Abstract
The primary focus of research on the amygdala has been on the detection of and response to emotion but the amygdala also sometimes responds to new or unexpected stimuli without specific emotional content. Very little is currently known about why the amygdala responds to some new stimuli but not others. Here we investigated the conditions that are necessary and sufficient for the expression of novelty specific amygdala responses by presenting novel and repeated images to human participants and varying the content of these images while measuring blood-oxygenation level (BOLD) dependent responses. In Experiment 1 we presented novel and repeated emotional and neutral images. Both emotional and neutral images of humans evoked more activity when novel than when repeated. In Experiment 2 we presented novel and repeated images of humans and scenes. Images of humans but not scenes evoke more activity when novel than when repeated. Our results suggest that the amygdala plays a stimulus-specific role in the brain’s novelty detection network. Surprisingly, emotion was not necessary for amygdalar novelty responses, but the presence of a human representation was important. Amygdala responses evoked by novel faces may reflect our need to use others’ faces as clues for important events in the environment.
Keywords: human, amygdala, novelty, emotion, faces, fMRI
1 Introduction1
Activity in the brain’s novelty detection network is thought to represent an early stage in memory encoding, focusing attention on unexpected stimuli or events (Tulving et al., 1996). The amygdala plays an important role in the formation of new memories for emotional events (Canli et al., 2000). Although often overlooked in novelty detection studies (Daselaar et al., 2006; Menon et al., 2000; Tulving et al., 1996; Yamaguchi et al., 2004), the amygdala often responds to novel stimuli much like other regions (i.e. hippocampus, parahippocampal gyrus). For example, Schwartz and colleagues presented blocks of faces and found that the amygdala responded maximally when the faces in a given block were presented only once (Schwartz et al., 2003). In contrast, Yamaguchi and colleagues showed that the hippocampus but not the amygdala was activated by novel presentations of animals, buildings and landscapes, suggesting that novelty per se does not drive amygdala responses (Yamaguchi et al., 2004).
Although larger blood-oxygenation level dependent (BOLD) responses have been observed in the human amygdala for stimuli that are novel to the observer, we still lack a basic understanding of the conditions that are necessary and sufficient for such responses. We hypothesized that novelty-specific amygdala responses are not evoked by all stimuli, but are dependent on characteristics of the novel stimulus.
We used functional magnetic resonance imaging (fMRI) to determine which stimulus characteristics are most important for novelty-specific amygdala responses. We presented novel and repeated images and systematically varied the content of these images based on the previously established framework of amygdala functions. In Experiment 1 we sought to determine if emotional content played an important role in amygdalar novelty responding. In Experiment 2 we sought to determine if human representations were necessary for novelty-specific amygdala responses. Finally, we investigated the temporal properties of novelty-specific amygdalar responses by comparing the responses evoked by novel and repeated stimuli across trials.
2 Materials and Methods
2.1 Participants
Fifty-three neurologically healthy undergraduate students (Age: M = 20.78, SD = 2.90) at the University of Wisconsin-Milwaukee participated in this experiment and received $20 for participation, as well as extra credit in their psychology classes and a picture of their brain. Thirty-three were female. Three participants were excluded from the study because of computer/recording failures. Ten participants were excluded because of excessive head motion. Of the remaining participants, 20 individuals (14 female) participated in Experiment 1, and 20 individuals (12 female) participated in Experiment 2. All participants gave informed consent, and the protocol was approved by the Institutional Review Boards for human subject research at the University of Wisconsin-Milwaukee and the Medical College of Wisconsin.
2.2 Procedure
Stimuli were presented using the software package Presentation (Neurobehavioral Systems, Inc., Albany, CA), using a Dell laptop (model: Inspiron 9300, Dell Inc., Red Rock, TX). All participants saw a series of 20 eight second presentations of novel and repeated images while positioned in the fMRI scanner. Participants viewed the stimuli via a back projection system with prism glasses mounted to the head coil.
In the novel conditions, we presented a series of five different images, where each image was presented once. In the repeated conditions, we presented a single image six times. For the repeated conditions we included only trials where the image had been repeated. Therefore, the initial presentation of these stimuli was counted in the respective novel categories. In total, there were five trials of each stimulus type, corresponding to the experimental categories for each experiment. Repeated stimuli were counterbalanced across subjects.
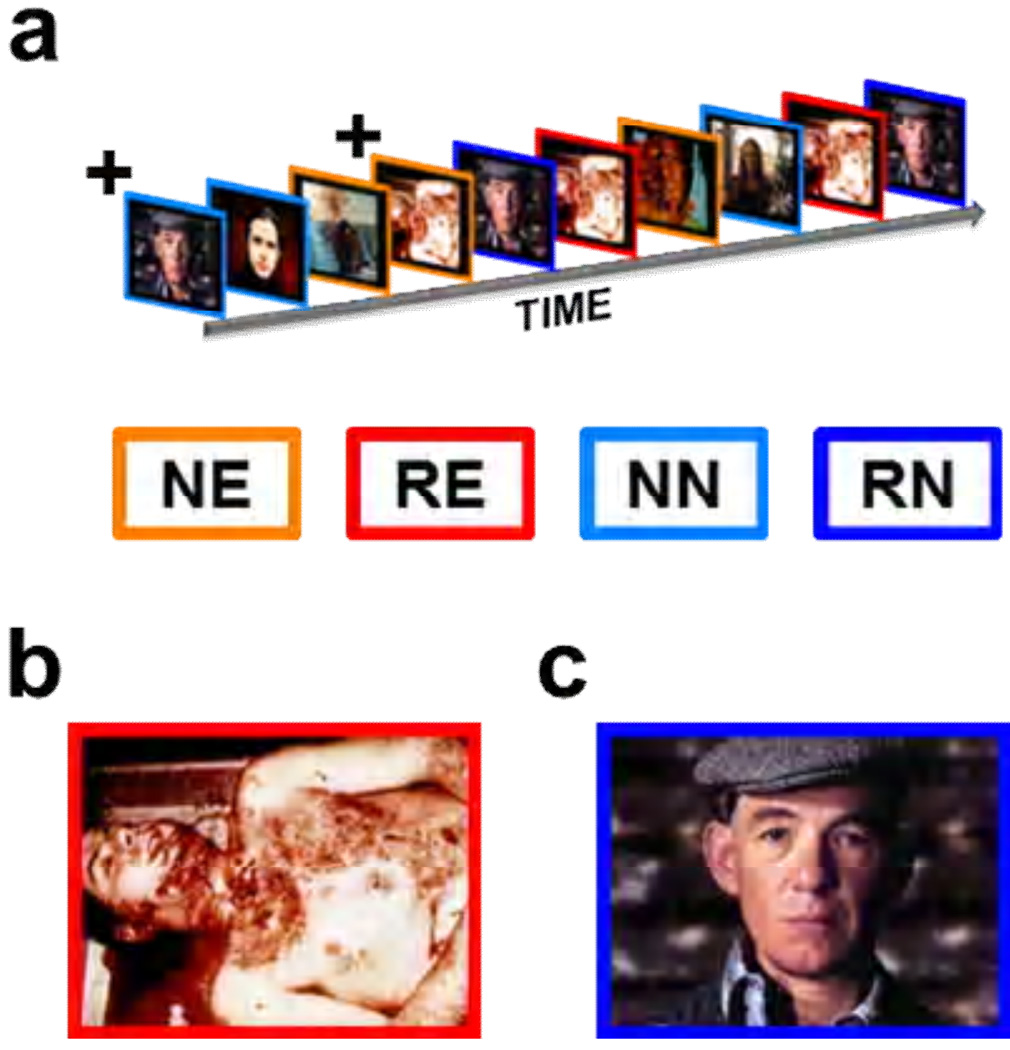
In Experiment 1, we manipulated the emotional content of the stimuli by presenting emotional (novel = NE; repeated = RE) and neutral (novel = NN; repeated = RN) images of humans from the International Affective Picture System (IAPS) database (Lang, 2005). See Figure 1 for design summary. Emotional images depicted mutilated human bodies. Neutral images depicted healthy individuals with their gaze directed toward the camera. Normative ratings of the images confirm that the emotional images were rated as more arousing and more emotionally negative than the neutral images (See Supplementary Table 3 for picture codes and normative ratings). Each picture was presented centrally, and presentations were separated by a 20 second average variable intertrial interval (ITI; ± 4 sec).
Figure 1. In Experiment 1 we presented emotional and neutral images while measuring BOLD activity.
(a) Images were presented sequentially in an event related design. (b,c)The images are representative of the emotional (b) and neutral (c) images shown to participants. All participants saw 5 presentations of novel emotional (NE) and 5 presentations of novel neutral (NN) images indicated by the orange and light blue outlines, respectively. In addition all saw 5 repetitions of one emotional (RE) and one neutral (RN) image, shown in red and blue respectively.
