Abstract
Exposure to methamphetamine during brain development impairs cognition in children and adult rodents. In mice, these impairments are greater in females than males. Adult female, but not male, mice show impairments in novel location recognition following methamphetamine exposure during brain development. In contrast to adulthood, little is known about the potential effects of methamphetamine exposure on cognition in adolescent mice. As adolescence is an important time of development and is relatively understudied, the aim of the current study was to examine potential long-term effects of neonatal methamphetamine exposure on behavior and cognition during adolescence. Male and female mice were exposed to methamphetamine (5 mg/kg) or saline once a day from postnatal day 11-20, the period of rodent hippocampal development. Behavioral and cognitive function was assessed during adolescence beginning on postnatal day 30. During the injection period, methamphetamine-exposed mice gained less weight on average compared to saline-exposed mice. In both male and female mice, methamphetamine exposure significantly impaired novel object recognition and there was a trend towards impaired novel location recognition. Anxiety-like behavior, sensorimotor gating, and contextual and cued fear conditioning were not affected by methamphetamine exposure. Thus, neonatal methamphetamine exposure affects cognition in adolescence and unlike in adulthood equally affects male and female mice.
Keywords: Methamphetamine, cognition, adolescence, hippocampus, postnatal
1. Introduction
Compared to other drugs of abuse, methamphetamine (MA) use among pregnant women has increased over the past decade [42]. This increase is concerning as exposure to MA during brain development increases the risk of being born small for gestational age [37,38] and the risk of being born with birth defects [14]. MA exposure is also associated with increased physiological stress, decreased arousal, and poor quality of movement during the first five days of life [21,39]. MA has persistent and long-term effects on cognition in childhood and adolescence. MA-exposed children show cognitive impairments in visual motor integration [7] and hippocampus-dependent spatial memory [27]. Consistent with hippocampal dysfunction, children between the ages of 3-16 exposed in utero to MA have a smaller hippocampus [8], a brain structure important for cognitive function [13,30]. Finally, school performance is delayed and physical ability impaired in MA-exposed children of up to 14 years of age (for a review, see [22]).
Animal studies examining the effects of MA exposure during brain development are consistent with the human studies. Rats exposed prenatally to MA show impaired righting reflexes from postnatal day (PND) 1-12, reduced motor coordination on the rotorod test on PND 23 [35], and impaired hippocampus-dependent spatial learning in the water maze in adulthood [36]. Some stages of rodent hippocampal development occur during the first three postnatal weeks, modeling human hippocampal development during the third trimester [9,10]. Postnatal exposure to MA also produces long-term cognitive impairments in adulthood. Rodents exposed to MA during this postnatal phase of hippocampal development show impaired spatial learning and memory in the water maze [1,15,18,34,45-47,51-53] and the Barnes maze [49], impaired novel location and novel object recognition [1,33], and reduced sensorimotor gating in a pre-pulse inhibition test [1]. The long-term cognitive effects of MA exposure during hippocampal development are more severe in female than male mice [1,33]. While adult female MA-exposed mice show both novel location and novel object recognition impairments, adult male MA-exposed mice show only novel object recognition impairments [1,33]. Female mice show a slower metabolism of MA following a single exposure on PND 11, which might contribute to their increased susceptibility to develop cognitive impairments in adulthood following neonatal MA treatment [2].
MA exposure during hippocampal development also affects other measures of hippocampal function in adulthood. Rodents exposed to MA during postnatal hippocampal development show reduced levels of the dendritic marker microtubule-associated protein-2 [2], altered expression of brain derived neurotrophic factor [34], decreased dendritic spine density [50], and alterations in muscarinic acetylcholine receptor levels [33] in the hippocampus in adulthood.
Animal models of MA exposure during hippocampal development have typically focused on the long-term effects of MA in adulthood and relatively little research has focused on the potential effects of MA exposure on cognition in adolescence. Rats exposed to MA (5 mg/kg four times a day) during hippocampal development show impaired spatial learning and memory in the water maze during adolescence (at both PND 30 and PND 40; [47]). In terms of brain development, behavioral patterns, and hormonal changes, PND 30 in rodents is considered to model the beginning of adolescence in humans [24,40]. However, cognitive performance on tests other than the water maze has not been assessed and no studies have been performed in mice. Therefore, in this study we investigated the effects of MA exposure during hippocampal development on performance in a variety of behavioral and cognitive tests in adolescent male and female mice.
2. Materials and methods
2.1. Animals
Three- to 5-month-old male and female C57BL/6J mice were bred in our colony using breeding cages containing one male and two female mice. Female mice were singly housed from the first sign of pregnancy. Lab chow (PicoLab Rodent Diet 20, #5053; PMI Nutrition International, St. Louis, MO) and water were given ad libitum. On PND21, pups were weaned and group housed with five mice per cage according to sex. They were provided soft foods during the injection period and two weeks after weaning to maintain stable weight gain. The mice were kept on a 12 hr light/dark schedule (lights on at 06:00). Behavioral testing took place during the light cycle. All procedures conformed to the standards of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the Oregon Health and Science University.
