Summary
Background
In many organisms, germ cells are segregated from the soma through the inheritance of the specialized germ plasm, which contains mRNAs and proteins that specify germ cell fate and promote germline development. Whereas germ plasm assembly has been well characterized, mechanisms mediating germ plasm inheritance are poorly understood. In the Drosophila embryo, germ plasm is anchored to the posterior cortex and nuclei that migrate into this region give rise to the germ cell progenitors, or pole cells. How the germ plasm interacts with these nuclei for pole cell induction and is selectively incorporated into the forming pole cells is not known.
Results
Live imaging of two conserved germ plasm components, nanos mRNA and Vasa protein, revealed that germ plasm segregation is a dynamic process involving active transport of germ plasm RNA-protein complexes coordinated with nuclear migration. We show that centrosomes accompanying posterior nuclei induce release of germ plasm from the cortex and recruit these components by dynein-dependent transport on centrosome-nucleated microtubules. As nuclei divide, continued transport on astral microtubules partitions germ plasm to daughter nuclei, leading to its segregation into pole cells. Disruption of these transport events prevents incorporation of germ plasm into pole cells and impairs germ cell development.
Conclusions
Our results indicate that active transport of germ plasm is essential for its inheritance and ensures the production of a discrete population of germ cell progenitors endowed with requisite factors for germline development. Transport on astral microtubules may provide a general mechanism for the effective segregation of cell fate determinants.
Introduction
The segregation of the germline from somatic tissues is a fundamental event in animal development. In many organisms, germline progenitor cells are specified early in embryogenesis through the inheritance of a specialized cytoplasm known as the germ plasm. The germ plasm contains RNA- and protein-rich granules that are characteristic of germ cells throughout the animal kingdom and are thought to contain determinants that promote germ cell fate and suppress somatic fate [1]. The role of germ plasm in germ cell formation and function has been well demonstrated in Drosophila [2]. Here, the germ plasm is localized at the posterior of the early embryo, thereby restricting germ cell formation to the posterior pole.
The Drosophila embryo develops initially as a syncytium, in which divisions of the embryonic nuclei are not followed by cytokinesis. During nuclear division cycles 8–10, the nuclei migrate to the cortex, where they continue to divide. Shortly after reaching the cortex, nuclei that have migrated into the germ plasm initiate a budding of the plasma membrane that encapsulates them and the surrounding cytoplasm to form the “pole cells,” or germ cell progenitors. The remaining cortical nuclei are enclosed during cycle 14 to form the somatic cells [3]. Germ plasm is both necessary and sufficient to induce germ cells, as mutations that disrupt germ plasm assembly prevent germ cell formation while ectopically localized germ plasm can induce production of germ cells at the new site [2]. How the germ plasm directs posterior nuclei to initiate pole cell formation is not known, however.
In addition to determinants of germ cell formation, the germ plasm also contains RNAs that are incorporated into pole cells where they function in germ cell development [4]. One of these, nanos (nos) mRNA, is essential for both abdominal and germline development. Although present throughout the syncytial embryo, nos is highly enriched in the germ plasm where it is selectively translated to produce a Nos protein gradient that directs abdominal segmentation [5, 6]. nos mRNA also becomes incorporated into the germ cells and is required for their mitotic and transcriptional quiescence, survival, and migration to the gonad [4, 7]. We have previously shown that germ plasm localization of nos is essential for its accumulation and function in germ cells [8]. Similar roles for nos homologs in germline development have been demonstrated in a variety of invertebrates and vertebrates. Moreover, transcripts from the Xenopus, C. elegans, and zebrafish nos homologs have also been shown to be enriched in the germ plasm, suggesting that germ plasm association is a conserved mechanism for ensuring the passage of nos to the germ cells [7].
Germ plasm assembly occurs during oogenesis, initiated by the kinesin-dependent localization and subsequent translation of oskar (osk) mRNA at the oocyte posterior [2, 9]. Osk is both necessary and sufficient for recruitment of additional germ plasm components such as the DEAD-box helicase Vasa (Vas) to form electron-dense organelles called polar granules, as well as for the localization of nos mRNA [2, 9]. nos accumulates at the posterior late in oogenesis, through a mechanism involving diffusion within the ooplasm and entrapment by the germ plasm [10]. FRAP and cytoskeletal disruption experiments have shown that nos, along with germ plasm components like Vas, becomes stably anchored to the actin cytoskeleton during oogenesis and that this actin-based anchoring mechanism maintains germ plasm localization in the newly fertilized embryo [11, 12].
How germ plasm RNAs like nos become incorporated into germ cells remains poorly understood. Elegant cytological and ultrastructural studies performed over forty years ago showed that polar granules cluster around posterior nuclei and appear to concentrate on mitotic microtubules during nuclear divisions [13, 14]. Perinuclear localization of germ plasm is indeed a conserved feature of most germ cells [15]. In situ hybridization experiments detected nos and other germ plasm-localized RNAs concentrated around posterior nuclei during pole cell budding [16, 17], but how this distribution arises and whether the apparent association of germ plasm components with nuclei is required for germ cell segregation or is a consequence thereof remains unknown. Thus far, it is not known whether the segregation of germ plasm RNAs to the germ cells occurs through passive engulfment as the cortical actin cytoskeleton is remodeled during germ cell formation [18] or whether this segregation occurs by an active process.
To elucidate the mechanism by which germ plasm components required for germ cell specification and germ cell function are sequestered away from the soma and into germ cells, we monitored the dynamics of fluorescently labeled nos mRNA and Vas protein at high resolution during early embryogenesis. Using pharmacological and genetic manipulations, we show that centrosomes associated with the posterior nuclei trigger the release of actin-anchored germ plasm components from the cortex. Detached germ plasm particles then undergo rapid microtubule and dynein-dependent transport toward proximal nuclei. Persistence of this transport during nuclear divisions prior to the completion of pole cell formation successively segregates the germ plasm to daughter nuclei, and ultimately into the forming germ cells. We provide evidence that active transport is essential for germ plasm inheritance and ensures segregation of the germline fate determinants away from the somatic nuclei and into the germ cells. The finding that nos mRNA is transported actively during embryogenesis contrasts with its passive transport in the oocyte and reveals cell-type specific contexts for mRNA localization mechanisms.
Results
Live imaging of nos mRNA in early embryos
We visualized the process of germ plasm incorporation into pole cells by using 4-D multi-photon microscopy to image depths in excess of 80 μm at the posterior of the embryo for several hours without detectable photobleaching or phototoxicity. Here, and in experiments described below, nos mRNA is labeled in vivo with GFP (nos*GFP), using the transgenic MS2 tagging system that we have previously established for Drosophila [10]. Tagged nos mRNA is present at levels comparable to the wild-type mRNA (data not shown) and confers wild-type nos activity. In the newly fertilized embryo, nos*GFP particles are distributed in a cap covering the posterior pole (Figure 1A; also see [10]). As embryogenesis proceeds and nuclei arrive at the posterior cortex, these particles coalesce in perinuclear, ring-like structures. Subsequently, when the posterior nuclei divide and initiate pole bud formation, the rings of nos appear to divide and segregate with daughter nuclei (Figure 1A and Movie S1.1). This striking redistribution of nos mRNA prior to pole bud formation suggests that nos and possibly other germ plasm components may become incorporated into pole cells through their association with posterior nuclei.
