shifting have been validated as specific biomarkers of the limbic and prefrontal loops respectively using imaging or lesions studies in multiple species, including humans [19], primates [22] and mice [11]. We are not aware of any studies identifying a substrate for Response shifting.
| Element in the behavioral hierarchy | Emotions / motivations | Goals / Goal directed behavior | Motor programs / Fixed action patterns | Muscle movements | Eye movements |
|---|---|---|---|---|---|
| Cortico-striatal loop indicated by lesion studies | OFC (limbic) loop | DLPFC loop | Premotor branch of the motor loop | Primary motor branch of the motor loop | SEF loop |
| Failure to inhibit an inappropriate element before it begins (pre-emptive disinhibition) | Inappropriately elicited motivations; Labile fleeting emotional states (e.g. bursts of anger in intermittent explosive disorder) | Inappropriate single executions of goal directed behavior – i.e. impulsive behaviors. E.g. the setting of a fire in pyromania | Inappropriate single responses (e.g. stimulus bound behaviors; automatic touching; echopraxia, echolalia). | Inappropriate single muscle movements (e.g. some simple tics in GTS). | Single inappropriate saccades. |
| Failure to inhibit an ongoing element when completed (post-emptive disinhibition) | Failure of satiation; Persistent repetitive emotional states (e.g. obsessions). | Repetitive persistence of goals once they have been met (e.g. compulsive behaviors; circumscribed interests). | Inappropriate repetitive identical patterns of motor output – i.e. stereotypies. | Inappropriate repetition of individual muscle movements. | Continuous eye movements (e.g. eye rolling stereotypies) |
| Neuropsychological biomarker (post-emptive disinhibition) | Affective shifting IDED reversals | Set shifting IDED EDS | Response shifting Bias-corrected two-choice gambling | ||
|
| |||||
| OCD Fingerprint | Impaired | Impaired | |||
| Autism Fingerprint | Impaired | Impaired | |||
| TTM Fingerprint | Impaired | ||||
The control of behavior is effected by a series of corticostriatal loops [4–6,66] which are conserved throughout the vertebrates [62,72]. Each loop is comprised of a “direct pathway” that activates behavior, and an “indirect pathway” that inhibits it [51,61]. Separate loops serve separate functions, and each of the fundamental layers of hierarchy in behavioral control appears to be subserved by a different loop [51,61] (Table 1). Accordingly, each loop can be associated with a neuropsychological deficit or neurological sign, some of which have associated lesion-validated tasks (e.g. [22]) and are potential biomarkers [36]. The corticostriatal loops have been generally implicated in ARBs across disorders [1,3,8,18,20,43,53,67,71]. However, dysfunction in different loops may be responsible for fundamentally distinct symptoms [1,5,8,18,25,26,73]. While authors may differ on fine details, the consistent theme in these accounts is that ARB is the result of disinhibition of the indirect pathway, and the form of repetition seen in spontaneous symptoms reflects the component of behavior each loop sequences [1,8,18,25,26,69,73]. (Although see [9] for an alternative hypothesis).
Inhibition acts either: 1) to prevent behavioral elements from beginning, thus suppressing behavioral elements that are out of context (e.g. performing a feeding goal during mating behavior) or out of sequence; or 2) to terminate behavioral elements, either to hand over control to the next element in a sequence, or to allow early termination of the hierarchy (e.g. terminating feeding to initiate escape). Thus we view inappropriate or out-of-context single elements (e.g. explosive rage at the motivational level, or echopraxia at the motor program level) as failures of pre-emptive inhibition; and excessive repetition of elements when they should be terminated (e.g. compulsive behaviors at the goal level, or stereotypies at the motor program level) as failures of postemptive inhibition. Therefore these hypotheses (Table 1) are easily tested by relating specific ARBs to neuropsychological tasks that assess disinhibition at different levels of behavioral control.
Although the majority of ARBs involve some degree of both pre- and postemptive disinhibition, relevant ARB research has mostly focused on measures of postemptive inhibition, particularly affective-, set-, or response- shifting neuropsychological tasks. Similarly, the majority of experiments typically assess broad measures of inhibitory function against overarching scores of ARB severity. While these studies consistently find relationships between ARB and disinhibited behavioral control [26] (e.g. in autism [46,59], TTM [70]), fine-grained analyses of different types of ARB against different types of shifting deficit are rare. Nevertheless examples can be found. In autism for instance, as we predict, stereotypies correlate with impaired response-shifting; compulsive behaviors correlate with set-shifting deficits; and these relationships are partially dissociated [73,74]. Similarly, comparisons of TTM (compulsive hair pulling) and OCD typically find evidence of limbic dysfunction (including affective shifting) in OCD but not TTM (e.g. [12,13]), which we would predict as obsessions are fundamentally affective in nature, but plucking in TTM is associated with somatosensory rather than affective experiences [77].
Thus shifting deficits serve as biomarkers of different forms of ARB, regardless of disorder, and different disorders are associated with different combinations or `fingerprints' of these biomarkers, reflective of their symptomatology. Accordingly, we tested the hypotheses that: stereotypies in mice are biologically equivalent to stereotypies in schizophrenia and autism, and thus will show only response-shifting deficits; that barbering in mice is dissociable and biologically equivalent to compulsive behavior in autism, TTM and other disorders, and thus will show set-shifting deficits; and that neither behavior is biologically equivalent to obsessions in OCD, and thus neither will show affective shifting deficits.
2. Materials and methods
2.1. Subjects
Subjects were C57BL/6 mice, bred from C57BL/6J progenitors. To test our predictions in a range of mice, and to avoid unnecessarily breeding mice, we selected a heterogeneous set of mice from our colony, that varied in sex (11 females, 13 males; 10 cages total), age (5 to 15 months), housing density (1–4 mice per cage), and body weight. We then accounted for this variability in our analyses by blocking by cage (as mice from the same cage share these variables in common, cage acts like a matched pair). We therefore avoided the risk of false positive or negative results due to the particular arbitrary environment of our lab [64,65,78] and instead tested our hypotheses in a wide population. All procedures were approved by our Institutional Animal Care and Use Committee.