2.2. Injections
(d)-MA hydrocholoride (5 mg/kg), obtained from the Research Triangle Institute (Research Triangle Park, NC) through the National Institute on Drug Abuse drug supply program, was diluted with 0.9% sodium chloride (saline (SA)) to the appropriate concentration. This dose was selected based on our previous findings that it causes long-term cognitive impairments in adult mice exposed during hippocampal development [1,33]. Male and female pups were weighed and given a single intra-peritoneal injection of MA (n = 24) or SA (n = 27) daily at 10:00 from PND 11-20 (injection volume of 0.1mL). A within-litter injection design was used to balance the number of MA and SA injections within a litter and across sexes. This design has been used in prior postnatal MA investigations [1,12,16,31,33,45,47,49,53].
2.3. Behavioral testing
Beginning on PND 28, mice were individually housed. Behavioral testing began on PND 30. All mice were tested consecutively using the following sequence of tests: open field, elevated zero maze, novel location and novel object recognition, pre-pulse inhibition, and contextual and cued fear conditioning. This order of testing was used to begin with the least stressful assessment and end with those thought to be most stressful. The open field and elevated zero maze tests were performed as previously described [5].
2.4. Novel location novel object recognition
Mice were individually habituated to a lit open arena with clear Plexiglas walls (40.6 cm × 40.6 cm; Hamilton-Kinder, Poway, CA) for five min on three consecutive days. On the fourth day, the mice were trained in five consecutive ten min trials. The first three were familiarization trials with three objects placed within the arena (one in each of three corners). For the novel location recognition test (fourth trial), one object was relocated to a novel location in the arena. The same object was moved for all mice. For the novel object recognition test (fifth trial), one object was replaced with a novel object. The same object was replaced for all mice. There was a four min inter-trial interval between all trials. The time spent exploring each object during all trials was analyzed using a multiple body point video tracking system that can track the nose-point of a mouse, as described ([4]; Ethovision XT, Noldus, Leesburg, VA). Exploration of an object was defined when the nose of the mouse was in a zone containing the object and the center point of the mouse was not. Subsequently, the percent time the animal explored a particular object out of the total time exploring all three objects was calculated. The difference between the percent time spent exploring the object in the novel location (trial four) and the percent time spent exploring the same object in its original location (trial three) was calculated to measure hippocampus-dependent novel location recognition. The percent time exploring the novel object in trial five was calculated to measure hippocampus-independent novel object recognition. Additional details about the test are available elsewhere [4,5].
2.5. Pre-pulse inhibition
Mice were placed in an enclosure within a startle monitor sound-attenuated chamber and startle response amplitudes were measured with a force transducer (Hamilton-Kinder, Poway, CA). Following a 5 min acclimation period, mice were exposed to 3, 40 ms acoustic stimuli (110 db). The testing phase consisted of 20 ms pre-pulses (70-80 db) followed by 50 ms delays and 40 ms acoustic stimuli (110 db). The same pattern of acoustic stimuli and testing with pre-pulses then occurred with a 120 db stimulus. Random inter-trial intervals were used between trials (15-30 sec). PPI was calculated using the following formula: % response = 100 × ((S-PS)/S), where S was the mean startle amplitude without a pre-pulse and PS was the mean startle response following a pre-pulse. Thus, a 100% response in the PPI test indicates complete inhibition of the startle response during pre-pulse trials.
2.6. Fear conditioning
Contextual and cued fear conditioning tests were performed as previously described [44]. Briefly, in contextual fear conditioning, the mice learn to associate the environment (fear conditioning chamber) with a mild foot shock (unconditioned stimulus (US)). During training, the US was paired with a tone, allowing assessment of cued fear conditioning. Testing 24 hours later consisted of two phases: contextual testing where the mouse was placed in the same training context in the absence of the US, and cued testing where the mouse was placed in a different context in the absence of the US but in the presence of the tone. Re-exposure to either the context or tone results in freezing behavior [44].
2.7. Statistical analysis
All statistical analyses were performed using SPSS (Chicago, IL) software. As the pups were not individually tagged during the injection period, repeated measures ANOVA with litter as the experimental unit was used to assess the amount of weight gained each day during the injection period. Performance on all of the behavioral and cognitive tests was assessed using a linear mixed model with sex and treatment as the fixed effects and litter as the random effect. This statistical model allowed us to examine the effects of treatment and sex on performance on the behavioral and cognitive tests after accounting for the potential effects of litter. Six mice were removed from the novel location and novel object recognition analysis due to influential outlier scores (greater than two standard deviations from the mean) or failure to explore the objects during any of the trials (less than a total of 2 seconds of exploration over the 5 trials). All analyses were conducted with a two-tailed significance alpha level of 0.05.