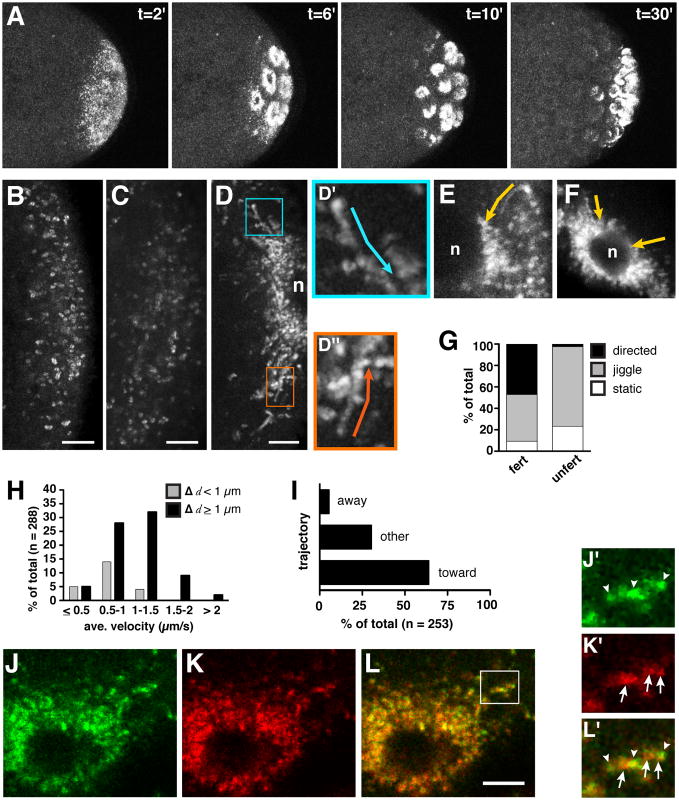
Figure 1. Live imaging of nos mRNA and Vas protein during pole cell formation.
(A) Two-photon excitation time-lapse imaging of the posterior region of an embryo expressing nos*GFP (anterior toward the left). Representative time points from Movie S1.1 are shown, where t=0 corresponds to the beginning of the time-series. (B–D) High resolution confocal time-lapse imaging of the posterior cortex of embryos expressing nos*GFP (anterior toward the left). 20–25 sequential frames spanning 6–8 seconds are superimposed so that moving particles appear as a trail of dots. (B) 0–1 hour old embryo (frames from Movie S1.2); (C) 1–2 hour old unfertilized embryo; (D) 1–2 hour old embryo with nucleus (n) at the posterior cortex (frames from Movie S1.3 Part 1); (D′ and D″) higher magnification of boxed regions in D showing sustained particle runs, with trajectories indicated by arrows. (E) Trail image showing nos*GFP detaching from the posterior cortex of a 1–2 hour embryo and traveling directly (arrow) toward a nucleus (n). Anterior is left. (F) Trail image of nos*GFP moving toward a nucleus at the anterior of a 1–2 hour old osk-bcd3′UTR embryo. (G) Percentage of particles undergoing movement in similarly aged 1–2 hour old fertilized embryos (fert; n=255 particles from 14 embryos) and unfertilized eggs (unfert; n=311 particles from 6 embryos). (H) Quantitation of average velocities and run lengths (Δ d) of motile particles (n=23 embryos). (I) Directionality of nos*GFP movement relative to nuclei labeled with H2AvD-mRFP (n=10 embryos) (see Movie S1.4). (J–L) Trail images from time-lapse analysis (Movie S1.5) of embryos expressing nos*GFP (J, green) and mCherry-Vas (K, red) show significant overlap (L, merge) as particles accumulate around a nucleus. Channels were imaged simultaneously under conditions where cross-talk is not detectable. (J′–L′) High power image of the region indicated by the box in (L). nos and Vas display heterogenous intensity profiles (arrowheads indicate predominant nos signal, arrows indicate predominant Vas signal). Scale bars: (B–D) 5 μm; (L) 2.5 μm. See also Figure S1 and Movies S1.1, S1.2, S1.3, S1.4, and S1.5.
To determine how nos is redistributed from the cortical anchor to its perinuclear location, we performed live imaging of nos*GFP particles at high temporal and spatial resolution. During the first hour of embryogenesis, and in unfertilized eggs, nos*GFP particles appear largely static or exhibit only a slight jiggling motion, as though attached to the posterior cortex (Figure 1B and Movie S1.2). Indeed, these immobile particles resemble nos*GFP particles anchored at the posterior cortex of the oocyte [19]. The dynamics of nos particles change dramatically as embryogenesis proceeds. Imaging of embryos between 1 and 2 hours post-fertilization revealed that nos particles detach from the cortex and initiate linearly directed movements, accumulating around nuclei that have migrated to the posterior (Figures 1D, 1E and Movie S1.3 Part 1). Analysis of carefully staged embryos showed that nos*GFP particles become motile approximately 1.25 hours into embryogenesis and that motility persists for about 30 minutes, throughout the process of pole cell budding.
Individual nos*GFP particles were monitored over time (see Experimental Procedures for details) and characterized as “static” if they showed no significant movement during the time sequence, “jiggling” if they showed only local fluctuations of ≤1 particle length in any direction, or “directed” if they displayed sustained runs of ≥ 0.5 μm in one direction. Within any 30 second time period after the onset of motility, nearly 50% of visible nos*GFP particles undergo directed runs, while approximately 40% jiggle, and a small percentage remain static (Figure 1G). By contrast, in aged unfertilized eggs, where nuclear divisions, nuclear migration, and pole cell formation do not occur, nos remains tethered at the posterior cortex (Figure 1C and 1G). Directed movement of nos*GFP is often rapid and long-range. The average velocities of individual particles range from 0.11–2.57 μm/s (mean = 1.01 ± 0.44 μm/s), with some runs exceeding 10 microns (Figure 1H). Complex behaviors including pauses, direction changes, and direction reversals were also observed. The velocities and types of movement exhibited by nos*GFP are similar to those previously reported for bcd and osk mRNAs engaged in microtubule-dependent transport during oogenesis [19, 20].
Germ plasm components transit rapidly toward posterior nuclei
The lack of nos motility in aged unfertilized eggs indicates that the onset of nos motility is not dictated by an inherent timing mechanism and suggests that it is linked to the arrival of nuclei at the posterior cortex. To begin to determine whether motility is required for the accumulation of nos around nuclei, we tracked individual nos*GFP particles relative to nuclei labeled with an RFP marker (Movie S1.4). Particle runs were scored as “toward” if their vector was directed to an RFP-labeled nucleus within the same focal plane, “away” if a particle juxtaposed to a nucleus moved away from that nucleus, or “other” if particle displacement could not be correlated to the position of a nucleus. We found that nearly 65% of runs are directed toward posterior nuclei (Figure 1I). This directional bias is likely an underestimate, since many trajectories in the “away” and “other” categories probably represent movement toward nuclei in other focal planes.