2.2. Experimental design
The ARBs we examined develop spontaneously in mice. We examined our experimental population twice. First we tested for the predicted correlations between ARB and affective- and set-shifting deficits using the IDED task [31,73]; then we tested for the predicted correlations ARB and response-shifting deficits using the bias-corrected two-choice gambling task [30,73]. We used the same mice in both experiments in order to test whether these classes of ARB were dissociable.
2.3. Abnormal Repetitive Behaviors
2.3.1. Barbering (Compulsive Behavior)
The most common ARB in mice that meets the definition of compulsive behavior (flexible goal-directed behaviors pursuing an inappropriately repeated goal [26]) is `barbering'. Barbering is particularly common in the C57BL/6 strain [34]. However, only some individuals in a cage barber, and importantly barbering is unrelated to social dominance [27]. The behavior is extremely goal-directed, and flexible in form and orientation [68], and as such meets the criteria for compulsive behavior, but not stereotypy [26]. Spontaneous excessive plucking in several other species, [14,60], and excessive plucking in genetically manipulated mice (e.g. [37,39]), have been proposed as homologues to TTM or OCD; and barbering does indeed show many epidemiological and phenomenological parallels to focused plucking in TTM [26,34]. Mice at least 5 months old were used because barbering develops post-puberty [27,34].
Seven barbers were identified at the time of performing the IDED task following methods described in [34] - these mice are considered barbers in the categorical analyses shown in Fig 2. However two more mice subsequently developed the behavior. We therefore adopted a second more sensitive measure, where the severity of barbering was quantified as the mean percentage of body area denuded from cagemates. Following [34], hair loss was drawn for each mouse in the cage on a standardized mouse body map, and the proportion of body area denuded by each barbering mouse calculated by custom written software [34]. Attributing barbering scores requires the comparison of hair loss patterns within a cage at a single timepoint. Therefore to ensure a fair comparison between cagemates, barbering was quantified at set dates for all cages showing hair loss, and the date following completion of the IDED task by all the mice in the cage was used. Nine mice were assigned barbering scores.
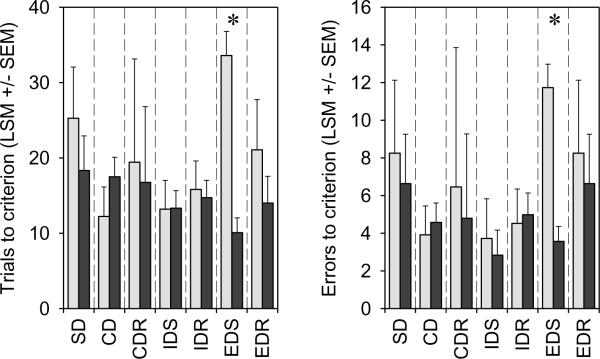
Fig. 2. Barbers show set-shifting deficits in the IDED task compared with control mice.
Barbers (light grey bars); Control mice (dark grey bars). Set-shifting deficits are measured by the number of trials, or the number of errors, taken to complete the ExtraDimensional Shift (EDS) stage of the task. Barbers were unimpaired on any of the other stages of the task, which measure other learning and executive processes including compound learning ability, error detection, and affective shifts [22] [15]; indicating an extremely specific impairment. Note that each stage of the task was analyzed separately so that each stage was partialled only for stages proceeding it in the task (hence the dashed lines separating stages on the graph). Both panels show LSM ± SE, and * = significant at p <0.005. Neither analysis was transformed.
2.3.2. Stereotypies
Following completion of the gambling task by all mice in the cage, stereotypy was quantified following methods described in [29,80], and confirmed in pilot work. We separated cage mates, allowed three days acclimatization, recorded the first two hours of night-time behavior using infra-red video. These videos were scored using one-zero recording of 30 second intervals, for inactivity, general activity, maintenance behaviors, and stereotypies (bar mouthing, route tracing, twirling, and jumping; for detailed descriptions see [29,80]). As described in [29] each 30 interval was coded as containing stereotypy (by the presence of any stereotypy) or not, and as active (by the presence of any behavior other than inactive) or not. Stereotypy was then quantified as the proportion of active intervals in which stereotypy was observed.
2.4. Neuropsychological biomarkers
2.4.1. IDED task
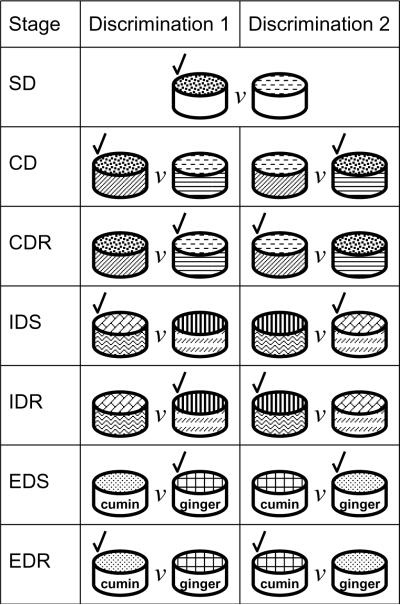
We worked with each cage in turn, performing the IDED task following [31], which was originally modified from a rat paradigm described in [10]. Mice were first feed deprived to 85–90% of their ad libitum feeding weights, and trained to dig in metal bowls for food reward (¼ of a honey-nut Cheerio) in a U-maze [31]. Each mouse was then presented with a series of discriminations (stages) where the outer texture, the inner digging medium and the odor of the cups were varied to cue reward (Fig. 1). The criterion for completing each stage was 8/10 consecutive trials correct. Sessions continued for 1 hour or until mice showed off-task behaviors (at which point further data becomes meaningless) [31].
Fig. 1. An example of the IDED task.
