Abstract
Background
The oxidant/antioxidant balance in lung tissue is hypothesised to contribute to chronic obstructive pulmonary disease (COPD) risk. Observational studies consistently report higher antioxidant status associated with lower COPD risk, but few randomised studies have been reported.
Methods
A post-hoc analysis of 38,597 women without chronic lung disease at baseline was conducted in the Women’s Health Study (WHS) to test the effect of vitamin E on risk of incident chronic lung disease. The WHS was a randomised, double-blind, placebo-controlled, factorial trial of vitamin E (600 IU every other day) and aspirin (100 mg every other day) in female health professionals aged ≥45. Using Cox proportional hazards models, the effect of randomised vitamin E assignment on self-reported, physician-diagnosed chronic lung disease was evaluated.
Results
During 10 years of follow-up (376,710 person-years), 760 first occurrences of chronic lung disease were reported in the vitamin E arm compared to 846 in the placebo arm (Hazard Ratio [HR] 0.90; 95% confidence interval [CI] 0.81–0.99; p=0.029). This 10% reduction in the risk of incident chronic lung disease was not modified by cigarette smoking, age, randomised aspirin assignment, multivitamin use, or dietary vitamin E intake (minimum P for interaction = 0.19). Current cigarette smoking was a strong predictor of chronic lung disease risk (HR 4.17; 95% CI 3.70–4.70; versus never smokers).
Conclusions
In this large, randomised trial, assignment to 600 IU of vitamin E led to a 10% reduction in the risk of chronic lung disease in women.
Key words (MeSH): pulmonary disease, chronic obstructive, antioxidants, tocopherols, intervention studies, randomised controlled trial
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is characterised by progressive, irreversible airflow limitation and comprises a significant public health burden, with increasing trends in incidence and prevalence.[1] COPD prevalence in the U.S. adult population is 3–4% and, worldwide COPD prevalence is about 10%.[1,2] COPD was the 5th leading cause of death in the U.S. in 2001 and is expected to become the 3rd leading cause of death by 2020, largely due to population ageing and increasing cumulative cigarette smoke exposure, the primary risk factor for COPD.[1,3]
Factors that may contribute to rising COPD incidence include: obesity, dietary patterns, environmental and occupational exposures, and improved diagnostic and screening programmes.[1,4–6] Several lines of evidence support the hypothesis that diet plays a role in COPD aetiology.[4,6,7] Observational studies of diet or nutritional status biomarkers and randomised trials of diet or nutritional supplements have investigated the relation of antioxidants, notably vitamin E, and lung outcomes. Observational studies investigating the association of dietary intake and pulmonary function consistently report that higher intake of nutrients with antioxidant properties is associated with better pulmonary outcomes, but causal inferences are limited by concerns about confounding and other biases.[8,9] Studies comparing COPD patients to healthy individuals report lower plasma and peripheral skeletal muscle vitamin E (αtocopherol) concentrations in patients, and a lower risk of death from respiratory disease with higher serum α-tocopherol concentration, but whether nutrition contributed to the onset of COPD is less clear.[10–12]
Randomised trials of diet change or vitamin E supplements in clinical populations have reported mixed results. COPD patients who increased intake of antioxidant-rich foods had improved pulmonary function over three years while those on usual diets experienced continuous lung function decline.[13] Studies of α-tocopherol treatment in COPD patients report mainly negative results, although conclusions are limited by an incomplete understanding of potential to benefit, the short duration of studies, and case heterogeneity.[14–16] Very few large, randomised studies of non-diseased individuals have been completed. In the Heart Protection Study (HPS), which included participants with coronary disease, other occlusive arterial disease, or diabetes, a post-hoc analysis found no effect of vitamin E supplements on the occurrence of respiratory-related death, on COPD/asthma hospitalization rates, or on pulmonary function measured by spirometry at the end of the study.[17] In the Alpha-Tocopherol and Beta-Carotene (ATBC) study, a study of male cigarette smokers, there was no effect of α-tocopherol on the incidence of chronic bronchitis or COPD symptoms.[16]
Using data from the Women’s Health Study (WHS), a large study of apparently healthy women aged ≥ 45 years, we tested the hypothesis that supplementation with 600 IUs of α-tocopherol every other day decreases the occurrence rate of chronic lung disease.
METHODS
Additional details in the online supplement.
