Abstract
The anterior cingulate cortex (ACC) and opioid receptors have been suggested to play a role in attributing incentive motivational properties to drug-related cues. We examined whether blockade of ACC opioid receptors would reduce cue-induced ethanol-seeking behavior in mice. We show that intra-ACC opioid receptor blockade disrupted expression of an ethanol-induced conditioned place preference, suggesting that endogenous opioid modulation in the ACC may be critical for maintaining the cue’s conditioned rewarding effects.
Keywords: Ethanol, cue-induced drug seeking, incentive motivation, opioid receptors, anterior cingulate cortex, conditioned place preference
Drug-associated cues can exert strong motivational influences, increasing drug-seeking behaviors that potentially lead to relapse (Everitt & Robbins, 2005; See, 2002). Human brain imaging studies have identified several neural areas activated by drug-related cues, including the anterior cingulate cortex (ACC), which has been hypothesized to reflect incentive motivational effects of drug-related cues (e.g., Childress et al., 1999; Grüsser et al., 2004; Myrick et al., 2004) and to regulate conditioned cue relapse (See, 2002). The ACC is also a key site of opioid action in human as well as rodent prefrontal cortex (e.g., Jones et al., 1991; Vogt et al., 1995; Vogt et al., 2001). Interestingly, opioid receptor binding in the ACC is highly correlated with craving during early abstinence in alcohol- (Williams et al., 2009) and cocaine- (Gorelick et al., 2005) dependent subjects. Also, antagonism of opioid receptors reduces cue-induced craving and reactivity in alcoholics (O’Malley et al., 2002; Monti et al., 1999). Thus, it is possible that the ACC’s role in cue-induced drug seeking is mediated, at least in part, by opioid signaling within the ACC.
General support for this hypothesis comes from rodent models that show changes in ACC Fos expression following behaviors induced by cues previously paired with cocaine (Crawford et al., 1995; Neisewander et al., 2000), morphine (Harris & Aston Jones, 2003) and ethanol (Dayas et al., 2007). Further, animal studies have shown that opioid receptor blockade modulates cue-induced ethanol-seeking behavior (Ciccocioppo et al., 2002; Cunningham et al., 1995, 1998; Dayas et al., 2007; Katner et al., 1999; Kuzmin et al., 2003; Marinelli et al., 2009; Middaugh & Bandy, 2000). For example, a non-selective opioid antagonist (naloxone) was previously shown to facilitate extinction, but not acquisition or initial expression, of cue-induced ethanol-seeking behavior in mice (Cunningham et al., 1995; 1998) using the conditioned place preference (CPP) procedure, a well-established model of cue-induced seeking behavior (Cunningham et al., 2006; Tzschentke, 2007). The authors hypothesized that opioid receptor blockade altered the conditioned motivation that normally maintained cue-induced seeking behavior during testing (Cunningham et al., 1998). Later studies showed that naloxone’s effect was attributable, in part, to actions at opioid receptors located in the ventral tegmental area (VTA), but not in nucleus accumbens (Bechtholt & Cunningham, 2005). Additional studies identified a crucial role for dopamine receptors in the amygdala (Gremel & Cunningham, 2009), suggesting that ethanol CPP might depend on opioid receptor modulation of VTA dopamine neurons leading to downstream release and activation of BLA. In addition, the BLA is activated by cortical glutamatergic afferents, including those from the ACC (e.g., Gabbott et al., 2005), raising the possibility that amygdala activation during cue-induced ethanol-seeking behavior is also influenced by the ACC.
Taken together with previous work suggesting a role for the ACC in conditioned behavior (e.g., Cardinal et al., 2002) and the high density of opioid receptors in the ACC (Vogt et al., 1995, 2001), we hypothesized that expression of cue-induced ethanol-seeking behavior depends on opioid signaling within the ACC. To examine this, we infused the opioid receptor antagonist methylnaloxonium into the ACC immediately before testing expression of an ethanol-induced CPP.