3. Results
3.1. Weight gain
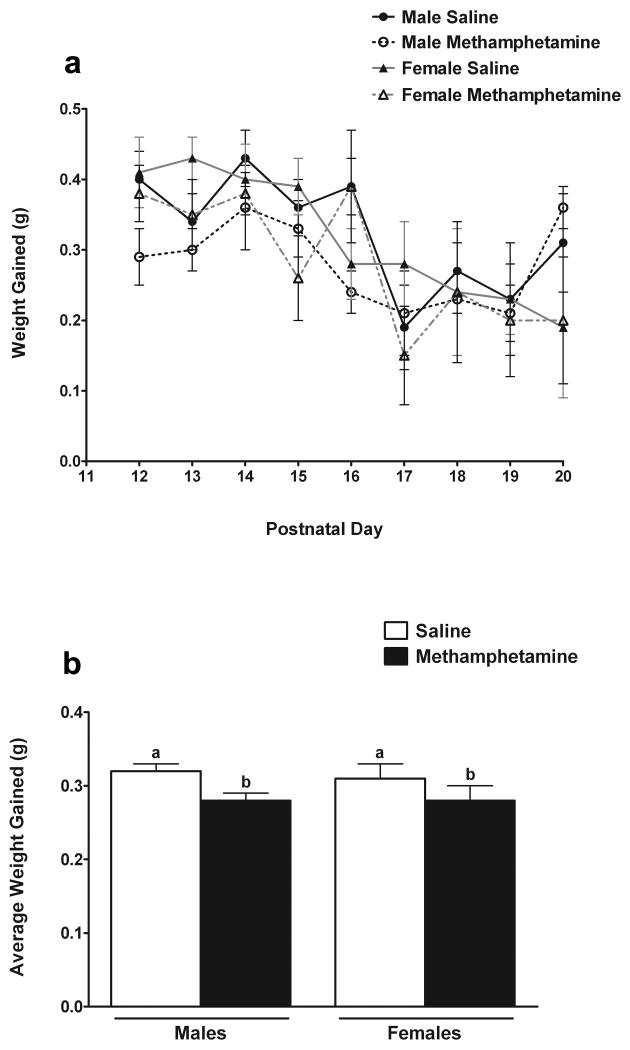
MA- and SA-injected pups were weighed each day during the injection period to monitor weight gain. Repeated measures ANOVA showed an effect of postnatal day on the amount of weight gained from the previous day (main effect of day; F (1, 29) = 5.20, p = 0.03). Post hoc tests showed that all of the mice tended to gain more weight at the beginning of the injection period and the amount of weight gained from the previous day declined toward the end of the injection period. The repeated measures ANOVA also showed an effect of treatment on weight gained from the previous day (main effect of treatment; F (1, 29) = 6.82, p = 0.01). MA-treated mice gained on average less weight each day than SA-treated mice, regardless of sex (F (1, 31) = 7.35, p = 0.01; Figure 1). There was no effect of sex on weight gained from the previous day during the injection period (F (1, 29) = 0.10, p > 0.70).
Figure 1.
Pup weight gain during the injection period (postnatal day 11-20). Regardless of sex, MA-exposed pups gained less weight each day from the previous day during the injection period than SA-exposed pups. (A) Weight gained each day from the previous day across the injection period. (B) Weight gained each day from the previous day averaged across all days of the injection period. aSA-exposed mice, bMA-exposed mice.
3.2. Exploration and measures of anxiety in the open field and elevated zero maze
Distance moved and the percent time spent in the center versus the periphery of the arena was determined in the open field test. Percent time spent in the center versus the periphery of the arena was used as a measurement of anxiety-like behavior. Mice that spend more time in the center of the open field arena are considered to be less anxious [11]. There was no effect of MA treatment on distance moved or percent time spent in the center of the arena. There was also no effect of sex on distance moved or percent time spent in the center of the arena (Table 1).
Table 1. Explorative and anxiety-like behavior in the open field and elevated zero maze1.
| Treatment | Sex | Test | Distance moved (cm) | Percent time in open area/areas |
|---|---|---|---|---|
| SA | Males | Open Field | 4660.49 ± 254.95 | 10.69 ± 1.44 |
| MA | Males | Open Field | 4247.40 ± 289.68 | 9.29 ± 1.78 |
| SA | Females | Open Field | 5163.67 ± 314.43 | 11.12 ± 1.68 |
| MA | Females | Open Field | 4936.13 ± 180.81 | 12.83 ± 1.45 |
| SA | Males | Zero Maze | 1735.52 ± 64.08 | 17.23 ± 1.47 |
| MA | Males | Zero Maze | 1666.08 ± 91.50 | 14.69 ± 1.63 |
| SA | Females | Zero Maze | 2033.54 ± 89.42a | 16.65 ± 1.38 |
| MA | Females | Zero Maze | 1892.94 ± 73.71a | 20.75 ± 2.34 |
Data are expressed as mean ± S.E.M. SA = saline, MA = methamphetamine.