Study Design
The WHS, a randomised, double-blind, placebo-controlled, two-by-two factorial trial assessed risks and benefits of vitamin E supplements (600 IU every other day; Natural Source Vitamin E Association, Washington, DC, USA) and/or aspirin (100 mg every other day; Bayer AG, Leverkusen, Germany) in the primary prevention of cardiovascular disease and cancer. Full details of the study design are published elsewhere.[18] The study was registered with clinicaltrials.gov (NCT00000479).
Eligibility criteria included: age ≥45 years, healthcare professional, U.S. residence, no previous history of coronary heart disease, cerebrovascular disease, cancer (except non-melanoma skin cancer), or other major chronic illness; no more than weekly use of vitamins E, A, or β-carotene supplements; no history of adverse aspirin effects; less than weekly use of aspirin or non-steroidal anti-inflammatory drug (NSAID), or willingness to forgo; no use of anticoagulants or corticosteroids. A 3-month placebo-only run-in period identified likely long-term compliers. Of these, 39,876 women remained willing and eligible and were randomised into WHS between April 1993 and January 1996.[18]
Questionnaire Data
Mailed questionnaires collected baseline data on anthropometric, demographic, lifestyle, and clinical characteristics. Follow-up questionnaires, completed twice during the first year and annually thereafter, assessed study supplement compliance, new disease occurrence and diagnosis date, personal characteristics and habits, non-study aspirin, vitamin and NSAID use, and side effects. Compliance, defined as taking two-thirds of study supplements, was similar (78.9% and 71.6% at 5 and 10 years, respectively) between active and placebo groups.[19] Non-trial vitamin E supplement use ≥ 4 days/month was 10.0% and 10.9% at 5 and 10 years, respectively.[19]
Chronic Lung Disease Ascertainment
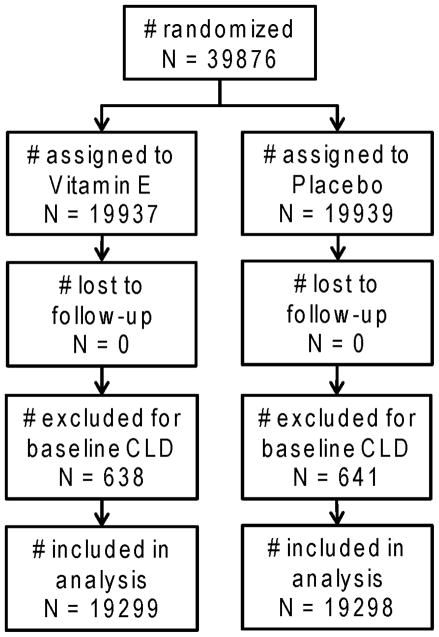
Chronic lung disease (CLD) was not a pre-specified trial endpoint. Occurrence of self-reported MD-diagnosed CLD was ascertained on questionnaires beginning 12 months after study enrolment. A multipart question asked participants “have you ever been diagnosed by a physician as having any of the following?”, and choices included “other chronic lung disease (e.g. emphysema, chronic bronchitis, bronchiectasis)” as well as “asthma.” For each diagnosis, date of diagnosis was reported. Thereafter, annual questionnaires asked about diagnoses occurring since the prior questionnaire, including diagnosis date. Incident cases were ascertained through March 31, 2004 (scheduled trial end). Prevalent CLD was defined as CLD diagnosis prior to trial enrolment. Women with prevalent CLD were excluded from the analysis (figure 1).
Figure 1.
Flow diagram of the vitamin E component of the Women’s Health Study chronic lung disease analysis
Statistical Analysis
1,279 women reported prevalent CLD (638 in vitamin E group; 641 in placebo group), thus 38,597 participants were available for analyses. All analyses followed the intention-to-treat principle. Cumulative incidence of CLD by study arm was assessed using Kaplan-Meier methods and log-rank tests to compare survival curves. Cox proportional hazards models estimated hazard ratios. Further models considered whether the effect of vitamin E on incident CLD was modified by smoking status, age, body mass index, multivitamin use, alcohol intake, baseline asthma, history of cholesterol levels ≥240mg/dL, or randomised aspirin assignment. Effect modification was tested for statistical significance using likelihood ratio tests comparing models with and without interaction terms. Data management and analyses were completed in SAS (SAS Institute Inc., Cary, NC).