DBA/2J adult male mice (N = 127) were obtained from Jackson Laboratory at 6 weeks of age. Surgeries were performed at 7-9 weeks of age. Initially mice were housed 4 per cage; after surgery mice were housed 2 per cage. Mice were maintained at 20-24°C on a 12 h light dark cycle (lights on at 7:00 am) with food and water available ad libitum. All procedures were conducted in accordance with The National Institute of Health (NIH) “Principles of Laboratory Animal Care” and the Oregon Health & Science University IACUC approved the protocol.
Mice were anesthetized with an IP injection (4 ml/kg) of an anesthetic cocktail containing ketamine (30.0 mg/ml) and xylazine (3.0 mg/ml). The NSAID analgesic Meloxicam (0.2 mg/kg SC) was used to manage post-operative pain. A single guide cannula (10 mm, 25 gauge) was implanted midline anchored to the skull with stainless steel screws, 1 mm above the ACC (from Bregma: anterior = 0.74; lateral = 0.00; ventral = −1.0) (Paxinos & Franklin, 2001). Given the midline location of the ACC and its small size (which made bilateral cannulation difficult), we decided to give one larger midline infusion rather than two smaller bilateral infusions. Mice were given 4-9 days of recovery (counterbalanced within infusion groups) before beginning the experimental procedure.
Ethanol (20% v/v, saline vehicle) was prepared from a 95% stock solution and administered IP at a dose of 2 g/kg (injection volume, 12.5 ml/kg). These parameters were chosen on the basis of many studies reporting robust CPP to tactile stimuli in DBA/2J mice at this dose and concentration (e.g., Groblewski et al., 2008). Methylnaloxonium (Naloxone methiodide; Sigma, St. Louis, MO) was dissolved in artificial cerebral spinal fluid (aCSF) containing glucose. Methylnaloxonium, a quaternary derivative of naloxone, was used because it does not cross the blood-brain barrier and it has been shown to diffuse away from the injection site more slowly than naloxone (Schroeder, Weinger, Vakassian, & Koob, 1991). Doses of 0.750 and 0.375 μg/0.2 μl were chosen based on previous work showing reduced expression of an ethanol-induced CPP when these doses were infused into mouse VTA (Bechtholt & Cunningham, 2005).
The apparatus and procedure have previously been described in greater detail (Cunningham et al., 2006). In brief, 12 identical place conditioning chambers (30 × 15 × 15 cm) were enclosed in sound and light attenuated chambers. Infrared light sources and photodetectors (mounted 2.2 cm above the floor at 5-cm intervals) were used to measure general activity (beam crosses) and the time spent on each side of the box. Two distinct interchangeable floor halves were used as tactile conditioned stimuli (CSs). The grid floor consisted of stainless steel rods (2.3 mm diameter) mounted 6.4 mm apart in acrylic rails. The hole floor was made from perforated 16-gauge stainless steel with 6.4-mm round holes on 9.5-mm staggered centers. These two textures have repeatedly been shown to produce approximately equal preference for each tactile floor cue, allowing for unbiased detection of CPP (Cunningham et al., 2003).
An unbiased conditioned place preference procedure was used (e.g., Cunningham et al., 2003), which consisted of 3 phases: habituation (one session), conditioning (eight sessions) and testing (one session). During habituation, mice received an IP saline injection before placement on a smooth paper floor for 5 min to reduce the novelty associated with handling and injections. Mice underwent four conditioning trials (CS+ and CS− trial types) alternating trial type across days, where they were given either saline or ethanol (2g/kg) injections immediately before being placed in the apparatus for 5 min. Mice in the Grid+ conditioning subgroup received ethanol paired with the grid floor (CS+) and saline paired with the hole floor (CS−). Conversely, mice in the Grid− conditioning subgroup received ethanol paired with the hole floor (CS+) and saline paired with the grid floor (CS−). The order of CS exposure was counterbalanced within groups. After conditioning, all mice received a place preference test involving 15-min exposure to a half hole/half grid floor. The order of CS exposure during conditioning and position (left vs. right) of grid floor during testing was counterbalanced within groups.