For full details see [22,31,58]. Digging cups are depicted varying in either outer texture, inner digging medium, or odor. In each stage two possible discriminations were presented at random on any trial, representing the possible combinations of the two stimulus dimensions varied in that stage. In any stage only one dimension cued reward, and one stimulus within the dimension indicated the correct choice (indicated by a tick mark). SD, Simple Discrimination; CD, Compound Discrimination; CDR Compound Discrimination Reversal; IDS, IntraDimensional Shift; IDR, IntraDimensional Reversal; EDS, ExtraDimensional Shift; EDR, ExtraDimensional Reversal. The consistent cuing of reward in a single dimension through stages SD-IDR leads to the formation of an abstract rule, or `set', that must be overcome to solve the EDS stage. Set-shifting deficits are apparent as a selective impairment in completing the EDS stage. Affective shifting deficits are apparent as poor performance on reversal stages.
In the first 5 stages (Simple discrimination, Compound discrimination, Compound Discrimination Reversal, IntraDimensional shift, IntraDimensional reversal) one dimension (e.g. odor) cued reward, one dimension varied at random (e.g. texture), and one remained constant (Fig. 1). This forms an abstract rule, termed a `set', whereby the subject generalizes across each successive task focusing their attention on this one dimension. Set formation is confirmed by the IDS stage [22,31,58,73]. In the sixth stage (EDS, ExtraDimensional Shift) the previously random dimension remained constant, the previously constant dimension cued reward, and the previously cuing dimension varied randomly. Impaired set-shifting is apparent as persistent attending to the previously cuing dimension, measured simply as the number of trials to criterion or the number of errors to criterion on the EDS stage [22,31,58,73]. EDS is followed by a final reversal. Conversely, each reversal stage represents an affective shift. In primates [22] OFC versus DLPFC lesions uniquely impair reversal versus EDS stages respectively. The same double dissociation is seen in rats [15] and mice [11].
2.4.2. Bias corrected two choice gambling task
Following completion of the IDED task for all cages we then performed the bias corrected two choice gambling task with each cage in turn, adapting [30]. The `bias correction' refinement of the task ensures proper measurement of response-shifting, and avoids complications that can otherwise arise in animals [30]. Three mice died in between the experiments. Mice were again feed deprived to 85–90% of their ad libitum feeding weights. The task was performed in a T-maze. Cups were placed in either arm of the maze, each containing three honey-nut Cheerios under a wire screen and 1 inch of wood shavings. A ¼ Cheerio reward was placed above the wire screen in one of the cups at the start of each trial, such that each cup was rewarded with a probability = the proportion of responses to the other cup in the previous twenty trials. This randomization procedure eliminates side biases that can confound this paradigm [30]. One hundred trials were performed with each mouse over a series of sessions. Thus although reward is unpredictable, maintaining a 50:50 choice ratio between the two arms of the maze will maximize reward. The mouse can do so by generating either a random or patterned sequence of responses. Repetitive patterned sequences indicate impaired response-shifting. The unpredictable location of the reward, and adjusting reward probabilities trial-by-trial (rather than session-by-session) avoids superstitious conditioning of alternative strategies, such as spontaneous alternation, or session-cued reversal.
The sequence of responses was analyzed using 3rd order Markov Chain analysis, which yields the probability of sequential independence (i.e. 0 = a completely predictable sequence; 1 = a completely random sequence). Impaired response shifting was calculated as the (1 – the probability of sequential independence) = probability of sequential non-independence, (i.e. the probability that the sequence was not generated at random); and logit transformed to yield an unbounded variable suitable for regression analysis.
In both tasks, by terminating the session at the onset of off-task behaviors we ensured that neither stereotypy nor barbering behavior interfered with either task (and indeed, none was observed in the apparatus).
2.5. Statistical analysis
All analyses were performed using GLM in Minitab 14 for Windows. Assumptions of GLM (normality of error, homogeneity of variance, linearity) were confirmed graphically post hoc, and transformations (specified in figure legends) applied as needed. All analyses were blocked by cage nested within sex to control for confounds of sex, age, parentage, etc. [31,64,65].
Approximately half the mice in this experiment were overtrained between IDR and EDS as part of a separate experiment. Overtraining was independent of barbering status. Nevertheless overtraining status was included in all IDED analyses to eliminate any effect of this variable. To test for effects of barbering status on task performance, barbering status was included as an independent variable, each task stage was examined in turn as the dependent variable, and the mouse's scores on proceeding stages of the task were included to eliminate confounding processes measured by these stages [31]. Barbering severity and stereotypy were correlated with IDED task performance using similar procedures.
Barbering severity and stereotypy were correlated with response-shifting in GLMs blocked by cage nested within sex. The total number of trials of previous task experience was included as a controlling variable, as was the mouse's performance on IDED simple and compound discrimination, to control for general learning abilities. These latter three variables were not necessary for significance, but improved the fit of the model.
Stereotypy and barbering severity, and stereotypy and barbering status, were compared using GLMs blocked by cage and sex, and the same controlling variables as the corresponding analyses above. Because barbering and stereotypy are both quantitative variables, these analyses avoided the loss of power inherent in treating them as qualitative (e.g. if we had formed high-and-low groups of mice for each behavior). Type III SS are used throughout to ensure that all results generalize across the other factors in the model.