RESULTS
Baseline characteristics in the 38,597 participants, summarized in table 1, were balanced between the vitamin E and placebo arms. Thus, participants in both arms were similar on age, smoking, body mass index, multivitamin use, dietary intake of vitamin E, alcohol intake, history of asthma diagnosis, and percent with cholesterol ≥240 mg/dL (Table 1). The mean age of study participants was 54.5 years, and women were followed on average for 9.8 years (376,710 person-years; 188,578 person-years in the vitamin E arm, 188,132 person-years in the placebo arm).
TABLE 1.
Baseline characteristics of Women’s Health Study participants by vitamin E randomization
| Vitamin E (N=19299)* | Placebo (N=19298)† | |
|---|---|---|
| Characteristic | No. (%)‡ | No. (%)‡ |
| Demographic/lifestyle | ||
| Age, years‡ | 54.5 (7.0) | 54.6 (7.0) |
| <55 | 11714 (60.7) | 11679 (60.5) |
| 55–64 | 5654 (29.3) | 5677 (29.4) |
| ≥ 65 | 1931 (10.0) | 1942 (10.1) |
| Cigarette smoking | ||
| Current | 2434 (12.6) | 2491 (12.9) |
| Past | 6937 (36.0) | 6823 (35.4) |
| Never | 9909 (51.4) | 9968 (51.7) |
| Average duration, yearsठ| 18.8 (12.5) | 18.9 (12.6) |
| Body mass index, kg/m2‡ | 26.0 (5.0) | 26.0 (5.0) |
| <25.0 | 9598 (50.8) | 9670 (51.1) |
| 25.0-<30.0 | 5880 (31.1) | 5837 (30.8) |
| ≥ 30.0 | 3404 (18.0) | 3411 (18.0) |
| Nutrition | ||
| Multivitamin Use | ||
| Never | 2521 (13.2) | 2553 (13.4) |
| Past only | 10927 (57.4) | 10982 (57.7) |
| Current | 5574 (29.3) | 5499 (28.9) |
| Vitamin E intake, mg/day | ||
| Diet only‡ | 6.6 (5.0) | 6.6 (5.3) |
| Diet + Supplements‡ | 63.2 (143.0) | 62.6 (140.9) |
| Alcohol intake | ||
| Rare/never | 8743 (45.3) | 8590 (44.5) |
| 1–3/month | 2531 (13.1) | 2553 (13.2) |
| 1–6/week | 6048 (31.4) | 6194 (32.1) |
| 1+/day | 1970 (10.2) | 1959 (10.2) |
| Medical conditions | ||
| Asthma diagnosis | 1104 (5.7) | 1105 (5.7) |
| Cholesterol ≥ 240 mg/dL | 5615 (29.1) | 5688 (29.5) |
| Study aspirin assignment | 9638 (49.9) | 9654 (50.0) |
N total for each characteristic ranges from 18,882 to 19,299, given missing data in some variables
N total for each characteristic ranges from 18,918 to 19,298, given missing data in some variables
Continuous variables are presented as means (SD)
Average smoking duration for current and past smokers only
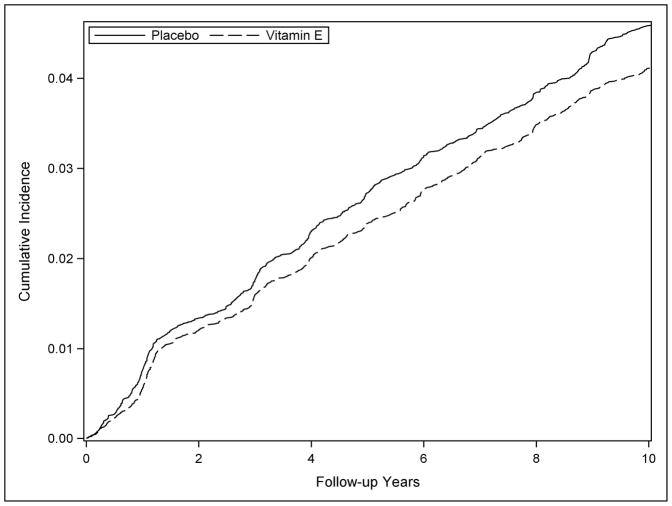
Participants reported 1,606 new diagnoses of chronic lung disease, corresponding to a cumulative incidence of 4.2%. Participants in the vitamin E arm reported 760 incident CLD diagnoses (cumulative incidence, 3.9%) compared to 846 occurrences in the placebo arm (cumulative incidence, 4.4%; figure 3), corresponding to a statistically significant 10% reduction in risk among participants randomised to vitamin E supplements (hazard rate (HR) 0.90; 95% confidence interval (CI) 0.81–0.99; p = 0.029). Comparing the cumulative CLD incidence by year of follow-up in the vitamin E and placebo groups (figure 2) the curves separate at about 1.5 years on study and continue to diverge until about 5 years of supplementation, maintaining a consistent separation thereafter. In contrast, the aspirin intervention had little or no association with risk of chronic lung disease; the hazard ratio was 0.98 (95% CI 0.89–1.08).