An 11 mm stylet was lowered to the injection site 24 – 36 h before the first intracranial infusion to minimize interference from potential behavioral effects of initial injector lowering. Immediately before testing, all mice were given a midline infusion via a microinfusion pump (Model A-74900-10; Cole Parmer, Vernon Hills, IL) of aCSF or methylnaloxonium (0.375 or 0.750 μg/0.2 μl) at a rate of 0.1 μl/min. Injectors were kept in place for an additional 30 sec to allow for diffusion of the drug away from the injection site. Then, to mimic the conditioning day procedure, animals were weighed, injected with saline and placed in the apparatus for the choice test session. Within 24 h of the final CPP test, all mice were euthanized with pentobarbital (150 mg/kg). Brains were postfixed in 2% w/v paraformaldehyde in phosphate-buffered saline (PBS), and then sucrose cryoprotected. Cannula and injector placement were determined by examining thionin stained 40 μm sections.
Time spent on the grid floor (mean sec/min) during the place preference test served as the primary dependent variable. In this unbiased design, the difference in time spent on the grid floor by the Grid+ and Grid− conditioning subgroups provides an index of CPP (Cunningham et al., 2003, 2006). Preliminary analyses of test data in consecutive 5-min blocks indicated drug treatment effects in all three blocks. Thus, the primary data analyses focused on performance averaged over all 15 min. An overall two-way (Dose × Conditioning Subgroup) analysis of variance (ANOVA) was followed by planned comparisons between the Grid+ and Grid− conditioning subgroups to determine whether place conditioning was significant within each dose group (Bonferroni corrected p values). Additional two-way ANOVAs were used to directly compare each methylnaloxonium dose group to the aCSF control group. Activity during the place preference test (counts/min) was assessed using one-way ANOVA (Dose). Conditioning trial activity data were analyzed by two-way (Dose × CS trial type) repeated-measures ANOVA (collapsed across trials).
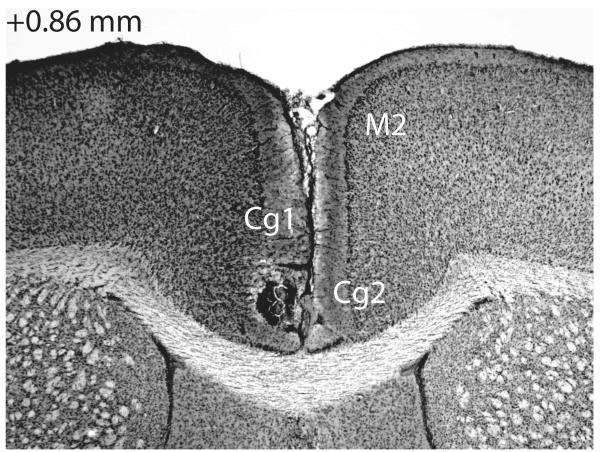
Data from one mouse were removed due to a procedural error. Data from five additional mice were discarded due to problems with histological determination of cannula placement. An example photomicrograph of the ACC injection site is shown in Figure 1. The majority of our placements were midline, but those that were slightly off-midline were equally distributed between the left and right hemispheres. Behavioral data were included in our analyses only for mice that showed a clearly identified injector tract in the ACC (n = 65; see Figure 2 caption for group n’s). Ten mice were excluded for incorrect cannula placements (injector tract in secondary motor cortex, n = 5; corpus callosum, n = 5). Further, midline infusions into the ACC through a chronic indwelling cannula in mice presented difficulties in assessing cannula placement. Since the injector only extended 1 mm beyond the cannula tip, and the cannula was positioned midline above the saggital vein, identifying the location of the injection itself was difficult due to the cannula- or histology-induced damage in the surrounding tissue. Additionally, a longer delay in extracting the brain may have resulted in an increased infection rate. Keeping inclusion criteria consistent with our previous work, we conservatively excluded 46 additional mice (Bechtholt & Cunningham, 2005; Gremel & Cunningham, 2009; Gremel & Cunningham, 2010). Less conservative data analyses that included these 46 mice yielded conclusions identical to those based on the primary data analyses (see next section).
