Abstract
A persistent negative energy balance results in bone loss. It is not clear whether the bone loss associated with chronic negative energy balance can be prevented. The objective of this study was to assess the efficacy of intermittent low dose parathyroid hormone (PTH) treatment in maintaining normal bone formation during severe energy restriction. Six-month-old male Fisher 344 rats were divided into 4 treatment groups: (1) baseline, (2) ad libitum (ad lib)-fed control, (3) energy-restricted (to consume 40% ad lib caloric intake), or (4) energy-restricted + low dose (1 μg/kg/d) PTH. Severe energy restriction for 14 days decreased body weight and serum leptin levels. Compared to ad lib-fed controls, energy-restricted rats had lower cancellous bone formation, higher osteoclast perimeter/bone perimeter and higher bone marrow adiposity in the proximal tibial metaphysis. Also, the energy-restricted rats had a lower periosteal bone formation rate at the tibia-fibula synostosis. Administration of PTH to energy-restricted rats had no effect on weight loss or osteoclast perimeter/bone perimeter. In contrast, energy-restricted rats treated with PTH had higher rates of cancellous and cortical bone formation compared to energy-restricted rats, and did not differ from the ad lib-fed control animals. Furthermore, PTH treatment maintained normal bone marrow adiposity. In conclusion, rapid weight loss in adult male rats was accompanied by decreased bone formation and increased bone marrow adiposity and these changes were prevented by low dose PTH treatment. Taken together, the results suggest that the energy cost of bone formation in adult rats is low and PTH therapy is effective in preventing the reduced bone formation associated with rapid weight loss.
Keywords: bone remodeling, energy restriction, osteoporosis, obesity
INTRODUCTION
Energy availability and accrual of bone mass during growth are closely coupled. Bone mass was positively associated with weight gain in growing mice fed normal and high fat diets [1]. In contrast, energy restriction sufficient to decrease body weight gain reduced accrual of bone mass. The skeletal changes during moderate energy restriction in growing rodents were mediated by a combination of increased bone resorption and decreased bone formation [2], and lifelong energy restriction was reported to decrease peak bone mass [3–5].
Once peak bone mass has been achieved, the relationship between bone mass and energy availability is less clear cut. In skeletally mature rats, moderate weight gain had no positive effect on bone metabolism [6]. Similarly, weight maintenance, due to moderate energy restriction, had no negative effects on bone [6]. Thus, there may be differences between the responses of the growing and adult skeletons to changes in energy availability.
In adult humans, a negative energy balance resulting in rapid weight loss results in bone loss and increased risk for osteoporosis [7–11]. The bone loss has been variously attributed to decreased bone formation, increased bone resorption, and increased bone turnover with a net increase in bone resorption [12–15]. The lack of consistency among studies suggests that multiple factors can modify the skeletal response to energy restriction. Confounding variables include gender, age, ethnicity, body mass index, duration of energy restriction, individual macro and micronutrient deficiencies, metabolic effects of weight loss medications, and lifestyle factors, such as physical activity, alcohol consumption or cigarette smoking, known to influence bone metabolism [11].
Exercise has been evaluated and shown to have limited efficacy in reducing bone loss during weight loss [9, 11]. An alternative and less explored approach is the use of pharmacological agents to prevent the detrimental skeletal effects of a negative energy balance. Intermittent (typically once daily) administration of parathyroid hormone (PTH) is bone anabolic [16–19]. Approved for treatment of postmenopausal osteoporosis, PTH was shown to be effective in stimulating bone formation in rat models for a variety of metabolic bone diseases. These included disuse, chronic alcohol abuse, and deficiencies in estrogen, androgen and growth hormone [16, 19–22]. To our knowledge, the efficacy of PTH in preventing the detrimental skeletal effects of weight loss has not been investigated. Therefore, the major goals of this study were to 1) determine the effects of rapid weight loss due to severe energy restriction on bone formation, and 2) assess the efficacy of intermittent PTH on blunting the expected decrease in bone formation.
MATERIALS AND METHODS
Animals
Six-month-old male Fisher 344 rats (Harlan Sprague-Dawley, Indianapolis, IN) were housed in individual cages on a 12:12-h light-dark cycle. The animals were maintained in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the experimental protocol was approved by the Institutional Animal Care and Use Committee.
Experimental Design
The rats were fed a diet (Rodent Blox 8604, HarlanTeklad, Madison, WI) which contained 1.5% calcium, 1% phosphorous and 5 IU/g vitamin D. The rats were randomized by weight into 4 treatment groups: (1) baseline sacrificed at treatment initiation (n = 10), (2) ad libitum (ad lib)-fed (n = 10), (3) energy-restricted to consume 40% of the ad lib caloric intake (n = 10), or (4) energy-restricted + PTH (n = 7). Recombinant human PTH (1—34; 1 μg/kg/day; Eli Lilly, Indianapolis, IN) or vehicle (0.1ml 2% heat-inactivated rat serum in acidified saline) was administered daily via sc injection for 14 days. The short duration of study minimized the confounding effects of a change in body mass on bone metabolism [15]. The rats were weighed every 4 days and at necropsy. Fluorochromes were administered 1 day prior to treatment initiation (20 mg/kg oxytetracycline; Sigma Chemical, St Louis, MO) and 10 and 2 days (calcein, 10 mg/kg; Sigma Chemical, St Louis, MO) prior to sacrifice to label mineralizing bone for assessment of rates of bone formation. The baseline group was sacrificed on day 0, the start of the energy restriction.