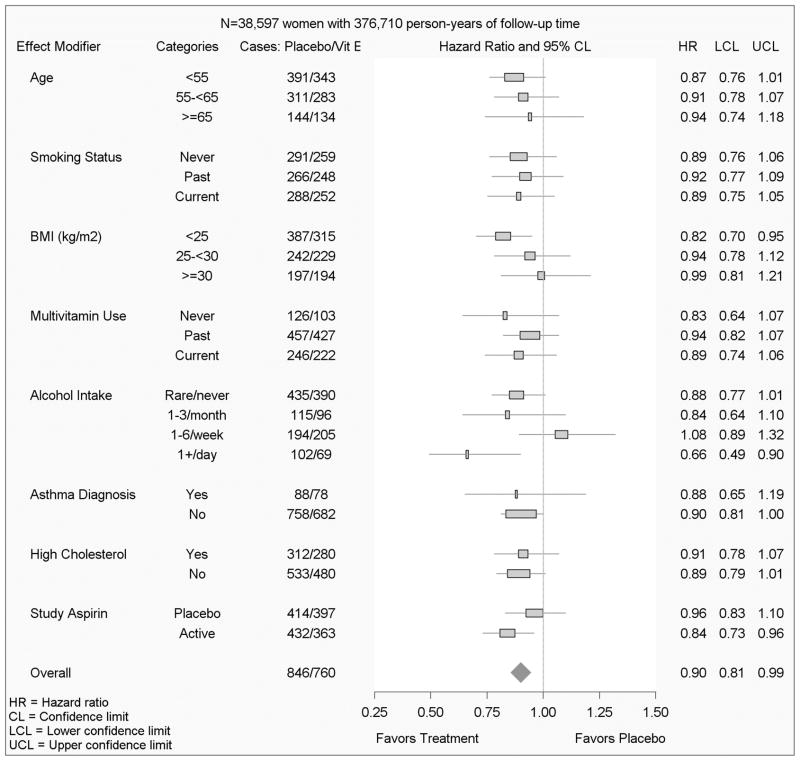
Figure 3.
Effect modification of the vitamin E / chronic lung disease effect in the Women’s Health Study
Figure 2.
Cumulative incidence of chronic lung disease during the randomized component of the Women’s Health Study
Cigarette smoking had a strong association with CLD incidence (current smoker vs. never smoker: HR 4.17; 95% CI 3.70–4.70; p = <0.0001). In addition, other known COPD risk factors were positively associated with the CLD outcome: older age at randomization (age ≥65 years vs. <55 years; HR 2.38; 95% CI 2.07–2.73; p = <0.0001), obesity (BMI ≥30.0 vs. <25.0; HR 1.60; 95% CI 1.41–1.81; p = <0.0001), asthma diagnosis prior to randomization (HR 1.94; 95% CI 1.65–2.28; p = <0.0001), and hypercholesterolemia (HR 1.42; 95% CI 1.28–1.57; p = <0.0001).
There was no statistical evidence that the effect of randomised vitamin E assignment on CLD risk was modified by age (p=0.86), smoking status (p=0.96), body mass index (p=0.25), multivitamin use (p=0.67), baseline asthma history (p=0.89), cholesterol ≥240 mg/dL (p=0.84), or by study aspirin assignment (p=0.19) (figure 3). Alcohol intake was borderline statistically significant (p=0.054) as a modifier of the effect of vitamin E on CLD, and women consuming one or more alcoholic drinks per day had the strongest vitamin E protective effect. In additional analyses, there was no evidence of effect modification by race, exercise frequency, hypertension, and baseline dietary intake of either vitamin E or C. For all models, controlling for randomised aspirin assignment did not alter the effect of vitamin E supplement assignment on CLD risk. An additional sensitivity analysis was conducted, censoring women who reported incident asthma from the CLD analysis; the association of vitamin E was similar with a 9% reduction in risk of CLD (HR 0.91, 95% CI: 0.81–1.03).