+The initial presentation of the repeated stimuli was counted as novel.
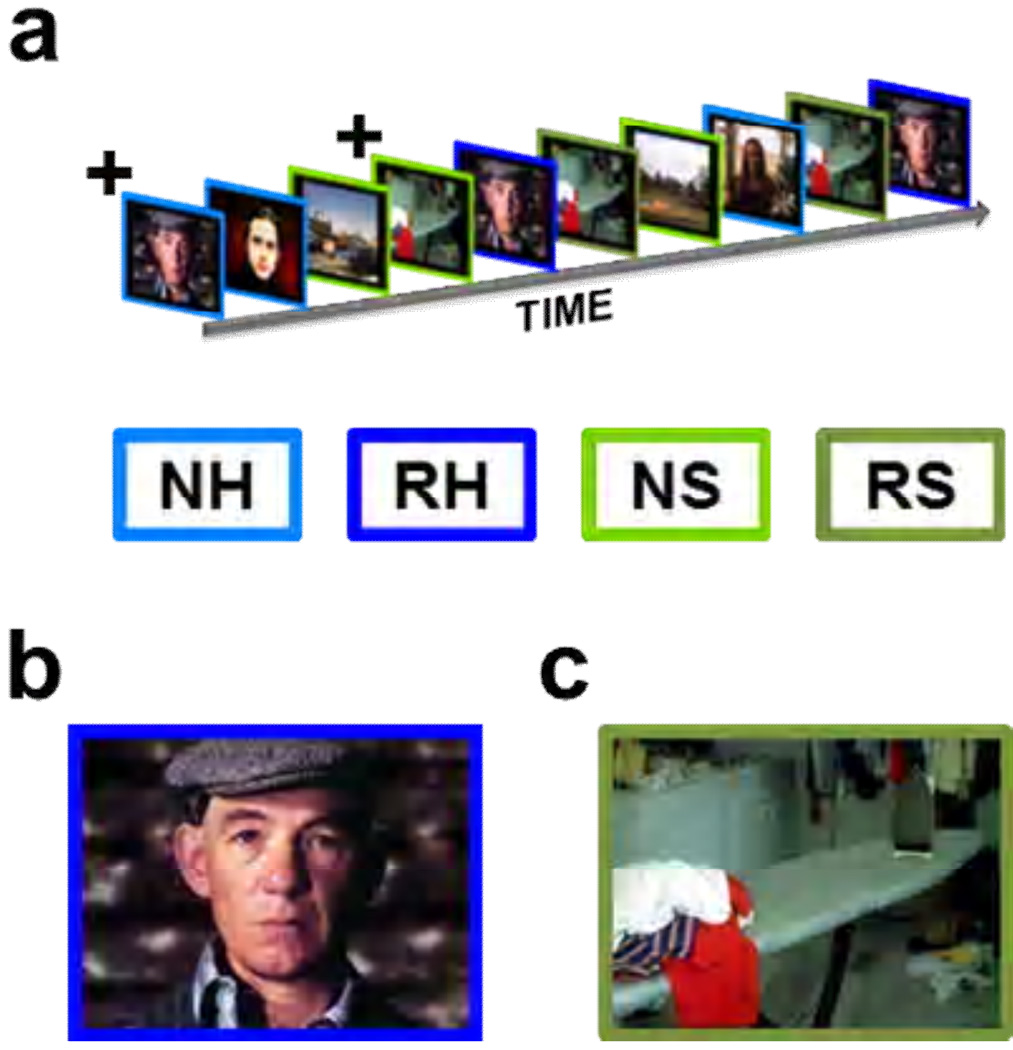
In Experiment 2, we presented images of humans (novel = NH; repeated = RH) and scenes (novel = NS; repeated = RS), all of which were emotionally neutral. This experiment was a partial replication of Experiment 1 because our images of humans were the same images used for the neutral images above. The scenes were chosen from the same picture database (Lang, 2005) and equated for arousal and valence ratings. As in Experiment 1, each picture was presented centrally, and presentations were separated by a 20 second average variable intertrial interval (ITI; ± 4 sec).
Prior to the experiment participants underwent a procedure where they rated the intensity of an electrical stimulus as part of a separate study. In order to ensure that the participants were attending to the stimuli, they were asked to continuously rate their expectancy of receiving this stimulation by manipulating a dial (Supplementary Figure 1). However at no time during the actual experiment did the individuals receive the stimulation. We also measured skin conductance responses throughout the experiments (See Supplementary Tables 4 and 5 and Supplementary Figure 2).
2.3 MRI
We conducted whole brain imaging using a 3T short bore GE Signa Excite MRI system. Functional images were acquired using a T2* weighted gradient-echo, echoplanar pulse sequence. Contiguous four millimeter sagittal slices (TR = 2sec; TE = 25ms; field of view = 24cm; flip angle = 90°) were collected during the experiment. Two hundred and ninety whole brain images were collected. High resolution spoiled gradient recalled (SPGR) acquisition images were collected to serve as a three-dimensional anatomical map for the functional images.
2.4 MRI segmentation
Subcortical segmentation was performed using the Freesurfer software package, which is freely available online and has been described previously (Fischl et al., 2002; Fischl et al., 2004). Freesurfer generated volumes were then realigned to native space using the Analysis of Functional NeuroImages software package (AFNI). These realigned volumes were then manually edited to conform to previously described standards (Morey et al., 2009).
2.5 Functional imaging data acquisition
Functional imaging data were reconstructed and processed using AFNI (Cox, 1996). fMRI data were passed through motion correction and edge detection algorithms, then registered to the fifth image in the timeseries. Raw fMRI data were manually inspected for large head movements. Images that contained discrete head movements were censored, and participants showing excessive movement (greater than 2mm displacement or more than 5 instances of discrete head movements) were excluded from further analyses. Head motion and dial movement regressors were included in the analysis as regressors of no interest. Timeseries data were deconvolved with stimulus canonicals using a least squares procedure, to yield average impulse response functions (IRFs).
2.6 Functional imaging data analysis
For whole brain analyses SPGR images were manually warped into Talairach space using anatomical markers. Images one through five of the IRFs were used to calculate percent area under the curve (%AUC). The %AUC maps were then registered to Talairach space and resampled to 1mm isotropic voxels using linear interpolation. Images were then blurred using a 4mm full-width at half-maximum Gaussian kernel. The resulting maps were used in the group level analyses. We used cluster thresholding (Forman et al., 1995) to correct for multiple comparisons across the voxels in the whole brain volume (p = 0.005; rmm = 2; xyz = 1; Volume = 228µL; corrected α = 0.05).
For the ROI analyses, image three from the IRF was registered to the unwarped SPGR data on a subject-by-subject basis and resampled to 1mm isotropic voxels using linear interpolation. The images used for the ROI analyses were not warped or blurred, in order to forego the distortion caused by these procedures. Because voxelwise data were not used in the group-level analyses, these steps were unnecessary. We chose an alpha level of 0.05 for all analyses.
2.7 Skin conductance responses
Skin conductance level (SCL) was recorded via two surface cup electrodes (silver/silver chloride, 8mm diameter, Biopac model EL258-RT, Goleta, CA) filled with electrolyte gel (Signa Gel, Parker laboratories Fairfield, NJ) attached to the bottom of the participants left foot approximately 2cm apart, and sampled at 200 Hz throughout the experiment. We sampled SCL during the 8 second stimulus period and the preceding two second baseline period. Raw values for each trial were normalized to that trial’s average baseline SCL and expressed as a percent change from that baseline value. SCR timecourse data were obtained by averaging the percent change values across participants and across trials at each timepoint during the stimulus period. Statistical tests were computed at each timepoint with an alpha level of 0.05. Monte Carlo simulations were used to correct for multiple comparisons across timepoints (See Supplementary Table 5 and Supplementary Figure 2).
2.8 SCR Monte Carlo simulations
Monte Carlo simulations were conducted to correct for multiple comparisons carried out on SCRs, in a manner similar to cluster thresholding of fMRI data. We generated 200,000 strings of random p-values that were the same length as the SCR samples (2000 values), using a random number generator. We then counted the number of sequential significant p-values (Tp = timepoint p-value), using a timepoint α (Tα) of 0.05, and determined the likelihood that a given stretch of sequential significant p-values could have arisen due to chance alone. We found that less than 0.002% of the simulations yielded sample lengths (length of sequential significant p-values) greater than or equal to 7 timepoints in length, which is equivalent to 35 ms in duration. Therefore to correct for multiple comparisons, we thresholded our data using a combination of individual timepoint p-value (Tα = 0.05) and sample length (γp > 7; See Supplementary Table 4).