3. Results
3.1. Compulsive Behavior
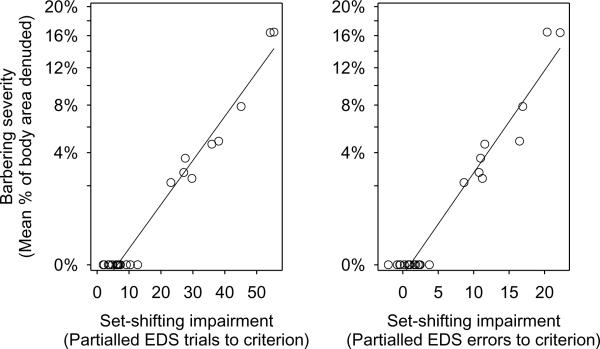
As predicted, barbers were impaired on the EDS stage compared with non-barbering cage-mates, showing impaired set-shifting measured either as trials (GLM: F1,7 = 24.49; p = 0.002) taken to complete the stage, or as errors made on the stage (GLM: F1,7 = 19.20; p = 0.003) (Fig. 2). There were no significant differences between barbers and non-barbers when we repeated identical analyses for the six other stages of the task (Fig. 2). Furthermore, the severity of barbering behavior was correlated with the degree of impaired set-shifting, measured as trials (GLM: partial r = 0.85; F1,7 = 18.7; p = 0.004) or errors (GLM: partial r = 0.73; F1,7 = 8.04; p = 0.025) taken to complete the EDS stage (Fig. 3). The severity of barbering behavior was uncorrelated with performance on the other stages of the task, with the exception of Compound Discrimination Reversal (stage CDR, Fig. 1), where the more severe barbers completed this stage the quickest. However barbers and non-barbers do not differ on CDR, suggesting that a tendency to persist in-set may aid severe barbers in making within-set reversals.
Fig. 3. Set-shifting deficits predict barbering severity.
The severity of barbering behavior, measured as the mean percentage of body area denuded from cage mates correlates with the severity of set-shifting deficit measured by the number of trials, or the number of errors, taken to complete the ExtraDimensional Shift (EDS) stage of the IDED task. With the exception of Compound Discrimination Reversal (CDR), which was negatively correlated, no other stage of the IDED task was correlated with barbering severity. EDS score is partialled for the other variables in the regression model. Barbering severity is angular transformed in both analyses.
3.2. Stereotypies
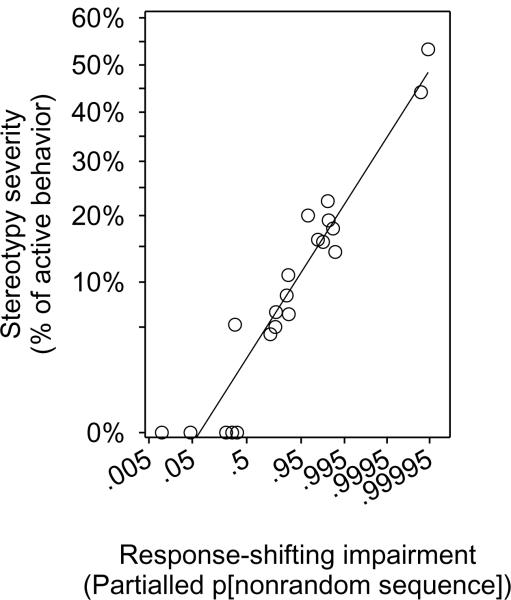
As predicted, the severity of stereotypy was correlated with impaired response-shifting (GLM: partial r = 0.84; F1,8 = 18.78; p = 0.003) (Fig. 4).
Fig. 4. Response-shifting deficits predict stereotypy.
The severity of stereotypy, measured as the mean proportion of active behavior spent stereotyping during the first two hours of the night, was positively correlated with the severity of response-shifting deficit, measured as the probability of non-random sequence generation in the bias-corrected two choice gambling task. The severity of response-shifting deficit was logit transformed so that partialling by blocking variables would not yield impossible values. Stereotypy was angular transformed.
3.3. Neuropsychological biomarkers classify mouse ARB by their fingerprint
Because these experiments were performed in the same animals we were able to confirm the double dissociation between the two ARBs and their related neuropsychological biomarkers. Thus, as predicted the two forms of ARB, and their associated biomarkers were doubly dissociated. Stereotypy severity did not differ between barbers and controls (GLM: F1,10 = 0.04: p =0.848) and stereotypy and barbering severity were uncorrelated (GLM: F1,10 = 1.00; p = 0.341). Response-shifting did not differ between barbers and controls (GLM: F1,8 = 0.32; p = 0.587), and barbering severity was uncorrelated with response-shifting (GLM: F1,8 = 0.85; p = 0.383). Stereotypy was uncorrelated with set-shifting measured as trials (GLM: F1,5 = 0.30; p = 0.609) or errors to criterion (GLM: F1,5 = 0.85; p = 0.798), or any other stage of the task measured as either trials or errors to criterion. Thus each class of ARB was uniquely related to a single neuropsychological biomarker (Table 1).
4. Discussion
These data confirm and extend previous results suggesting that animal and human stereotypies share similar mechanisms [26,28–30,76] by showing that: 1) compulsive behaviors are distinct from stereotypies in mice; and 2) that like humans, stereotypies are related to impaired response-shifting; while compulsive behaviors are related to impaired set-shifting. Given the specific involvement of the limbic cortico-striatal loop in OCD, a necessary (but not sufficient) feature of an OCD model will be biomarkers of OFC deficits (such as reversal learning). Thus the lack of reversal learning deficits correlated with either ARB suggests that: 1) both ARBs may not be associated with driving affective states or obsessions; 2) and that while barbering shows construct validity as a model of TTM and other OCSD involving set-shifting deficits, barbering does not model OCD per se (e.g. [39]).
Although difficult to validate, a spontaneous model (such as barbering or stereotypy) is generally far more powerful than an induced model (such as a genetically manipulated or drug-treated animal) for investigating gene-by-environment interactions, endophenotypes, biomarkers, and other complex developmental processes critical to the 4P's approach [34]. Induced models inevitably tell us little about the etiologies of the majority of human patients that do not share the model's etiology, while spontaneous models represent a breakthrough for disorders characterized by complex heterogeneous etiologies and distinct clinical subpopulations (such as autism, OCD and TTM). Our data illustrate that reverse translation of biomarkers or endophenotypes can validate spontaneous models of abnormal behavior, as proposed by other authors [36]. Indeed, the precision with which both barbering and stereotypy were each associated with a single neuropsychological biomarker represents a level of discriminant validity that exceeds current animal models of ARB, and rare in animal models of abnormal human behavior in general. These behaviors illustrate both possible outcomes of validation by reverse translation. In the case of barbering its epidemiology [34] and biomarker fingerprint is highly specific to TTM. Conversely, in the case of stereotypy its association with response shifting is not specific to a single disorder, but is specific to stereotypies seen in multiple disorders (e.g. autism and schizophrenia), providing a model of the symptom rather than and single disorder.