To determine whether nuclei that migrate to the posterior are uniquely able to recruit nos, we used the osk-bcd3′UTR transgene [21] to generate embryos with germ plasm containing nos*GFP localized ectopically at the anterior of the embryo in addition to its normal localization at the posterior. As cortical migration of nuclei proceeded, we monitored nos*GFP alternately at the anterior and posterior poles. Both pools of nos behave similarly, with the majority of particles transiting toward the most proximal nucleus (Figure 1F). However, the two domains of nos do not initiate movement simultaneously. Rather, the onset of nos motility at the anterior is delayed by several minutes. This difference in the onset of motility between anteriorly and posteriorly localized nos is consistent with the observation that nuclei arrive at the posterior cortex one division cycle earlier than they reach the rest of the cortex [3]. These results, as well as additional evidence presented below, indicate that all nuclei are competent to recruit nos mRNA at the cortex. Moreover, they strongly suggest that it is the arrival of the nuclei at the cortex that triggers the release of nos from its cortical anchor.
Co-transport of germ plasm components
Previous results have shown that nos*GFP and Vas are colocalized at the posterior in newly fertilized embryos [10]. We therefore investigated whether germ plasm components like Vas and Osk are also transported toward posterior nuclei. Live imaging of early embryos expressing GFP-tagged Vas or Osk showed that these germ plasm proteins behave similarly to nos, with posteriorly anchored particles transitioning to rapid, directed movement (Movie S1.3 Parts 2 and 3). To test whether nos retains its association with Vas throughout particle transport, we performed colocalization experiments in fixed embryos before, during, and after pole cell formation, using nos*GFP and mCherry-Vas. Quantitative analysis showed that the distributions of nos and Vas are most highly correlated during the period of pole cell formation, when germ plasm particles migrate and associate with posterior nuclei (Figure S1.1).
To determine whether the colocalization observed in static images is characteristic of co-migrating particles or represents accumulations of germ plasm components after transport, we performed rapid time-lapse imaging of nos*GFP and mCherry-Vas simultaneously. Throughout the transport process, most motile particles contain both nos mRNA and Vas, although the relative contributions of each vary among individual ribonucleoprotein complexes (RNPs). These somewhat heterogeneous RNPs transit toward nuclei and coalesce into larger perinuclear foci (Figures 1J–1L and Movie S1.5). Moreover, Vas transport occurs in embryos that lack nos mRNA (data not shown), suggesting that motility is a generalized property of germ plasm components that are partitioned into germ cells.
Centrosome nucleated microtubules mediate germ plasm transport
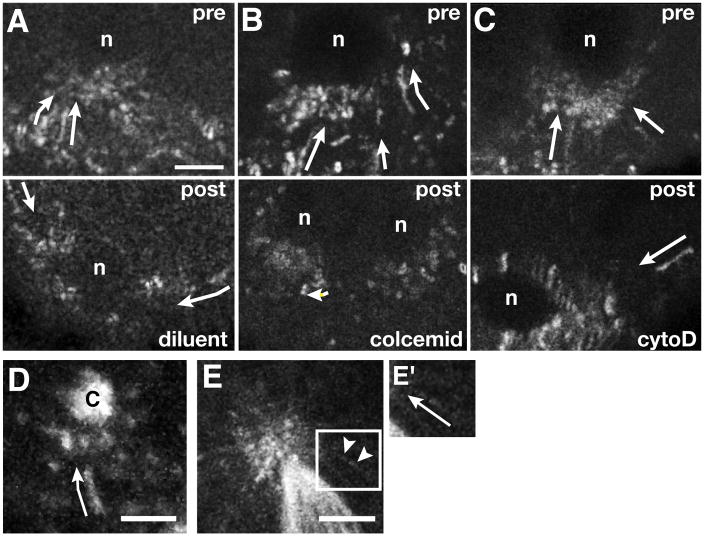
The kinetics of germ plasm transport we observe are similar to those previously reported for the microtubule-dependent active transport of mRNAs during oogenesis [references in 9, 22]. To test the microtubule dependence of nos transport during pole cell formation, we microinjected the microtubule-destabilizing drug colcemid into early embryos. Because microtubules are required for nuclear migration, we selected embryos in which nuclei had already reached the posterior cortex and injections were performed just after the onset of nos motility. Colcemid injection resulted in the immediate cessation of particle movement, whereas neither injection of the actin-destabilizing drugs cytochalasin D and latrunculin A, nor control injections of diluent impaired nos motility (Figures 2A–2C, Movie S2.1 and data not shown). Thus, microtubules, but not actin filaments, are required for transport of germ plasm components.
Figure 2. Transport of nos*GFP is microtubule-dependent.
Trail images from time-lapse movies of embryos with nos*GFP at the posterior cortex (anterior is up). (A–C) Pharmacological disruption experiments. Embryos were imaged immediately prior to (pre) and within 5 minutes after (post) injection of diluent (A), colcemid (B), or cytochalasin D (cytoD, C). Arrows indicate directed particle runs. (D) Trail image of nos*GFP (arrow) traveling toward a centrosome (c) labeled with GFP-Cnn. Six consecutive frames spanning two seconds of developmental time are superimposed. (E) Trail image of GFP-Vas moving on microtubules (arrowheads) labeled with GFP-α-tubulin. Time-lapse images spanning approximately 1.6 seconds are superimposed. The complete time sequence is shown in Movie S2.2. (E′) Magnification of the Vas particle in (E), with the trajectory indicated by the arrow. Scale bars: (A) 2.5 μm; (D) and (E) 5 μm. See also Movies S2.1 and S2.2.
Which microtubules provide the tracks for germ plasm transport toward nuclei? Polar granules detected cytologically appear to be concentrated around the spindle poles during pole bud divisions [13, 14]. In addition, experiments using aphidicolin to dissociate centrosomes from nuclei showed that centrosomes alone can migrate to the cortex and initiate pole bud formation [23]. We therefore asked whether microtubules nucleated by the centrosomes could provide direct tracks for germ plasm RNP transport. As a first step, we first visualized nos*GFP or GFP-Vas together with centrosomes and microtubules, immunostained with anti-Centrosomin (Cnn) and anti-tubulin antibodies, at various points throughout pole cell formation. In addition, we performed high resolution time-lapse imaging of nos*GFP or GFP-Vas together with centrosomes labeled with GFP-Cnn or microtubules labeled with GFP-α-tubulin.
As interphase nuclei and their associated centrosomes approach the posterior cortex, nos*GFP and GFP-Vas begin to coalesce around the most proximal centrosomes and their microtubules (Figure 3A and data not shown). At the posterior, germ plasm components become further enriched around the astral microtubules of interphase nuclei (Figure 3B). Live imaging during this period revealed nos*GFP particles transiting directly toward centrosomes and accumulating in their immediate vicinity (Figure 2D). As mitosis proceeds, the association of nos and Vas with astral microtubules is maintained, leading to the partitioning of both RNA and protein to daughter nuclei (Figures 3C–3E, Figure 4A). In cases where only one of the two centrosomes associated with a nucleus is proximal to the germ plasm, nos and Vas accumulation is limited to that pole, leading to their asymmetric segregation during the subsequent division (Figures 3D and 4A). By time-lapse imaging, we detected movement of nos*GFP and GFP-Vas particles along astral microtubules, even after spindle formation, suggesting that continued trafficking on astral microtubules may ensure partitioning of germ plasm components to pole cells (Figure 2E, Movie S2.2, and data not shown).
