Abstract
Platelet transfusion is one of the most crucial therapeutic approaches in Medicine. However, severe and fatal adverse reactions may develop. In addition to their important function in hemostasis, platelets’ role in inflammation has become more evident. Recently, platelets are also recognized as the main source of circulating soluble CD40 ligand (sCD40L, (CD154)), which plays significant roles in hemostasis, platelet activation, clot stability, interactions with other cells, and upregulation of different mediators.
In this review, we will briefly highlight the importance of platelet transfusion, its role in inflammatory and thrombotic transfusion reactions, and visit the most recent findings on sCD40L.
Platelets: a Brief Overview of Recent Novel Findings
The significant role of platelets, anucleate cell fragments derived from megakaryocytes, in maintaining normal hemostasis is well known. During the last decade, platelet involvement in inflammation, innate immunity and host defense has become evident. Platelets recruit white blood cells by exposing P-selectin on their surface and also initiate signal transduction in neutrophils and endothelial cells via trans-cellular mechanisms involving lipids [1]. Involvement of other mediators (e.g., IL-1β, IL-6, IL-8) [2] and receptors (e.g., Toll like receptors [TLR]) [3] in platelet function support their complex role in immunity and inflammation, as well as hemostasis. In addition, platelets are recognized as the main source of circulating soluble CD40 ligand (sCD40L) (formally known as CD154), a member of the tumor necrosis family of cytokines that is a powerful activator of CD40 bearing immune and structural cells [4].
The role of sCD40L in platelet function has been of great interest in recent years. Multiple studies demonstrated that platelet sCD40L plays vital roles in platelet interactions with other cells, including neutrophils, T cells, and endothelial cells [5–8]. sCD40L involvement in platelet-mediated neutrophil activation is implicated as one of the mechanisms of transfusion-related acute lung injury (TRALI) [5]. During the early phases of the immune response when the number of CD40 positive T cells is still low, platelet-derived sCD40L provides early stimulation to B cells [6]. In vitro, sCD40L is able to stimulate immunoglobulin production in human B cells in the absence of any additional cellular elements. Platelet: T cell interaction was also demonstrated in a mouse model of Listeria monocytogenes infection, where platelet-derived sCD40L enhances cytotoxic T cell activity and survival in a dose-dependent manner [8].
Platelet sCD40L also has a significant role in hemostasis by binding the major fibrinogen receptor on platelet (GPIIb-IIIa) and contributing to platelet activation and clot stability [9]. Platelet sCD40L interacts with CD40 on endothelial cells leading to increased endothelial tissue factor, decreased thrombomodulin expression, and upregulation of mediators such as IL-8, MCP-1, adhesion molecules, and metalloproteinases [2].
Platelet Transfusion
Therapeutic platelet transfusions are widely accepted as indicated in patients with severe thrombocytopenia and/or platelet dysfunction associated with active serious bleeding (WHO grade of ≥ 2) [10]. Prophylactic platelet transfusions are widely employed based upon the patient’s underlying illness and the perceived clinical assessment of bleeding risk. However, due to decreased supply and increased concerns about the risks of platelet transfusion, the concept of prophylactic platelet transfusion is being challenged as perhaps being without evidence base, and potentially doing more harm than good for some patients.
Identifying an appropriate platelet count trigger for platelet transfusion has been one of the major challenges in weighing the risk to benefit ratio of prophylactic transfusions. A platelet count of ≤5000/μL is associated with substantial increases in spontaneous hemorrhage in chronically thrombocytopenic patients with an intact vascular system [11]. Four randomized trials failed to demonstrate significant differences in bleeding risks comparing prophylactic platelet transfusion triggers of 10,000 versus 20,000/μL [12–15]. Consequently, a platelet count of 10,000/μL is now widely recommended as a trigger for prophylactic platelet transfusion in patients with thrombocytopenia due to bone marrow disorders, chemotherapy, or hematopoietic progenitor cell transplantation [16, 17].
Platelet transfusion dose effects on posttransfusion platelet counts and interval-to-next transfusion have been evaluated in three prospective studies [18–20]. All results were interpreted as favoring higher doses of platelet transfusions. Although platelet transfusion rate decreased with larger doses, there were no differences in hemorrhagic events. A platelet dose of 0.07 × 1011/kg was recommended for stable thrombocytopenic patients and 0.15 × 1011/kg for patients with acute platelet consumption [14]. However, a recent large trial found no difference in bleeding episodes and minimal increase in transfusion dosing employing half the traditional dose of platelets (Platelet Dose Trail, “PLADO Trial”) [21].