Comparison of barbering with the Hoxb8 knockout mouse [37] as models of TTM illustrates these points well. Hoxb8 knockouts exhibit an extreme barbering phenotype that is unlike TTM behaviorally (e.g. the mice show tissue damage, which is not normally seen in TTM), epidemiologically (Hoxb8 knockout mice show no female sex bias, and no influence of reproductive and stress risk factors; while barbering resembles TTM in all these regards), and biologically (the OFC circuit loop is implicated in this model, and the mice also shows skeletal deformations unseen in TTM). Thus the Hoxb8 mouse shows only superficial convergent validity to TTM, and clearly fails in terms of discriminant validity (i.e. the model shows many features the disorder does not). Conseqeuntly a treatment developed in this mouse would run the risk of treating the specific manipulation in the mouse, rather than the general developmental physiology in the human (and hence failing to translate). Furthermore, the 100% penetrance of the Hoxb8 phenotype precludes the investigation of gene-by-environment interactions, and hence 4P's modeling in general. Thus, unlike spontaneous barbering, it cannot tell us much if anything can about Predicting, Preventing or Personalizing our approach to the disorder.
These findings reach far beyond animal modeling. For instance, if behaviors that commonly occur in laboratory mice and other animals as a consequence of the captive environment [27,29,50,80] are homologues of human ARB, then the welfare of these animals may be impaired [26]. This possibility is also troubling for scientists – these biomarkers and other reported correlates of ARB, are likely to affect many widely used behavioral measures [24]. Thus strains that show a high prevalence of ARBs, or animals raised under conditions likely to induce ARBs, may introduce variability into experiments, compromising power, and potentially contributing to poor between-laboratory replicability and external validity [24,79]. Finally, as the different human ARBs are typically treated very differently, distinguishing classes of animal ARB may help guide and refine the treatment of animal ARBs [25,26]. This is particularly important as many (e.g. [57]) but not all (notably [52]) veterinary authors conceptualize all animal ARB as a homogenous entity analogous to human OCD.
Although they allow a degree of precision and validity rare in high-throughput methods, these neuropsychological measures are laborious and time consuming, and their cost was the delay between each stage of the study. Thus ideally all measures would have been taken in close proximity, and there would be little opportunity for ARBs to develop or animals to die. However this issue is unlikely to have affected the results, as it would have weakened correlations, and produced false negatives, rather than the rather precise predicted pattern of positive results. Spontaneously occurring ARBs are difficult to work with as their development is unpredictable. One solution is to take a cross-section of the population at a single age. Instead we opted to employ a heterogeneous population, and control for this variability using blocking factors and controlling variables. This approach more closely models heterogeneous human populations, and avoids false negative or positive results due to the arbitrary age chosen [32]. Of these variables, cage was the most important (and necessary) blocking factor to include, but this is to be expected, as cage captured the variance due to multiple environmental features.
Several previous studies have found correlations between stereotypy and the response-shifting impairment biomarker [28,30] or broader measures of impaired response flexibility, such as impaired extinction [28,29,76] in multiple species. However, this relationship has not been seen previously in mice using the gambling task [54] and extinction [42]. Both these studies used CD1 mice, potentially pointing to additional complexities in this relationship – though given the generality of this relationship in multiple species, CD1 mice may be an important exception warranting further study.
The most important limitation of the study is interpretational. Thus, currently the association of ARB with these biomarkers is purely correlational – thus it is unclear whether ARB reflects pathological disinhibition of behavioral control induced by an abnormal environment, or merely the extremes of normal function expressed in an abnormal environment. Although circumstantial evidence [24] and preliminary data [28] point to pathology, this is an empirical issue to be addressed in future studies [24]. Resolving this point will clarify the interpretation of the current results in terms of animal modeling and animal welfare, as well as their implications for the validity of data from research animals performing ARB.