2.9 Electrical stimulation
As part of another experiment, participants were given presentations of an electrical stimulation before the experiment began. Electrical stimulation was administered via an AC (60 Hz) source (Contact Precision Instruments, Model SHK1, Boston, MA) through two surface cup electrodes (silver/silver chloride, 8mm diameter, Biopac model EL258-RT, Goleta, CA) filled with electrolyte gel (Signa Gel, Parker laboratories Fairfield, NJ). The electrodes were placed on the skin over the subject’s right tibial nerve over the right medial malleolus. Participants were given several half-second presentations of the shock. They rated the shock on a scale from zero (no sensation) to ten (painful but tolerable). Intensity was increased gradually in mA until participants rated the sensation as a ten.
2.10 Shock expectancy
In order to ensure that the participants attended to the stimuli, they continuously rated their expectancy of receiving the electrical stimulation. To do so, participants controlled a visual analog scale on the computer screen using dial. The analog scale was anchored with 0 and 100. Participants were instructed to move the cursor to 0 if they were absolutely sure that they would not receive an electrical stimulation, to move the cursor to 100 if they were absolutely sure that they would receive a stimulation, and to keep the cursor near 50 if they felt like there was an equal probability of receiving or not receiving a stimulation. Responses were recorded throughout the experiment and sampled at 40 Hz (See Supplementary Figure 1). An alpha level of 0.05 was used for all analyses.
3 Results
3.1 Novelty-specific BOLD responses in the amygdala and hippocampus are not dependent on emotion
We began by investigating emotion as a potential mediating factor for two reasons. First, the amygdala is important for the perception of and response to emotional stimuli (Adolphs et al., 1994; Cheng et al., 2003; Williams et al., 2001). Second, amygdala activity at encoding correlates with subsequent memory for emotional scenes, suggesting that amygdala activity may help facilitate memory for emotional events (Canli et al., 2000). Amygdalar novelty responses may facilitate the encoding of emotional stimuli, which would provide a link between amygdala activity and memory performance. To test this we presented novel and repeated emotional and neutral images while measuring BOLD activity.
Based on the novelty/encoding hypothesis and the link between amygdala activity and facilitated recognition for emotional scenes, we predicted that there would be larger amygdala responses to novel emotional images than repeated emotional images, but similar magnitude amygdala responses to novel and repeated neutral images. To determine the effects of novelty and emotion on brain activity, we performed a mixed effects ANOVA on BOLD intensity using novelty and emotion as fixed factors and subject as a random factor. The most striking finding of the whole brain BOLD analysis was a robust main effect for novelty bilaterally in the amygdala and hippocampus, but no main effect for emotion and no novelty by emotion interaction in these structures (Figure 3a–b. See Supplementary Table 1 full list of activations).
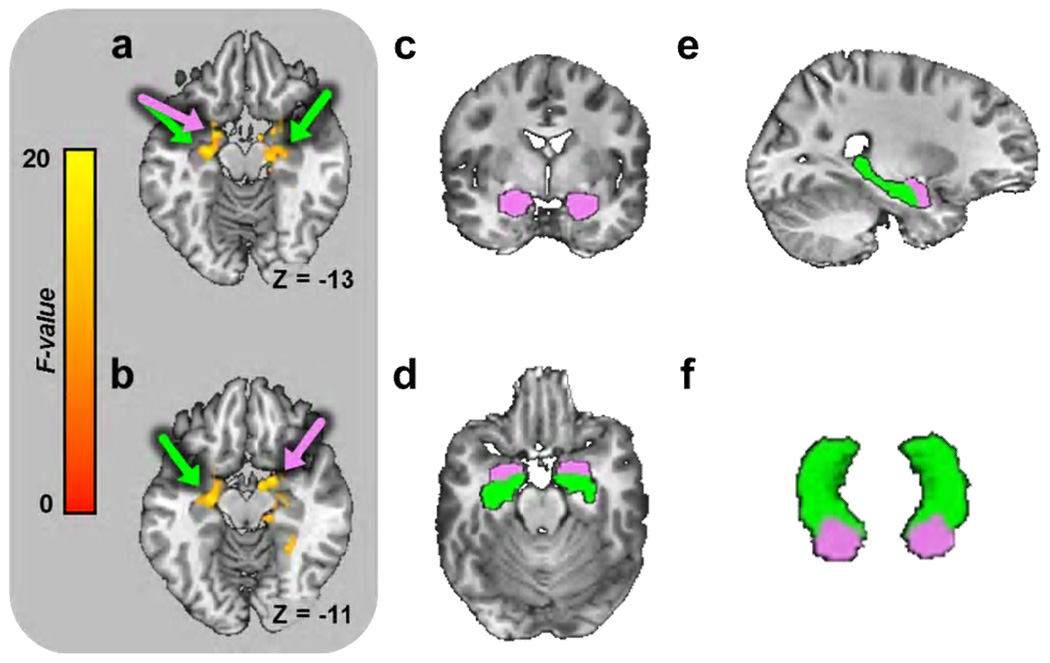
Figure 3. Whole brain BOLD analysis reveals significant main effect for novelty bilaterally in the amygdala and hippocampus.
(a,b) Axial slices showing main effect for novelty in amygdala and hippocampus (Data from Experiment 1). Colors indicate size of F-statistic depicted on brain slice and correspond to the colors on the scale to the left. Arrows indicate areas where clusters overlap with the amygdala (pink) and the hippocampus (green). (c–f) We used automated subcortical segmentation to identify the amygdala (pink) and hippocampus (green) on a subject-by-subject bases. (c–f) Representation of a sample subject’s segmentation in coronal (c), axial (d), sagittal (e), and 3-d views (f).
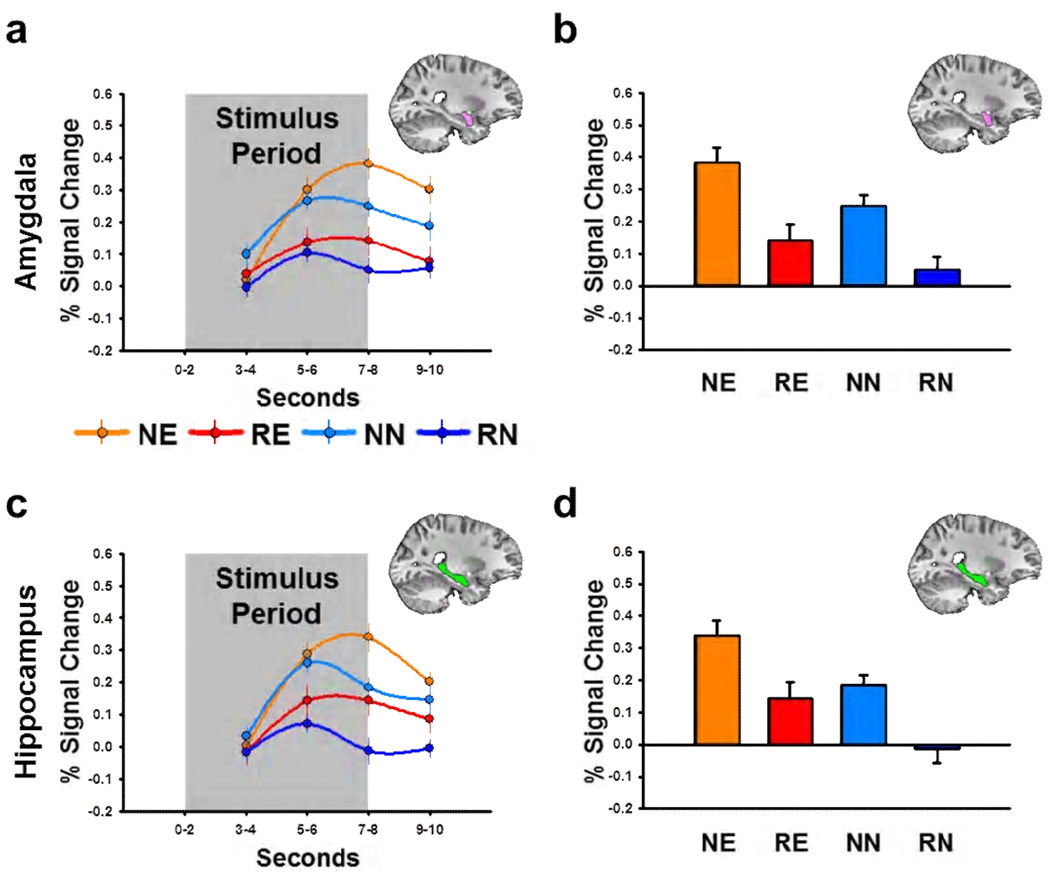
To characterize these effects, we sampled the stimulus evoked BOLD responses within the amygdala and hippocampus. To independently sample activity within these two structures, we created anatomical regions of interest (ROIs; See Figure 3c–f) for the amygdala and hippocampus on a subject-by-subject basis, using an automated subcortical segmentation algorithm (Fischl et al., 2002; Fischl et al., 2004). These automatically generated anatomical ROIs were then manually edited to conform to previously described standards (Morey et al., 2009). We sampled the BOLD data from the final two seconds of the stimulus period (Seconds 7–8), which corresponded to the overall peak of the impulse response functions (See Figure 4a and 4c). We performed a novelty by emotion by laterality ANOVA on these values, with left/right hemisphere as a repeated measure. Consistent with the whole brain analysis, novel stimuli evoked a robust BOLD response in the amygdala and hippocampus whether they are emotional or neutral (Novelty main effect: Amygdala, F(1,76) = 19.71, p = 3.02E-5; Hippocampus, F(1,76) = 15.32, p = 1.97E-4; See Figure 4.). In addition, emotional stimuli evoked more activity than neutral stimuli in both structures (Emotion main effect: Amygdala, F(1,76) = 5.18, p = 0.025; Hippocampus, F(1,76) = 9.61, p =0.003). However, the novelty effects are similar for emotional and neutral stimuli (Novelty × Emotion interaction: Amygdala, F(1,76) = 0.19, p = 0.667; Hippocampus, F(1,76) < 0.01, p = 0.986). Also, this pattern of responses is consistent across hemispheres (Laterality main effect, Amygdala, F(1,76) = 1.24, p = 0.269; Hippocampus, F(1,76) = 0.01, p = 0.92).