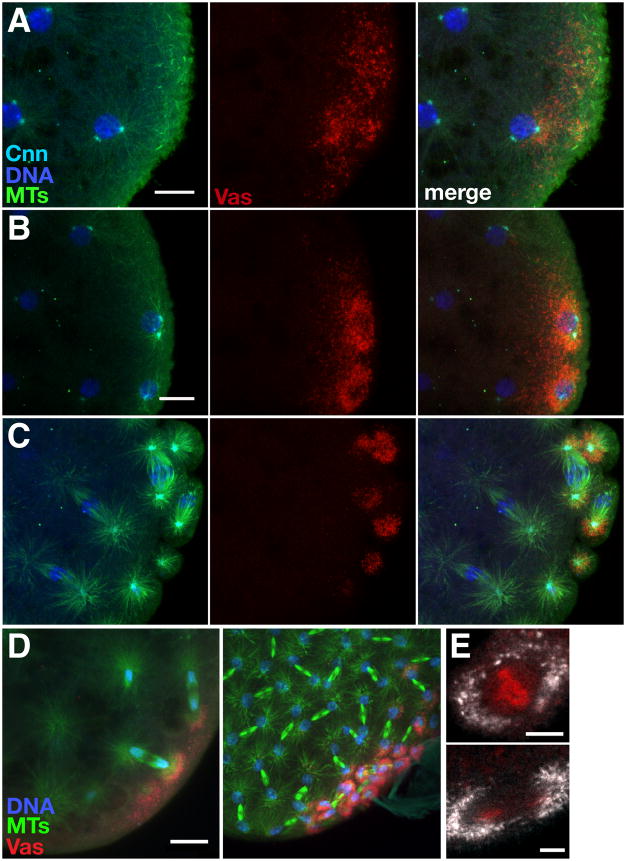
Figure 3. Recruitment and segregation of germ plasm by centrosomal microtubules.
(A–C) Confocal Z-series projections showing mCherry-Vas (Vas, red) together with microtubules (MTs, green), centrosomes (Cnn, cyan) and DNA (blue) at the posterior of embryos fixed prior to (A, B) and during (C) pole cell formation. Anterior is toward the left. Vas released from the cortex accumulates around astral microtubules as nuclei reach the posterior cortex (A) and remains associated with microtubules throughout nuclear divisions and pole cell formation (B, C). We have not yet been able to resolve whether the perinuclear ring appearance of the germ plasm (also Figure 1A) reflects the 3-dimensional organization of astral microtubules or whether there is a transient spreading of germ plasm around the nuclear periphery. (D) Anti-Vas (Vas, red) and anti-tubulin (MTs, green) immunofluorescence. Z-series projections spanning 20 μm, showing asymmetric accumulation of Vas around posterior nuclei (blue) with only one astral MT array in proximity to the posterior pole, restricting pole cell formation to the posterior. (E) Distribution of nos*GFP (white) relative to nuclei labeled with H2AvD-mRFP during metaphase (top) and anaphase (bottom). Scale bars: (A, B, D) 10 μm; (E) 5 μm.
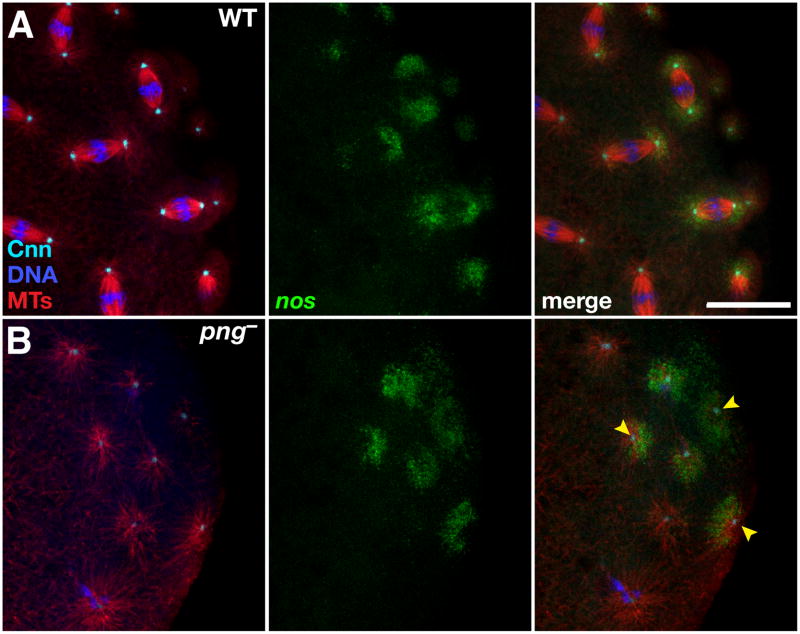
Figure 4. Centrosomes are sufficient for release and recruitment of nos mRNA from the cortex.
(A,B) Immunofluorescence detection of microtubules (MTs, red) and centrosomes (Cnn, cyan) in (A) wild-type (WT) or (B) png3318 (png−) embryos expressing nos*GFP (green). Free centrosomes (arrowheads) and their microtubule arrays are sufficient to recruit nos. Scale bar: 20 μm. See also Figure S4.
To further test the sufficiency of centrosomes to recruit germ plasm [23], we used a mutation in pan gu (png), which encodes a cell cycle kinase that promotes entry into mitosis [24], to genetically isolate centrosomes from nuclei. In embryos from png mutant females, DNA replication is uncoupled from mitosis, resulting in formation of giant polypoloid nuclei. Centrosomes continue to divide, nucleate microtubules, and migrate independently of nuclei [24]. Immunostaining of png mutant embryos with anti-tubulin and anti-Cnn antibodies confirmed the presence of free centrosomes with their associated microtubules at the cortex (Figure 4). Moreover, centrosomes that enter the germ plasm are competent to recruit both nos*GFP and Vas and to form anucleate pole cells (Figures 4B and S4). In vivo imaging of nos*GFP in png mutant embryos showed particles of nos associating with multiple, small round structures, which appear to be isolated centrosomes (data not shown). Taken together, these results indicate that centrosome-nucleated microtubules provide tracks for the transport of germ plasm components to posterior nuclei and for the segregation of germ plasm during subsequent divisions. Moreover, in both imaging of fixed embryos and time-lapse studies, nos and Vas transport appears to occur selectively on astral microtubules, although we cannot rule out the possibility of occasional movement on spindle microtubules.