Figure 1.
Photomicrograph showing thionin-stained coronal section of intra-ACC injection site. For reference, the location of the cingulate cortex, area 1 (Cg1), cingulate cortex area 2 (Cg2), and the secondary motor cortex (M2) are indicated by their abbreviations. Number in the upper left hand corner indicates the distance from bregma in millimeters of the section (Paxinos & Franklin, 2001).
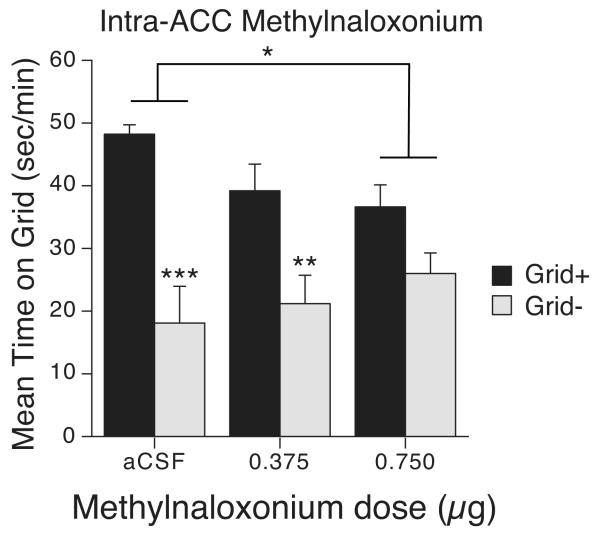
Figure 2.
Intra-ACC methylnaloxonium disrupts cue-induced ethanol-seeking behavior. Mean time (s/min; + SEM) spent on the grid floor during a 15-min test after intra-ACC methylnaloxonium. Mice in the Grid+ conditioning group were given ethanol paired with the grid floor (aCSF n = 10; 0.375 μg methylnaloxonium n = 13; 0.750 μg methylnaloxonium n = 13). Mice in the Grid− conditioning group were given ethanol paired with the hole floor (aCSF n = 9; 0.375 μg methylnaloxonium n = 9; 0.750 μg methylnaloxonium n = 11) Brackets indicate a significant Dose × Conditioning group interaction. *p < 0.02 **p < 0.01 ***p < 0.0001
Methylnaloxonium-infused mice spent 58.4 ± 4.2% (0.750 μg) and 68 ± 5.3% (0.375 μg) time on the previously drug-paired floor compared to 74.4 ±5 .3% for aCSF-infused mice. Control (aCSF) mice showed a strong place preference, as indicated by a large difference in time spent on the grid floor between mice in the Grid+ and Grid− conditioning subgroups (see Figure 2). However, blockade of ACC opioid receptors dose-dependently interfered with CPP expression. Two-way (Dose × Conditioning Subgroup) ANOVA yielded a significant main effect of Conditioning Subgroup [F(1, 59) = 35.7, p < 0.001] and a trend towards a significant interaction [F(2, 59) = 3.0, p = 0.06]. Planned comparisons between the Grid+ and Grid− subgroups showed significant CPP in mice infused with aCSF or the low methylnaloxonium dose (Bonferroni corrected p’s < 0.007). However, mice infused with the high (0.750 μg) methylnaloxonium dose did not show a significant CPP. Additional comparisons between pairs of dose groups (i.e., Dose × Conditioning Subgroup ANOVAs) revealed a significant difference in CPP magnitude only between aCSF and the high methylnaloxonium dose group [F(1,39) = 6.8, p < 0.02], confirming that this dose disrupted expression of ethanol-induced CPP. Analysis of data from an additional 15 min of testing during the same test session showed a similar pattern of group differences (data not shown), although CPP was generally weaker during the second half of the session, presumably due to extinction (aCSF: 69.6 ± 5.4%; 0.375 μg: 58.4 ± 6.1%; 0.750 μg: 58.2 ± 4.6%).