5. Acknowledgements
We thank undergraduate interns, and Aria Prater and Sandra Weisker. Project design and infrastructure: JPG, JAM and JDM. Bias-corrected gambling task, barbering scoring, and supporting software: JPG. IDED task and stereotypy scoring HW and JPG. Project management: CMT and BD. Funding: NIMH grant # 5 R03 MH 63907-2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- [1].Albin RL, Mink JW. Recent advances in Tourette syndrome research. Trends Neurosci. 2006;29:175. doi: 10.1016/j.tins.2006.01.001. [DOI] [PubMed] [Google Scholar]
- [2].Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–75. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- [3].Albin RL, Young AB, Penney JB. The Functional Anatomy of Disorders of the Basal Ganglia. Trends Neurosci. 1995;18:63–4. [PubMed] [Google Scholar]
- [4].Alexander GE, Crutcher MD. Functional Architecture of Basal Ganglia Circuits - Neural Substrates of Parallel Processing. Trends Neurosci. 1990;13:266–71. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- [5].Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalmocortical circuits: parallel substrates for motor, oculomotor, `prefrontal' and `limbic' functions. Prog Brain Res. 1990;85:119–46. [PubMed] [Google Scholar]
- [6].Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- [7].American Psychiatric Association . Diagnostic and statistical manual of mental disorders. xxvii. American Psychiatric Association; Washington, DC, USA: 1994. p. 886. [Google Scholar]
- [8].Aouizerate B, Guehl D, Cuny E, Rougier A, Bioulac B, Tignol J, Burbaud P. Pathophysiology of obsessive-compulsive disorder: A necessary link between phenomenology, neuropsychology, imagery and physiology. Prog Neurobiol. 2004;72:195–221. doi: 10.1016/j.pneurobio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- [9].Berridge KC, Aldridge JW, Houchard KR, Zhuang XX. Sequential super-stereotypy of an instinctive fixed action pattern in hyper-dopaminergic mutant mice: a model of obsessive compulsive disorder and Tourette's. BMC Biol. 2005;3 doi: 10.1186/1741-7007-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double Dissociation of the Effects of Medial and Orbital Prefrontal Cortical Lesions on Attentional and Affective Shifts in Mice. J Neurosci. 2008;28:11124–30. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bohne A, Keuthen NJ, Tuschen-Caffier B, Wilhelm S. Cognitive inhibition in trichotillomania and obsessive-compulsive disorder. Behav Res Ther. 2005;43:923–42. doi: 10.1016/j.brat.2004.06.014. [DOI] [PubMed] [Google Scholar]
- [13].Bohne A, Savage CR, Deckersbach T, Keuthen NJ, Jenike MA, Tuschen-Caffier B, Wilhelm S. Visuospatial abilities, memory, and executive functioning in trichotillomania and obsessive-compulsive disorder. Journal Of Clinical And Experimental Neuropsychology. 2005;27:385–99. doi: 10.1080/13803390490520418. [DOI] [PubMed] [Google Scholar]
- [14].Bordnick PS, Thyer BA, Ritchie BW. Feather picking disorder and trichotillomania: An avian model of human psychopathology. Journal of Behavior Therapy & Experimental Psychiatry. 1994;25:189–96. doi: 10.1016/0005-7916(94)90019-1. [DOI] [PubMed] [Google Scholar]
- [15].Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends Neurosci. 2002;25:340. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- [16].Caldwell GW, Ritchie DM, Masucci JA, Hageman W, Yan Z. The new pre-preclinical paradigm: Compound optimization in early and late phase drug discovery. Curr Top Med Chem. 2001;1:353–66. doi: 10.2174/1568026013394949. [DOI] [PubMed] [Google Scholar]
- [17].Campbell KM, de Lecea L, Severynse DM, Caron MG, McGrath MJ, Sparber SB, Sun L-Y, Burton FH. OCD-like behaviors caused by a neuropotentiating transgene targeted to cortical and limbic D1+ neurons. J Neurosci. 1999;19:5044–53. doi: 10.1523/JNEUROSCI.19-12-05044.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci Biobehav Rev. 2005;29:399. doi: 10.1016/j.neubiorev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- [19].Cools R, Clark L, Robbins TW. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J Neurosci. 2004;24:1129–35. doi: 10.1523/JNEUROSCI.4312-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Crider A. Perseveration in schizophrenia. Schizophrenia Bulletin. 1997;23:63–74. doi: 10.1093/schbul/23.1.63. [DOI] [PubMed] [Google Scholar]
- [21].Crusio WE, Goldowitz D, Holmes A, Wolfer D. Standards for the publication of mouse mutant studies. Genes Brain and Behavior. 2009;8:1–4. doi: 10.1111/j.1601-183X.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- [22].Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- [23].Frith CD, Done DJ. Stereotyped responding by schizophrenic-patients on a 2-choice guessing task. Psychol Med. 1983;13:779–86. doi: 10.1017/s0033291700051485. [DOI] [PubMed] [Google Scholar]
- [24].Garner JP. Stereotypies and other Abnormal Repetitive Behaviors: potential impact on validity, reliability, and replicability of scientific outcomes. ILAR Journal. 2005;46:106–17. doi: 10.1093/ilar.46.2.106. [DOI] [PubMed] [Google Scholar]
- [25].Garner JP. Box 10.2. Implications of Recognizing Mechanistic Differences in Abnormal Repetitive Behaviour. In: Rushen J, Mason G, editors. Stereotypic Animal Behaviour: Fundamentals and Applications to Welfare. CABI; Wallingford, England, UK: 2006. pp. 293–4. [Google Scholar]
- [26].Garner JP. Ch 5. Perseveration and stereotypy - systems-level insights from clinical psychology. In: Rushen J, Mason G, editors. Stereotypic Animal Behaviour: Fundamentals and Applications to Welfare. CABI; Wallingford, England, UK: 2006. pp. 121–52. [Google Scholar]
- [27].Garner JP, Dufour B, Gregg LE, Weisker SM, Mench JA. Social and husbandry factors affecting the prevalence and severity of barbering (`whisker trimming') in laboratory mice. Appl Anim Behav Sci. 2004;89:263–82. [Google Scholar]