Measures significantly higher for females than males for respective test.
Next, distance moved and the percent of time in the open anxiety-provoking areas versus closed areas of the elevated zero maze were measured. Similar to the open field, there was no effect of MA treatment on distance moved or percent time in the open areas of the elevated zero maze. Female mice moved a greater distance in the elevated zero maze than male mice, irrespective of treatment (main effect of sex; F (1, 26.20) = 10.48, p < 0.01; Table 1). There was no effect of sex on percent time in the open areas of the maze.
3.3 Novel location and novel object recognition
There was no effect of MA treatment or sex on the total amount of time spent exploring the objects over the five trials in the novel location and novel object recognition test (Figure 2A). There was also no effect of MA treatment or sex on the average distance moved in the novel location novel object test. However, when the percent time spent exploring an object in a novel versus familiar location was used as a measure of novel location recognition [4], there was a trend for MA-exposed mice to show reduced novel location recognition compared to SA-exposed mice (main effect of treatment; F (1, 22.23) = 3.38, p = 0.079; Figure 2B). There was no effect of sex on novel location recognition.
Figure 2.
Novel location and novel object recognition test performance. (A) There was no difference between the groups in the total amount of time spent exploring the 3 objects in the arena for each trial of the test. (B) There was a trend of MA-exposed mice to spend less time exploring the object in the new location versus the old location compared to SA-exposed mice (p = 0.079). (C) MA-exposed mice spent less time exploring the novel object compared to SA-exposed mice. aSA-exposed mice, bMA-exposed mice.
The percent time spent exploring a novel object was used as a measure of novel object recognition [4]. MA-treated mice showed reduced novel object recognition compared to SA-treated mice (main effect of treatment; F (1, 25.02) = 7.98, p < 0.01; Figure 2C). There was no effect of sex on novel object recognition.
3.4. Sensorimotor gating in the pre-pulse inhibition test
There was no effect of MA treatment or sex on the baseline acoustic startle response or PPI with the 110 db stimulus (data not shown). For the 120 db stimulus trials, there was no effect of MA treatment or sex on the baseline acoustic startle response. Male mice showed increased PPI compared to female mice for the 120 db stimulus (main effect of sex; F (1, 47) = 10.77, p < 0.01; Table 2), but there was no difference between the SA-treated or MA-treated mice in PPI during the 120 db stimulus.
Table 2. Baseline acoustic startle response and pre-pulse inhibition1a.
| Treatment | Sex | Baseline startle (N) | Pre-pulse inhibition |
|---|---|---|---|
| SA | Males | 2.86 ± 0.22 | 59.63 ± 3.67b |
| MA | Males | 2.68 ± 0.25 | 52.47 ± 4.65b |
| SA | Females | 3.01 ± 0.25 | 36.25 ± 4.97 |
| MA | Females | 2.84 ± 0.25 | 45.46 ± 5.10 |
Data are expressed as mean ± S.E.M. SA = saline, MA = methamphetamine.
Only 120 db trials are shown as there were no sex differences in the 110 db trials.
Males significantly higher than females.
3.5. Contextual and cued fear conditioning
The first two minutes of the training (prior to tone-US presentation) were analyzed to exclude potential locomotor or anxiety-like effects contributing to freezing behavior (baseline freezing behavior). There was no difference between the treatment groups or sexes in baseline percent freezing during the training phase (Table 3).
Table 3. Percent freezing in contextual and cued fear conditioning1.
| Treatment | Sex | Baseline | Contextual test | Pre-tone cued test | During tone cued test |
|---|---|---|---|---|---|
| SA | Males | 13.44 ± 2.50 | 39.98 ± 4.57 | 19.83 ± 4.11 | 61.22 ± 4.07 |
| MA | Males | 10.56 ± 2.41 | 41.65 ± 4.52 | 17.41 ± 2.69 | 57.72 ± 4.78 |
| SA | Females | 10.17 ± 2.34 | 33.60 ± 4.74 | 12.00 ± 1.88 | 58.67 ± 4.51 |
| MA | Females | 10.19 ± 2.37 | 36.48 ± 4.32 | 21.28 ± 3.14 | 66.86 ± 4.18 |
Data are expressed as mean percentages ± S.E.M. SA = saline, MA = methamphetamine.