Tissue Collection
For tissue collection, the ratswere weighed, anesthetized with CO2, and decapitated. Trunk blood was collected and serum stored at −20°C for leptin evaluation. Seminalvesicles (a biomarker for changes in androgen levels) were excised and weighed. The right tibiae were removed, cleaned of tissue and tibial length was measured using aprecision caliper. The tibiae were then placed in 70% ethanol for histomorphometric evaluation.
Serum Measurements
Serum leptin levels were determined by immunoassay as described [23].
Bone Histomorphometry
Cancellous Bone Histomorphometry
For histomorphometric evaluation of cancellous bone, proximal tibiae were dehydrated and embedded without demineralizationto preserve fluorochrome labels as described [19]. Longitudinal sections (5 μm thick) were cut using a vertical bed microtome (Leica 2065). An area of 2.8 mm2,1 mm distal to the growth plate, was analyzed as described [19]. Static bone measurements consisted of bone area/tissue area (%) and the derived architectural indices of trabecular thickness (μm), trabecular number (mm−1), and trabecular separation (μm). Measurements of bone marrow adiposity included adipocyte area/tissue area (%), adipocyte density (#/mm2), and adipocyte size (μm2). These were determined as described and validated [23]. Additionally, osteoclast perimeter/bone perimeter (%) and osteoblast perimeter/bone perimeter (%) were measured as described [20]. Fluorochrome-based indices of bone formation, including mineralizing perimeter/bone perimeter (percentage of cancellous bone perimeter with a double fluorochrome label + single fluorochrome label, %), and mineral apposition rate (μm/d) were also measured, and bone formation rate was calculated using perimeter (μm2/μm/d), bone area (%/d), and tissue area (%/d) referents [19].
Cortical Bone Histomorphometry
For measurement of cortical bone, sections were cut proximal to the tibia-fibula synostosisand prepared as described [19]. Cross-sectional area (mm2), cortical bone area (mm2), medullary area (mm2), and periosteal mineralizing (double-labeled) perimeter (mm), mineral apposition rate (μm/d), and bone formation rate (mm2 x 10−3/day) were determined.
All cancellous and cortical histomorphometric measurements were performed using the OsteoMeasure Analysis System (OsteoMetrics, Atlanta GA).
Statistical Analysis
One-way ANOVA followed by a Bonferroni post-hoc test was used to evaluate differences among treatment groups. If ANOVA assumptions of homogeneity of variance were not met, a Kruskal-Wallis followed by a Tamhane post hoc test was used. A paired t-test was used to compare baseline and terminal body weight within a treatment group. Differences were considered significant at p<0.05. All data are expressed as mean ± SE.
RESULTS
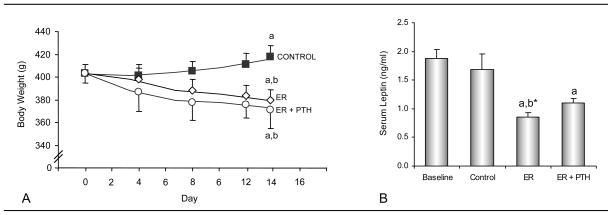
The effects of severe energy restriction and treatment with PTH on body weight as a function of time and on serum leptin levels at termination of treatment are shown in Figure 1. Whereas body weight increased in control (+5%) rats, energy restriction to 40% of ad lib food intake resulted in weight loss in energy-restricted (−4.5%) and PTH-treated energy-restricted (−4.2%) rats. At the completion of the 2 week intervention, ad lib control rats were heavier than energy-restricted rats or PTH-treated energy-restricted rats. Serum leptin levels were lower in energy-restricted rats compared to animals sacrificed at baseline irrespective of PTH treatment. PTH treatment had no effect on weight loss or serum leptin levels in energy-restricted rats. Significant differences among groups were not detected for seminal vesicle weight (data not shown) suggesting that short duration energy restriction did not result in reduced circulating androgen levels [24].
Figure 1.
Effects of energy restriction (ER) and energy restriction + PTH on body weight (A) and serum leptin levels (B) in 6-month-old male rats. Values are mean ± SE. aDifferent from Baseline, P<0.05; a* P<0.1; bDifferent from Control, P<0.05; b* P<0.1.
The effects of energy restriction and treatment with PTH on tibia length and cancellous bone mass and architecture in the proximal tibial metaphysis are shown in Table 1. Significant differences in tibial length were not detected among baseline and treatment groups. In concordance, the oxytetracycline label administered 1 day prior to treatment initiation was located withinthe calcified growth-plate cartilage (not shown), indicating that minimal longitudinal bone growth had occurred during the experiment. As a consequence, longitudinal growth rate, as normally determined by sequential fluorochrome labeling, was too low to measure. Significant differences between baseline and treatment groups were not detected for either cancellous bone area/tissue area or any of the architectural endpoints evaluated (trabecular number, trabecular thickness, and trabecular separation). However, there was a tendency (p=0.08) for the PTH-treated energy-restricted rats to have a higher cancellous bone area/tissue area than baseline rats. Significant differences between ad lib-fed control and energy-restricted rats were not detected for any of the endpoints evaluated. PTH-treated energy-restricted rats had greater bone area/tissue area compared to energy-restricted rats. Significant differences in trabecular number, trabecular thickness, or trabecular separation were not detected between energy-restricted and PTH-treated energy-restricted rats.
Table 1.