- [28].Garner JP, Mason G, Smith R. Stereotypic route-tracing in experimentally-caged songbirds correlates with general behavioural disinhibition. Anim Behav. 2003;66:711–27. [Google Scholar]
- [29].Garner JP, Mason GJ. Evidence for a relationship between cage stereotypies and behavioural disinhibition in laboratory rodents. Behav Brain Res. 2002;136:83–92. doi: 10.1016/s0166-4328(02)00111-0. [DOI] [PubMed] [Google Scholar]
- [30].Garner JP, Meehan CL, Mench JA. Stereotypies in caged parrots, schizophrenia and autism: evidence for a common mechanism. Behav Brain Res. 2003;145:125–34. doi: 10.1016/s0166-4328(03)00115-3. [DOI] [PubMed] [Google Scholar]
- [31].Garner JP, Thogerson CM, Würbel H, Murray JD, Mench JA. Animal Neuropsychology: Validation of the Intra-Dimensional Extra-Dimensional set shifting task in mice. Behav Brain Res. 2006;173:53–61. doi: 10.1016/j.bbr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- [32].Garner JP, Thorgersen CM, Mench JA, Würbel H. Standardization and the Red Queen: applying methodologies from ethology, neuropsychology, and field biology to problems in high-throughput behavioral methods. In: Mendl M, Bradshaw JWS, Burman OHP, Butterworth A, Harris MJ, Held SDE, Jones SM, Littin KE, Main DCJ, Nicol CJ, Parker RMA, Paul ES, Richards G, Sherwin CM, Statham PTE, Toscano MJ, Warriss PD, editors. Proceedings of the 40th International Congress of the International Society for Applied Ethology. Vol. 11. Bristol, UK: 2006. [Google Scholar]
- [33].Garner JP, Wayne CM, Würbel H, Mench JA. Barbering (whisker trimming) in laboratory mice involves the same brain systems as compulsive behaviors in trichotillomania, autism and other obsessive-compulsive spectrum disorders. In: Ferrante V, editor. Proceedings of the 37th International Congress of The International Society for Applied Ethology. Vol. 75. Fondazione Iniziative Zooprofilattiche e Zootecniche; Abano Terme, Italy: 2003. [Google Scholar]
- [34].Garner JP, Weisker SM, Dufour B, Mench JA. Barbering (fur and whisker trimming) by laboratory mice as a model of human trichotillomania and Obsessive-Compulsive Spectrum Disorders. Comp Med. 2004;54:216–24. [PubMed] [Google Scholar]
- [35].Gerlai R, Clayton NS. Analysing hippocampal function in transgenic mice: An ethological perspective. Trends Neurosci. 1999;22:47–51. doi: 10.1016/s0166-2236(98)01346-0. [DOI] [PubMed] [Google Scholar]
- [36].Gould TD, Gottesman II. Psychiatric endophenotypes and the development of valid animal models. Genes Brain And Behavior. 2006;5:113–9. doi: 10.1111/j.1601-183X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- [37].Greer JM, Capecchi MR. Hoxb8 is required for normal grooming behavior in mice. Neuron. 2002;33:23–34. doi: 10.1016/s0896-6273(01)00564-5. [DOI] [PubMed] [Google Scholar]
- [38].Grier JW. Biology of animal behavior. Times Mirror/Mosby College Pub.; St. Louis: 1984. p. xix.p. 693.p. 54. [Google Scholar]
- [39].Hill RA, McInnes KJ, Gong ECH, Jones MEE, Simpson ER, Boon WC. Estrogen Deficient Male Mice Develop Compulsive Behavior. Biological Psychiatry. 2007;61:359–66. doi: 10.1016/j.biopsych.2006.01.012. [DOI] [PubMed] [Google Scholar]
- [40].Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3:711–6. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- [41].Kuhlmann J. Alternative strategies in drug development: clinical pharmacological aspects. Int J Clin Pharmacol Ther. 1999;37:575–83. [PubMed] [Google Scholar]
- [42].Latham N, Mason G. Frustration and perseveration in stereotypic captive animals: Is a taste of enrichment worse than none at all? Behav Brain Res. 2010;211:96–104. doi: 10.1016/j.bbr.2010.03.018. [DOI] [PubMed] [Google Scholar]
- [43].Lewis MH, Bodfish JW. Repetitive behavior disorders in autism. Mental Retardation & Developmental Disabilities Research Reviews. 1998;4:80–9. [Google Scholar]
- [44].Lewis MH, Tanimura Y, Lee LW, Bodfish JW. Animal models of restricted repetitive behavior in autism. Behav Brain Res. 2007;176:66–74. doi: 10.1016/j.bbr.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, Stevens KE, Maccaferri G, McBain CJ, Sussman DJ, WynshawBoris A. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- [46].Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of Autistic Disorder. Journal of Autism and Developmental Disorders. 2005;35:445–60. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- [47].Lucey JV, Burness CE, Costa DC, Gacinovic S, Pilowsky LS, Ell PJ, Marks IM, Kerwin RW. Wisconsin Card Sorting Test (WCST) errors and cerebral blood flow in obsessive-compulsive disorder (OCD) British Journal of Medical Psychology. 1997;70:403–11. doi: 10.1111/j.2044-8341.1997.tb01916.x. [DOI] [PubMed] [Google Scholar]
- [48].Lyon M, Robbins T. The action of central nervous system stimulant drugs: a general theory concerning amphetamine effects. Current Developments In Psychopharmacology. 1975;2:79–163. [Google Scholar]
- [49].Mason G. Ch 11. Stereotypic behaviour in captive animals: Fundamentals, and implications for welfare and beyond. In: Rushen J, Mason G, editors. Stereotypic Animal Behaviour: Fundamentals and Applications to Welfare. CABI; Wallingford, England, UK: 2006. [Google Scholar]
- [50].Mason GJ. Stereotypies: a critical review. Anim Behav. 1991;41:1015–37. [Google Scholar]
- [51].McHaffie JG, Stanford TR, Stein BE, Coizet V, Redgrave P. Subcortical loops through the basal ganglia. Trends Neurosci. 2005;28:401. doi: 10.1016/j.tins.2005.06.006. [DOI] [PubMed] [Google Scholar]
- [52].Mills DS, Luescher UA. Ch 10. Veterinary and pharmacological approaches to Abnormal Repetitive Behaviour. In: Rushen J, Mason G, editors. Stereotypic Animal Behaviour: Fundamentals and Applications to Welfare. CABI; Wallingford, England, UK: 2006. pp. 285–323. [Google Scholar]