Figure 4. Novel emotional and novel neutral stimuli drive BOLD activity in the amygdala and hippocampus.
(a,c) Line graphs represent BOLD timecourse in the amygdala (a) and hippocampus (c) during Experiment 1. (b,d) Bar graphs represent the percent signal change in the amygdala (b) and hippocampus (d) during the last two seconds of the stimulus period. All data points represent mean±SEM. (NE = novel emotional, RE = repeated emotional, NN = novel neutral, RN = repeated neutral)
3.2 Novel faces but not novel scenes activate the amygdala
In Experiment 1, we showed that the amygdala responds to novel stimuli whether they are emotional or neutral. In contrast, several previous novelty studies have failed to observe significant effects for the amygdala (Daselaar et al., 2006; Menon et al., 2000; Tulving et al., 1996; Yamaguchi et al., 2004). This lack of effect may be due to the heterogeneous collection of stimuli used in these previous studies (e.g., words, animals, landscapes, etc.). All of our stimuli depicted humans. Similarly, other studies showing novelty-evoked BOLD responses in the amygdala used images of humans as well (Schwartz et al., 2003; Wright et al., 2003). Thus, the presence of humans/faces in the stimulus may be a key stimulus attribute, necessary for the expression of amygdalar novelty responses, although for a recent counterpoint see Blackford et al. (2010). To test this we presented novel and repeated images of humans and scenes while measuring BOLD activity.
To determine the effects of novelty and picture content on brain activity, we performed a mixed effects ANOVA on BOLD intensity using novelty and picture content as fixed factors and subject as a random factor (See Supplementary Table 2 full list of activations.). In this whole brain BOLD analysis we did not observe a significant novelty by picture content interaction in the amygdala or hippocampus. But because we had a priori hypotheses about the amygdala and hippocampus and because we used an anatomical ROI approach in Experiment 1, continued that approach here.
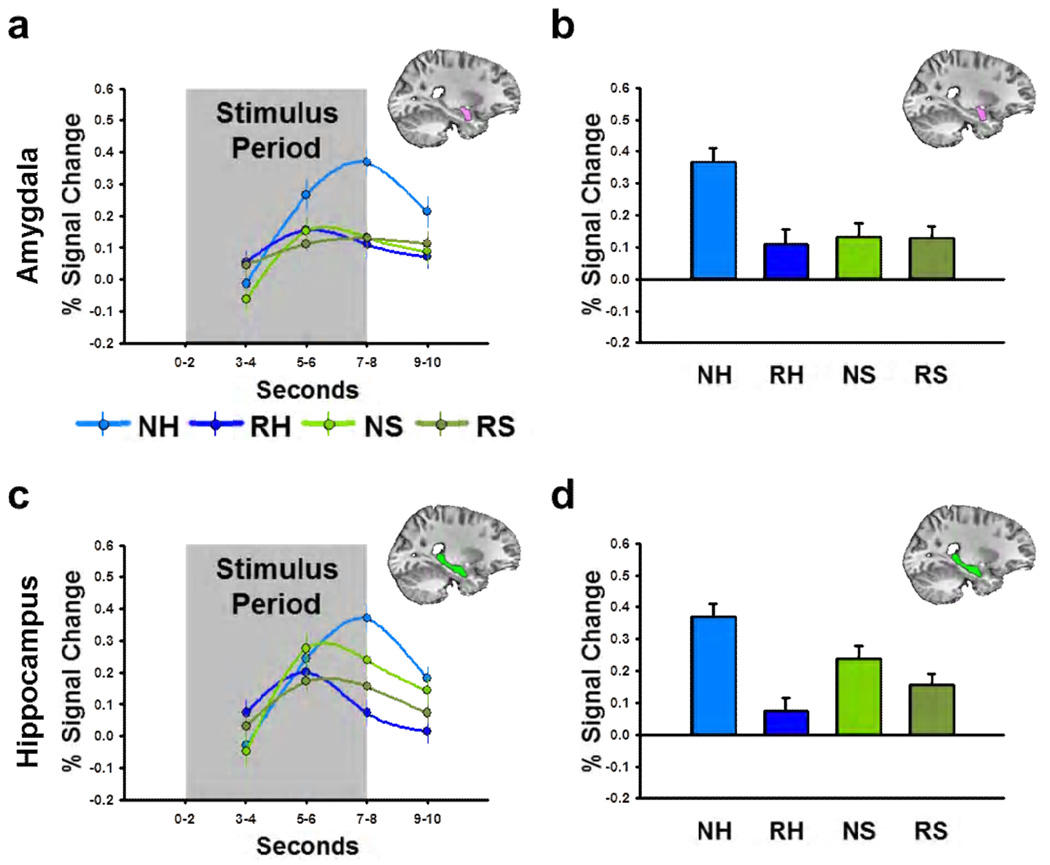
We created anatomical ROIs for the amygdala and hippocampus on a subject-by-subject basis, using the same process as the first experiment. We then sampled the BOLD data within the amygdala and hippocampus using these ROIs (Seconds 7–8; See Figure 5a and 5c). Next we performed a novelty by picture content by laterality ANOVA on these values, with left/right hemisphere as a repeated measure. Figure 5 shows that novel humans evoke larger magnitude BOLD responses in the amygdala than repeated humans but novel scenes evoke similar magnitude BOLD responses as repeated scenes (Novelty × Picture Content interaction: F(1,76) = 5.19, p = 0.026; post hoc Bonferroni-corrected t-tests: Novel vs. Repeated Humans, t(38) = 2.99, p = 0.005; Novel vs. Repeated Scenes, t(38) = 0.04, p = 0.970). In contrast, novel humans and novel scenes both evoke larger magnitude hippocampal responses than repeated humans and scenes (Novelty Main Effect: F(1,76) = 11.75, p = 0.001), although the effect size may be larger for humans (Novelty × Picture Content interaction: F(1,76) = 3.71, p = 0.058). This pattern of responses was consistent across hemispheres for both structures (Laterality Main Effect: Amygdala, F(1,76) = 1.52, p = 0.222; Hippocampus, F(1,76) = 0.01, p = 0.940).
Figure 5. Novel faces but not novel scenes drive amygdala BOLD.
(a,c) Line graphs represent BOLD timecourse in the amygdala (a) and hippocampus (c) during Experiment 2. (b,d) Bar graphs represent the percent signal change in the amygdala (b) and hippocampus (d) during the last two seconds of the stimulus period. All data points represent mean±SEM. (NH = novel human, RH = repeated human, NH = novel scene, RH = repeated scene)
3.3 Neural activity in the amygdala and hippocampus decreases after a single stimulus presentation
The results from these two experiments demonstrate novelty-specific responses in the amygdala and hippocampus, consistent with several previous studies (Schwartz et al., 2003; Wright et al., 2003). Furthermore, they strongly suggest that amygdalar novelty responses are specific to stimuli that contain biologically relevant information, such as images of conspecifics. However, because responses were averaged across several stimulus repetitions, it is difficult to determine how the BOLD responses decrease across repeated stimulus presentations. If the results are driven by novelty, then the magnitude of the BOLD response should not depend on the number of times a given stimulus is repeated. That is, we would expect to see a large initial response followed by an immediate decrease to a stable baseline. If the results are driven by a more gradual process like habituation, then the magnitude of the BOLD response should depend on the number of times a given stimulus is repeated. That is, we would expect to see a large initial response, followed by a gradual decay toward baseline (Groves and Thompson, 1970). Habituation is commonly evoked to explain decreases in the magnitude of amygdala responses, but is rarely tested specifically (Britton et al., 2008; Buchel et al., 1998; Fischer et al., 2003). To distinguish between effects driven by novelty and effects driven by habituation, we reexamined the novelty effect from Experiment 1 while explicitly accounting for the number of stimulus repetitions.