Effects of energy restriction (ER) and energy restriction + PTH on tibial length, cancellous bone in the proximal tibial metaphysis, and cortical bone in the tibial diaphysis of 6-month-old male rats.
| Endpoint | Baseline | Control | ER | ER + PTH | ANOVA P< |
|---|---|---|---|---|---|
| Tibia length (cm) | 4.4 ± 0.0 | 4.3 ± 0.1 | 4.4 ± 0.0 | 4.4 ± 0.1 | NS |
| Proximal tibia (cancellous bone) | |||||
| Bone area/tissue area (%) | 25.0 ± 1.0 | 23.8 ± 2.7 | 21.5 ± 2.0 | 29.7 ± 1.3a*,c | 0.048 |
| Trabecular number (mm−1) | 3.3 ± 0.1 | 3.1 ± 0.2 | 2.9 ± 0.2 | 3.5 ± 0.2 | 0.050 |
| Trabecular thickness (μm) | 77 ± 3 | 75 ± 4 | 74 ± 4 | 85 ± 3 | NS |
| Trabecular separation (μm) | 234 ± 11 | 258 ± 30 | 287 ± 25 | 204 ± 13 | 0.093 |
| Tibial diaphysis (cortical bone) | |||||
| Cross-sectional area (mm2) | 3.54 ± 0.15 | 3.46 ± 0.12 | 3.48 ± 0.10 | 3.35 ± 0.11 | NS |
| Cortical area (mm2) | 2.56 ± 0.14 | 2.36 ± 0.12 | 2.51 ± 0.08 | 2.37 ± 0.13 | NS |
| Marrow area (mm2) | 0.91 ± 0.13 | 1.10 ± 0.07 | 0.97 ± 0.07 | 0.99 ± 0.06 | NS |
Values are mean ± SE
Different from Baseline, P<0.05;
P<0.1
Different from ER, P<0.05
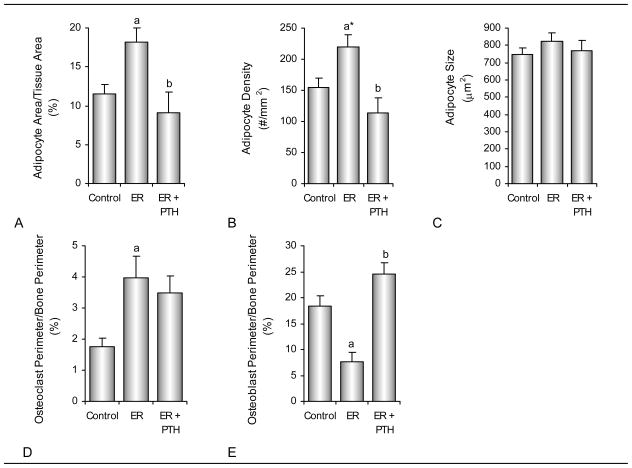
The effects of energy restriction and treatment with PTH on bone marrow adiposity, osteoclast perimeter/bone perimeter, and osteoblast perimeter/bone perimeter are shown in Figure 2. Bone marrow adipocyte area/tissue area was greater in energy-restricted rats compared to either ad lib-fed or PTH-treated energy-restricted rats. Adipocyte density tended to be greater (P=0.05) or was greater in energy-restricted compared to ad lib-fed and PTH-treated energy-restricted rats, respectively. There was no difference among groups in adipocyte size and adipocyte area/tissue area and adipocyte density did not differ between PTH-treated energy-restricted rats and ad lib controls. Osteoclast perimeter/bone perimeter was greater in energy-restricted rats compared ad lib-fed rats. Osteoblast perimeter/bone perimeter was lower in energy-restricted rats compared to either ad lib-fed or PTH-treated energy-restricted rats.
Figure 2.
Effects of energy restriction (ER) and energy restriction + PTH on bone marrow adiposity, osteoclasts, and osteoblasts in proximal tibial metaphysis of 6-month-old male rats. Shown are adipocyte area/tissue area (A), adipocyte density (B), adipocyte size (C), osteoclast perimeter/bone perimeter (D), and osteoblast perimeter/bone perimeter (E). Values are mean ± SE. aDifferent from Control, P<0.05; a* P<0.1; bDifferent from ER, P<0.05.
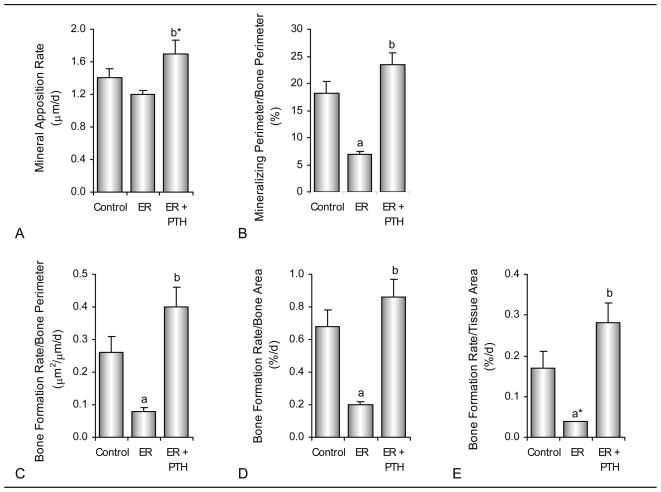
The effects of energy restriction and treatment with PTH on dynamic cancellous bone histomorphometry are shown in Figure 3. Significant differences between ad lib fed control and energy-restricted rats were not detected for mineral apposition rate. However, mineralizing perimeter and bone formation rate (perimeter, bone area, or tissue area referents) were lower in the energy-restricted rats compared to the ad lib control rats. Energy-restricted rats treated with PTH tended (p=0.08) to have higher mineral apposition rate and bone formation rate (tissue referent), and had higher mineralizing perimeter (bone perimeter referent) and bone formation rate (bone perimeter and bone area referents) compared to energy-restricted rats. Mineralizing perimeter, mineral apposition rate, and bone formation rate in energy-restricted rats treated with PTH did not differ from ad lib control rats.
Figure 3.