Flisberg et al. assessed the efficacy of the transfused platelets utilizing rotational thromboelastometry immediately after a single transfusion [22]. Compared to the pretransfusion data, the clot formation time decreased by 32% (P = 0.005) and the maximum clot strength increased by 47% (P = 0.005) with a mean increase in platelet count of 12 × 109/L. This provides good evidence that transfused platelets are functional immediately after transfusion.
Platelet Transfusion Reactions
Transfusion reactions are more common with platelet transfusions than with red blood cell transfusions [23, 24]. This varies with leukoreduction, ABO matching and degree of supernatant depletion after storage. Some reports demonstrate decreased reactions with apheresis single donor transfusions, but in general do not account for ABO mismatching, storage duration and other variables that are potentially important to reaction rates. The clinical characteristics of acute reactions may include febrile non-hemolytic transfusion reactions (FNHTR) (most common: fever, rigors), allergic reactions (rash and urticaria predominate), transfusion-associated sepsis, and TRALI. Pre-storage leukoreduced platelets reduce the risk of febrile reactions to as high as 14% of patients who received filtered transfusions [25, 26] or 1% or less when platelet transfusions are ABO identical. However, removing the supernatant of transfused platelets before transfusion by simple saline washing reduces febrile complications significantly to <0.1% [27].
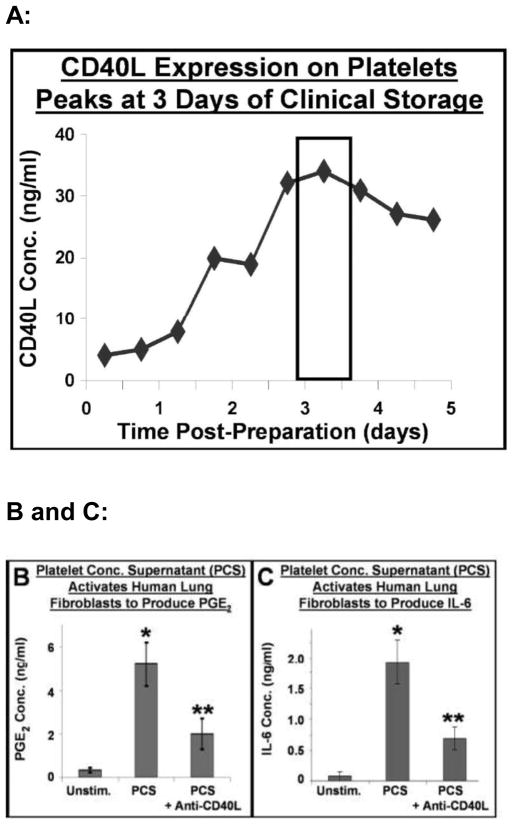
Our group demonstrated that platelets collected and stored for transfusion under blood bank conditions release significant amounts of sCD40L into the supernatant and express substantial increases in surface CD40L (Fig. 1/panel A) [28–31]. Platelet sCD40L stimulates upregulation of cyclooxygenase (Cox-2) and in vitro production of inflammatory mediators by human fibroblasts, including IL-6 and PGE2 [28–30]. The production of PGE2 (the main inducer of fever in humans) was induced by a dilution of 1:200 of stored platelet supernatants and this effect was abrogated by anti-CD40L pre-treatment (Fig. 1/panels B and C).
Figure 1.
Panel A: The concentration of sCD40L in the storage supernatant of washed platelets prepared for transfusion is greatest at 3 or 4 days of storage. Soluble CD40L was measured by standard sandwich ELISA. Panels B and C: Human lung fibroblasts were stimulated with medium (unstimulated), PCS (platelet concentrate supernatant) or CD40L-depleted PCS. After 24 hours, the medium was analyzed for PGE2 and IL-6 content. Unstimulated cells produced low levels of PGE2 and IL-6, while PCS caused great amounts of these mediators to be produced by the fibroblasts. These high levels of production were reduced when CD40L was neutralized in the PCS via an anti-CD40L antibody. Mean +/− SEM, n = 9, * p < 0.001 PCS vs. Unstimulated, ** p < 0.001 PCS + anti-CD40L vs. PCS alone. (Reprint permission granted by Blumberg et al. and Springer Science + Business Media [43].)