DISCUSSION
In this large, randomised, double-blind, placebo-controlled trial in apparently healthy women 600 IU of vitamin E on alternate days reduced the risk of self-reported newly diagnosed chronic lung disease by approximately 10%. There was no statistically significant difference in the magnitude of the effect of vitamin E by age, smoking status, randomised aspirin assignment, multivitamin use, or asthma history, and the protective effect was slightly stronger in women consuming ≥ 1 drink/day. Randomised assignment to aspirin had no association with the risk of CLD.
Two prior randomised trials investigated vitamin E supplementation in relation to lung outcomes, and both reported no effect of intervention.[16,17] In the Heart Protection Study (HPS) 20,536 adults aged 40–80 with prevalent coronary artery disease (CAD) were randomised to a combined intervention of 600 IUs of vitamin E, 250 mg vitamin C, and 20 mg β-carotene or placebo daily for 5 years.[17] HPS differed from the WHS in important ways. HPS enroled participants with clinically diagnosed CAD, occlusive arterial disease, or diabetes and 75% of participants were men.[17] WHS comprised women without cardiovascular disease history. Study duration (5 years in HPS vs. 10 yrs in WHS) and supplement formulation (HPS, combined supplement vs. WHS, vitamin E alone) also differed. Finally, the outcomes in HPS, pulmonary function measured by spirometry, death due to respiratory illness, and COPD or asthma-related hospitalizations differed from the WHS, which investigated incidence of chronic lung disease diagnosis.[17]
In the Alpha-tocopherol Beta-carotene Cancer Prevention Study (ATBC) 29,133 male cigarette smokers aged 50–69 were randomised to receive 50 mg vitamin E and/or 20 mg β-carotene or placebo daily for 4 years.[16] ATBC differed substantially from the WHS in the supplement studied, the trial duration and the population studied.[16] ATBC reported no effect of vitamin E on COPD-related symptoms, a substantially different endpoint than incidence of chronic lung disease diagnosis.
Strengths of the WHS include the large number of participants, the large number of self-reported, MD-diagnosed CLD outcomes, high adherence rate, and high follow-up rate. CLD is associated with ageing, thus the minimum age required for study enrolment, 45 years, yielded a population at risk for incident CLD risk.
Several limitations deserve mention. While the size of this trial was adequate to detect a statistically significant small to moderate effect of vitamin E supplementation on incident CLD, the trial was not specifically designed to test the studied hypothesis. Thus, outcome ascertainment was based solely on self-reported MD-diagnosis, a concern that is partly mitigated by the fact that participants were female health professionals. A validation study of self-reported COPD outcomes in female nurses found that 78% of self-reported COPD cases were confirmed with medical record review, suggesting self-report of lung disease by the female health professionals comprising the WHS is likely to have excellent validity.[20] While the question about outcome occurrence listed bronchiectasis, the low incidence of bronchiectasis in this age range leads to the reasonable assumption that most occurrences reported refer to COPD. Current cigarette smoking was a strong predictor of CLD in these data, providing evidence of face validity for the outcome ascertainment. Finally, if the outcome is misclassified (either by undercounting cases or by including false positives) the misclassification is likely to affect both arms of the trial equally, and the hazard ratio may therefore be an underestimate of the true effect size.
Outcome definition
The complexity of airway disease phenotypes raises substantial concern about misdiagnosis of COPD and asthma, particularly in women.[21] To address the possibility that women reporting a new asthma diagnosis actually had COPD, a sensitivity analysis was conducted. When women reporting incident asthma were excluded from the analysis, vitamin E was associated with a 9% reduction in the risk of CLD (HR 0.91, 95% CI: 0.81–1.03), similar to the findings in the full study group. There was little or no effect of vitamin E supplementation on incident self-reported MD-diagnosed asthma (HR 0.99; 95% CI 0.90–1.08; p=0.83). Kurth et al (2008) investigated the effect of randomized aspirin assignment on the risk of incident adult-onset asthma in the Women’s Health Study; women in the aspirin arm had a 10% lower risk of incident asthma compared to women in the placebo arm. When women developing chronic obstructive pulmonary disease over follow-up were censored from the analysis, the findings were similar.[22] The effect of aspirin on incident asthma, and the lack of effect of aspirin on incident CLD supports the notion of differentiation in the self-reported diagnoses.