Effects of energy restriction (ER) and energy restriction + PTH on dynamic cancellous bone histomorphometry in proximal tibial metaphysis of 6-month-old male rats. Shown are mineral apposition rate (A), mineralizing perimeter/bone perimeter (B) and bone formation rate calculated using bone perimeter (C), bone area (D) and tissue area (E) referents. Values are mean ± SE. aDifferent from Control, P<0.05; a* P<0.1; bDifferent from ER, P<0.05; b* P<0.1.
The effects of energy restriction and treatment with PTH on cortical bone mass and architecture in the tibial diaphysis are shown in Table 1. Significant differences among treatment groups were not detected for cross sectional area, cortical bone area, or medullary area.
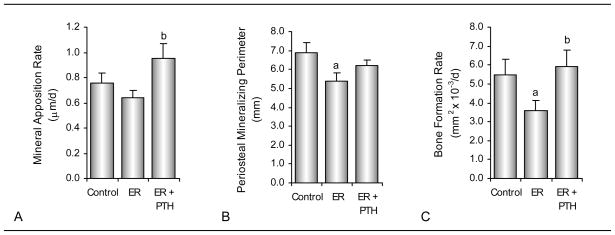
The effects of energy restriction and treatment with PTH on dynamic periosteal bone measurements are shown in Figure 4. Mineral apposition rate did not differ between ad lib control and energy-restricted rats. However, PTH treatment resulted in a higher mineral apposition rate in energy-restricted rats compared to energy restriction alone. Periosteal mineralizing perimeter and bone formation rate were lower in energy-restricted rats compared to ad lib control rats. Periosteal bone formation rate were higher in energy-restricted PTH-treated rats compared to the energy-restricted rats. Significant differences in periosteal mineralizing perimeter and bone formation rate were not detected between energy-restricted PTH-treated rats and ad lib control rats.
Figure 4.
Effects of energy restriction and energy restriction + PTH on dynamic cortical bone histomorphometry in tibial diaphysis of 6-month-old male rats. Shown are mineral apposition rate (A), mineralizing perimeter (B) and bone formation rate (C). Values are mean ± SE. aDifferent from Control, P<0.05; bDifferent from ER, P<0.05.
DISCUSSION
Rapid weight loss was induced in 6-month-old male rats by severe energy restriction. The reduction in energy intake to 40% of normal is similar to levels of energy restriction often used to induce rapid weight loss in human subjects [25] and larger than the energy restriction typically used (60–80% of normal) to slow weight gain in growing rodents [3–5, 26]. Weight loss was accompanied by lower indices of bone formation and higher osteoclast perimeter/bone perimeter and bone marrow adiposity. PTH treatment had no effect on weight loss in energy-restricted rats but maintained normal levels of cancellous and cortical bone formation. In addition, PTH treatment prevented the increase in bone marrow adiposity associated with energy restriction.
Fluorochrome labeling provides an estimate of bone formation during the interval between sequential labels. However, the double fluorochrome labeling used in this study may underestimate the inhibitory effect of a negative energy balance on bone formation because the initial label was administered only 5 days following initiation of energy restriction. In spite of this limitation, mineralizing perimeter/bone perimeter, an index of osteoblast number, was found to be reduced in proximal tibial metaphysis and on the periosteal surface at the tibia-fibula synostosis. Furthermore, osteoblast perimeter/bone perimeter was similarly reduced in energy-restricted rats. In contrast, mineral apposition rate was not significantly altered at either sampling site, suggesting that energy restriction had no short-term effect on deposition and mineralization of bone matrix by active osteoblasts. In contrast, osteoclast perimeter/bone perimeter was increased in energy-restricted rats. Taken together, these observations suggest that severe energy restriction decreases bone formation and increases bone resorption by decreasing osteoblast perimeter and increasing osteoclast perimeter, respectively.
There is substantive evidence that skeletal growth is sensitive to changes in energy balance [27–29]. Although the precise relationship depends on genetic background and skeletal site, bone mass and body size in growing mice fed normal and high fat diets are highly correlated [1]. Additionally, suboptimal energy intake (for maximum weight gain) reduces peak bone mass in growing rodents [2, 5] and minipigs [30]. In girls, anorexia nervosa slows bone growth but need not drastically reduce adult stature because epiphyseal closure is delayed and bone growth continues, albeit at a slower rate and for a longer interval of time [31]. Increasing energy availability following energy deprivation results in at least partial growth recovery [32, 33]. This tight coupling between energy availability and growth may provide a survival advantage to cope with intervals of food scarcity.
To our knowledge, the energy cost of bone formation has not been established. The present study was performed in young adult rats exhibiting minimal skeletal growth. Increases in tibia length, cancellous bone area, or cross-sectional and cortical area were not observed between baseline and control rats sacrificed 14 days later. Also, tibial longitudinal growth rate, assessed using the very sensitive double fluorochrome labeling method, was below detection limit. PTH treatment maintained bone formation during energy restriction but did not accelerate weight loss or result in a further reduction in serum leptin levels, suggesting that the energy cost of bone remodeling in adult male rats is relatively low. We cannot, however, rule out the possibility that the energy cost of bone growth in young animals is higher and contributes to the skeletal sensitivity of growing rodents to moderate energy restriction.
A deficiency in one or more critical micronutrients, such as calcium or phosphorus, results in impaired bone growth even when energy requirements are met [34]. Although the energy-restricted diet was not adjusted in this study to compensate for reductions in micronutrient intake, the diet contained calcium, phosphorous and vitamin D in great excess of that required to maintain normal bone and mineral homeostasis [35]. Additionally, the observed efficacy of PTH to maintain bone formation rate argues against micronutrient deficiency as a contributing factor in impaired bone formation due to energy restriction.