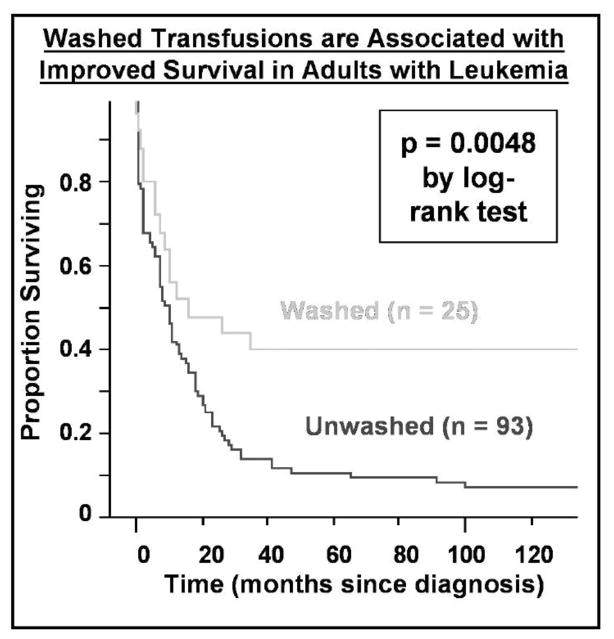
Typically, about 250 mL of platelet supernatant plasma is infused with each platelet transfusion, which, in an adult, exceeds by a factor of 10 the concentration needed to induce PGE2 production in vitro. Furthermore, platelet transfusions are repeated every day or so for weeks in patients with acute leukemia, for example, during induction therapy or stem cell transplantation. This repeated dosing may contribute significantly to adverse reactions to transfusion. Our group has reported preliminary data from a small randomized trial wherein washed platelet transfusions improved survival in adults with acute leukemia (Fig. 2) [32, 33].
Figure 2.
Washed transfusions are associated with improved survival in leukemia patients. A Kaplan-Meier plot of survival of 118 consecutive adult patients (ages 18–80) treated with curative intent for acute leukemia is shown according to whether patients received washed versus unwashed transfusions during their entire course. Patients receiving washed transfusions had significantly better survival, even after adjusting for other prognostic factors by proportional hazards analysis. (Reprint permission granted by Blumberg et al. and Springer Science + Business Media [43].)
Thrombosis after Platelet Transfusion
In a recent retrospective cohort study, Khorana et al, reported an association between red blood cell and platelet transfusions and increased risks for venous and arterial thrombosis and mortality in hospitalized cancer patients [34]. This multicenter study included 504,208 hospitalizations of cancer patients between 1995 and 2003. Three percent (15,237) of the patients in this analysis received at least 1 platelet transfusion. Based on multivariate analysis, an increased risk for venous thromboembolism was independently linked to platelet transfusion (OR, 1.20; 95% CI, 1.11 – 1.29). Similar results were seen for arterial thromboembolism (OR, 1.55; 95% CI, 1.40 – 1.71; P < .001). Platelet transfusions were also associated with a higher risk for death during hospitalization (OR, 2.40; 2.27 – 2.52; P < .001) [34]. Further studies with more rigorous clinical data are needed to investigate whether these associations are cause and effect.
Microparticles (MPs) and their association with thrombotic risks are a very active area of investigation. Platelet-derived MPs (PMPs) are small (<1 μm) vesiculated fragments that are normally detected in circulation in low concentrations. Higher concentrations were reported in thrombotic conditions, such as cerebrovascular events, unstable angina, and acute myocardial infarction [35, 36]. PMPs contain both pro- and anticoagulant proteins [36] and associate with fibrin during thrombotic events [37]. It was also reported that PMPs increase the adherence of monocytes to endothelial cells via upregulation of adhesion molecules on both cell types [38], which may play a role in atherogenesis [39].
In a recent report, Sugawara and colleagues demonstrated higher concentrations of PMPs in non-leukoreduced whole blood that were significantly reduced (by 72%) in prestorage leukoreduced whole blood [40]. In addition, PMPs increased by 2 logs in the non-leukoreduced blood over a period of 35 days but remain at stable levels in prestorage leukoreduced blood. Similar results on platelet concentrates (PC) were reported by a Japanese group [41]. High levels of PMPs were associated with 203 allergic transfusion reactions reported in 137 patients following pretransfusion-leukoreduced PC transfusions.