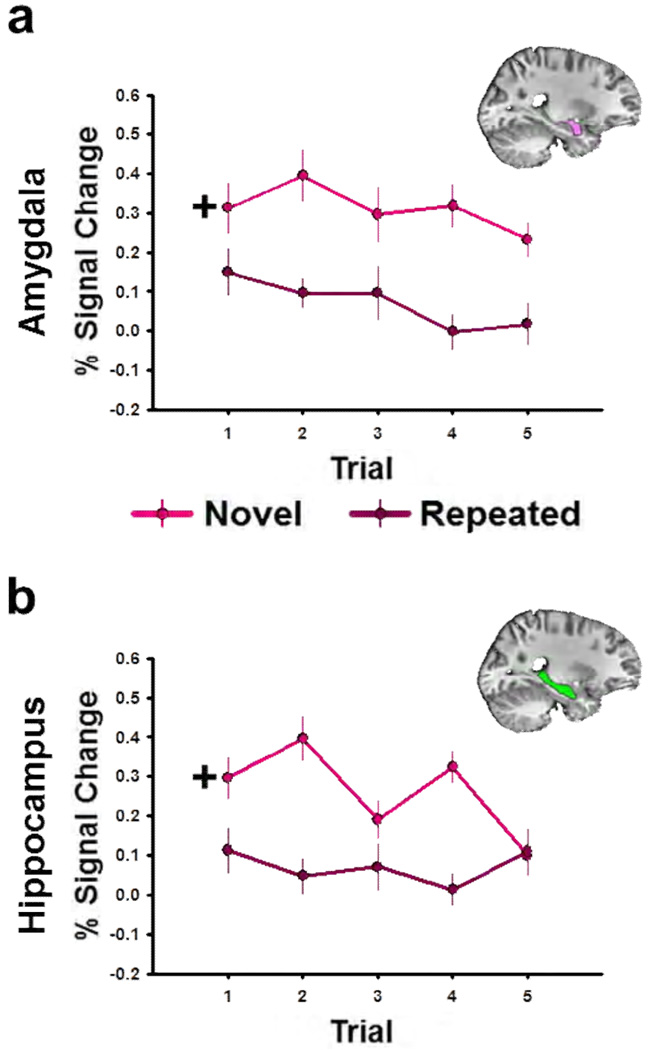
To understand the temporal characteristics of the novelty evoked responses in the amygdala and hippocampus, we reanalyzed the data from the Experiment 1 to determine the BOLD response on each of the trials. Because we did not observe a novelty by emotion interaction in the previous analyses, we collapsed across emotion. Thus we were able to deconvolve the single subject BOLD data with separate canonicals for each trial, using the same least squares procedure as before. This allowed us to determine the effects of trial number on the BOLD response in the amygdala and hippocampus. To do this we performed an ANOVA on the BOLD activity (Seconds 7–8) within the anatomical amygdala and hippocampal ROIs. The most important thing to understand about this analysis is that the initial presentation of the to-be-repeated image is counted as novel. This is important because the initial data point for the repeated condition is actually the second presentation (first repetition) of the repeated image. Again, if our effects are driven by a rapid process, we should see a main effect for novelty but no novelty by trial interaction. If our effects are driven by a more gradual habituation process, we should see a small difference on the beginning trials followed by a larger difference on subsequent trials, which would yield a novelty × trial interaction. Novel stimuli evoke a larger magnitude BOLD response in the amygdala (Novelty main effect: F(1,190) = 29.58, p = 1.63E-7; See Figure 6) and the hippocampus (Novelty main effect: F(1,190) = 26.33, p = 7.08E-7). In both structures the novelty effect is consistent across hemispheres (Laterality main effect: Amygdala, F(1,190) = 1.85, p = 0.176; Hippocampus, F(1,190) = 0.02, p = 0.883) and present even on early trials. In the amygdala this response seems to be consistent across trials (Trial main effect: F(4,190) =1.04, p = 0.390; Novelty × Trial interaction: F(4,190) = 0.47, p = 0.790). However there may be a trend towards habituation to the novel stimuli in the hippocampus (Trial main effect: F(4,190) = 1.03, p = 0.393; Novelty by Trial interaction: F(4,190) = 2.27, p = 0.063).
Figure 6. BOLD responses in the amygdala and hippocampus decrease after single stimulus presentation.
(a,b) Graphs depict BOLD response in the amygdala (a) and hippocampus (b) across trials during Experiment 1. Data points reflect the mean±SEM BOLD response evoked by the novel (dark red) and the repeated (light red) images during each trial
+ Note that for the repeated images, Trial 1 is actually the second image presentation, because on the first presentation the image is novel.
3.4 Humans and scenes evoke BOLD responses in distinct cortical areas
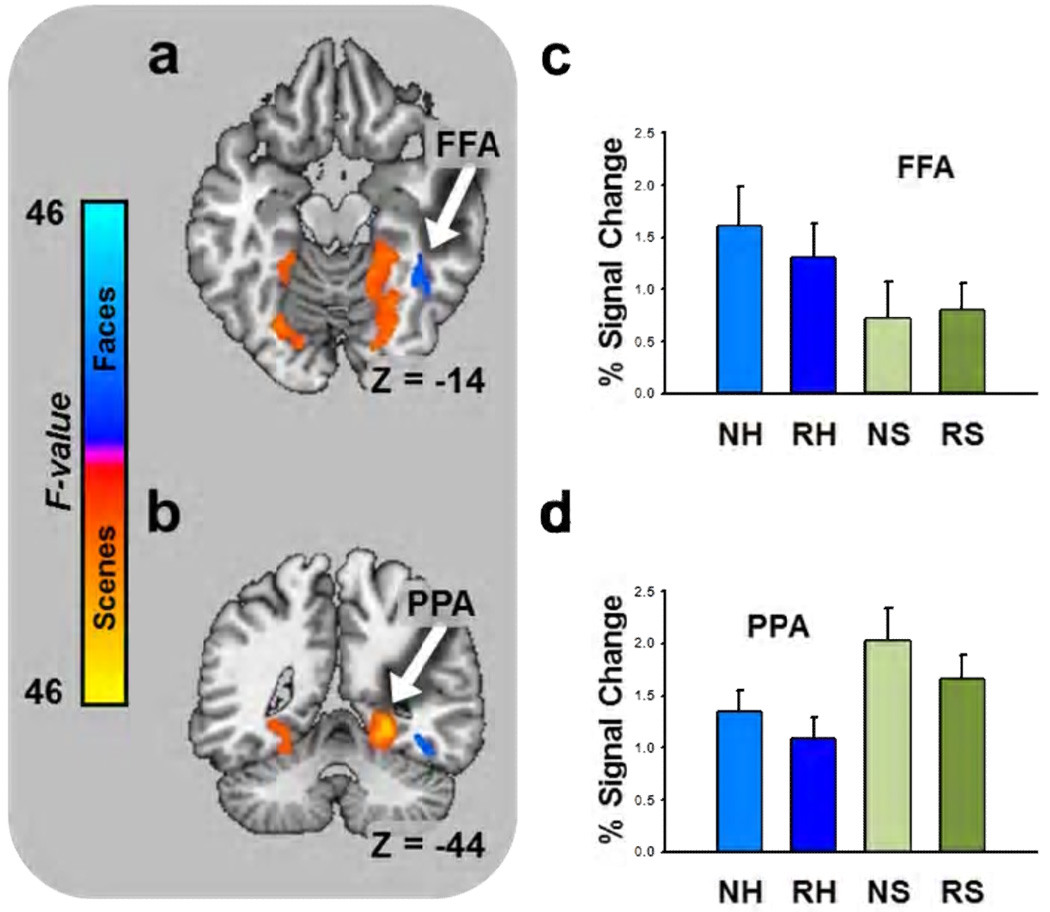
In Experiment 2, we presented images of humans and images of scenes. Based on previous work (Epstein, 2008; Kanwisher and Yovel, 2006), we expected to find activations in areas of the cortex that corresponded to the domains of the different image types. Specifically, we expected to observe larger responses to faces in the fusiform face area (FFA) and larger responses to scenes in the parahippocampal place area (PPA). As a manipulation check we performed an exploratory whole brain mixed effects ANOVA on BOLD intensity with novelty and picture content as fixed factors and subject as a random factor, using the methods from the first experiment. As expected we observed significant activations in FFA and PPA. The pattern of means in these areas is consistent with previous observations. That is, faces evoke larger magnitude BOLD responses than scenes in FFA and scenes evoke larger magnitude BOLD responses than faces in PPA (See Figure 7 and Supplementary Table 2).