Leptin plays an important role in bone metabolism and energy restriction results in decreased serum leptin levels [2], a finding reproduced in the current study. Leptin receptor-deficient (fa/fa) rats, in spite of morbid obesity, have reduced cancellous and cortical bone mass [36]. This finding suggests that leptin is bone anabolic in the rat. However, other studies suggest that leptin acts primarily as a permissive factor necessary for optimal bone growth and maturation [6, 37]. In support of the latter view, diet-induced changes in leptin levels within the normal physiological range had no effect on bone metabolism in skeletally mature rats [6].
The mechanism for the skeletal response to severe energy restriction in rats is likely multifactorial. We speculate that it involves disruption of growth hormone (GH) signaling. GH is believed to be the single most important determinant of postnatal growth, accounting for over 80% of variation in body size [38]. Energy restriction results in increased GH secretion, decreased circulating levels of IGF-I, and end-organ resistance to GH [7, 29, 39–41]. Hypophysectomy (HYPOX) results in hypophagia and, similar to energy restriction, greatly decreased body weight gain, suppressed bone formation, and increased bone marrow adiposity [23, 42]. Furthermore, PTH, possibly due to its ability to increase skeletal production of IGF-I, was able to reverse the inhibitory effects of HYPOX on bone formation without altering HYPOX-induced changes in growth or energy metabolism [20, 23]. Thus, the skeletal response to severe energy restriction and PTH observed in the present study is consistent with, but not proof for, deficient GH signaling.
Peripheral fat depots act as dynamic energy reservoirs to maintain circulating triglyceride and free fatty acid levels. These depots are depleted during negative energy balance [43]. In contrast, a negative energy balance results in increased bone marrow adiposity [44–48]. Also, during starvation bone marrow adipocytes sequester and esterify fatty acids [49–54]. These observations suggest that the bone marrow fat depot does not function as an important energy reservoir for peripheral tissues. Rather, recent studies suggest that bone marrow adipocytes act as a negative regulator of hematopoietic lineage cell differentiation [51, 55–57]. It is notable that PTH treatment prevented the increase in bone marrow adiposity during severe energy restriction. The mechanism for this action is not certain but there is evidence that PTH signaling plays a major role in the ontogeny of the bone marrow. Expression of a constitutively active PTH/PTHrP receptor in osteogenic cells delayed the appearance of adipocytes in bone marrow [58], whereas administration of PTH to rodents or humans expanded the number of bone marrow hematopoietic stem cells [59].
The bone anabolic effects of intermittent PTH have been studied extensively and human PTH (1–34) is approved by the Federal Drug Administration for the treatment of postmenopausal osteoporosis. In the present study, we evaluated the efficacy of 1 μg/kg/d PTH in preventing the reduction in bone formation in energy-restricted rats. Compared to the human therapeutic dose (20 μg/patient/d), much higher doses of PTH (40 – 400 μg/kg body weight/d) have typically been studied in rodents [60–62]. Based on differences in metabolism, Komatsu et al. [60] concluded that the rat equivalent of a human therapeutic dose of PTH is ~3 μg/kg/d. Dose-response studies have shown that rats are responsive to even lower doses of PTH [63] and, furthermore, high doses of the hormone in animal models interfere with the ability to assess interactions between PTH and lifestyle factors such as physical activity [64]. As a consequence, utilization of very high daily doses of the hormone may overestimate the efficacy of PTH. The much lower dose of PTH used in the present study, although not necessarily directly comparable to humans because of species differences in physiology, was previously shown to have a bone anabolic effect on cancellous bone in normal rats, and rat models for chronic alcohol abuse, growth hormone deficiency, and disuse [17, 23, 64].
In summary, rapid weight loss induced by severe energy restriction decreased bone formation and increased osteoclast perimeter/bone perimeter and bone marrow adiposity. Administration of low dose PTH maintained normal bone formation and bone marrow adiposity, without a further increase in osteoclast perimeter/bone perimeter, in the energy-restricted rats. These results suggest that PTH treatment may be an effective countermeasure to prevent detrimental skeletal side effects associated with rapid weight loss due to energy restriction.
Acknowledgments
Funding Support: This work was supported by National Institute of Health grants AA 011140 (to R.T Turner) and AR 054609 (to U.T. Iwaniec) and National Aeronautics and Space AdministrationGrant NAG9-1458 (to R.T. Turner).
Human recombinant PTH (1–34) was a gift from Eli Lilly, Indianapolis, IN.