Significant levels of sCD40L accumulate during platelet storage. High levels of sCD40L are independently associated with increased risk of death, myocardial infarction, and congestive heart failure [42]. Therefore, elevated levels of both of PMPs and sCD40L in stored platelets are a potential factor in the association of increased thrombosis following platelet transfusions. Interactions between platelets and endothelial cells, particularly through sCD40L and platelet membrane CD40L, represents a potential pathologic mechanism in these cases [43]. Recent data support a role for platelet sCD40L as a mediator of endothelial cell dysfunction, particularly in the coronary circulation [44]. CD40 on endothelial cells was demonstrated to be a key receptor facilitating vascular inflammation and early narrowing of arteries [45]. This is of great interest because transfusion is associated with a significant increase in myocardial infarction [46] and thrombosis in epidemiologic studies [34]. In addition, recent in vitro investigations of sCD40L-endothelial cell interactions demonstrate that endothelial cells exhibit decreased endogenous nitric oxide synthesis and increased oxidative stress after exposure to high (1 or 5 μg/ml) concentrations of sCD40L [44].
Inflammatory Reactions to Platelet Transfusion
One of the most common inflammatory reactions to platelet transfusion is fever with or without rigors (FNHTR). Traditionally, this reaction was attributed to the reaction of anti-white cell antibodies in the recipient’s plasma interacting with the leukocytes in the platelet concentrate. However, removal of leukocytes prior to transfusion, whether pre-storage or immediately pre-transfusion does not completely abrogate such reactions to platelet transfusion. Good evidence associates accumulated high concentrations of leukocyte-and platelet-derived cytokines in stored platelets with FNHTR [47]. Combined or individual leukoreduction and removal of supernatant from platelets prior to transfusion reduce these reactions significantly [47, 48].
Platelet Transfusion Alloimmunization and Refractoriness
Refractoriness to platelet transfusion is another immune mediated reaction, and in this case is primarily due to humoral alloimmunization to Human Leukocyte Antigens (HLA), platelet specific and ABO antigens. The American Society of Clinical Oncology has defined refractoriness to platelet transfusion as a corrected count increment (CCI) of <5,000/μL [16] within 1 hour of a platelet transfusion. Animal studies and multiple prospective randomized trails demonstrated that platelet leukoreduction reduces alloimmunization to HLA antigens and platelet transfusion significantly [49].
Providing leukoreduced, ABO identical platelet transfusions reduces the platelet transfusion refractoriness rate in our Institution setting to <1% (unpublished data of the authors). Leukoreduction is the most important method of preventing platelet transfusion refractoriness, as demonstrated by randomized trials. Providing ABO-identical platelets is considered a good first step in the management of a platelet-refractory patient [50], if ABO identical transfusions have not been uniformly given previously. If refractoriness remains following two sequential transfusions of ABO-identical platelets, HLA and platelet antibody tests should be performed [51]. In an early study prior to the advent of leukoreduction patients receiving ABO-identical platelets experienced less refractoriness (36% versus 75%) and required fewer platelet transfusions by half as compared with patients receiving ABO-unmatched platelets [52]. In the same small cohort study of leukemia patients, transfusion of ABO-nonidentical platelet led to shorter remissions and survival [53]. In another randomized trial prior to the advent of leukoreduction, recipients of ABO-incompatible platelets become platelet refractory at a higher rate than the ABO-identical recipients (69% versus 8%, respectively, P = .001) [54]. These results also demonstrated that transfusion of ABO-incompatible platelets stimulates the production of additional anti-HLA and platelet-specific alloantibodies
Platelet Transfusion and Acute Lung Injury
One of the most important, uncommon, but serious, immune-mediated adverse reactions to platelet transfusion is TRALI. TRALI is the leading etiology for transfusion-related fatalities reported to the FDA in the USA (around 50% of cases) [55]. Antibodies to HLA or Human Neutrophil Antigens (HNA), particularly prevalent in multiparous women, previously transfused patients, or organ transplant recipients, have been implicated in TRALI cases [56]. However, TRALI is routinely reported following transfusion recipients receiving blood from never transfused male donors[57].
Biologically active lipids (lysophosphatidylcholines [LysoPCs]) and other biomediators/cytokines, which are released and accumulate during platelet storage’ also have been implicated in the pathogenesis of TRALI [58, 59]. Silliman and colleagues, along with our group, demonstrated that sCD40L concentrations in platelet concentrates involved in cases of TRALI were significantly higher than transfusions which did not lead to TRALI (Fig. 3) [5].
Figure 3.

CD40L concentration in platelet concentrates is associated with transfusion-related acute lung injury (TRALI). Higher levels of CD40L in platelet transfusions implicated in cases of TRALI as opposed to non-implicated control platelet transfusions. (Reprint permission granted by Blumberg et al. and Springer Science + Business Media [43].)
