Alström Syndrome (ALMS) (MIM #203800) is a rare, complex, autosomal recessive genetic disorder affecting multiple organs and systems. Patients present with cone-rod dystrophy leading to blindness, hearing impairment, severe insulin resistance, acanthosis nigricans, and type 2 diabetes (T2DM), obesity, dilated cardiomyopathy (DCM) in infancy, or adolescent/adult onset restrictive cardiomyopathy. Hormonal imbalances, hyperlipidemia, short adult stature with scoliosis, liver steatosis and cirrhosis, pulmonary fibrosis, and renal failure is progressive in adolescents and adults. Low testosterone levels are frequently observed in males, but hyperandrogenism and hirsutism is often reported in female patients. Secondary sexual characteristics such as axillary and pubic hair are usually normal in both males and females. Variable complications can include hypothyroidism, delay of developmental milestones, seizures, gastrointestinal and urological dysfunction.1,2
The gradual onset of the classic symptoms throughout childhood often delays the diagnosis until adolescence or adulthood. Treatment is challenging and the dermatological phenotypes are often overlooked.
ALMS is caused by mutations in ALMS1 (chr 2p13)3,4. Cellular localization studies have shown that the ALMS1 protein is ubiquitously expressed and localizes to centrosomes and basal bodies of ciliated cells.5,6 and roles in intracellular transport and ciliary & centrosomal function have been suggested.6
A 12.5 year (y) old female presented with classic phenotypic characteristics of ALMS, along with severe presentation of gynecological and dermatological features of the syndrome.
There were some minor psychomotor delays in development: sitting (9 months (m)), walking (14 m), first spoken words (>1 y). She had several episodes of pneumonia during infancy and childhood. At 3 m, she had seizures, with the activity diminishing by the age of 4 y with medication. At 4 m, DCM was diagnosed. With low dose digoxin therapy, her heart function was stabilized. At age 12y, although there was no documented heart failure, edema developed in the hands and feet. She had nystagmus and photophobia during infancy with rapid deterioration of visual acuity after the age of 7–8 y. Ophthalmological and fundus examination revealed nystagmus and bilateral cone-rod retinopathy by ERG. She was blind by the age of 12 y at which time hearing impairment was diagnosed. TSH was 7.4 μU/ml with normal levels of fT4, confirming subclinical hypothyroidism.
Her hyperphagia and aggressive weight gain started at the age of 4–5 y. At the age of 10 y, she had abdominal obesity and her height was 133 cm. Her Body Mass Index (BMI kg/m2) was 34.2 and BMI-SDS: 2+3SDS. Peak glycemia on oral glucose tolerance testing (OGTT) was 7.4 mmol/l, and peak insulin was >400 μIU/ml. T2DM (postprandial hyperglycemia up to 12 mmol/L, HbA1C 9.1%), and higher levels of lipids was diagnosed.
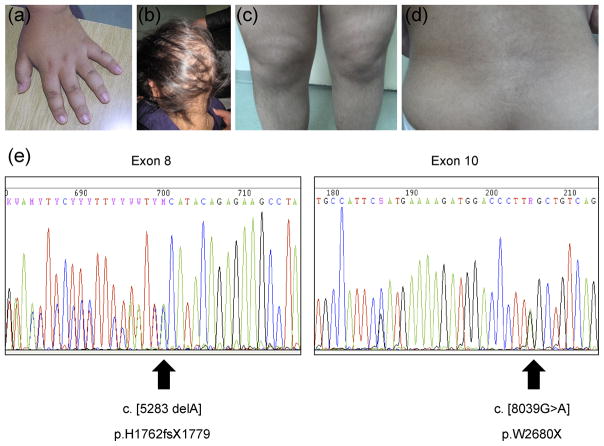
Acanthosis nigricans was present in the nuchal and axillary region and on the hands (Fig. 1a). Lipids were elevated (cholesterol 10.9 mmol/L, triglycerides 31.6 mmol/L). She had moderate elevation of hepatic enzymes and abdominal ultrasonography showed fatty liver.
Figure 1.
Menarche occurred at age 11.5 y, with regular menstruation for 6 m, but she has subsequently developed oligomenorrhea at age 12.5y. Follicle stimulating hormone (FSH) was 1.9 mIU/mL (0.6–3.4), Luteinizing hormone (LH) was 4.6 (1.7–5.0), testosterone was 2.9 nmol/L (0.19–2.67). Dehydroepiandrosterone sulfate (DHEAS) was 7.4 nmol/L (0.9–5.2), suggesting androgen excess. Cortisol values at 8 a.m was 12.1 μg/dL and was suppressed by a short Dexametasone test to 2 md/dL, thus Cushing syndrome was excluded from the differential diagnosis. She has had frequent urinary and renal infections and lung infections during her adolescence.
At the age of 10y her hair gradually became sparse and slow-growing, and partial alopecia was visible (Fig 1b). Hair loss at the beginning was limited to thinning at the front, sides and crown without itching or pain. She maintained the frontal hairline, eyebrows, and hair on other parts of the body. Hirsutism, affecting the chin, back of the neck, back (Fig 1c), gluteal region, and both arms and legs (Fig 1d) progressed as the patient approached pubertal age.
Genetic analysis revealed novel compound heterozygous mutations in ALMS1 (c.5283delA; p.H1762fs X1779 and c.8039G>A; p.W2680X (Fig 1e). Both mutations result in a premature termination codon and truncation of the resulting protein.
Several phenotypes observed in this patient, including alopecia, acanthosis nigricans, and hyperandrogenism (hirsutism) are of interest for dermatology specialists. There are other rare disorders characterized by dermatological phenotypes in combination with ocular alterations (Table 1), but the association of alopecia, hirsutism, and acanthosis nigricans with nystagmus, photophobia, cone-dystrophy, or blindness is unique to ALMS.