Figure 7. Humans and scenes evoke BOLD responses in domain-specific cortical areas.
(a) Axial slice showing main effect for faces in the fusiform face area. (b) Coronal slice showing main effect for scenes in the parahippocampal place area. (a,b) Colors indicate size of F-statistic depicted on brain slice and correspond to the colors on the scale to the left. Bar graphs represent the percent signal change in the structures marked by the arrows (Data from Experiment 2). (c) Faces evoke more activity than scenes in the fusiform face area. (d) Scenes evoke more activity than faces in the parahippocampal place area. (a: FFA = fusiform face area; c: NE = novel emotional, RE = repeated emotional, NN = novel neutral, RN = repeated neutral; b: PPA = parahippocampal place area; d: NH = novel human, RH = repeated human, NH = novel scene, RH = repeated scene).
3.5 Behavioral Results
3.5.1 Shock expectancy
As part of a separate experiment, participants received presentations of an electrical stimulation prior to the data were collected. To ensure that participants were attending to the stimuli, we told them to continuously rate their expectancy of receiving an electrical stimulation using an onscreen visual analog scale.
In the first experiment we performed a novelty by emotion by trial ANOVA on these values, with trial as a repeated measure. Participants indicated that the stimulation was more likely to occur during presentations of the emotional stimuli than during the neutral stimuli (Emotion main effect: F(1,76) = 13.31; p = 0.0005; See Supplementary Figure 1). Participants showed a decrease in expectancy across trials (Trial main effect: F(4,76) = 2.64; p = 0.034), which seemed to be smaller for emotional stimuli (Emotion by Trial interaction: F(4,76) = 2.53; p = 0.041). Results suggest that participants are more likely to expect aversive outcomes when presented with emotional pictures.
In the second experiment we performed a novelty by picture content by trial ANOVA on these values, with trial as a repeated measure. Participants indicated that the stimulation was more likely to occur during presentations of the novel stimuli than during the repeated stimuli (Novelty main effect: F(1,76) = 21.72; p = 1.3E-5). Participants showed a decrease in expectancy across trials (F(4,76) = 3.75; p = 0.005), which seemed to be similar for both stimulus types (F(4,76) = 1.065; p = 0.374).
3.5.2 Skin conductance responses
In the first experiment we performed ANOVAs at each timepoint of the stimulus period using novelty and emotion as factors. We observed a significant novelty by emotion interaction during the second half of the stimulus period (See Supplementary Table 5 and Supplementary Figure 2a). Results suggest that autonomic responses evoked by emotional stimuli habituate with repeated stimulus presentations.
In the second experiment we performed ANOVAs at each timepoint of the stimulus period using novelty and picture content as factors. We observed a significant main effect for novelty during the second half of the experiment (See Supplementary Table 5 and Supplementary Figure 2b). Results suggest that neutral stimuli generally evoke autonomic responses that habituate with repeated stimulus presentations. However, results from the first experiment suggest that neutral stimuli fail to evoke autonomic responses when presented in the same context as emotional stimuli.
4 Discussion - The amygdala plays a stimulus specific role in the detection of novel stimuli
We show that the amygdala is sensitive to stimulus novelty, but only when certain types of stimuli are used. Surprisingly, these findings do not depend on the emotional content of the images. Novel emotional and neutral images of humans each evoke robust amygdala responses. Our findings are original because we show that neutral scenes do not evoke a novelty response in the amygdala. Remarkably, amygdala activity does not seem to gradually habituate with repeated stimulus presentations. Instead, activity diminishes after a single presentation and this difference remains consistent across subsequent trials.
In Experiment 1, we measured the effects of novelty and emotion on amygdala activity and found a clear superiority for novelty. It should be noted that our lack of emotion effect could be due to a lack of power, given the limited number of trials (See below for a more detailed discussion). Even so, the fact that we observe such a robust effect for novelty is surprising because many think that the primary function of the amygdala is to detect and respond to emotional stimuli (Öhman and Mineka, 2001). For example, patients with amygdala lesions fail to recognize fearful facial expressions (Adolphs et al., 1994). These patients also show impairments in social learning and emotional empathy tasks (Hurlemann et al., 2010). Fear-relevant stimuli are also more easily associated with an aversive stimulus (Öhman and Soares, 1993) and capture attention in complex visual arrays (Öhman et al., 2001). In addition to fearful stimuli, the amygdala is also sensitive to other emotional facial expressions (Adolphs, 2008; Britton et al., 2006) such as anger (Whalen et al., 2001) and surprise (Kim et al., 2003). Furthermore, amygdala responses can be elicited by either emotional faces and scenes (Britton et al., 2006; Canli et al., 2000; Hariri et al., 2002; Irwin et al., 1996; Stark et al., 2004) although expressions tend to evoke more activity than scenes (Britton et al., 2006; Hariri et al., 2002).In Experiment 2, we observed an amygdalar novelty effect for images of humans but not scenes, which is consistent with observations that the amygdala receives highly processed visual information (McDonald, 1998) and is sensitive to images of faces (Adolphs, 2008) and body gestures (Hadjikhani and de Gelder, 2003). Also, the amygdala and fusiform gyrus are often coactivated during the presentation of faces (Britton et al., 2008), and lesions of the amygdala decrease face evoked fusiform gyrus activity (Vuilleumier et al., 2004). Lesions of the amygdala impair the ability to recognize fear from a fearful expression, which may stem from an impairment in the ability to orient one’s gaze towards the eyes of a fearful expression (Adolphs et al., 2005). Likewise, amygdala responses in normal individuals predict gaze shifts toward fearful eyes (Gamer and Buchel, 2009), and are larger when individuals view subjects with large pupils (Demos et al., 2008). Amygdala responses are smaller for familiar faces than for unfamiliar faces (Gobbini et al., 2004; Leibenluft et al., 2004). Taken together, these results suggest that the amygdala responds to novel instances of particular classes of stimuli, such as human faces. These results may also help to explain why some studies have reported novelty effects (Daselaar et al., 2006; Grunwald et al., 1998; Knight, 1996) while others failed to report novelty effects in the amygdala (Schwartz et al., 2003; Wright et al., 2003).
Our results however differ from those of a recent study by Blackford and colleagues (2010). These two studies differ because they used stimuli that did not contain a representation of a human. Even so, they show that novel common and uncommon stimuli each activate the amygdala. In addition, they show that novel uncommon stimuli activate the amygdala more than novel common stimuli. In light of these recent findings, it is difficult to conclude that amygdalar novelty responses are exclusive stimuli depicting humans, especially given that to do so would require testing an infinite number of different stimulus types. What we can say from our data is that there are certain types of stimuli that do not evoke such a response, and that more research is needed to determine the key feature or features that distinguish between stimuli that do and stimuli that do not evoke an amygdalar novelty response. One possibility is that Blackford and colleagues used stimuli with strong foreground objects, whereas we used scenes where the focus was on the background content. Future studies should investigate the difference between amygdala novelty responses evoked by foreground and background content.
Another way that our study differs from previous novelty studies is that we used an event related design, which allowed us to determine the response magnitude on each presentation of the novel and repeated stimuli. Because of this we are able to show that novel images of humans evoke a response in the amygdala that diminishes even after a single repetition. Because previous experiments studying novelty-specific amygdala responses used block designs (Blackford et al., 2010; Schwartz et al., 2003; Wright et al., 2003; Wright et al., 2006), this kind of temporal resolution was not possible. These results may also explain why previous experiments show such rapid habituation of amygdala responses (Britton et al., 2008; Buchel et al., 1998; Fischer et al., 2003; Wright et al., 2003). These previous reports of amygdala habituation may be due partially to an initial response to stimulus novelty.
We believe that the novelty effects in the current experiment represent an initial evaluation of human faces for evidence of threat in the environment. In contrast, previous work in our lab employing non-face stimuli in a fear conditioning paradigm has shown that amygdala BOLD arises only on trials where the subject demonstrates a conditioned response (Cheng et al., 2006; Cheng et al., 2003; Cheng et al., 2007). Interestingly, the amygdala BOLD in these two types of studies may arise from different neural processes in the amygdala, possibly from different nuclei (Davis et al., 2010; Goossens et al., 2009). The amygdala receives multimodal sensory inputs via projections to the basolateral nuclei (Sah et al., 2003), which play a crucial role in associating environmental stimuli with biologically significant outcomes (LeDoux, 2000). These structures project to the central nucleus (Sah et al., 2003), which initiates defensive behaviors via projections to the hypothalamus and brainstem (LeDoux, 2000). Interestingly, these separate nuclei may make independent contributions to BOLD activity, which is often classified as generally as “amygdala BOLD.” Activity from the basolateral nuclei may lead to the detection of BOLD responses related to stimulus processing whereas activity of the central nucleus may lead to the detection of BOLD responses related to response expression.