In a recent in vivo transfusion study in a rat model, Vlaar et al. [57] found that transfusion of aged platelet concentrates caused mild lung inflammation in healthy rats and increased pulmonary and systemic coagulopathy in endotoxin pretreated rats. Pulmonary injury was also induced following transfusion of aged platelet supernatants, which contains high levels of mediators that enhance neutrophil priming activity. These results support the hypothesis that TRALI can develop in the absence of HLA and HNA antibodies in donor or recipient.
Summary
Platelet transfusion is crucial in treating thrombocytopenic patients with life threatening hemorrhage. However, platelets as prepared for transfusion contain significant amounts of inflammatory mediators and microparticles, which are thought to play a role in a variety of serious or even fatal transfusion reactions. ABO identical transfusions and leukoreduction have been demonstrated to be of importance in multiple epidemiologic and randomized clinical trials. Further research is needed, but partial or complete removal of stored platelet concentrate supernatant may substantially reduce the risks of adverse events for most or perhaps all platelet transfusion-dependent patients.
Supplementary Material
Acknowledgments
This review was supported by ES01247, HL095467, and RC1 HL100051.
Footnotes
Conflict-of-interest disclosure: M. A. R. receives research support from CSL Behring. All other authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mohanty D. Current concepts in platelet transfusion. Asian J Transfus Sci. 2009 Jan;3(1):18–21. doi: 10.4103/0973-6247.45257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slupsky JR, Kalbas M, Willuweit A, Henn V, Kroczek RA, Muller-Berghaus G. Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb Haemost. 1998 Dec;80(6):1008–14. [PubMed] [Google Scholar]
- 3.Andonegui G, Kerfoot SM, McNagny K, Ebbert KV, Patel KD, Kubes P. Platelets express functional Toll-like receptor-4. Blood. 2005 Oct 1;106(7):2417–23. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 4.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998 Feb 5;391(6667):591–4. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 5.Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps R, et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006 Oct 1;108(7):2455–62. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elzey BD, Grant JF, Sinn HW, Nieswandt B, Waldschmidt TJ, Ratliff TL. Cooperation between platelet-derived CD154 and CD4+ T cells for enhanced germinal center formation. J Leukoc Biol. 2005 Jul;78(1):80–4. doi: 10.1189/jlb.1104669. [DOI] [PubMed] [Google Scholar]
- 7.Elzey BD, Schmidt NW, Crist SA, Kresowik TP, Harty JT, Nieswandt B, et al. Platelet-derived CD154 enables T-cell priming and protection against Listeria monocytogenes challenge. Blood. 2008 Apr 1;111(7):3684–91. doi: 10.1182/blood-2007-05-091728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cognasse F, Hamzeh-Cognasse H, Lafarge S, Chavarin P, Cogne M, Richard Y, et al. Human platelets can activate peripheral blood B cells and increase production of immunoglobulins. Exp Hematol. 2007 Sep;35(9):1376–87. doi: 10.1016/j.exphem.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Prasad KS, Andre P, He M, Bao M, Manganello J, Phillips DR. Soluble CD40 ligand induces beta3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc Natl Acad Sci U S A. 2003 Oct 14;100(21):12367–71. doi: 10.1073/pnas.2032886100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrence JB, Yomtovian RA, Dillman C, Masarik SR, Chongkolwatana V, Creger RJ, et al. Reliability of automated platelet counts: comparison with manual method and utility for prediction of clinical bleeding. Am J Hematol. 1995 Apr;48(4):244–50. doi: 10.1002/ajh.2830480408. [DOI] [PubMed] [Google Scholar]
- 11.Slichter SJ, Harker LA. Thrombocytopenia: mechanisms and management of defects in platelet production. Clin Haematol. 1978 Oct;7(3):523–39. [PubMed] [Google Scholar]