Proposed mechanisms
Prior studies document the presence of vitamin E in the lung compartment and the mechanisms of delivery of vitamin E to alveolar type II cells in the lung.[23] Vitamin E transport to the type II cells is hypothesised to occur via high-density lipoproteins because type II cells have no physical contact with plasma and interact only with interstitial fluid lipoproteins, which are predominantly HDL lipoproteins.[23] Thus, the concentration of HDL cholesterol in the plasma and in the interstitial fluid predicts the amount of vitamin E available to the lung compartment to combat oxidative stress. Prior studies have reported that HDL cholesterol and apolipoprotein A-I levels are positively associated with FEV1, even after adjusting for serum antioxidant concentrations, a finding that may reflect the delivery of vitamin E to lung tissues.[24] Thus, a higher HDL cholesterol level is hypothesised to deliver a greater effective dose of vitamin E to the lung compartment.
Given that HDL cholesterol levels are 20-25% higher in women compared to men in all age groups, differences in the biologically effective dose of vitamin E may contribute to the difference in findings between women in WHS and the predominantly male participants in the HPS and ATBC.[25] Sex-related differences in the effect of vitamin E supplementation on all-cause mortality have been reported, with stronger protective effects of low-dose vitamin E supplementation evident in study populations comprised of ≥ 75% women.[26]
Alcohol intake is proposed to reduce CVD risk by raising HDL cholesterol levels, and a threshold of ≥ 1 drink per day is associated with both higher HDL cholesterol levels and attenuation in CVD risk.[27,28] Given the important role of HDL cholesterol in transporting vitamin E to the lung, exploratory analyses considered alcohol intake as a modifier of the effect of vitamin E on CLD. Among variables considered as possible effect modifiers, alcohol intake was marginally statistically significant (p=0.054), and the preventive effect of vitamin E on CLD was greatest in women consuming the highest level of alcohol (≥ 1 drink/day). These preliminary findings are consistent with the hypothesis that delivery of vitamin E to lung tissue may vary by plasma levels of HDL cholesterol.
Under our hypothesis, the effect of antioxidant supplementation was expected to be stronger in participants with a higher oxidative burden, for example, in current cigarette smokers. Contrary to expectation, there was no evidence of effect modification by smoking. If supplementation with vitamin E acts through other systemic mechanisms, for example by improving immune system function (Meydani 2005), then a general effect of vitamin E would be supported. [29]
Efficacy and Safety Considerations
There has been substantial discussion of the efficacy and safety of vitamin E supplementation in the scientific literature.[30] Potential harmful effects include an increased risk of all cause mortality, susceptibility to bleeding, and haemorrhagic stroke.[31–33] The meta-analysis linking high-dose vitamin E supplementation to increased risk of mortality, however, has been criticised for its methodology and a recent paper suggested that vitamin E has beneficial effects on ischemic stroke risk. [33–35] Thus, the design of future vitamin E supplementation trials must carefully consider information about risks and benefits, and recommendations may need to be tailored to specific populations.
Summary and Conclusion
The WHS was comprised of female health professionals aged ≥45, the majority of whom were of European descent. Healthy women taking 600 IU vitamin E supplements every other day were 10% less likely to report a new chronic lung disease diagnosis during the study period. Any decisions about use of vitamin E as a preventive must consider information about vitamin E associated risks and bioavailability.[22,26,30,32,34,35] Given that there are few prevention strategies for emphysema and chronic bronchitis, further study of vitamin E in relation to chronic obstructive pulmonary disease is of public health interest.
Acknowledgments
We are indebted to the participants in the Women’s Health Study for their outstanding commitment and cooperation, to the entire staff of the Women’s Health Study for their expert assistance, and to the Division of Preventive Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
FUNDING
This study was funded by grants from the National Institutes for Health, USA, grants NIH HL071022 (PAC); NIH HL043851 and CA47988 (Women’s Health Study). Funding sources had no involvement in study design, data collection, analyses, or interpretation, writing of the report, or decision to submit the report for publication.
Footnotes
ETHICS APPROVAL
The Women’s Health Study was approved by the institutional review board of Brigham and Women’s Hospital and monitored by an external data and safety monitoring board.