Table 1.
Disorders with dermatological and pigmentary retinopathy or cone-rod dystrophy in combination
| Disorder | Dermatological phenotype | Ocular phenotype | Comment |
|---|---|---|---|
| Alström Syndrome (ALMS) a MIM 203800 |
Acanthosis nigricans Alopecia Hirsutism |
Cone-rod dystrophy Photophobia Nystagmus Blindness Subcapsular cataracts |
Severe systemic involvement. Mutations in ALMS1, ALMS1 expressed in the basal bodies of ciliated cells. Combination of features seen only in ALMS. |
| Alopecia areata | Alopecia areata | Lens involvement and degenerative changes, pigmentary clumping and abnormal vascular changes | Normal population |
| MULIBREY nanism (Muscle, liver, brain, eye nanism) MIM 253250 |
Acanthosis nigricans Cutaneous nevi (limbs) |
Pigmentary retinopathy Macular changes-Yellow dots on macula Hypoplasia of choroid Astigmatism Strabismus |
Mutations in b TRIM37 – proxisomal protein |
| Lipodystrophy with congenital cataracts and neurodegeneration MIM 606721 |
Acanthosis nigricans | Pigmentary retinopathy Congenital cataract Nystagmus Ocular dysmetria |
|
| HJDM (Hypotrichosis congenital with Juvenile Macular Dystrophy) MIM 601553 |
Alopecia, Congenital hypotrichosis, Fusiform beading of hair shaft, Pili torti | Juvenile macular (cone-rod) dystrophy | Mutations in c CDH3, a gene encoding P- cadherin, which is expressed in retinal pigment epithelium and in hair follicles. |
| Oliver McFarlane syndrome MIM 275400 |
Frontal alopecia Very long eyelashes and eyebrows |
Pigmentary retinopathy Ring iris heterochromia Nystagmus |
Mental retardation, dwarfism |
| Cone-rod congenital amaurosis MIM 204110 |
Congenital hypertrichosis, Hirsutism | Cone-rod dystrophy | |
| Joubert syndrome 10 (JBTS10) MIM 300804 |
Hirsutism | Pigmentary retinopathy | Mutations in d OFD1, e(CXORF5) a ciliary protein disorder |
| Mucopolysaccharidosis type IIIC(Sanfilippo syndrome) MIM 252930 |
Hirsutism, Coarse hair | Pigmentary retinopathy | Mutations in f HGSNAT |
| Edwards syndrome MIM 268020 |
Acanthosis nigricans | Pigmentary retinopathy | Similar to ALMS, but with mental retardation |
Mendelian Inheritance in Man (http://www.ncbi.nlm.nih.gov/omim);
Tripartite motif-containing 37;
Cadherin-3 (P-cadherin);
Oral-facial-digital syndrome 1;
Chromosome X open reading frame 5;
heparin acetyl-CoA:alpha-glucosaminide N-acetyltransferase
This case illustrates the necessity to consider the range of possibilities for diagnosis in young onset hyperandrogenism, particularly with obesity and other concomitant pathological conditions. Although there is still significant lack of knowledge on the specific mechanism that causes all of the complex features of ALMS, therapeutic intervention with weight loss and medication can alleviate some of the secondary consequences for the patient.
Acknowledgments
Funding: M.K., E.S.-A, and R.K. are supported in part by grant #13-873/3-05 of the Ministry of Health, Republic of Macedonia. J.D.M. is supported by the National Institutes of Health HD036878.
References
- 1.Marshall JD, Bronson RT, Collin GB, et al. New Alström syndrome phenotypes based on the evaluation of 182 cases. Arch Intern Med. 2005;165(6):675–83. doi: 10.1001/archinte.165.6.675. [DOI] [PubMed] [Google Scholar]
- 2.Marshall JD, Beck S, Maffei P, et al. Alström Syndrome. Eur J Hum Genet. 2007;15:1193–1202. doi: 10.1038/sj.ejhg.5201933. [DOI] [PubMed] [Google Scholar]
- 3.Collin GB, Marshall JD, Ikeda A, et al. Mutations in ALMS1 cause obesity, type 2 diabetes and neurosensory degeneration in Alstrom syndrome. Nat Genet. 2002;31(1):74–78. doi: 10.1038/ng867. [DOI] [PubMed] [Google Scholar]
- 4.Hearn T, Renforth GL, Spalluto C, et al. Mutation of ALMS1, a large gene with a tandem repeat encoding 47 amino acids, causes Alstrom syndrome. Nat Genet. 2002;31 (1):79–83. doi: 10.1038/ng874. [DOI] [PubMed] [Google Scholar]
- 5.Hearn T, Spalluto, Phillips VJ, et al. Subcellular Localization of ALMS1 Supports Involvement of Centrosome and Basal Body Dysfunction in the Pathogenesis of Obesity, Insulin Resistance, and Type 2 Diabetes. Diabetes. 2005;54:1581–7. doi: 10.2337/diabetes.54.5.1581. [DOI] [PubMed] [Google Scholar]
- 6.Collin GB, Cyr E, Bronson R, et al. Alms1-disrupted mice recapitulate human Alström syndrome. Hum Mol Genet. 2005;14(16):2323–33. doi: 10.1093/hmg/ddi235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.den Dunnen JT, Antonarakis E. Nomenclature for the description of human sequence variations. Hum Genet. 2001;109:121–124. doi: 10.1007/s004390100505. [DOI] [PubMed] [Google Scholar]