- [53].Mink JW. The Basal Ganglia and Involuntary Movements: Impaired Inhibition of Competing Motor Patterns. Arch Neurol. 2003;60:1365–8. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- [54].Nam-Mi Gross A, Garner JP, Würbel H. Effects of environmental enrichment on cage stereotypy and recurrent perseveration in mice. In: Hausberger M, editor. XXXI International Ethological Conference; Rennes, France. 2009. p. 254. [Google Scholar]
- [55].National Institute of Mental Health . The National Institute of Mental Health Strategic Plan. 2008. [Google Scholar]
- [56].Norman DA, Shallice T, Davidson RJ. Attention to action: willed and automatic control of behaviour. In: Schwartz GE, Shapiro D, editors. Consciousness and self-regulation: advances in research and theory. vol. 4. Plenum Press; New York: 1986. pp. 1–18. [Google Scholar]
- [57].Overall KL. Clinical Behavioral Medicine for Small Animals. Mosby; St. Louis: 1997. p. 544. [Google Scholar]
- [58].Owen AM, Roberts AC, Hodges JR, Summers BA, Polkey CE, Robbins TW. Contrasting Mechanisms of Impaired Attentional Set-Shifting in Patients With Frontal Lobe Damage or Parkinsons Disease. Brain. 1993;116:1159–75. doi: 10.1093/brain/116.5.1159. [DOI] [PubMed] [Google Scholar]
- [59].Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biological Psychiatry. 2007;61:482–6. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- [60].Rapoport JL, Ryland DH, Kriete M. Drug Treatment of Canine Acral Lick - an Animal Model of Obsessive-Compulsive Disorder. Archives of General Psychiatry. 1992;49:517–21. doi: 10.1001/archpsyc.1992.01820070011002. [DOI] [PubMed] [Google Scholar]
- [61].Redgrave P, Prescott TJ, Gurney K. The basal ganglia: a vertebrate solution to the selection problem? Neuroscience. 1999;89:1009. doi: 10.1016/s0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- [62].Reiner A, Medina L, Veenman CL. Structural and functional evolution of the basal ganglia in vertebrates. Brain Res Rev. 1998;28:235–85. doi: 10.1016/s0165-0173(98)00016-2. [DOI] [PubMed] [Google Scholar]
- [63].Rettew DC, Cheslow DL, Rapoport JL, Leonard HL. Neuropsychological test performance in trichotillomania: A further link with obsessive-compulsive disorder. Journal of Anxiety Disorders. 1991;5:225–35. [Google Scholar]
- [64].Richter H, Garner JP, Auer C, Kunert J, Würbel H. Systematic variation improves reproducibility of animal experiments. Nat Methods. 2010;7:167–8. doi: 10.1038/nmeth0310-167. [DOI] [PubMed] [Google Scholar]
- [65].Richter H, Garner JP, Würbel H. Environmental standardization: cure or cause of poor reproducibility in animal experiments? Nat Methods. 2009;6:257–61. doi: 10.1038/nmeth.1312. [DOI] [PubMed] [Google Scholar]
- [66].Rolls ET. Neurophysiology and cognitive functions of the striatum. Revue Neurologique. 1994;150:648–60. [PubMed] [Google Scholar]
- [67].Rosenberg DR, Keshavan MS. Toward a neurodevelopmental model of obsessive-compulsive disorder. Biological Psychiatry. 1998;43:623–40. doi: 10.1016/s0006-3223(97)00443-5. [DOI] [PubMed] [Google Scholar]
- [68].Sarna JR, Dyck RH, Whishaw IQ. The Dalila effect: C57BL6 mice barber whiskers by plucking. Behav Brain Res. 2000;108:39–45. doi: 10.1016/s0166-4328(99)00137-0. [DOI] [PubMed] [Google Scholar]
- [69].Sheppard DM, Bradshaw JL, Purcell R, Pantelis C. Tourette's and comorbid syndromes: Obsessive compulsive and attention deficit hyperactivity disorder. A common etiology? Clinical Psychology Review. 1999;19:531–52. doi: 10.1016/s0272-7358(98)00059-2. [DOI] [PubMed] [Google Scholar]
- [70].Stanley MA, Hannay HJ, Breckenridge JK. The neuropsychology of trichotillomania. Journal of Anxiety Disorders. 1997;11:473–88. doi: 10.1016/s0887-6185(97)00024-8. [DOI] [PubMed] [Google Scholar]
- [71].Stein DJ, O'Sullivan RL, Hollander E. The neurobiology of trichotillomania. In: Stein DJ, Christenson GA, Hollander E, editors. Trichotillomania. American Psychiatric Press, Inc; Washington, DC, US: 1999. pp. 43–61. [Google Scholar]
- [72].Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp Brain Res. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- [73].Turner M. Towards an executive dysfunction account of repetitive behaviour in autism. In: Russell J, editor. Autism As an Executive Disorder. Oxford University Press; New York, NY, USA: 1997. pp. 57–100. [Google Scholar]
- [74].Turner M. Annotation: Repetitive behaviour in autism: A review of psychological research. J Child Psychol Psychiatry Allied Disciplines. 1999;40:839–49. [PubMed] [Google Scholar]
- [75].Van de Weerd HA, Vandenbroek FAR, Beynen AC. Removal of Vibrissae in Male-Mice Does Not Influence Social-Dominance. Behav Processes. 1992;27:205–8. doi: 10.1016/0376-6357(92)90177-F. [DOI] [PubMed] [Google Scholar]
- [76].Vickery SS, Mason GJ. Stereotypy in caged bears correlates with perseverative responding on an extinction task. Appl Anim Behav Sci. 2005;91:247–60. [Google Scholar]
- [77].Woods DW, Flessner C, Franklin ME, Wetterneck CT, Walther MR, Anderson ER, Cardona D. Understanding and treating trichotillomania: What we know and what we don't know. Psychiatric Clinics Of North America. 2006;29:487–+. doi: 10.1016/j.psc.2006.02.009. [DOI] [PubMed] [Google Scholar]
- [78].Würbel H. Behaviour and the standardization fallacy. Nat Genet. 2000;26:263. doi: 10.1038/81541. [DOI] [PubMed] [Google Scholar]
- [79].Würbel H. Ideal homes? Housing effects on rodent brain and behaviour. Trends Neurosci. 2001;24:207–11. doi: 10.1016/s0166-2236(00)01718-5. [DOI] [PubMed] [Google Scholar]
- [80].Würbel H, Stauffacher M, vonHolst D. Stereotypies in laboratory mice - Quantitative and qualitative description of the ontogeny of `wire-gnawing' and `jumping' in Zur:ICR and Zur:ICR nu. Ethology. 1996;102:371–85. [Google Scholar]