Aspects of these results also replicate previous studies. First, our stimuli evoked more hippocampal activity when novel than when repeated. Consistent with previous research, this suggests that the hippocampus is sensitive to novelty in general. Second, hippocampal responses to novel stimuli showed a trend toward habituation in the first experiment. The hippocampus may be sensitive to the novelty of other aspects of the experimental procedure (Nyberg, 2005), which is consistent with other studies showing second-order novelty effects in the hippocampus (Strange and Dolan, 2001; Yamaguchi et al., 2004). In addition to replicating work on the hippocampus, we also replicate work suggesting that there are cortical areas that selectively process faces (Kanwisher and Yovel, 2006) and scenes (Epstein, 2008).
Our data, consistent with previously published papers, suggest that the amygdala and hippocampus may be doing different things during the presentation of novel stimuli. The hippocampus responds to novelty in general and the amygdala responds to the novelty of certain types of stimuli, such as faces. It is unclear how these brain structures interact when we encounter new people. The amygdala seems to contribute to emotion discrimination by directing attention to the eye region of faces, which is necessary for detection of fear (Adolphs et al., 2005; Gamer and Buchel, 2009). One possibility is that the bulk of this processing occurs on the initial stimulus presentation, and is triggered by novelty inputs from the hippocampus. According to this hypothesis, novelty signals from the hippocampus and facial-recognition signals from higher level visual processing areas converge on the amygdala, and this convergence of information triggers the increase in amygdala activity that we observed. Although we did not directly test this hypothesis, it is supported by our data and can be tested by measuring the functional connectivity between the amygdala and hippocampus during novel and repeated presentations of faces.
The presence of a human in a novel image is sufficient to evoke a novelty-specific amygdala response, and emotional content is not necessary for these responses. However, it is currently unclear whether emotional content is sufficient to evoke such responses when the novel image does not depict a human subject. A recent study by Larson and colleagues found that novel spider images evoke larger magnitude amygdala responses than novel neutral images (Larson et al., 2006), however without a condition where the images are repeated, it is difficult to tell whether this effect is driven by novelty. In addition, a study by Weierich and colleagues (2010) suggests that novel positive and negative images are able to evoke amygdalar novelty responses; however, without explicitly controlling for presence of a human representation, it is difficult to determine whether emotion in general is sufficient to evoke an amygdalar novelty response.
Again, our results suggest that novel neutral faces but not scenes activate the amygdala, but we are far from understanding what is special about faces. According to some, the amygdala has evolved to rapidly and automatically detect stimuli that have historically signalled threat in the environment (Isbell, 2006; Öhman and Mineka, 2001). Like faces, images of threatening animals like snakes and spiders also meet this criterion. Research suggesting that the amygdala responds to novel snakes or spiders would support this evolutionary hypothesis.
It’s also unclear which aspects of human representations are necessary and sufficient to evoke novelty responses in the amygdala. The amygdala is sensitive to emotional facial expressions (Adolphs, 2008; Britton et al., 2006; Kim et al., 2003; Whalen et al., 2001) and lesions to the amygdala impair patients’ ability to direct their gaze toward the eye regions of photographs (Adolphs et al., 2005). Therefore, images of just faces or more specifically images of just the eye region of the face may be sufficient to evoke an amygdalar novelty response. However, emotional body gestures also evoke amygdala activity (Hadjikhani and de Gelder, 2003), suggesting that images of bodies may be sufficient to evoke an amygdala novelty response. Finally, our results suggest that the amygdala responds to the novelty of faces independent of emotional content; however our study did not include happy faces. Given that neutral faces can be perceived as more ambiguous and evoke more amygdala activity than happy faces (Kukolja et al., 2008), it is possible that happy faces may not evoke an amygdala novelty response.
Amygdalar novelty responses are larger in individuals with inhibited temperament (Schwartz et al., 2003). However, it is currently unclear how they relate to other behaviors previously associated with amygdala activity. For instance, there is often a high correspondence between skin conductance responses and amygdala activity (Cheng et al., 2003; Williams et al., 2001). In our current experiments we measured skin conductance responses, but the patterns did not correspond to the amygdala data (See Supplementary Figure 2).
One limitation to this study is that the original experiment was part of another separate fear conditioning experiment. As a result, subjects were exposed to an aversive electrical stimulation prior to their fMRI scan. Because of this exposure, it is difficult to say whether these results are generalizable to contexts where the aversive stimulation is not present. This is an empirical question that needs to be answered with additional studies. However, there are several reasons why we believe that these results are not dependent upon previous exposure to an aversive electrical stimulation. First, the basic novelty effect has been demonstrated in studies where an aversive electrical stimulation was never presented (Schwartz et al., 2003; Wright et al., 2003; Wright et al., 2006). Our study adds to this literature by showing that the amygdalar novelty response is not evoked by all stimulus types. Given that uncertainty about aversive outcomes has been shown to upregulate amygdala activity (Sarinopoulos et al., 2010), it is unlikely that the exposure to electrical stimulation prior to the experiment accounts for the specificity of amygdalar novelty responses that we show here. In addition, the pattern of shock expectancy in these experiments did not correspond to the pattern of amygdala BOLD, suggesting that differences in shock expectancy across stimulus types are not sufficient to explain our findings. First, individuals in Experiment 1 generally believed the shock was more likely during emotional stimuli; however these individuals showed larger BOLD responses to novel emotional and neutral stimuli. Second, individuals believed that the shock was more likely on earlier trials, but our novelty effect in the amygdala was consistent across trials. Finally, subjects in Experiment 2 expected the shock more during novel stimuli than repeated stimuli generally, but showed larger amygdala responses only to the novel faces.
Another limitation to our experiment is that we included only 5 trials for each stimulus types. Therefore, our marginal emotion effect in Experiment 1, and our lack of a novelty effect for scenes in Experiment 2 may have been due to type II error. With regard to emotion, this is a valid concern. We originally expected an effect for emotion in Experiment 1, and the pattern of amygdala activity shows marginally more activity for emotional stimuli. There is no doubt that amygdala activity contributes emotional processing, and that our design is not completely sensitive to this activity. However, given that we see such a striking effect for novelty at this level of sensitivity, our results suggest that novelty is a powerful driver of amygdala activity. With regard to scenes, it is unlikely that our lack of a novelty effect is due to a lack of sensitivity. If this were the case, there should have been marginally larger responses to novel scenes coupled with a large amount of error. Instead amygdala responses are roughly equivalent for novel and repeated scenes, and significantly smaller than amygdala responses to novel humans.
The novelty/encoding hypothesis suggests that novelty processing modulates encoding by prioritizing the to-be-encoded information (Tulving et al., 1996). It is unclear whether amygdala novelty responses facilitate encoding. In Experiment 2 we attempted to address this question by measuring subsequent memory for the test stimuli, but the small number of stimuli made recognition too easy and participants performed at ceiling levels. However, by identifying stimulus sets that both evoke and fail to evoke amygdala novelty responses, our results provide a tool for subsequent researchers to address this question.
Our difficulty finding behavioral outputs that correspond to amygdalar novelty responses further suggest that these responses represent a sensory evaluation of the stimuli. We believe that the primary function of the amygdala is to allow the individual to rapidly initiate defensive behaviors in dangerous situations, but in order to do so the amygdala must keep the organism vigilant by allocating attentional resources to stimuli that potentially signal biologically significant outcomes (Davis and Whalen, 2001; Holland and Gallagher, 1999).
Faces offer a snapshot of the outside world as viewed through the eyes of another, which can alert an individual to biologically significant events that may have otherwise gone unperceived (Whalen, 2007). We believe that all novel faces, independent of emotional content, engage the basolateral nuclei of the amygdala, which evaluate the face and engage the central nucleus if and only if the face signals a biologically significant outcome.
Supplementary Material
Figure 2. In Experiment 2 we presented images of humans and scenes while measuring BOLD activity.
(a) As in Experiment 1, Images were presented sequentially in an event related design. (b,c)The images are representative of the human images (b) and scene images (c) shown to participants. All participants saw 5 presentations of novel human (NH) and 5 presentations of novel scene (NS) images, which are indicated by the light blue and light green outlines, respectively. In addition all saw 5 repetitions of one human (RH) and one scene (RS) image, shown with dark blue and dark green outlines, respectively.