References Cited
- 1.Iwaniec UT, Dube MG, Boghossian S, Song H, Helferich WG, Turner RT, Kalra SP. Body mass influences cortical bone mass independent of leptin signaling. Bone. 2009;44:404–12. doi: 10.1016/j.bone.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devlin M, Cloutier A, Thomas N, Panus D, Lotinun S, Pinz I, Baron R, Rosen C, Bouxsein M. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brochmann EJ, Duarte ME, Zaidi HA, Murray SS. Effects of dietary restriction on total body, femoral, and vertebral bone in SENCAR, C57BL/6, and DBA/2 mice. Metabolism. 2003;52:1265–73. doi: 10.1016/s0026-0495(03)00194-x. [DOI] [PubMed] [Google Scholar]
- 4.Hamrick MW, Ding KH, Ponnala S, Ferrari SL, Isales CM. Caloric restriction decreases cortical bone mass but spares trabecular bone in the mouse skeleton: implications for the regulation of bone mass by body weight. J Bone Miner Res. 2008;23:870–8. doi: 10.1359/jbmr.080213. [DOI] [PubMed] [Google Scholar]
- 5.Tatsumi S, Ito M, Asaba Y, Tsutsumi K, Ikeda K. Life-long caloric restriction reveals biphasic and dimorphic effects on bone metabolism in rodents. Endocrinology. 2008;149:634–41. doi: 10.1210/en.2007-1089. [DOI] [PubMed] [Google Scholar]
- 6.Turner RT, Iwaniec UT. Moderate weight gain does not influence bone metabolism in skeletally mature female rats. Bone. 2010;47:631–635. doi: 10.1016/j.bone.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grinspoon SK, Baum HB, Kim V, Coggins C, Klibanski A. Decreased bone formation and increased mineral dissolution during acute fasting in young women. J Clin Endocrinol Metab. 1995;80:3628–33. doi: 10.1210/jcem.80.12.8530611. [DOI] [PubMed] [Google Scholar]
- 8.Park HA, Lee JS, Kuller LH, Cauley JA. Effects of weight control during the menopausal transition on bone mineral density. J Clin Endocrinol Metab. 2007;92:3809–15. doi: 10.1210/jc.2007-1040. [DOI] [PubMed] [Google Scholar]
- 9.Prouteau S, Benhamou L, Courteix D. Relationships between serum leptin and bone markers during stable weight, weight reduction and weight regain in male and female judoists. Eur J Endocrinol. 2006;154:389–95. doi: 10.1530/eje.1.02103. [DOI] [PubMed] [Google Scholar]
- 10.Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA. Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Res. 2005;20:455–63. doi: 10.1359/JBMR.041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapses SA, Riedt CS. Bone, body weight, and weight reduction: what are the concerns? J Nutr. 2006;136:1453–6. doi: 10.1093/jn/136.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucey AJ, Paschos GK, Cashman KD, Martinez JA, Thorsdottir I, Kiely M. Influence of moderate energy restriction and seafood consumption on bone turnover in overweight young adults. Am J Clin Nutr. 2008;87:1045–52. doi: 10.1093/ajcn/87.4.1045. [DOI] [PubMed] [Google Scholar]
- 13.Rector RS, Loethen J, Ruebel M, Thomas TR, Hinton PS. Serum markers of bone turnover are increased by modest weight loss with or without weight-bearing exercise in overweight premenopausal women. Appl Physiol Nutr Metab. 2009;34:933–41. doi: 10.1139/H09-098. [DOI] [PubMed] [Google Scholar]
- 14.Redman LM, Rood J, Anton SD, Champagne C, Smith SR, Ravussin E. Calorie restriction and bone health in young, overweight individuals. Arch Intern Med. 2008;168:1859–66. doi: 10.1001/archinte.168.17.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsiftsis DD, Mylonas P, Mead N, Kalfarentzos F, Alexandrides TK. Bone mass decreases in morbidly obese women after long limb-biliopancreatic diversion and marked weight loss without secondary hyperparathyroidism. A physiological adaptation to weight loss? Obes Surg. 2009;19:1497–503. doi: 10.1007/s11695-009-9938-z. [DOI] [PubMed] [Google Scholar]
- 16.Gabet Y, Kohavi D, Muller R, Chorev M, Bab I. Intermittently administered parathyroid hormone 1–34 reverses bone loss and structural impairment in orchiectomized adult rats. Osteoporos Int. 2005;16:1436–43. doi: 10.1007/s00198-005-1876-6. [DOI] [PubMed] [Google Scholar]
- 17.Iwaniec UT, Trevisiol CH, Maddalozzo GF, Rosen CJ, Turner RT. Effects of low-dose parathyroid hormone on bone mass, turnover, and ectopic osteoinduction in a rat model for chronic alcohol abuse. Bone. 2008;42:695–701. doi: 10.1016/j.bone.2007.12.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosekilde L, Sogaard CH, McOsker JE, Wronski TJ. PTH has a more pronounced effect on vertebral bone mass and biomechanical competence than antiresorptive agents (estrogen and bisphosphonate)--assessed in sexually mature, ovariectomized rats. Bone. 1994;15:401–8. doi: 10.1016/8756-3282(94)90816-8. [DOI] [PubMed] [Google Scholar]
- 19.Turner RT, Evans GL, Cavolina JM, Halloran B, Morey-Holton E. Programmed administration of parathyroid hormone increases bone formation and reduces bone loss in hindlimb-unloaded ovariectomized rats. Endocrinology. 1998;139:4086–91. doi: 10.1210/endo.139.10.6227. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt IU, Dobnig H, Turner RT. Intermittent parathyroid hormone treatment increases osteoblast number, steady state messenger ribonucleic acid levels for osteocalcin, and bone formation in tibial metaphysis of hypophysectomized female rats. Endocrinology. 1995;136:5127–34. doi: 10.1210/endo.136.11.7588250. [DOI] [PubMed] [Google Scholar]