The first three minutes of the contextual fear conditioning test were analyzed for group differences in percent time freezing. There were no differences between the treatment groups or sexes in the percent time freezing during the contextual fear conditioning test (Table 3). For the cued fear conditioning test, the first three minutes (prior to tone onset) and last three minutes (during tone presentation) were analyzed separately. Similar to the contextual fear conditioning results, there was no difference between the treatment groups or sexes in the percent time freezing during either phase of the cued fear conditioning test (Table 3). When the pre- and post-tone freezing behavior was analyzed as a repeated measure ANOVA, there were also no effects of treatment or sex (data not shown).
4. Discussion
The results of this study show that MA exposure during hippocampal development reduces postnatal weight gain, reduces novel location recognition, and impairs novel object recognition in both adolescent male and female mice. To the best of our knowledge, this is the first study to examine the effects of postnatal MA exposure on cognitive function in multiple tests during adolescence.
The effects of MA exposure on cognition during adolescence are similar to those we previously found in adulthood, but the mediating effects of sex differed in the two age groups. Similar to what we found in adolescence, adult MA-exposed male and female mice were impaired in the novel object recognition task [1,33]. However, both male and female mice showed modest (p = 0.079) novel location recognition impairments during adolescence in the current study, whereas only female mice showed novel location recognition impairments in adulthood [1,33], suggesting recovery of this cognitive deficit in the males with age. The mechanism underlying the increased susceptibility in female mice is not yet well understood. It might relate to the slower rate of MA metabolism in female than male mice [2]. In addition, male sex hormones may be protective against the MA-induced insults in the brain. Indeed, androgens can attenuate other brain challenges [26,28]. However, the protective effects of androgens may be insufficient during adolescence when mice are going through puberty. Increases in gonadotropin-releasing hormone receptor expression, which is coupled with increased responsiveness to follicle-stimulating hormone and luteinizing hormone, occur earlier in female than male rats [3]. Thus, the slower hormonal development in male rodents may relate to delayed maturation of cognitive function and delayed neuroprotection of gonad hormones in the males. By adulthood, in contrast, androgens might be protective due to their higher levels and longer presence since the MA insult, thus ameliorating MA-induced impairments in novel location recognition observed in only adult females.
As litter can sometimes [17], but not always [33], affect outcome measures in developmental studies, we analyzed performance on all of the behavioral and cognitive tests using a linear mixed model, which assesses the effects of treatment and sex after accounting for potential correlations between pups within the same litter. The use of this conservative statistical model may be one reason why the novel location recognition test did not reach statistical significance. Indeed, when the novel location recognition data was analyzed using a 2-way ANOVA, the effect of treatment became statistically significant, suggesting that the difference in novel location recognition between the MA- and SA-treated adolescent mice is biologically relevant and important.
We did not find any effects of MA exposure on PPI performance in adolescent mice. Studies from our lab in adult C57Bl6/J wild-type mice exposed to MA from PND 11-20 have shown PPI impairments [1], but they were not seen in human apoE3 or apoE4 mice [33]. Together, these data suggest that the effects of neonatal MA exposure on PPI performance are age- and genotype-dependent.
We did not find any effects of developmental MA exposure in adolescent mice on contextual or cued fear conditioning, which are considered to be dependent on the hippocampus and amygdala, respectively [23,25,41]. Previous reports have shown that psychostimulant drugs of abuse can affect fear conditioning in adulthood. A low pre-test dose of cocaine enhanced both contextual and cued fear conditioning and a high dose impaired this conditioning when mice were tested in the absence of the drug [54]. Pre-treatment with MA also enhanced freezing in a contextual fear conditioning paradigm in adult rats [43]. However, in the current investigation there were no effects of developmental MA exposure on contextual or cued fear conditioning in adolescence. Thus, fear conditioning behavior may be less sensitive to the effects of MA exposure during hippocampal development due to the involvement of other brain regions such as the amygdala.
In addition to long-term effects of MA during brain development on cognition in adolescence, MA also has acute effects on cognition in the adolescent brain. MA use during adolescence impairs executive function in the Stroop interference task, fine motor speed on the Grooved Pegboard task, and spatial organization in the WAIS/WISC-IV matrices test when the participants are tested during adolescence [20]. Furthermore, the effects of MA on sequential reaction time are mediated by sex [20]. The effects of acute or long-term (chronic) drug exposure may also differ in the adolescent versus adult brain. Indeed, the results from our current study demonstrate age-dependent interactions between MA exposure and sex. More studies are warranted to examine the effects of drugs of abuse other than MA on adolescent brain function as they may drastically differ from those seen in adults.