COMPETING INTERESTS
None
COPYRIGHT
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence on a worldwide basis to the BMJ Publishing Group Ltd, and its Licensees to permit this article (if accepted) to be published in Thorax and any other BMJPGL products and to exploit all subsidiary rights, as set out in our licence, http://thorax.bmj.com/site/about/licence.pdf .
Reference List
- 1.Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27:397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- 2.Buist AS, Vollmer WM, McBurnie MA. Worldwide burden of COPD in high- and low-income countries. Part I. The burden of obstructive lung disease (BOLD) initiative. Int J Tuberc Lung Dis. 2008;12:703–8. [PubMed] [Google Scholar]
- 3.Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat Med. 1998;4:1241–3. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 4.Varraso R, Fung TT, Barr RG, Hu FB, Willett W, Camargo CA., Jr Prospective study of dietary patterns and chronic obstructive pulmonary disease among US women. Am J Clin Nutr. 2007;86:488–95. doi: 10.1093/ajcn/86.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sood A, Qualls C, Arynchyn A, Beckett WS, Gross MD, Steffes MW, et al. Obesity-Asthma Association: Is It Explained by Systemic Oxidant Stress? Chest. 2009;108:744–53. doi: 10.1378/chest.09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smit HA, Grievink L, Tabak C. Dietary influences on chronic obstructive lung disease and asthma: a review of the epidemiological evidence. Proc Nutr Soc. 1999;58:309–19. doi: 10.1017/s0029665199000427. [DOI] [PubMed] [Google Scholar]
- 7.Romieu I, Trenga C. Diet and obstructive lung diseases. Epidemiol Rev. 2001;23:268–87. doi: 10.1093/oxfordjournals.epirev.a000806. [DOI] [PubMed] [Google Scholar]
- 8.Hu G, Cassano PA. Antioxidant nutrients and pulmonary function: the Third National Health and Nutrition Examination Survey (NHANES III) Am J Epidemiol. 151:975–81. doi: 10.1093/oxfordjournals.aje.a010141. [DOI] [PubMed] [Google Scholar]
- 9.Grievink L, Smit HA, Ocke MC, van't Veer P, Kromhout D. Dietary intake of antioxidant (pro)-vitamins, respiratory symptoms and pulmonary function: the MORGEN study. Thorax. 1998;53:166–71. doi: 10.1136/thx.53.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKeever TM, Lewis SA, Smit HA, Burney P, Cassano PA, Britton J. A multivariate analysis of serum nutrient levels and lung function. Respir Res. 2008;9:67–76. doi: 10.1186/1465-9921-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gosker HR, Bast A, Haenen GR, Fischer MA, van d V, Wouters EF, et al. Altered antioxidant status in peripheral skeletal muscle of patients with COPD. Respir Med. 2005;99:118–25. doi: 10.1016/j.rmed.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Wright ME, Lawson KA, Weinstein SJ, Pietinen P, Taylor PR, Virtamo J, et al. Higher baseline serum concentrations of vitamin E are associated with lower total and cause-specific mortality in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 2006;84:1200–7. doi: 10.1093/ajcn/84.5.1200. [DOI] [PubMed] [Google Scholar]
- 13.Keranis E, Makris D, Rodopoulou P, Martinou H, Papamakarios G, Daniil Z, et al. Impact of dietary shift to higher antioxidant foods in COPD: A Randomized Trial. Eur Respir J. 2010;36:774–80. doi: 10.1183/09031936.00113809. [DOI] [PubMed] [Google Scholar]
- 14.Daga MK, Chhabra R, Sharma B, Mishra TK. Effects of exogenous vitamin E supplementation on the levels of oxidants and antioxidants in chronic obstructive pulmonary disease. J Biosci. 2003;28:7–11. doi: 10.1007/BF02970125. [DOI] [PubMed] [Google Scholar]
- 15.Nadeem A, Raj HG, Chhabra SK. Effect of vitamin E supplementation with standard treatment on oxidant-antioxidant status in chronic obstructive pulmonary disease. Indian J Med Res. 2008;128:705–11. [PubMed] [Google Scholar]
- 16.Rautalahti M, Virtamo J, Haukka J, Heinonen OP, Sundvall J, Albanes D, et al. The effect of alpha-tocopherol and beta-carotene supplementation on COPD symptoms. Am J Respir Crit Care Med. 1997;156:1447–52. doi: 10.1164/ajrccm.156.5.96-11048. [DOI] [PubMed] [Google Scholar]