- 21.Sibonga JD, Iwaniec UT, Shogren KL, Rosen CJ, Turner RT. Effects of parathyroid hormone (1–34) on tibia in an adult rat model for chronic alcohol abuse. Bone. 2007;40:1013–20. doi: 10.1016/j.bone.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Wronski TJ, Yen CF, Qi H, Dann LM. Parathyroid hormone is more effective than estrogen or bisphosphonates for restoration of lost bone mass in ovariectomized rats. Endocrinology. 1993;132:823–31. doi: 10.1210/endo.132.2.8425497. [DOI] [PubMed] [Google Scholar]
- 23.Menagh PJ, Turner RT, Jump DB, Wong CP, Lowry MB, Yakar S, Rosen CJ, Iwaniec UT. Growth hormone regulates the balance between bone formation and bone marrow adiposity. J Bone Miner Res. 2010;25:757–68. doi: 10.1359/jbmr.091015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakley GK, Schutte HD, Jr, Hannon KS, Turner RT. Androgen treatment prevents loss of cancellous bone in the orchidectomized rat. J Bone Miner Res. 1991;6:325–30. doi: 10.1002/jbmr.5650060403. [DOI] [PubMed] [Google Scholar]
- 25.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118:2583–91. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devlin M, Cloutier A, Thomas N, Panus D, Lotinun S, Pinz I, Baron R, Rosen C, Bouxsein M. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010 doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baek K, Barlow AA, Allen MR, Bloomfield SA. Food restriction and simulated microgravity: effects on bone and serum leptin. J Appl Physiol. 2008;104:1086–93. doi: 10.1152/japplphysiol.01209.2007. [DOI] [PubMed] [Google Scholar]
- 28.Ferguson VL, Greenberg AR, Bateman TA, Ayers RA, Simske SJ. The effects of age and dietary restriction without nutritional supplementation on whole bone structural properties in C57BL/6J mice. Biomed Sci Instrum. 1999;35:85–91. [PubMed] [Google Scholar]
- 29.Munoz MT, Argente J. Anorexia nervosa in female adolescents: endocrine and bone mineral density disturbances. Eur J Endocrinol. 2002;147:275–86. doi: 10.1530/eje.0.1470275. [DOI] [PubMed] [Google Scholar]
- 30.Maier GW, Kreis ME. Limited nutritional energy supply differentially impairs growth and bone mineralization of the developing lumbar vertebrae in minipigs. Bone. 2005;36:512–20. doi: 10.1016/j.bone.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Prabhakaran R, Misra M, Miller KK, Kruczek K, Sundaralingam S, Herzog DB, Katzman DK, Klibanski A. Determinants of height in adolescent girls with anorexia nervosa. Pediatrics. 2008;121:e1517–23. doi: 10.1542/peds.2007-2820. [DOI] [PubMed] [Google Scholar]
- 32.Boyer PM, Compagnucci GE, Olivera MI, Bozzini C, Roig MC, Compagnucci CV, Alippi RM. Bone status in an animal model of chronic sub-optimal nutrition: a morphometric, densitometric and mechanical study. Br J Nutr. 2005;93:663–9. doi: 10.1079/bjn20041331. [DOI] [PubMed] [Google Scholar]
- 33.Hosea HJ, Taylor CG, Wood T, Mollard R, Weiler HA. Zinc-deficient rats have more limited bone recovery during repletion than diet-restricted rats. Exp Biol Med (Maywood) 2004;229:303–11. doi: 10.1177/153537020422900404. [DOI] [PubMed] [Google Scholar]
- 34.Rader JI, Baylink DJ, Hughes MR, Safilian EF, Haussler MR. Calcium and phosphorus deficiency in rats: effects on PTH and 1,25-dihydroxyvitamin D3. Am J Physiol. 1979;236:E118–22. doi: 10.1152/ajpendo.1979.236.2.E118. [DOI] [PubMed] [Google Scholar]
- 35.Kwiecinski GG, Petrie GI, DeLuca HF. Vitamin D is necessary for reproductive functions of the male rat. J Nutr. 1989;119:741–4. doi: 10.1093/jn/119.5.741. [DOI] [PubMed] [Google Scholar]
- 36.Tamasi JA, Arey BJ, Bertoline DR, Feyen JH. Characterization of bone structure in leptin receptor-deficient Zucker (fa/fa) rats. J Bone Miner Res. 2003;18:1605–1611. doi: 10.1359/jbmr.2003.18.9.1605. [DOI] [PubMed] [Google Scholar]
- 37.Iwaniec UT, Boghossian S, Lapke PD, Turner RT, Kalra SP. Central leptin gene therapy corrects skeletal abnormalities in leptin-deficient ob/ob mice. Peptides. 2007;28:1012–9. doi: 10.1016/j.peptides.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutter NB, Bustamante CD, Chase K, Gray MM, Zhao K, Zhu L, Padhukasahasram B, Karlins E, Davis S, Jones PG, Quignon P, Johnson GS, Parker HG, Fretwell N, Mosher DS, Lawler DF, Satyaraj E, Nordborg M, Lark KG, Wayne RK, Ostrander EA. A single IGF1 allele is a major determinant of small size in dogs. Science. 2007;316:112–5. doi: 10.1126/science.1137045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douyon L, Schteingart DE. Effect of obesity and starvation on thyroid hormone, growth hormone, and cortisol secretion. Endocrinol Metab Clin North Am. 2002;31:173–89. doi: 10.1016/s0889-8529(01)00023-8. [DOI] [PubMed] [Google Scholar]
- 40.Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008;8:77–83. doi: 10.1016/j.cmet.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thissen JP, Underwood LE, Ketelslegers JM. Regulation of insulin-like growth factor-I in starvation and injury. Nutr Rev. 1999;57:167–76. doi: 10.1111/j.1753-4887.1999.tb06939.x. [DOI] [PubMed] [Google Scholar]
- 42.Kidder LS, Schmidt IU, Evans GL, Turner RT. Effects of growth hormone and low dose estrogen on bone growth and turnover in long bones of hypophysectomized rats. Calcif Tissue Int. 1997;61:327–35. doi: 10.1007/s002239900343. [DOI] [PubMed] [Google Scholar]