Germ plasm transport is required for germ cell formation and function
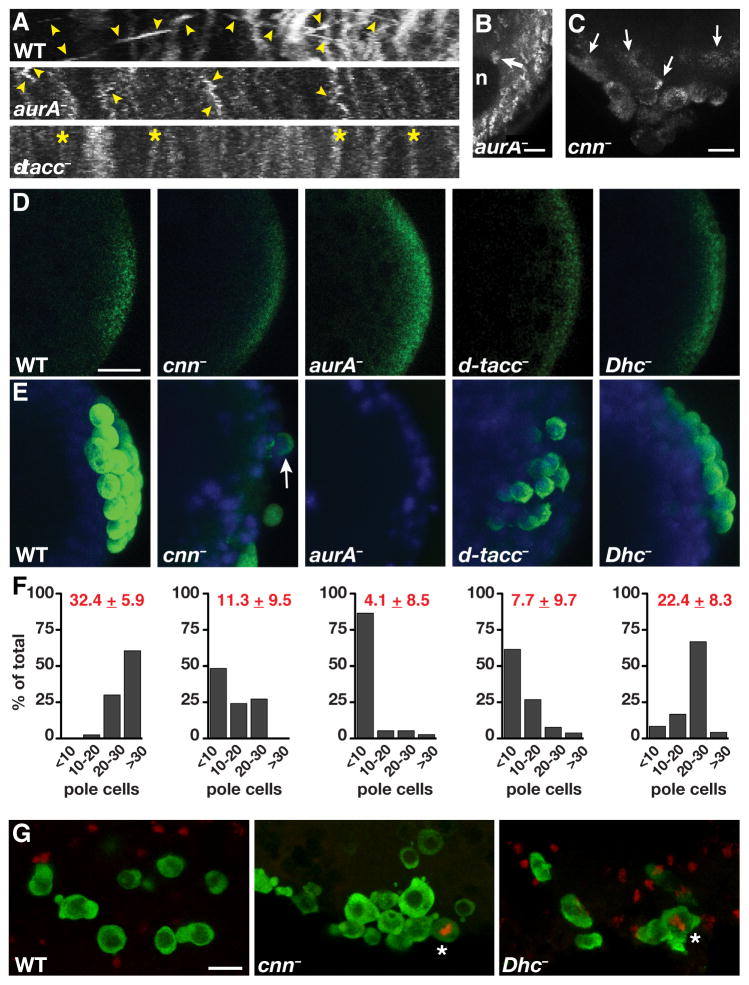
We examined the effect of disrupting germ plasm transport by taking advantage of mutations in centrosome components including Cnn, Aurora A kinase (AurA), and Drosophila transforming acidic coiled-coil protein (D-TACC). These factors interact with each other through defined pathways to regulate centrosome maturation as well as overall microtubule length and stability [25]. In many of the embryos from cnn, aurA, and d-tacc mutant females, mitotic defects prevent the cortical migration of nuclei and nuclear distribution is compromised. However, in each case approximately 50–60% of the mutant embryos complete nuclear migration, and in these embryos, nos transport is generally inefficient. Particles of nos*GFP appear less motile and often fail to associate with posterior nuclei (Figures 5A and 5B). In some embryos, nos transport initiates properly but subsequently arrests, resulting in a pool of nos that is never incorporated into pole cells as well as in defective pole cell formation (Figure 5C).
Figure 5. Centrosomal microtubules mediate transport of germ plasm into pole cells for germ cell specification.
(A) Representative kymographs from time-lapse images of 1–2 hour old mutant embryos expressing nos*GFP: aurA− (aurA87Ac-3/aurA3); dtacc− (dtacc1/Df(3R)110). Images were generated from a 300 × 200 pixel region of interest at the embryo posterior (ROI) and show particle movement over 35 frames, approximately 12 seconds of developmental time. The x axis spans 18.83 μm. Numerous linear runs (arrowheads) are detected throughout the imaging period in wild-type embryos. nos particle motility is severely reduced in aurA mutants, although some short localized runs are observed (arrowheads), and is eliminated in this d-tacc mutant embryo (asterisk). (B) Trail image of a 1–2 hour old aurA− embryo showing reduced nos particle movement and minimal accumulation of nos around a nucleus (n; arrow). Twenty consecutive frames spanning 6.6 seconds are superimposed. (C) Z-series projection (30 μm) from a time-lapse sequence of a cnn− (cnnHK21) embryo showing inefficient and irregular incorporation of nos*GFP into malformed pole cells (arrows). (D, E) Anti-Vas immunofluorescence (green) and DAPI-stained nuclei (blue) in 0–2 hour old (D) or 3–5 hour old (E) embryos. Note that a large number of unfertilized d-tacc embryos lack detectable localized Vas (data not shown). Vas is present at the posterior of mutant embryos prior to pole cell formation (D) but it often fails to associate with posterior nuclei and pole cells are often absent or severely reduced in number (E). Arrow indicates a pole cell with little germ plasm. (F) Quantification of pole cell number is shown below the corresponding genotypes in (E). The mean ± SD is indicated in red for each genotype: WT (n=40 embryos); cnn− (n=33 embryos); aurA− (n=37 embryos); d-tacc− (n= 26 embryos); Dhc− (Dhc6–10/Dhc6-6, n=24 embryos). (G) Confocal Z-series projections of migrating germ cells from stage 10–13 wild-type (WT), cnn−, and Dhc− (Dhc6–10/Dhc6-6) embryos, immunostained for Vas (green) and for the mitotic marker phospho-histone H3 Ser10 (red). Mitotically active germ cells (asterisks) were not detected in wild-type embryos (n = 37) but were found in cnn− (n = 3/25) and Dhc− (n=3/30) embryos. Similar results were obtained with d-tacc mutant embryos (n = 2/28) although these embryos were more difficult to stage due to developmental defects (data not shown). aurA mutant embryos could not be properly analyzed due to severe earlier developmental defects. Scale bars: (B) 2.5 μm; (C, D, G) 10 μm.
Whereas germ plasm is properly localized at the posterior initially, impaired transport to posterior nuclei in cnn, aurA, and d-tacc mutant embryos is accompanied by a marked reduction in pole cell number as compared to wild-type (Figures 5D–5F). Moreover, pole cells that do form often have greatly reduced germ plasm (Figure 5E). The inefficient incorporation of germ plasm into pole cells may therefore decrease the concentration of factors required for normal germ cell development. Indeed, migrating germ cells that are positive for the mitotic marker phospho-histone H3 were detected at low frequency in mutant embryos, indicating that the mitotic quiescence characteristic of wild-type germ cells is not maintained (Figure 5G). Together, these results provide evidence that normal centrosome organization and microtubule integrity are necessary not only for germ plasm recruitment and incorporation into pole cells but also for germ cell specification.