- 12.Heckman KD, Weiner GJ, Davis CS, Strauss RG, Jones MP, Burns CP. Randomized study of prophylactic platelet transfusion threshold during induction therapy for adult acute leukemia: 10,000/microL versus 20,000/microL. J Clin Oncol. 1997 Mar;15(3):1143–9. doi: 10.1200/JCO.1997.15.3.1143. [DOI] [PubMed] [Google Scholar]
- 13.Zumberg MS, del Rosario ML, Nejame CF, Pollock BH, Garzarella L, Kao KJ, et al. A prospective randomized trial of prophylactic platelet transfusion and bleeding incidence in hematopoietic stem cell transplant recipients: 10,000/L versus 20,000/microL trigger. Biol Blood Marrow Transplant. 2002;8(10):569–76. doi: 10.1053/bbmt.2002.v8.pm12434952. [DOI] [PubMed] [Google Scholar]
- 14.Rebulla P, Finazzi G, Marangoni F, Avvisati G, Gugliotta L, Tognoni G, et al. The threshold for prophylactic platelet transfusions in adults with acute myeloid leukemia. Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto. N Engl J Med. 1997 Dec 25;337(26):1870–5. doi: 10.1056/NEJM199712253372602. [DOI] [PubMed] [Google Scholar]
- 15.Wandt H, Frank M, Ehninger G, Schneider C, Brack N, Daoud A, et al. Safety and cost effectiveness of a 10 × 10(9)/L trigger for prophylactic platelet transfusions compared with the traditional 20 × 10(9)/L trigger: a prospective comparative trial in 105 patients with acute myeloid leukemia. Blood. 1998 May 15;91(10):3601–6. [PubMed] [Google Scholar]
- 16.Schiffer CA, Anderson KC, Bennett CL, Bernstein S, Elting LS, Goldsmith M, et al. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001 Mar 1;19(5):1519–38. doi: 10.1200/JCO.2001.19.5.1519. [DOI] [PubMed] [Google Scholar]
- 17.British Committee for Standards in Haematology BTTFCPK. Guidelines for the use of platelet transfusions. Br J Haematol. 2003 Jul;122(1):10–23. doi: 10.1046/j.1365-2141.2003.04468.x. [DOI] [PubMed] [Google Scholar]
- 18.Goodnough LT, Kuter DJ, McCullough J, Slichter SJ, DiPersio J, Romo J, et al. Prophylactic platelet transfusions from healthy apheresis platelet donors undergoing treatment with thrombopoietin. Blood. 2001 Sep 1;98(5):1346–51. doi: 10.1182/blood.v98.5.1346. [DOI] [PubMed] [Google Scholar]
- 19.Norol F, Bierling P, Roudot-Thoraval F, Le Coeur FF, Rieux C, Lavaux A, et al. Platelet transfusion: a dose-response study. Blood. 1998 Aug 15;92(4):1448–53. [PubMed] [Google Scholar]
- 20.Klumpp TR, Herman JH, Gaughan JP, Russo RR, Christman RA, Goldberg SL, et al. Clinical consequences of alterations in platelet transfusion dose: a prospective, randomized, double-blind trial. Transfusion. 1999 Jul;39(7):674–81. doi: 10.1046/j.1537-2995.1999.39070674.x. [DOI] [PubMed] [Google Scholar]
- 21.Slichter SJ, Kaufman RM, Assmann SF, McCullough J, Triulzi DJ, Strauss RG, et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med. 2010 Feb 18;362(7):600–13. doi: 10.1056/NEJMoa0904084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flisberg P, Rundgren M, Engstrom M. The effects of platelet transfusions evaluated using rotational thromboelastometry. Anesth Analg. 2009 May;108(5):1430–2. doi: 10.1213/ane.0b013e31819bccb7. [DOI] [PubMed] [Google Scholar]
- 23.Heddle NM, Klama LN, Griffith L, Roberts R, Shukla G, Kelton JG. A prospective study to identify the risk factors associated with acute reactions to platelet and red cell transfusions. Transfusion. 1993 Oct;33(10):794–7. doi: 10.1046/j.1537-2995.1993.331094054613.x. [DOI] [PubMed] [Google Scholar]
- 24.Spiess BD, Royston D, Levy JH, Fitch J, Dietrich W, Body S, et al. Platelet transfusions during coronary artery bypass graft surgery are associated with serious adverse outcomes. Transfusion. 2004 Aug;44(8):1143–8. doi: 10.1111/j.1537-2995.2004.03322.x. [DOI] [PubMed] [Google Scholar]
- 25.Mangano MM, Chambers LA, Kruskall MS. Limited efficacy of leukopoor platelets for prevention of febrile transfusion reactions. Am J Clin Pathol. 1991 May;95(5):733–8. doi: 10.1093/ajcp/95.5.733. [DOI] [PubMed] [Google Scholar]