- 17.MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20, 536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 18.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women's Health Study. J Womens Health Gend Based Med. 2000;9:19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 19.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 20.Barr RG, Herbstman J, Speizer FE, Camargo CA., Jr Validation of self-reported chronic obstructive pulmonary disease in a cohort study of nurses. Am J Epidemiol. 2002;155:965–71. doi: 10.1093/aje/155.10.965. [DOI] [PubMed] [Google Scholar]
- 21.Tinkelman DG, Price DB, Nordyke RJ, Halbert RJ. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma. 2006;43:75–80. doi: 10.1080/02770900500448738. [DOI] [PubMed] [Google Scholar]
- 22.Schurks M, Glynn RJ, Rist PM, Tzourio C, Kurth T. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702. doi: 10.1136/bmj.c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolleck I, Sinha P, Rustow B. Vitamin E as an antioxidant of the lung: mechanisms of vitamin E delivery to alveolar type II cells. Am J Respir Crit Care Med. 2002;166:S62–S66. doi: 10.1164/rccm.2206019. [DOI] [PubMed] [Google Scholar]
- 24.Cirillo DJ, Agrawal Y, Cassano PA. Lipids and pulmonary function in the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2002;155:842–8. doi: 10.1093/aje/155.9.842. [DOI] [PubMed] [Google Scholar]
- 25.Cheung BM, Li M, Ong KL, Wat NM, Tam S, Pang RW, et al. High density lipoprotein-cholesterol levels increase with age in American women but not in Hong Kong Chinese women. Clin Endocrinol (Oxf) 2009;70:561–8. doi: 10.1111/j.1365-2265.2008.03361.x. [DOI] [PubMed] [Google Scholar]
- 26.Gerss J. The association of vitamin E supplementation and mortality - finally consistent results of statistical analysis. RE: The questionable association of vitamin E supplementation and mortality - inconsistent results of different meta-analytic approaches. Cell Mol Biol (Noisy-le-grand) 2010;56:OL1266–OL1267. [PubMed] [Google Scholar]
- 27.De Oliveira E Silva ER, Foster D, McGee HM, Seidman CE, Smith JD, Breslow JL, et al. Alcohol consumption raises HDL cholesterol levels by increasing the transport rate of apolipoproteins A-I and A-II. Circulation. 2000;102:2347–52. doi: 10.1161/01.cir.102.19.2347. [DOI] [PubMed] [Google Scholar]
- 28.Foerster M, Marques-Vidal P, Gmel G, Daeppen JB, Cornuz J, Hayoz D, et al. Alcohol drinking and cardiovascular risk in a population with high mean alcohol consumption. Am J Cardiol. 2009;103:361–8. doi: 10.1016/j.amjcard.2008.09.089. [DOI] [PubMed] [Google Scholar]
- 29.Meydani SN, Han SN, Wu D. Vitamin E and immune response in the aged: molecular mechanisms and clinical implications. Immunol Rev. 2005;205:269–84. doi: 10.1111/j.0105-2896.2005.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell SJ, Grochoski GT. How safe is vitamin E supplementation? Crit Rev Food Sci Nutr. 2008;48:760–74. doi: 10.1080/10408390701719355. [DOI] [PubMed] [Google Scholar]
- 31.Miller ER, III, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 32.Violi F, Pignatelli P, Basili S. Nutrition, supplements, and vitamins in platelet function and bleeding. Circulation. 2010;121:1033–44. doi: 10.1161/CIRCULATIONAHA.109.880211. [DOI] [PubMed] [Google Scholar]
- 33.Schurks M, Glynn RJ, Rist PM, Tzourio C, Kurth T. Effects of vitamin E on stoke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:C5702. doi: 10.1136/bmj.c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerss J, Kopcke W. The questionable association of vitamin E supplementation and mortality--inconsistent results of different meta-analytic approaches. Cell Mol Biol (Noisy -le-grand) 2009;55(Suppl):OL1111–OL1120. [PubMed] [Google Scholar]
- 35.Berry D, Wathen JK, Newell M. Bayesian model averaging in meta-analysis: vitamin E supplementation and mortality. Clin Trials. 2009;6:28–41. doi: 10.1177/1740774508101279. [DOI] [PubMed] [Google Scholar]