Dynein-dependence of nos transport
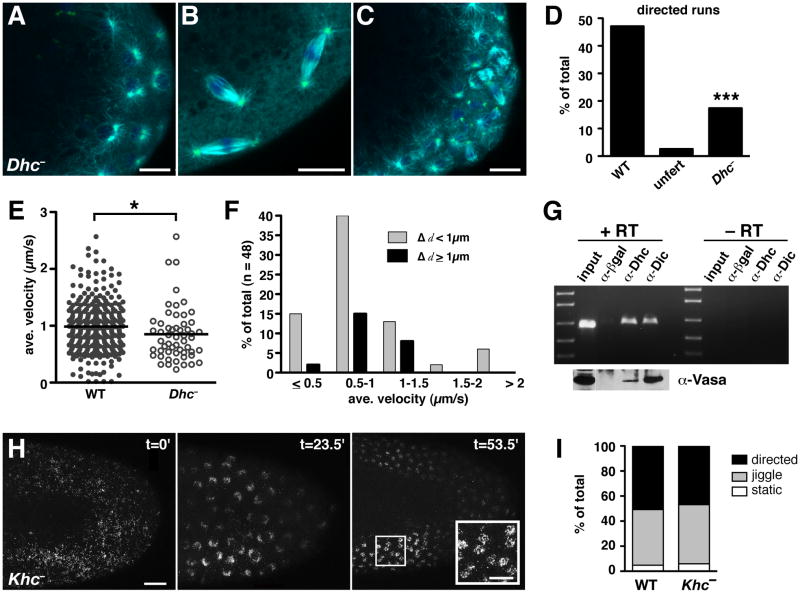
The rapid transit of germ plasm RNPs suggests that molecular motors are required for their transport. Moreover, use of centrosome-nucleated microtubules predicts that germ plasm transport should be mediated by a minus-end directed motor. We therefore tested a requirement for the dynein motor complex, which has previously been implicated in transport of a number of mRNAs within the Drosophila oocyte [9]. We made use of hypomorphic mutations in the ATPase motor domain of dynein heavy chain, Dhc 64C, which impair dynein function but permit oogenesis and production of fertilized eggs [26]. Microtubule organization is not entirely wild-type in embryos from Dhc mutant females, but the overall structure of the mitotic spindle with its astral microtubules is intact (Figures 6A–6C). Dhc mutant embryos also exhibit some nuclear migration defects, leading us to limit the analysis of nos particle movement to embryos with nuclei near the posterior cortex. Although we did observe embryo-to-embryo variability that is likely due to the hypormorphic nature of the alleles, all Dhc− allelic combinations examined showed a decrease in the fraction of nos*GFP or GFP-Vas particles undergoing directed movement (Figures 6D, S6, Movie S6.1, and data not shown). Of the motile nos*GFP particles, single-particle analysis indicates that the average velocity is modestly, but significantly, reduced in Dhc− mutant embryos relative to wild-type embryos. In addition, these slower particles travel shorter distances (Figures 6E, 6F, S6A–S6E). Thus, when combined with the decreased number of particles engaged in directed movement, this effect of Dhc mutation results in less nos associating with nuclei and a significant reduction in the amount of nos incorporated into pole cells (Movie S6.1). Like centrosomal protein mutants, Dhc mutants form fewer pole cells and exhibit germ cell specification defects (Figures 5E–G). Dynein dependence is further supported by the cessation of GFP-Vas motility after acute inhibition of dynein function by heat shock-induced overexpression of p50/dynamitin (Figure S6F).
Figure 6. Germ plasm transport requires dynein, but not kinesin, during pole cell formation.
(A–C) Immunofluorescence detection of microtubules (cyan), centrosomes (green), and DNA (blue) at the posterior of Dhc6–10/Dhc6-6 (Dhc−) embryos. (A, B) 0–1 hour old embryos with centrosomes and astral microtubules visible during interphase (A) and mitosis (B). (C) 1–2 hour old embryo. (D) Quantitation of motile nos*GFP particles in 30 second time-lapse images from 1–2 hour old wild-type (WT) embryos (n = 255 particles from 14 embryos), similarly aged unfertilized eggs (n = 311 particles from 6 eggs), and 1–2 hour old Dhc6–10/Dhc6–12 (Dhc−) embryos (n = 235 particles from 6 embryos). ***p < 0.001 as determined by the two-tailed Student’s t-test. (E) Scatter plot comparing the particle velocities in wild-type and Dhc6–10/Dhc6–12 embryos, with each data point representing a single motile particle. The solid line indicates the mean value (wild-type = 1.01 ± 0.44 μm/s; Dhc− = 0.89 ± 0.48 μm/s). *p ≤ 0.05 as determined by the Wilcoxon rank-sum test. (F) Distribution of the average velocities and run lengths (Δd) of nos*GFP particles in Dhc6–10/Dhc6–12 mutant embryos. (G) Co-immunoprecipitation analysis. RT-PCR detects nos following immunoprecipitiation of embryo extracts with antibodies for dynein heavy chain (α-Dhc), dynein intermediate chain (α-Dic), or a control antibody to β-galactosidase (α-βgal). Reactions were performed with (+) or without (−) reverse transcriptase (RT). Specific enrichment of nos in anti-Dhc and anti-Dic immunoprecipitates was confirmed under nonsaturating RT-PCR conditions (Figure S6G). Immunoblotting for Vas is shown below the corresponding samples in (G). (H) Time course showing the distribution of GFP-Vas in the posterior half of a Khc27 germline clone embryo (Khc−, anterior to the left). Before nuclear migration (t=0) Vas is dispersed over the entire cortex. Following nuclear migration and divisions (t=23.5′ and t=53.5′), Vas associates with nuclei. (I) Percentage of GFP-Vas particles undergoing movement in 1–2 hour old wild-type (WT) embryos (n = 189 particles from 4 embryos) and Khc− embryos (n = 755 particles from 4 embryos). Scale bars: (A–C) 10 μm; (H) 20 μm, inset 10 μm. See also Figure S6 and Movies S6.1 and S6.2.
A direct requirement for dynein in germ plasm transport predicts that germ plasm components should be physically associated with the dynein motor complex. We therefore immunoprecipitated dynein complexes from embryos 1–2 hours post-fertilization, using monoclonal antibodies for Dhc or the cargo-binding subunit, dynein intermediate chain (Dic), and analyzed the immunoprecipitates for germ plasm components. Both nos mRNA and Vas protein are immunoprecipitated by anti-Dhc and anti-Dic antibodies, but not by a control antibody (Figures 6G, S6G, and S6H). This biochemical evidence, together with the effects of disrupting dynein function on germ plasm motility and pole cell formation, strongly implicates a direct role for dynein in the transport of germ plasm for its inheritance by pole cells.
Kinesin is required for posteriorly localized germ plasm assembly but not for transport of germ plasm to nuclei
In many contexts, cargoes interact with both minus- and plus-end directed motors, sometimes simultaneously [27, 28]. To determine whether kinesin plays a role in germ plasm transport in addition to dynein, we analyzed the distribution and motility of germ plasm using GFP-Vas in embryos from Kinesin heavy chain (Khc) null germline clones. Posterior localization of osk during oogenesis is kinesin-dependent, and osk localizes indiscriminately around the oocyte cortex in Khc mutant ovaries. Moreover, Osk and Vas proteins also accumulate ectopically, suggesting that germ plasm assembly occurs around the entire cortex in these oocytes [29]. Whether the ectopic germ plasm persists to embryogenesis has not been determined. Examination of Khc mutant embryos showed that prior to nuclear migration, particles of GFP-Vas are dispersed over the cortex of the entire embryo (Figure 6H). Later, these particles accumulate indiscriminately around nuclei that have migrated to the cortex, resulting in the association of Vas with numerous nuclei far from the posterior pole (Figure 6H). Quantification of the frequency of movement, velocity, and run lengths of individual GFP-Vas particles shows that transport toward cortical nuclei is largely unaffected by the loss of kinesin (Figures 6I, S6I, S6J, and Movie S6.2). The dispensability of kinesin for transport of germ plasm properly assembled at the posterior pole was confirmed by microinjection of an immunoblocking antibody previously shown to impair Khc function (Figure S6K). Together, these results demonstrate that dynein, but not kinesin, is required for germ plasm transport within the embryo. Moreover, they provide further evidence that germ plasm transport is dictated by the most proximate nuclei and their associated microtubules.