- 26.Blumberg N, Heal JM, Gettings KF, Phipps RP, Masel D, Refaai MA, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010 Jun 18; doi: 10.1111/j.1537-2995.2010.02748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vo TD, Cowles J, Heal JM, Blumberg N. Platelet washing to prevent recurrent febrile reactions to leucocyte-reduced transfusions. Transfus Med. 2001 Feb;11(1):45–7. doi: 10.1046/j.1365-3148.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- 28.Phipps RP, Kaufman J, Blumberg N. Platelet derived CD154 (CD40 ligand) and febrile responses to transfusion. Lancet. 2001 Jun 23;357(9273):2023–4. doi: 10.1016/s0140-6736(00)05108-4. [DOI] [PubMed] [Google Scholar]
- 29.Blumberg N, Phipps RP, Kaufman J, Heal JM. The causes and treatment of reactions to platelet transfusions. Transfusion. 2003 Feb;43(2):291–2. doi: 10.1046/j.1537-2995.2003.t01-2-00362.x. author reply 2. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman J, Spinelli SL, Schultz E, Blumberg N, Phipps RP. Release of biologically active CD154 during collection and storage of platelet concentrates prepared for transfusion. J Thromb Haemost. 2007 Apr;5(4):788–96. doi: 10.1111/j.1538-7836.2007.02412.x. [DOI] [PubMed] [Google Scholar]
- 31.Blumberg N, Gettings KF, Turner C, Heal JM, Phipps RP. An association of soluble CD40 ligand (CD154) with adverse reactions to platelet transfusions. Transfusion. 2006 Oct;46(10):1813–21. doi: 10.1111/j.1537-2995.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- 32.Blumberg N, Heal JM, Rowe JM. A randomized trial of washed red blood cell and platelet transfusions in adult acute leukemia [ISRCTN76536440] BMC Blood Disord. 2004 Dec 10;4(1):6. doi: 10.1186/1471-2326-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blumberg N, Heal JM, Liesveld JL, Phillips GL, Rowe JM. Platelet transfusion and survival in adults with acute leukemia. Leukemia. 2008 Mar;22(3):631–5. doi: 10.1038/sj.leu.2404920. [DOI] [PubMed] [Google Scholar]
- 34.Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai MA, Lyman GH. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008 Nov 24;168(21):2377–81. doi: 10.1001/archinte.168.21.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holme PA, Orvim U, Hamers MJ, Solum NO, Brosstad FR, Barstad RM, et al. Shear-induced platelet activation and platelet microparticle formation at blood flow conditions as in arteries with a severe stenosis. Arterioscler Thromb Vasc Biol. 1997 Apr;17(4):646–53. doi: 10.1161/01.atv.17.4.646. [DOI] [PubMed] [Google Scholar]
- 36.Miyazaki Y, Nomura S, Miyake T, Kagawa H, Kitada C, Taniguchi H, et al. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood. 1996 Nov 1;88(9):3456–64. [PubMed] [Google Scholar]
- 37.Siljander P, Carpen O, Lassila R. Platelet-derived microparticles associate with fibrin during thrombosis. Blood. 1996 Jun 1;87(11):4651–63. [PubMed] [Google Scholar]
- 38.Barry OP, Pratico D, Savani RC, FitzGerald GA. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest. 1998 Jul 1;102(1):136–44. doi: 10.1172/JCI2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nomura S, Suzuki M, Katsura K, Xie GL, Miyazaki Y, Miyake T, et al. Platelet-derived microparticles may influence the development of atherosclerosis in diabetes mellitus. Atherosclerosis. 1995 Aug;116(2):235–40. doi: 10.1016/0021-9150(95)05551-7. [DOI] [PubMed] [Google Scholar]
- 40.Sugawara A, Nollet KE, Yajima K, Saito S, Ohto H. Preventing platelet-derived microparticle formation--and possible side effects-with prestorage leukofiltration of whole blood. Arch Pathol Lab Med. May;134(5):771–5. doi: 10.5858/134.5.771. [DOI] [PubMed] [Google Scholar]
- 41.Nomura S, Okamae F, Abe M, Hosokawa M, Yamaoka M, Ohtani T, et al. Platelets expressing P-selectin and platelet-derived microparticles in stored platelet concentrates bind to PSGL-1 on filtrated leukocytes. Clin Appl Thromb Hemost. 2000 Oct;6(4):213–21. doi: 10.1177/107602960000600406. [DOI] [PubMed] [Google Scholar]