As noted earlier, an overall two-way (Dose × Conditioning Subgroup) ANOVA that included both the 65 mice with cannula located in ACC as well as the 46 mice eliminated by our conservative histological criteria yielded conclusions similar to those from our primary analyses [significant main effect of Conditioning Subgroup: F(1,105) = 69.8, p < 0.0001; significant interaction: F(2,105) = 5.3, p < 0.007]. Averaged over all 111 mice, mean (± SEM) percent times spent on the ethanol-paired floor were 75.3±3.5, 64.4±3.8 and 59.6±2.9 for the aCSF, 0.375 μg and 0.750 μg groups, respectively.
To assess anatomical specificity, we also analyzed CPP in the 10 mice that were excluded for incorrect cannula placement. Although low numbers of mice in each conditioning subgroup precluded a complete factorial analysis of these data, we were able to compare percent time spent on the ethanol paired floor between aCSF treated mice (66.9±11.9%, n = 6) and mice in the high dose group (71.9±2.3%, n = 3). One-way ANOVA indicated no significant difference between these two groups. These data suggest that methylnaloxonium had no effect on CPP when it was infused either above or below the ACC.
All groups showed a similar level of activity (mean counts/min ± SEM) during the 15-min test session (aCSF = 51.7 ± 3.8; 0.375 μg = 53.0 ± 3.5; 0.750 μg = 61.6 ± 4.8; F = 1.7, p = 0.18). Additionally, all groups showed similar levels of activity during conditioning. Activity levels collapsed across trials showed similar stimulation to ethanol [main effect of drug: F(1, 61) = 809.9, p < 0.001] (aCSF = 184.0 ± 4.2; 0.375 μg = 186.6 ± 4.4; 0.750 μg = 177.2 ± 5.0), as well as similar activity on saline trials (aCSF = 71.4 ± 2.0; 0.375 μg = 71.8 ± 2.0; 0.750 μg = 66.9 ± 2.1). These results indicate that the effect of intra-ACC methylnaloxonium on CPP expression cannot be attributed to activity differences during conditioning or testing (Gremel & Cunningham, 2007).
To the best of our knowledge, we present the first data demonstrating a functional role for ACC opioid receptors in the modulation of cue-induced drug-seeking behavior. Specifically, we identify the first cortical site modulating ethanol-induced CPP by showing that blockade of ACC opioid receptors disrupts expression of ethanol CPP in mice. Thus, these data suggest that endogenous opioid activation of the ACC is involved either in memory retrieval or in the expression of cue-induced incentive motivational effects that influence drug-seeking behavior (Cunningham et al., 2011).
Although the role of the ACC in appetitive-conditioned behaviors has been extensively studied (Cardinal et al., 2002), previous studies of ACC opioid receptors have focused primarily on their role in pain processing (e.g., Petrovic et al., 2002). However, a recent study showed a graded effect of naloxone on hedonic processing of rewards in humans, as indexed by a reduction in pleasure ratings for larger rewards and a corresponding attenuation of associated brain activity in the rostral ACC (Petrovic et al., 2008). This study also showed that negative outcomes (i.e., loss of reward) were rated as more unpleasant under naloxone, an effect that was associated with increased activity in caudal ACC. Overall, these data suggest a potential role of ACC opioid receptors in the processing of both rewarding and aversive hedonic events, a possibility that receives further support from our finding that site-specific blockade of ACC opioid receptors interferes with responding to a conditioned stimulus previously paired with ethanol. In contrast to our previous studies with systemic naloxone (Cunningham et al., 1995, 1998), intra-ACC opioid receptor blockade disrupted the initial expression of CPP. Moreover, in contrast to systemic naloxone, intra-ACC methylnaloxonium did not gradually induce avoidance of the cue previously paired with ethanol. However, the latter effect of naloxone required repeated systemic exposure to the antagonist over several long-duration (60-min) tests, a procedure that would be difficult to duplicate using site-specific infusions whose efficacy presumably diminishes as the drug diffuses away from the injection site.