Discussion
Production of functional germ cells is essential to species survival. In a wide variety of animals, a small population of germ cell precursors becomes distinct from the somatic tissue through the inheritance of a specialized, maternally provided germ plasm. Little is known, however, about the mechanisms that ensure the transmission of germ plasm mRNAs and proteins to germ cells. We have uncovered a dynamic mechanism for germ plasm inheritance involving release of germ plasm RNPs from the posterior cortical actin anchor coordinated with their dynein-dependent transport to centrosomes that are associated with posterior nuclei. Transport of these RNPs occurs primarily, if not exclusively, on astral microtubules throughout the mitotic cycle. Our results suggest that directed transport of germ plasm components during pole bud formation ensures the production of a discrete population of germ cell progenitors and partitions factors required for germline development during subsequent divisions. Through this process, germline fate determinants are segregated away from somatic nuclei.
Pole cell formation is highly sensitive to the dosage of germ plasm components, as mutations that reduce the accumulation of germ plasm at the posterior pole result in fewer pole cells [21, 30]. We find that pole cell formation is similarly reduced when germ plasm transport is disrupted, as it is in Dhc mutants or in mutants that affect centrosome function. Although the molecular mechanism by which germ plasm promotes pole cell formation is unknown, our results suggest that directed transport of germ plasm components toward the small subset of nuclei that are the first to arrive at the posterior pole provides the requisite concentration of one or more factors necessary to impart germline fate and induce pole cell formation. In addition, because the first divisions of the nascent pole cells occur before budding is complete, the persistence of germ plasm transport toward centrosomes during these divisions would ensure that factors required for germline development, such as nos, are maintained within pole buds, segregated to daughter nuclei, and ultimately incorporated into the forming germ cells.
Germ plasm produced ectopically in osk-bcd3′UTR and Khc mutant embryos is transported to nearby nuclei, indicating that nuclei are not pre-determined to recruit germ plasm. Thus, the release of germ plasm from its actin-based anchor and the onset of germ plasm motility must be tightly coordinated with the arrival of nuclei at the posterior cortex to target germ plasm specifically to these nuclei and prevent the mis-specification of cell fate. Egg activation triggers the release of bcd mRNA from the anterior cortex, probably through a generalized activation-dependent restructuring of the cortical actin cytoskeleton [19]. This event does not release nos and Vas, however. Nor is germ plasm release scheduled by an intrinsic timing mechanism, as we show here. Consistent with our observation that nos release is delayed at the anterior in osk-bcd3′UTR embryos, formation of ectopic germ cells at the anterior lags behind pole cell formation at the posterior in these animals. Moreover, centrosomes isolated from nuclei, either pharmacologically [23] or genetically (this study) are sufficient to trigger germ plasm release from the posterior. Our data thus support a model whereby centrosomes and/or centrosome-nucleated microtubules associated with migrating nuclei trigger germ plasm release from the cortical anchor.
Astral microtubules provide the tracks along which germ plasm RNPs travel upon their initial release from the cortex. During mitosis in the syncytial embryo, astral microtubules appear to secure the partitioning of germ plasm RNPs to daughter nuclei. The preferential association with astral microtubules may also prevent the dilution of inductive signals during asymmetric division events, when only one aster is proximal to the germ plasm. The apparent specificity for astral microtubules suggests that the RNP-motor complexes may include factors that recognize particular microtubule-associated proteins or modifications that distinguish these microtubules as preferred tracks [31].
The observed dynein-dependent transport of nos during pole cell formation contrasts with its diffusion-based mode of localization during oogenesis [10]. Given that dynein-dependent transport of bcd mRNA to the oocyte anterior is ongoing during late oogenesis [12], it is essential that nos be excluded from interaction with the dynein transport machinery. nos may reside in a dynein-associated transport complex that is inactive or incompatible with the various oocyte microtubule sub-populations [22]. Alternatively, the composition of the nos RNP in the oocyte may simply preclude its association with the dynein motor complex. The observed co-transport of nos and Vas in the embryo suggests that nos becomes linked to dynein through its packaging into a complex with Vas and other germ plasm components. Whether germ plasm RNPs are coupled to dynein motors while they are anchored at the posterior or only after their release remains a subject for future investigation. A similar switch between motor-independent and motor-dependent modes of germ plasm mRNA translocation may occur in Xenopus, although the role of motors in Xenopus germ plasm inheritance is not yet clear [32–34].
Recent in situ hybridization studies have now identified over 50 mRNAs that are localized at the posterior of the Drosophila embryo and incorporated into pole cells [35, 36]. Further characterization of a subset of these mRNAs showed that they accumulate near posterior nuclei suggesting that they may be transported similarly to nos. Determining whether the different transcripts are co-transported will require the development of methods to simultaneously visualize multiple RNAs and germ plasm proteins. However, packaging of even subsets of RNAs together into germ plasm RNPs competent for dynein-mediated transport would greatly simplify the difficulty of partitioning a complex pool of transcripts to pole cells.
Experimental Procedures
Experimental procedures are provided in the Supplemental material online.
Supplementary Material
Acknowledgments
We are grateful to T. Weil whose observation of nos*GFP particle motility during embryogenesis initiated this project, J. Brechbiel for characterizing nos-(ms2)18 expression levels, and J. Goodhouse and S. Thiberge, respectively, for assistance with confocal and two-photon microscopy. We thank D. Glover, P. Lasko, T. Megraw, T. Orr-Weaver, J. Raff, W. Saxton, R. Warrior, M. Welte, and the Bloomington Stock Center for fly stocks; T. Hays, T. Kaufman, P. Lasko, R. Lehmann, P. Schedl, E. Wieschaus, and the Drosophila Studies Hybridoma Bank for antibodies; and P. Lasko for the GFP-Vas plasmid. We are also grateful to G. Deshpande, J. Lee, H. Lipshitz, N. Siddiqui, and T. Schüpbach for comments on the manuscript; and to T. Weil, G. Deshpande and members of the Gavis lab for discussions and advice. This work was supported by NIH Center grant P50GM071508 to the Lewis-Sigler Institute, and by a Johnson & Johnson Imaging Center Award and NIH grant GM067758 to E.R.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Strome S, Lehmann R. Germ versus soma decisions: lessons from flies and worms. Science. 2007;316:392–393. doi: 10.1126/science.1140846. [DOI] [PubMed] [Google Scholar]