- 42.Prasad KS, Andre P, Yan Y, Phillips DR. The platelet CD40L/GP IIb-IIIa axis in atherothrombotic disease. Curr Opin Hematol. 2003 Sep;10(5):356–61. doi: 10.1097/00062752-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Blumberg N, Spinelli SL, Francis CW, Taubman MB, Phipps RP. The platelet as an immune cell-CD40 ligand and transfusion immunomodulation. Immunol Res. 2009 Jan 29; doi: 10.1007/s12026-009-8106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen C, Chai H, Wang X, Jiang J, Jamaluddin MS, Liao D, et al. Soluble CD40 ligand induces endothelial dysfunction in human and porcine coronary artery endothelial cells. Blood. 2008 Oct 15;112(8):3205–16. doi: 10.1182/blood-2008-03-143479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donners MM, Beckers L, Lievens D, Munnix I, Heemskerk J, Janssen BJ, et al. The CD40-TRAF6 axis is the key regulator of the CD40/CD40L system in neointima formation and arterial remodeling. Blood. 2008 May 1;111(9):4596–604. doi: 10.1182/blood-2007-05-088906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rao SV, Jollis JG, Harrington RA, Granger CB, Newby LK, Armstrong PW, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004 Oct 6;292(13):1555–62. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 47.Heddle NM, Klama L, Singer J, Richards C, Fedak P, Walker I, et al. The role of the plasma from platelet concentrates in transfusion reactions. N Engl J Med. 1994 Sep 8;331(10):625–8. doi: 10.1056/NEJM199409083311001. [DOI] [PubMed] [Google Scholar]
- 48.Hetland G, Mollnes TE, Bergh K, Hogasen K, Bergerud UE, Solheim BG. Effect of filtration and storage of platelet concentrates on the production of the chemotaxins C5a, interleukin 8, tumor necrosis factor alpha, and leukotriene B4. Transfusion. 1998 Jan;38(1):16–23. doi: 10.1046/j.1537-2995.1998.38198141493.x. [DOI] [PubMed] [Google Scholar]
- 49.van Marwijk Kooy M, van Prooijen HC, Moes M, Bosma-Stants I, Akkerman JW. Use of leukocyte-depleted platelet concentrates for the prevention of refractoriness and primary HLA alloimmunization: a prospective, randomized trial. Blood. 1991 Jan 1;77(1):201–5. [PubMed] [Google Scholar]
- 50.Slichter SJ. Algorithm for managing the platelet refractory patient. J Clin Apher. 1997;12(1):4–9. doi: 10.1002/(sici)1098-1101(1997)12:1<4::aid-jca2>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 51.Slichter SJ, Davis K, Enright H, Braine H, Gernsheimer T, Kao KJ, et al. Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood. 2005 May 15;105(10):4106–14. doi: 10.1182/blood-2003-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heal JM, Rowe JM, McMican A, Masel D, Finke C, Blumberg N. The role of ABO matching in platelet transfusion. Eur J Haematol. 1993 Feb;50(2):110–7. doi: 10.1111/j.1600-0609.1993.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 53.Heal JM, Kenmotsu N, Rowe JM, Blumberg N. A possible survival advantage in adults with acute leukemia receiving ABO-identical platelet transfusions. Am J Hematol. 1994 Feb;45(2):189–90. doi: 10.1002/ajh.2830450219. [DOI] [PubMed] [Google Scholar]
- 54.Carr R, Hutton JL, Jenkins JA, Lucas GF, Amphlett NW. Transfusion of ABO-mismatched platelets leads to early platelet refractoriness. Br J Haematol. 1990 Jul;75(3):408–13. doi: 10.1111/j.1365-2141.1990.tb04356.x. [DOI] [PubMed] [Google Scholar]
- 55.Boshkov LK. Transfusion-related acute lung injury and the ICU. Crit Care Clin. 2005 Jul;21(3):479–95. doi: 10.1016/j.ccc.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 56.Muller JY. [TRALI: from diagnosis to prevention] Transfus Clin Biol. 2005 Jun;12(2):95–102. doi: 10.1016/j.tracli.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 57.Vlaar AP, Hofstra JJ, Kulik W, van Lenthe H, Nieuwland R, Schultz MJ, et al. Supernatant of stored platelets causes lung inflammation and coagulopathy in a novel in vivo transfusion model. Blood. Aug 26;116(8):1360–8. doi: 10.1182/blood-2009-10-248732. [DOI] [PubMed] [Google Scholar]
- 58.Silliman CC, Bjornsen AJ, Wyman TH, Kelher M, Allard J, Bieber S, et al. Plasma and lipids from stored platelets cause acute lung injury in an animal model. Transfusion. 2003 May;43(5):633–40. doi: 10.1046/j.1537-2995.2003.00385.x. [DOI] [PubMed] [Google Scholar]
- 59.Toy P, Lowell C. TRALI--definition, mechanisms, incidence and clinical relevance. Best Pract Res Clin Anaesthesiol. 2007 Jun;21(2):183–93. doi: 10.1016/j.bpa.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.