Our findings are generally consistent with previous studies suggesting a potential role for the ACC in place conditioning based on a positive correlation between Fos expression in ACC and drug-induced CPP (e.g., Crawford et al., 1995; Harris & Aston-Jones, 2003). Moreover, these data are consistent with a previous study showing that reversible inactivation of the ACC (using tetrodotoxin) reduces conditioned-cue reinstatement of an extinguished cocaine-seeking response (McLaughlin & See, 2003). However, our study is at odds with one study that showed mixed effects of quinolinic acid induced ACC lesions on CPP in rats. That study showed no effect of ACC lesions on morphine or cocaine-induced CPP, but lesions did interfere with CPP induced by a competitive NMDA receptor antagonist (CGP37849) (Tzschentke & Schmidt, 1999). There are several possible reasons for this apparent discrepancy (e.g., conditioning drug, species), including the possibility of post-lesion compensations in other brain areas or neurotransmitter systems.
In contrast, results from other lesion studies have implicated the ACC in conditioned behaviors, and suggest a role in discriminating between reward-predictive and neutral cues (Bussey et al., 1997; Cardinal et al., 2003; Parkinson et al., 2000). This hypothesis can explain the present results because blockade of ACC opioid receptors might have impaired ability to differentially associate ethanol with CS+ but not with CS−. However, in a recent study of cue-induced alcohol seeking in a self-administration procedure, an opioid antagonist-induced decrease in responding was not easily attributed to an inability to discriminate between cues. Instead, systemic opioid receptor blockade selectively altered responding only after exposure to a reward-predictive cue (S+) and not after exposure to a neutral cue (S−) (Dayas et al., 2007). In self-administration procedures in general, cue-induced alcohol seeking behavior is selectively attenuated with administration of opioid antagonists (e.g., Ciccociopo et al., 2002; Dayas et al., 2007; Katner et al., 1999; Marinelli et al., 2009). When considered together with findings from self-administration studies, the present results generally support the idea that ACC opioid receptors mediate predictive information about learned cue-drug associations.
Although a non-specific antagonist was used in the present study, it is tempting to speculate about the specific opioid receptor subtype responsible for the disruption of ethanol CPP. Within the ACC, delta receptors seem to be localized to cortical neurons, while mu receptors are localized to both cortical neurons and thalamic afferent axons (Vogt 1995; Vogt et al., 2001). Studies of reinstatement behavior after extinction of ethanol self-administration have suggested that the delta opioid receptor subtype mediates reinstatement induced by both context (i.e., static apparatus cues) and discrete cues (i.e., a phasic light-noise stimulus), while the mu opioid receptor subtype may have a more limited role only in context-induced reinstatement (Marinelli et al., 2009). Assuming that the mechanisms underlying stimulus control of CPP are more like those mediating context-induced reinstatement, these data suggest that activation of either of these opioid receptor subtypes within the ACC or on thalamic input might be involved in the expression of ethanol-induced CPP. Future studies examining specific receptor subtypes in and on pathways to the ACC could provide important information related to their potential roles in the memory and motivational processes that underlie performance of ethanol-induced CPP.
In summary, we found that blockade of opioid receptors in the ACC reduced cue-induced ethanol-seeking behavior in mice. The identification of a cortical site of opioid action adds to previous findings suggesting VTA opioid receptor involvement in cue-control over ethanol-seeking behaviors (Bectholt & Cunningham, 2005). Further, the ACC is anatomically connected to the amygdala and nucleus accumbens (e.g., Gabbot et al., 2005) in which we have previously identified specific receptor mechanisms governing the expression of ethanol CPP (Gremel & Cunningham, 2009). Overall, these data suggest a corticostriatal circuit regulates the expression of cue-induced ethanol-seeking behavior. Importantly, our findings shed new light on potential neural mechanisms underlying the conditioned motivational processes that contribute to addiction and relapse.