- 43.Caserta F, Tchkonia T, Civelek VN, Prentki M, Brown NF, McGarry JD, Forse RA, Corkey BE, Hamilton JA, Kirkland JL. Fat depot origin affects fatty acid handling in cultured rat and human preadipocytes. Am J Physiol Endocrinol Metab. 2001;280:E238–47. doi: 10.1152/ajpendo.2001.280.2.E238. [DOI] [PubMed] [Google Scholar]
- 44.Batista JN, Santolaria F, Gonzalez-Reimers E, Brito-Barroso ML, Jorge-Hernandes JA, Marsa L, Hernandez-Nieto L. Evaluation of marrow cellularity in alcoholism and hepatic cirrhosis, by aspiration, biopsy and histomorphometric. Drug Alcohol Depend. 1988;22:27–31. doi: 10.1016/0376-8716(88)90033-6. [DOI] [PubMed] [Google Scholar]
- 45.Bohm J. Gelatinous transformation of the bone marrow: the spectrum of underlying diseases. Am J Surg Pathol. 2000;24:56–65. doi: 10.1097/00000478-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94:2129–36. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui Q, Wang Y, Saleh KJ, Wang GJ, Balian G. Alcohol-induced adipogenesis in a cloned bone-marrow stem cell. J Bone Joint Surg Am. 2006;88 (Suppl 3):148–54. doi: 10.2106/JBJS.F.00534. [DOI] [PubMed] [Google Scholar]
- 48.Duque G. Bone and fat connection in aging bone. Curr Opin Rheumatol. 2008;20:429–34. doi: 10.1097/BOR.0b013e3283025e9c. [DOI] [PubMed] [Google Scholar]
- 49.Abella E, Feliu E, Granada I, Milla F, Oriol A, Ribera JM, Sanchez-Planell L, Berga LI, Reverter JC, Rozman C. Bone marrow changes in anorexia nervosa are correlated with the amount of weight loss and not with other clinical findings. Am J Clin Pathol. 2002;118:582–8. doi: 10.1309/2Y7X-YDXK-006B-XLT2. [DOI] [PubMed] [Google Scholar]
- 50.Bathija A, Davis S, Trubowitz S. Bone marrow adipose tissue: response to acute starvation. Am J Hematol. 1979;6:191–8. doi: 10.1002/ajh.2830060303. [DOI] [PubMed] [Google Scholar]
- 51.Gimble JM, Robinson CE, Wu X, Kelly KA. The function of adipocytes in the bone marrow stroma: an update. Bone. 1996;19:421–8. doi: 10.1016/s8756-3282(96)00258-x. [DOI] [PubMed] [Google Scholar]
- 52.Hussain MM, Mahley RW, Boyles JK, Lindquist PA, Brecht WJ, Innerarity TL. Chylomicron metabolism. Chylomicron uptake by bone marrow in different animal species. J Biol Chem. 1989;264:17931–8. [PubMed] [Google Scholar]
- 53.Tavassoli M. Differential response of bone marrow and extramedullary adipose cells to starvation. Experientia. 1974;30:424–5. doi: 10.1007/BF01921701. [DOI] [PubMed] [Google Scholar]
- 54.Tran MA, Dang TL, Berlan M. Effects of catecholamines on free fatty acid release from bone marrow adipose tissue. J Lipid Res. 1981;22:1271–6. [PubMed] [Google Scholar]
- 55.Dorshkind K, Montecino-Rodriguez E, Signer RA. The ageing immune system: is it ever too old to become young again? Nat Rev Immunol. 2009;9:57–62. doi: 10.1038/nri2471. [DOI] [PubMed] [Google Scholar]
- 56.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–63. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Price EA. Aging and erythropoiesis: current state of knowledge. Blood Cells Mol Dis. 2008;41:158–65. doi: 10.1016/j.bcmd.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 58.Kuznetsov SA, Riminucci M, Ziran N, Tsutsui TW, Corsi A, Calvi L, Kronenberg HM, Schipani E, Robey PG, Bianco P. The interplay of osteogenesis and hematopoiesis: expression of a constitutively active PTH/PTHrP receptor in osteogenic cells perturbs the establishment of hematopoiesis in bone and of skeletal stem cells in the bone marrow. J Cell Biol. 2004;167:1113–22. doi: 10.1083/jcb.200408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garrett RW, Emerson SG. The role of parathyroid hormone and insulin-like growth factors in hematopoietic niches: physiology and pharmacology. Mol Cell Endocrinol. 2008;288:6–10. doi: 10.1016/j.mce.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 60.Komatsu DE, Brune KA, Liu H, Schmidt AL, Han B, Zeng QQ, Yang X, Nunes JS, Lu Y, Geiser AG, Ma YL, Wolos JA, Westmore MS, Sato M. Longitudinal in vivo analysis of the region-specific efficacy of parathyroid hormone in a rat cortical defect model. Endocrinology. 2009;150:1570–9. doi: 10.1210/en.2008-0814. [DOI] [PubMed] [Google Scholar]
- 61.Lane NE, Thompson JM, Strewler GJ, Kinney JH. Intermittent treatment with human parathyroid hormone (hPTH[1–34]) increased trabecular bone volume but not connectivity in osteopenic rats. J Bone Miner Res. 1995;10:1470–7. doi: 10.1002/jbmr.5650101007. [DOI] [PubMed] [Google Scholar]
- 62.Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt JA, Westmore MS, Linda Y, Nold JB. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for 2 years and relevance to human safety. Toxicol Pathol. 2002;30:312–21. doi: 10.1080/01926230252929882. [DOI] [PubMed] [Google Scholar]
- 63.Turner RT, Evans GL, Lotinun S, Lapke PD, Iwaniec UT, Morey-Holton E. Dose-response effects of intermittent PTH on cancellous bone in hindlimb unloaded rats. J Bone Miner Res. 2007;22:64–71. doi: 10.1359/jbmr.061006. [DOI] [PubMed] [Google Scholar]
- 64.Turner RT, Lotinun S, Hefferan TE, Morey-Holton E. Disuse in adult male rats attenuates the bone anabolic response to a therapeutic dose of parathyroid hormone. J Appl Physiol. 2006;101:881–6. doi: 10.1152/japplphysiol.01622.2005. [DOI] [PubMed] [Google Scholar]