As part of hippocampal development occurs during the third trimester of human fetal gestation but the first 3 postnatal weeks in rodents [9,10], we exposed mice to MA during the early postnatal period. This model removes the potential confound of altered maternal care due to drug administration and withdrawal of the mother, as early life maternal care can greatly affect cognitive function [6,29]. Postnatal exposure to MA also controls for potential variations in the dose of MA that each pup would receive in utero depending on the size of the litter. Finally, this model allows a within litter control, as littermates receive different treatments, providing a clean and accurate way to assess the effects of MA exposure during hippocampal development in rodents. Humans likely will also be exposed to MA during the first or first and second trimesters. Previous studies in rodents have shown differential long-term effects of MA exposure during only the prenatal period versus only during the postnatal period. For example, rats exposed to MA during postnatal development compared to prenatal exposure showed altered nociception in adulthood, suggesting a unique effect of MA exposure during postnatal brain development [19]. Prenatal MA exposure improved performance in the water maze in adulthood [32] (but see [36]) whereas postnatal MA exposure impaired water maze performance in adulthood [1,15,18,34,45-47,51-53]. In another study, adolescent rats exposed to a high dose of MA prenatally showed reduced open field activity and rearing, but these same rats did not show water maze impairments in young adulthood compared to controls [48]. As the current study aimed to assess the effects of MA exposure during hippocampal development, we exposed the mice to MA during this postnatal period.
In conclusion, the results from this study show that exposure to MA during a time period equivalent to the third trimester of human fetal gestation reduces novel location recognition and impairs novel object recognition in both male and female mice during adolescence. Future studies with this rodent model of MA exposure during brain development are warranted to better understand the mechanisms underlying the effects of developmental MA exposure on brain function in adolescence.
Acknowledgments
The authors would like to thank Dr. Theodore Benice, Catherine Dayger, and Timothy Pfankuch for their help with the breeding, injections, and testing of the mice in this study. We would also like to thank Dr. Brian Piper for his thoughtful input in this study. This work was supported by a National Service Research Award F31DA026243 from the National Institute on Drug Abuse, an Achievement Rewards for College Scientists (ARCS) Scholarship, and the developmental account of Dr. Raber.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jessica A. Siegel, Email: siegelj@ohsu.edu.
Byung S. Park, Email: parkb@ohsu.edu.
Jacob Raber, Email: raberj@ohsu.edu.
References
- 1.Acevedo SF, de Esch IJ, Raber J. Sex- and histamine-dependent long-term cognitive effects of methamphetamine exposure. Neuropsychopharmacology. 2007;32:665–72. doi: 10.1038/sj.npp.1301091. [DOI] [PubMed] [Google Scholar]
- 2.Acevedo SF, Pfankuch T, van Meer P, Raber J. Role of histamine in short- and long-term effects of methamphetamine on the developing mouse brain. J Neurochem. 2008;107:976–86. doi: 10.1111/j.1471-4159.2008.05673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becu-Villalobos D, Gonzalez Iglesias A, Diaz-Torga G, Hockl P, Libertun C. Brain sexual differentiation and gonadotropins secretion in the rat. Cell Mol Neurobiol. 1997;17:699–715. doi: 10.1023/A:1022542221535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benice TS, Raber J. Object recognition analysis in mice using nose-point digital video tracking. J Neurosci Methods. 2008;168:422–30. doi: 10.1016/j.jneumeth.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137:413–23. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 6.Bredy TW, Lee AW, Meaney MJ, Brown RE. Effect of neonatal handling and paternal care on offspring cognitive development in the monogamous California mouse (Peromyscus californicus) Horm Behav. 2004;46:30–8. doi: 10.1016/j.yhbeh.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Chang L, Cloak C, Jiang CS, Farnham S, Tokeshi B, Buchthal S, Hedemark B, Smith LM, Ernst T. Altered neurometabolites and motor integration in children exposed to methamphetamine in utero. Neuroimage. 2009;48:391–7. doi: 10.1016/j.neuroimage.2009.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–7. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-Based Method For Translating Neurodevelopment From Laboratory Species To Humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- 11.Clement Y, Calatayud F, Belzung C. Genetic basis of anxiety-like behaviour: a critical review. Brain Res Bull. 2002;57:57–71. doi: 10.1016/s0361-9230(01)00637-2. [DOI] [PubMed] [Google Scholar]