Acknowledgements
This research was supported by NIH-NIAAA grants AA016041, AA007468, and AA007702. Experiments within this manuscript comply with the current laws of the United States of America.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bechtholt AJ, Cunningham CL. Ethanol-induced conditioned place preference is expressed through a ventral tegmental area dependent mechanism. Behav Neurosci. 2005;119:213–223. doi: 10.1037/0735-7044.119.1.213. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Everitt BJ, Robbins TW. Dissociable effects of cingulate and medial frontal cortex lesions on stimulus-reward learning using a novel Pavlovian autoshaping procedure for the rat: implications for the neurobiology of emotion. Behav Neurosci. 1997;111:908–919. doi: 10.1037//0735-7044.111.5.908. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Lachenal G, Halkerston KM, Rudarakanchana N, Hall J, Morrison CH, Howes SR, Robbins TW, Everitt BJ. Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats. Behav Neurosci. 2002;116:553–567. doi: 10.1037//0735-7044.116.4.553. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Marbini HD, Toner AJ, Bussey TJ, Robbins TW, Everitt BJ. Role of the anterior cingulate cortex in the control over behavior by Pavlovian conditioned stimuli in rats. Behav Neurosci. 2003;117:566–587. doi: 10.1037/0735-7044.117.3.566. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien C. Limbic activation during cue-induced cocaine craving. American Journal of Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford CA, McDougall SA, Bolanos CA, Hall S, Berger SP. The effects of the kappa agonist U-50,488 on cocaine-induced conditioned and unconditioned behaviors and Fos immunoreactivity. Psychopharmacology (Berl) 1995;120:392–399. doi: 10.1007/BF02245810. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Dickinson SD, Okorn DM. Naloxone facilitates extinction but does not affect acquisition or expression of ethanol-induced conditioned place preference. Experimental and Clinical Psychopharmacology. 1995;3:330–343. [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nature Protocols. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Groblewski PA, Voorhees CM. Place conditioning. In: Olmstead MC, editor. Animal models of drug addiction. Humana Press; Totowa, NJ: 2011. pp. XX–XX. [Google Scholar]
- Cunningham CL, Henderson CM, Bormann NM. Extinction of ethanol-induced conditioned place preference and conditioned place aversion: effects of naloxone. Psychopharmacology (Berl) 1998;139:62–70. doi: 10.1007/s002130050690. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Patel P. Rapid induction of Pavlovian approach to an ethanol-paired visual cue in mice. Psychopharmacology (Berl) 2007;192:231–241. doi: 10.1007/s00213-007-0704-4. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, Weiss F. Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biological Psychiatry. 2007;61:979–989. doi: 10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Warner TA, Jays PRL, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- George M, Anton R, Bloomer C, Teneback C, Drobes D, Lorberbaum J, Nahas Z, Vincent D. Activation of Prefrontal Cortex and Anterior Thalamus in Alcoholic Subjects on Exposure to Alcohol-Specific Cues. Archives of General Psychiatry. 2001;58:345. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino M, Endres CJ, Dannals RF, Frost JJ. Imaging brain mu-opioid receptors in abstinent cocaine users: time course and relation to cocaine craving. Biol Psychiatry. 2005;57:1573–1582. doi: 10.1016/j.biopsych.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Gremel C,M, Cunningham C,L. Role of test activity in ethanol-induced disruption of place preference expression in mice. Psychopharmacology (Berl) 2007;191:195–202. doi: 10.1007/s00213-006-0651-5. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Involvement of amygdala dopamine and nucleus accumbens NMDA receptors in ethanol-seeking behavior in mice. Neuropsychopharmacology. 2009;34:1443–1453. doi: 10.1038/npp.2008.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groblewski PA, Bax LS, Cunningham CL. Reference-dose place conditioning with ethanol in mice: empirical and theoretical analysis. Psychopharmacology (Berl) 2008;201:97–106. doi: 10.1007/s00213-008-1251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Enhanced morphine preference following prolonged abstinence: association with increased Fos expression in the extended amygdala. Neuropsychopharmacology. 2003;28:292–299. doi: 10.1038/sj.npp.1300037. [DOI] [PubMed] [Google Scholar]