- 2.Mahowald AP. Assembly of the Drosophila germ plasm. Int Rev Cytol. 2001;203:187–213. doi: 10.1016/s0074-7696(01)03007-8. [DOI] [PubMed] [Google Scholar]
- 3.Foe VE, Alberts BM. Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J Cell Sci. 1983;61:31–70. doi: 10.1242/jcs.61.1.31. [DOI] [PubMed] [Google Scholar]
- 4.Santos AC, Lehmann R. Germ cell specification and migration in Drosophila and beyond. Curr Biol. 2004;14:R578–589. doi: 10.1016/j.cub.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Gavis ER, Lehmann R. Localization of nanos RNA controls embryonic polarity. Cell. 1992;71:301–313. doi: 10.1016/0092-8674(92)90358-j. [DOI] [PubMed] [Google Scholar]
- 6.Lehmann R, Nusslein-Volhard C. The maternal gene nanos has a central role in posterior pattern formation of the Drosophila embryo. Development. 1991;112:679–691. doi: 10.1242/dev.112.3.679. [DOI] [PubMed] [Google Scholar]
- 7.Ewen-Campen B, Schwager EE, Extavour CG. The molecular machinery of germ line specification. Mol Reprod Dev. 2010;77:3–18. doi: 10.1002/mrd.21091. [DOI] [PubMed] [Google Scholar]
- 8.Gavis ER, Chatterjee S, Ford NR, Wolff LJ. Dispensability of nanos mRNA localization for abdominal patterning but not for germ cell development. Mech Dev. 2008;125:81–90. doi: 10.1016/j.mod.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becalska AN, Gavis ER. Lighting up mRNA localization in Drosophila oogenesis. Development. 2009;136:2493–2503. doi: 10.1242/dev.032391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forrest KM, Gavis ER. Live imaging of endogenous RNA reveals a diffusion and entrapment mechanism for nanos mRNA localization in Drosophila. Curr Biol. 2003;13:1159–1168. doi: 10.1016/s0960-9822(03)00451-2. [DOI] [PubMed] [Google Scholar]
- 11.Lantz VA, Clemens SE, Miller KG. The actin cytoskeleton is required for maintenance of posterior pole plasm components in the Drosophila embryo. Mech Dev. 1999;85:111–122. doi: 10.1016/s0925-4773(99)00096-9. [DOI] [PubMed] [Google Scholar]
- 12.Weil TT, Forrest KM, Gavis ER. Localization of bicoid mRNA in late oocytes is maintained by continual active transport. Dev Cell. 2006;11:251–262. doi: 10.1016/j.devcel.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Mahowald AP. Fine structure of pole cells and polar granules in Drosophila melanogaster. J Exp Zool. 1962;151:201–215. [Google Scholar]
- 14.Counce SJ. Developmental morphology of polar granules in Drosophila. Including observations on pole cell behavior and distribution during embryogenesis. J Morphol. 1963;112:129–145. [Google Scholar]
- 15.Strome S. WormBook. 2005. Specification of the germ line; pp. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavis ER, Curtis D, Lehmann R. Identification of cis-acting sequences that control nanos RNA localization. Dev Biol. 1996;176:36–50. doi: 10.1006/dbio.1996.9996. [DOI] [PubMed] [Google Scholar]
- 17.Raff JW, Whitfield WGF, Glover DM. Two distinct mechanisms localise cyclin B transcripts in syncytial Drosophila embryos. Development. 1990;110:1249–1261. doi: 10.1242/dev.110.4.1249. [DOI] [PubMed] [Google Scholar]
- 18.Warn RM, Smith L, Warn A. Three distinct distributions of F-actin occur during the divisions of polar surface caps to produce pole cells in Drosophila embryos. J Cell Biol. 1985;100:1010–1015. doi: 10.1083/jcb.100.4.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weil TT, Parton R, Davis I, Gavis ER. Changes in bicoid mRNA anchoring highlight conserved mechanisms during the oocyte-to-embryo transition. Curr Biol. 2008;18:1055–1061. doi: 10.1016/j.cub.2008.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimyanin VL, Belaya K, Pecreaux J, Gilchrist MJ, Clark A, Davis I, St Johnston D. In vivo imaging of oskar mRNA transport reveals the mechanism of posterior localization. Cell. 2008;134:843–853. doi: 10.1016/j.cell.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ephrussi A, Lehmann R. Induction of germ cell formation by oskar. Nature. 1992;358:387–392. doi: 10.1038/358387a0. [DOI] [PubMed] [Google Scholar]
- 22.St Johnston D. Moving messages: the intracellular localization of mRNAs. Nat Rev Mol Cell Biol. 2005;6:363–375. doi: 10.1038/nrm1643. [DOI] [PubMed] [Google Scholar]
- 23.Raff JW, Glover DM. Centrosomes, and not nuclei, initiate pole cell formation in Drosophila embryos. Cell. 1989;57:611–619. doi: 10.1016/0092-8674(89)90130-x. [DOI] [PubMed] [Google Scholar]
- 24.Shamanski FL, Orr-Weaver TL. The Drosophila plutonium and pan gu genes regulate entry into S phase at fertilization. Cell. 1991;66:1289–1300. doi: 10.1016/0092-8674(91)90050-9. [DOI] [PubMed] [Google Scholar]
- 25.Peset I, Vernos I. The TACC proteins: TACC-ling microtubule dynamics and centrosome function. Trends Cell Biol. 2008;18:379–388. doi: 10.1016/j.tcb.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 26.McGrail M, Hays TS. The microtubule motor cytoplasmic dynein is required for spindle orientation during germline cell divisions and oocyte differentiation in Drosophila. Development. 1997;124:2409–2419. doi: 10.1242/dev.124.12.2409. [DOI] [PubMed] [Google Scholar]
- 27.Welte MA, Gross SP. Molecular motors: a traffic cop within? HFSP J. 2008;2:178–182. doi: 10.2976/1.2956447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bullock SL. Translocation of mRNAs by molecular motors: think complex? Semin Cell Dev Biol. 2007;18:194–201. doi: 10.1016/j.semcdb.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Cha BJ, Serbus LR, Koppetsch BS, Theurkauf WE. Kinesin I-dependent cortical exclusion restricts pole plasm to the oocyte posterior. Nat Cell Biol. 2002;4:592–598. doi: 10.1038/ncb832. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi S, Sato K, Hayashi Y. The role of mitochondrial rRNAs and nanos protein in germline formation in Drosophila embryos. Zoolog Sci. 2005;22:943–954. doi: 10.2108/zsj.22.943. [DOI] [PubMed] [Google Scholar]
- 31.Verhey KJ, Gaertig J. The tubulin code. Cell Cycle. 2007;6:2152–2160. doi: 10.4161/cc.6.17.4633. [DOI] [PubMed] [Google Scholar]
- 32.King ML, Messitt TJ, Mowry KL. Putting RNAs in the right place at the right time: RNA localization in the frog oocyte. Biol Cell. 2005;97:19–33. doi: 10.1042/BC20040067. [DOI] [PubMed] [Google Scholar]
- 33.Savage RM, Danilchik MV. Dynamics of germ plasm localization and its inhibition by ultraviolet irradiation in early cleavage Xenopus embryos. Dev Biol. 1993;157:371–382. doi: 10.1006/dbio.1993.1142. [DOI] [PubMed] [Google Scholar]
- 34.Quaas J, Wylie C. Surface contraction waves (SCWs) in the Xenopus egg are required for the localization of the germ plasm and are dependent upon maternal stores of the kinesin-like protein Xklp1. Dev Biol. 2002;243:272–280. doi: 10.1006/dbio.2001.0564. [DOI] [PubMed] [Google Scholar]
- 35.Rangan P, DeGennaro M, Jaime-Bustamante K, Coux RX, Martinho RG, Lehmann R. Temporal and spatial control of germ-plasm RNAs. Curr Biol. 2009;19:72–77. doi: 10.1016/j.cub.2008.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lecuyer E, Yoshida H, Parthasarathy N, Alm C, Babak T, Cerovina T, Hughes TR, Tomancak P, Krause HM. Global analysis of mRNA localization reveals a prominent role in organizing cellular architecture and function. Cell. 2007;131:174–187. doi: 10.1016/j.cell.2007.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.