- 12.Crawford CA, Williams MT, Newman ER, McDougall SA, Vorhees CV. Methamphetamine exposure during the preweanling period causes prolonged changes in dorsal striatal protein kinase A activity, dopamine D2-like binding sites, and dopamine content. Synapse. 2003;48:131–7. doi: 10.1002/syn.10197. [DOI] [PubMed] [Google Scholar]
- 13.Deacon RM, Bannerman DM, Kirby BP, Croucher A, Rawlins JN. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav Brain Res. 2002;133:57–68. doi: 10.1016/s0166-4328(01)00451-x. [DOI] [PubMed] [Google Scholar]
- 14.Forrester MB, Merz RD. Risk of selected birth defects with prenatal illicit drug use, Hawaii, 1986-2002. J Toxicol Environ Health A. 2007;70:7–18. doi: 10.1080/15287390600748799. [DOI] [PubMed] [Google Scholar]
- 15.Grace CE, Schaefer TL, Graham DL, Skelton MR, Williams MT, Vorhees CV. Effects of inhibiting neonatal methamphetamine-induced corticosterone release in rats by adrenal autotransplantation on later learning, memory, and plasma corticosterone levels. Int J Dev Neurosci. 2010;28:331–42. doi: 10.1016/j.ijdevneu.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grace CE, Schaefer TL, Gudelsky GA, Williams MT, Vorhees CV. Neonatal methamphetamine-induced corticosterone release in rats is inhibited by adrenal autotransplantation without altering the effect of the drug on hippocampal serotonin. Neurotoxicol Teratol. 2010;32:356–61. doi: 10.1016/j.ntt.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–8. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- 18.Hruba L, Schutova B, Pometlova M, Rokyta R, Slamberova R. Effect of methamphetamine exposure and cross-fostering on cognitive function in adult male rats. Behav Brain Res. 2009;208:63–71. doi: 10.1016/j.bbr.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Hruba L, Vaculin S, Slamberova R. Effect of prenatal and postnatal methamphetamine exposure on nociception in adult female rats. Dev Psychobiol. 2010;52:71–7. doi: 10.1002/dev.20414. [DOI] [PubMed] [Google Scholar]
- 20.King G, Alicata D, Cloak C, Chang L. Neuropsychological deficits in adolescent methamphetamine abusers. Psychopharmacology (Berl) 2010;212:243–9. doi: 10.1007/s00213-010-1949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagasse LL, Wouldes T, Newman E, Smith LM, Shah RZ, Derauf C, Huestis MA, Arria AM, Grotta SD, Wilcox T, et al. Prenatal methamphetamine exposure and neonatal neurobehavioral outcome in the USA and New Zealand. Neurotoxicol Teratol. 2010 doi: 10.1016/j.ntt.2010.06.009. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lester BM, Lagasse LL. Children of addicted women. J Addict Dis. 2010;29:259–76. doi: 10.1080/10550881003684921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otto T, Poon P. Dorsal hippocampal contributions to unimodal contextual conditioning. J Neurosci. 2006;26:6603–9. doi: 10.1523/JNEUROSCI.1056-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–70. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 25.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 26.Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001;919:160–5. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- 27.Piper B, Corbett S, Olsen R, Raber J. Executive Function Profile in Children Exposed Prenatally to Nicotine, Alcohol, or Methamphetamine. Abstract Presented at the Society for Neuroscience Annual Meeting; San Diego, CA, U.S.A.. 2010. [Google Scholar]
- 28.Raber J, Bongers G, LeFevour A, Buttini M, Mucke L. Androgens protect against apolipoprotein E4-induced cognitive deficits. J Neurosci. 2002;22:5204–9. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Save E, Poucet B, Foreman N, Buhot MC. Object exploration and reactions to spatial and nonspatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behav Neurosci. 1992;106:447–56. [PubMed] [Google Scholar]
- 31.Schaefer TL, Skelton MR, Herring NR, Gudelsky GA, Vorhees CV, Williams MT. Short- and long-term effects of (+)-methamphetamine and (+/-)-3,4-methylenedioxymethamphetamine on monoamine and corticosterone levels in the neonatal rat following multiple days of treatment. J Neurochem. 2008;104:1674–85. doi: 10.1111/j.1471-4159.2007.05112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schutova B, Hruba L, Pometlova M, Deykun K, Slamberova R. Impact of methamphetamine administered prenatally and in adulthood on cognitive functions of male rats tested in Morris water maze. Prague Med Rep. 2008;109:62–70. [PubMed] [Google Scholar]