+The initial presentation of the repeated stimuli was counted as novel.
Acknowledgements
National Institute of Mental Health MH060668 and MH069558.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations. novel emotional image (NE); novel neutral image (NN); repeated emotional image (RE); repeated neutral image (RN); novel human image (NH); novel scene image (NS); repeated human image (RH); repeated scene image (RS); International Affective Picture System (IAPS); intertrial interval (ITI); skin conductance level (SCL); skin conductance response (SCR); region of interest (ROI); fusiform face area (FFA); parahippocampal place area (PPA)
References
- Adolphs R. Fear, faces, and the human amygdala. Curr Opin Neurobiol. 2008;18:166–172. doi: 10.1016/j.conb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Blackford JU, Buckholtz JW, Avery SN, Zald DH. A unique role for the human amygdala in novelty detection. Neuroimage. 2010;50:1188–1193. doi: 10.1016/j.neuroimage.2009.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Shin LM, Barrett LF, Rauch SL, Wright CI. Amygdala and fusiform gyrus temporal dynamics: responses to negative facial expressions. BMC Neurosci. 2008;9:44. [Google Scholar]
- Britton JC, Taylor SF, Sudheimer KD, Liberzon I. Facial expressions and complex IAPS pictures: common and differential networks. Neuroimage. 2006;31:906–919. doi: 10.1016/j.neuroimage.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. J Neurosci. 2000;20:RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Helmstetter FJ. Human amygdala activity during the expression of fear responses. Behav Neurosci. 2006;120:1187–1195. doi: 10.1037/0735-7044.120.5.1187. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human amygdala activity during Pavlovian fear conditioning: stimulus processing versus response expression. Behav Neurosci. 2003;117:3–10. doi: 10.1037//0735-7044.117.1.3. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Richards J, Helmstetter FJ. Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learn Mem. 2007;14:485–490. doi: 10.1101/lm.632007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: recollection, familiarity, and novelty. J Neurophysiol. 2006;96:1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Davis FC, Johnstone T, Mazzulla EC, Oler JA, Whalen PJ. Regional response differences across the human amygdaloid complex during social conditioning. Cereb Cortex. 2010;20:612–621. doi: 10.1093/cercor/bhp126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Demos KE, Kelley WM, Ryan SL, Davis FC, Whalen PJ. Human amygdala sensitivity to the pupil size of others. Cereb Cortex. 2008;18:2729–2734. doi: 10.1093/cercor/bhn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn Sci. 2008;12:388–396. doi: 10.1016/j.tics.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Wright CI, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Brain habituation during repeated exposure to fearful and neutral faces: a functional MRI study. Brain Res Bull. 2003;59:387–392. doi: 10.1016/s0361-9230(02)00940-1. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe Aj, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23 Suppl 1:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gamer M, Buchel C. Amygdala activation predicts gaze toward fearful eyes. J Neurosci. 2009;29:9123–9126. doi: 10.1523/JNEUROSCI.1883-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbini MI, Leibenluft E, Santiago N, Haxby JV. Social and emotional attachment in the neural representation of faces. Neuroimage. 2004;22:1628–1635. doi: 10.1016/j.neuroimage.2004.03.049. [DOI] [PubMed] [Google Scholar]
- Goossens L, Kukolja J, Onur OA, Fink GR, Maier W, Griez E, Schruers K, Hurlemann R. Selective processing of social stimuli in the superficial amygdala. Hum Brain Mapp. 2009;30:3332–3338. doi: 10.1002/hbm.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: a dual-process theory. Psychol Rev. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Grunwald T, Lehnertz K, Heinze HJ, Helmstaedter C, Elger CE. Verbal novelty detection within the human hippocampus proper. Proc Natl Acad Sci U S A. 1998;95:3193–3197. doi: 10.1073/pnas.95.6.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, de Gelder B. Seeing Fearful Body Expressions Activates the Fusiform Cortex and Amygdala. Current Biology. 2003;13:2201–2205. doi: 10.1016/j.cub.2003.11.049. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, Dziobek I, Gallinat J, Wagner M, Maier W, Kendrick KM. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin W, Davidson RJ, Lowe MJ, Mock BJ, Sorenson JA, Turski PA. Human amygdala activation detected with echo-planar functional magnetic resonance imaging. Neuroreport. 1996;7:1765–1769. doi: 10.1097/00001756-199607290-00014. [DOI] [PubMed] [Google Scholar]
- Isbell LA. Snakes as agents of evolutionary change in primate brains. J Hum Evol. 2006;51:1–35. doi: 10.1016/j.jhevol.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14:2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- Knight R. Contribution of human hippocampal region to novelty detection. Nature. 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Kukolja J, Schlapfer TE, Keysers C, Klingmuller D, Maier W, Fink GR, Hurlemann R. Modeling a negative response bias in the human amygdala by noradrenergic-glucocorticoid interactions. J Neurosci. 2008;28:12868–12876. doi: 10.1523/JNEUROSCI.3592-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Digitized photographs, instruction manual and affective ratings. Gainesville, FL: University of Florida; 2005. [Google Scholar]
- Larson CL, Schaefer HS, Siegle GJ, Jackson CAB, Anderle MJ, Davidson RJ. Fear Is Fast in Phobic Individuals: Amygdala Activation in Response to Fear-Relevant Stimuli. Biol Psychiatry. 2006;60:410–417. doi: 10.1016/j.biopsych.2006.03.079. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers' neural activation in response to pictures of their children and other children. Biol Psychiatry. 2004;56:225–232. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Menon V, White CD, Eliez S, Glover GH, Reiss AL. Analysis of a distributed neural system involved in spatial information, novelty, and memory processing. Hum Brain Mapp. 2000;11:117–129. doi: 10.1002/1097-0193(200010)11:2<117::AID-HBM50>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Pannu Hayes J, Wagner Ii HR, Lewis DV, LaBar KS, Styner M, McCarthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45:855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L. Any novelty in hippocampal formation and memory? Curr Opin Neurol. 2005;18:424–428. doi: 10.1097/01.wco.0000168080.99730.1c. [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: detecting the snake in the grass. J Exp Psychol Gen. 2001;130:466–478. doi: 10.1037//0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychol Rev. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Öhman A, Soares JJ. On the automatic nature of phobic fear: conditioned electrodermal responses to masked fear-relevant stimuli. J Abnorm Psychol. 1993;102:121–132. doi: 10.1037//0021-843x.102.1.121. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sarinopoulos I, Grupe DW, Mackiewicz KL, Herrington JD, Lor M, Steege EE, Nitschke JB. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cereb Cortex. 2010;20:929–940. doi: 10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants "grown up": adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Stark R, Schienle A, Walter B, Kirsch P, Blecker C, Ott U, Schafer A, Sammer G, Zimmermann M, Vaitl D. Hemodynamic effects of negative emotional pictures - a test-retest analysis. Neuropsychobiology. 2004;50:108–118. doi: 10.1159/000077948. [DOI] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ. Adaptive anterior hippocampal responses to oddball stimuli. Hippocampus. 2001;11:690–698. doi: 10.1002/hipo.1084. [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ, Craik FE, Habib R, Houle S. Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cerebral Cortex. 1996;6:71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Weierich MR, Wright CI, Negreira A, Dickerson BC, Barrett LF. Novelty as a dimension in the affective brain. Neuroimage. 2010;49:2871–2878. doi: 10.1016/j.neuroimage.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ. The uncertainty of it all. Trends Cogn Sci. 2007;11:499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Williams LM, Phillips ML, Brammer MJ, Skerrett D, Lagopoulos J, Rennie C, Bahramali H, Olivieri G, David AS, Peduto A, Gordon E. Arousal dissociates amygdala and hippocampal fear responses: evidence from simultaneous fMRI and skin conductance recording. Neuroimage. 2001;14:1070–1079. doi: 10.1006/nimg.2001.0904. [DOI] [PubMed] [Google Scholar]
- Wright CI, Martis B, Schwartz CE, Shin LM, Fischer H, McMullin K, Rauch SL. Novelty responses and differential effects of order in the amygdala, substantia innominata, and inferior temporal cortex. Neuroimage. 2003;18:660–669. doi: 10.1016/s1053-8119(02)00037-x. [DOI] [PubMed] [Google Scholar]
- Wright CI, Wedig MM, Williams D, Rauch SL, Albert MS. Novel fearful faces activate the amygdala in healthy young and elderly adults. Neurobiology of Aging. 2006;27:361–374. doi: 10.1016/j.neurobiolaging.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Hale LA, D'Esposito M, Knight RT. Rapid prefrontal-hippocampal habituation to novel events. J Neurosci. 2004;24:5356–5363. doi: 10.1523/JNEUROSCI.4587-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.