- Jones AK, Qi LY, Fujirawa T, Luthra SK, Ashburner J, Bloomfield P, Cunningham VJ, Itoh M, Fukuda H, Jones T. In vivo distribution of opioid receptors in man in relation to the cortical projections of the medial and lateral pain systems measured with positron emission tomography. Neurosci lett. 1991;126:25–28. doi: 10.1016/0304-3940(91)90362-w. [DOI] [PubMed] [Google Scholar]
- Kargo WJ, Nitz DA. Improvements in the signal-to-noise ratio of motor cortex cells distinguish early versus late phases of motor skill learning. J Neurosci. 2004;24:5560–5569. doi: 10.1523/JNEUROSCI.0562-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner SN, Magalong JG, Weiss F. Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology. 1999;20:471–479. doi: 10.1016/S0893-133X(98)00084-0. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharmacol Exp Ther. 2003;304:310–318. doi: 10.1124/jpet.102.041350. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Harding S, Li Z, Juzytsch W, Lê AD. Roles of opioid receptor subtypes in mediating alcohol-seeking induced by discrete cues and context. Eur J Neurosci. 2009;30:671–678. doi: 10.1111/j.1460-9568.2009.06851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Bandy AL. Naltrexone effects on ethanol consumption and response to ethanol conditioned cues in C57BL/6 mice. Psychopharmacology. 2000;151:321–327. doi: 10.1007/s002130000479. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM, Brown RA, Gulliver SB, Gordon A, Abrams DB. Naltrexone’s effect on cue-elicited craving among alcoholics in treatment. Alcohol Clin Exp Res. 1999;23:1386–1394. [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LTL, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. Journal of Neuroscience. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Academic Press; San Diego: 2001. [Google Scholar]
- Parkinson JA, Willoughby PJ, Robbins TW, Everitt BJ. Disconnection of the anterior cingulate cortex and nucleus accumbens core impairs Pavlovian approach behavior: further evidence for limbic cortical-ventral striatopallidal systems. Behav Neurosci. 2000;114:42–63. [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson K, Ingvar M. Placebo and Opioid Analgesia--Imaging a Shared Neuronal Network. Science. 2002;295:1737. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Pleger B, Seymour B, Kloppel S, De Martino B, Critchley H, Dolan R. Blocking Central Opiate Function Modulates Hedonic Impact and Anterior Cingulate Response to Rewards and Losses. Journal of Neuroscience. 2008;28:10509. doi: 10.1523/JNEUROSCI.2807-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder RL, Weinger MB, Vakassian l., Koob GF. Methylnaloxonium diffuses out of the rat brain more slowly than naloxone after direct intracerebral injection. Neuroscience Letters. 1991;12:173–177. doi: 10.1016/0304-3940(91)90678-m. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Functional heterogeneity of the rat medial prefrontal cortex: effects of discrete subarea-specific lesions on drug-induced conditioned place preference and behavioural sensitization. Eur J Neurosci. 1999;11:4099–4109. doi: 10.1046/j.1460-9568.1999.00834.x. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Wiley RG, Jensen EL. Localization of Mu and delta opioid receptors to anterior cingulate afferents and projection neurons and input/output model of Mu regulation. Experimental Neurology. 1995;135:83–92. doi: 10.1006/exnr.1995.1069. [DOI] [PubMed] [Google Scholar]
- Vogt LJ, Sim-Selley LJ, Childers SR, Wiley RG, Vogt BA. Colocalization of mu-opioid receptors and activated G-proteins in rat cingulate cortex. J Pharmacol Exp Ther. 2001;299:840–848. [PubMed] [Google Scholar]
- Williams TM, Davies SJ, Taylor LG, Daglish MR, Hammers A, Brooks DJ, Nutt DJ, Lingford-Hughes A. Brain opioid receptor binding in early abstinence from alcohol dependence and relationship to craving: an [11C]diprenorphine PET study. Eur Neuropsychopharmacol. 2009;19:740–748. doi: 10.1016/j.euroneuro.2009.06.007. [DOI] [PubMed] [Google Scholar]