- 33.Siegel JA, Craytor MJ, Raber J. Long-term effects of methamphetamine exposure on cognitive function and muscarinic acetylcholine receptor levels in mice. Behav Pharmacol. 2010;21:602–14. doi: 10.1097/FBP.0b013e32833e7e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skelton MR, Williams MT, Schaefer TL, Vorhees CV. Neonatal (+)-methamphetamine increases brain derived neurotrophic factor, but not nerve growth factor, during treatment and results in long-term spatial learning deficits. Psychoneuroendocrinology. 2007;32:734–45. doi: 10.1016/j.psyneuen.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slamberova R, Pometlova M, Charousova P. Postnatal development of rat pups is altered by prenatal methamphetamine exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:82–8. doi: 10.1016/j.pnpbp.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Slamberova R, Pometlova M, Syllabova L, Mancuskova M. Learning in the Place navigation task, not the New-learning task, is altered by prenatal methamphetamine exposure. Brain Res Dev Brain Res. 2005;157:217–9. doi: 10.1016/j.devbrainres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Smith L, Yonekura ML, Wallace T, Berman N, Kuo J, Berkowitz C. Effects of prenatal methamphetamine exposure on fetal growth and drug withdrawal symptoms in infants born at term. J Dev Behav Pediatr. 2003;24:17–23. doi: 10.1097/00004703-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Della Grotta S, et al. The infant development, environment, and lifestyle study: effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics. 2006;118:1149–56. doi: 10.1542/peds.2005-2564. [DOI] [PubMed] [Google Scholar]
- 39.Smith LM, Lagasse LL, Derauf C, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Grotta SD, et al. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol Teratol. 2008;30:20–8. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 41.Stafford JM, Lattal KM. Direct comparisons of the size and persistence of anisomycin-induced consolidation and reconsolidation deficits. Learn Mem. 2009;16:494–503. doi: 10.1101/lm.1452209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terplan M, Smith EJ, Kozloski MJ, Pollack HA. Methamphetamine use among pregnant women. Obstet Gynecol. 2009;113:1285–91. doi: 10.1097/AOG.0b013e3181a5ec6f. [DOI] [PubMed] [Google Scholar]
- 43.Tsuchiya K, Inoue T, Koyama T. Effect of repeated methamphetamine pretreatment on freezing behavior induced by conditioned fear stress. Pharmacol Biochem Behav. 1996;54:687–91. doi: 10.1016/0091-3057(96)00017-2. [DOI] [PubMed] [Google Scholar]
- 44.Villasana L, Rosenberg J, Raber J. Sex-dependent effects of (56)Fe irradiation on contextual fear conditioning in C57BL/6J mice. Hippocampus. 2009;20:19–23. doi: 10.1002/hipo.20659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vorhees CV, Inman-Wood SL, Morford LL, Broening HW, Fukumura M, Moran MS. Adult learning deficits after neonatal exposure to D-methamphetamine: selective effects on spatial navigation and memory. J Neurosci. 2000;20:4732–9. doi: 10.1523/JNEUROSCI.20-12-04732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vorhees CV, Skelton MR, Grace CE, Schaefer TL, Graham DL, Braun AA, Williams MT. Effects of (+)-methamphetamine on path integration and spatial learning, but not locomotor activity or acoustic startle, align with the stress hyporesponsive period in rats. Int J Dev Neurosci. 2009;27:289–98. doi: 10.1016/j.ijdevneu.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vorhees CV, Skelton MR, Williams MT. Age-dependent effects of neonatal methamphetamine exposure on spatial learning. Behav Pharmacol. 2007;18:549–62. doi: 10.1097/FBP.0b013e3282ee2abe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weissman AD, Caldecott-Hazard S. In utero methamphetamine effects: I. Behavior and monoamine uptake sites in adult offspring. Synapse. 1993;13:241–50. doi: 10.1002/syn.890130307. [DOI] [PubMed] [Google Scholar]
- 49.Williams MT, Blankenmeyer TL, Schaefer TL, Brown CA, Gudelsky GA, Vorhees CV. Long-term effects of neonatal methamphetamine exposure in rats on spatial learning in the Barnes maze and on cliff avoidance, corticosterone release, and neurotoxicity in adulthood. Brain Res Dev Brain Res. 2003;147:163–75. doi: 10.1016/j.devbrainres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Williams MT, Brown RW, Vorhees CV. Neonatal methamphetamine administration induces region-specific long-term neuronal morphological changes in the rat hippocampus, nucleus accumbens and parietal cortex. Eur J Neurosci. 2004;19:3165–70. doi: 10.1111/j.0953-816X.2004.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams MT, Moran MS, Vorhees CV. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology (Berl) 2003;168:329–38. doi: 10.1007/s00213-003-1433-y. [DOI] [PubMed] [Google Scholar]
- 52.Williams MT, Morford LL, Wood SL, Wallace TL, Fukumura M, Broening HW, Vorhees CV. Developmental D-methamphetamine treatment selectively induces spatial navigation impairments in reference memory in the Morris water maze while sparing working memory. Synapse. 2003;48:138–48. doi: 10.1002/syn.10159. [DOI] [PubMed] [Google Scholar]
- 53.Williams MT, Vorhees CV, Boon F, Saber AJ, Cain DP. Methamphetamine exposure from postnatal day 11 to 20 causes impairments in both behavioral strategies and spatial learning in adult rats. Brain Res. 2002;958:312–21. doi: 10.1016/s0006-8993(02)03620-x. [DOI] [PubMed] [Google Scholar]
- 54.Woolf NJ, Hernit MC, Butcher LL. Cholinergic and non-cholinergic projections from the rat basal forebrain revealed by combined choline acetyltransferase and Phaseolus vulgaris leucoagglutinin immunohistochemistry. Neurosci Lett. 1986;66:281–6. doi: 10.1016/0304-3940(86)90032-7. [DOI] [PubMed] [Google Scholar]